- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Biofilm and nitrogen fixation are two competitive strategies used by many plant-associated bacteria; however, the mechanisms underlying the formation of nitrogen-fixing biofilms
remain largely unknown. Here, we examined the roles of multiple signalling systems in the regulation of biofilm formation by root-associated diazotrophic _P. stutzeri_ A1501. Physiological
analysis, construction of mutant strains and microscale thermophoresis experiments showed that RpoN is a regulatory hub coupling nitrogen fixation and biofilm formation by directly
activating the transcription of _pslA_, a major gene involved in the synthesis of the Psl exopolysaccharide component of the biofilm matrix and _nifA_, the transcriptional activator of _nif_
gene expression. Genetic complementation studies and determination of the copy number of transcripts by droplet digital PCR confirmed that the regulatory ncRNA RsmZ serves as a signal
amplifier to trigger biofilm formation by sequestering the translational repressor protein RsmA away from _pslA_ and _sadC_ mRNAs, the latter of which encodes a diguanylate cyclase that
synthesises c-di-GMP. Moreover, RpoS exerts a braking effect on biofilm formation by transcriptionally downregulating RsmZ expression, while RpoS expression is repressed
posttranscriptionally by RsmA. These findings provide mechanistic insights into how the Rpo/Gac/Rsm regulatory networks fine-tune nitrogen-fixing biofilm formation in response to the
availability of nutrients. SIMILAR CONTENT BEING VIEWED BY OTHERS REGULATION OF EXTRACELLULAR MATRIX COMPONENTS BY AMRZ IS MEDIATED BY C-DI-GMP IN _PSEUDOMONAS OGARAE_ F113 Article Open
access 13 July 2022 PHENOTYPIC PLASTICITY AND A NEW SMALL MOLECULE ARE INVOLVED IN A FUNGAL-BACTERIAL INTERACTION Article Open access 28 September 2021 THE WSP CHEMOSENSORY SYSTEM MODULATES
C-DI-GMP-DEPENDENT BIOFILM FORMATION BY INTEGRATING DSF QUORUM SENSING THROUGH THE WSPR-RPFG COMPLEX IN _LYSOBACTER_ Article Open access 16 December 2022 INTRODUCTION The term ‘biofilm’ can
be defined as a community of microbes adhering to biotic or abiotic surfaces that is protected from environmental stresses by a self-produced extracellular matrix1,2. The extracellular
matrix, often referred to as extracellular polymeric substances, is composed of exopolysaccharides, proteins and extracellular DNA present in various concentrations depending on the
bacterial species3,4. The biofilm state provides potential advantages over the planktonic state, including increased resistance to antimicrobial agents, protection from environmental
stresses, and improved adaptation to nutrient deprivation5. Numerous investigations in recent decades have demonstrated that bacterial biofilm formation is a sequential process governed by
complex regulatory networks that differ from one bacterial species to another1,6. It is now well accepted that microbial biofilms are the most widely distributed and predominant mode of life
on Earth, influencing our lives tremendously in both positive and negative ways6,7,8,9. In general, as established in the model bacterium _Pseudomonas aeruginosa_, biofilm development
usually begins with attachment to a surface, followed by microcolony formation and production of the extracellular matrix responsible for the biofilm architecture10,11,12,13,14. Biofilm
formation has been studied intensively in the genus _Pseudomonas_, with an emphasis on genetic elements and molecular mechanisms; Gac/Rsm, c-di-GMP signalling and quorum-sensing (QS)
pathways were reported as the main mechanisms leading to biofilm formation15,16. The Gac/Rsm signalling pathway involves the GacS/GacA two-component regulatory system, the RNA-binding
protein RmsA, and its cognate regulatory non-coding RNAs (ncRNAs)17,18. The GacS/GacA two-component system activates the transcription of one or several genes for Rsm ncRNAs, which contain
multiple GGA motifs in exposed stem loops of their predicted secondary structures19. The GGA motifs allow Rsm ncRNAs to bind the RNA-binding proteins that act as global posttranscriptional
repressors, e.g., CsrA (in _Escherichia coli_) and RsmA (in _P. aeruginosa_), controlling important cellular processes, such as secondary metabolism (e.g., metabolism of pyocyanine or the QS
signal N-butyryl-homoserine lactone in _P. aeruginosa_), motility, and biofilm formation17,20. RsmA specifically recognises and binds to conserved GGA motifs in the 5′-untranslated region
(5′-UTR) of target mRNAs, thereby preventing ribosome access and protein translation17,21. RsmA controls biofilm formation through direct repression of various target genes, such as _pslA_
(involved in the synthesis of the exopolysaccharide Psl) and _sadC_ (involved in c-di-GMP synthesis)22,23. As a key biofilm regulatory molecule, the second messenger c-di-GMP is synthesised
by diguanylate cyclases (DGCs) that bear a GGDEF domain and is degraded by phosphodiesterases (PDEs) that harbour EAL or HD-GYP domains. _P. aeruginosa_ encodes several DGCs and PDEs; for
example, WspR/SadC/RoeA (DGC) and RocR/BifA (PDE), are absent in the _P. stutzeri_ A1501 genome, except for SadC and BifA, which modulate the level of c-di-GMP and influence
‘surface-associated behaviours’ by controlling polysaccharide syntheses16,24,25,26,27. The _P. aeruginosa_ biofilm matrix contains several polysaccharide components, including alginate,
pellicle (Pel) and Psl exopolysaccharides28. It has been shown that _pslA_ is the first gene in the _psl_ operon, which comprises 15 cotranscribed genes that are involved in the synthesis of
Psl29. Although current data relating to the roles of Psl are limited, Psl is a critical component of the _P. aeruginosa_ biofilm matrix, which functions as a scaffold, holding biofilm
cells together to initiate biofilm development30. In addition, evidence demonstrates that biofilm formation is controlled positively by RpoN but negatively by RpoS, suggesting global
antagonism between RpoN and RpoS, although there are contradictory reports31,32,33,34,35. Microbial biofilms are common on plant surfaces and have been associated with phytopathogenic
infections and colonisation by nitrogen-fixing rhizobacteria36,37. Because of dynamically fluctuating conditions in the rhizosphere, the ability of diazotrophic bacteria to form
nitrogen-fixing biofilms may confer many ecological advantages and thereby facilitate their physiological and metabolic adaptation to successfully survive in the rhizosphere, a
nitrogen-limited environment. An early study compared biofilm formation by a nitrogen-fixing strain of _Klebsiella pneumoniae_ with that of two other members of Enterobacteriaceae,
_Salmonella enteritidis_ and _E. coli_, and showed that the nitrogen-fixing strain formed the densest and most metabolically active biofilms38. Many nitrogen-fixing bacteria, such as those
of the genera _Rhizobium_, _Gluconacetobacter_ and _Azospirillum_, produce biofilms containing various exopolysaccharides39,40,41,42. For instance, _Sinorhizobium meliloti_ produces two
symbiosis-promoting exopolysaccharides, succinoglycan and galactoglucan, which function in host specificity and participate in early stages of a host plant infection, biofilm formation, and,
most importantly, protection from environmental stresses43,44,45. _Azospirillum_ cells are also capable of forming biofilms on both abiotic surfaces and in association with host plants46.
Previous studies have demonstrated that two response regulator proteins, TyrR and FlcA, were found to be involved in the transcriptional regulation of biofilm formation by _A. brasilense_
Sp7 via the production of capsular polysaccharides42,47. The root-associated bacterium _P. stutzeri_ A1501 is a rare example of a _Pseudomonas_ strain with nitrogen fixation ability48. _P.
stutzeri_ A1501 can survive in the soil, colonise the root surface, and endophytically invade the root tissues of host plants. During evolution, A1501 acquired a nitrogen fixation island
with a _nif_-specific regulatory system from a diazotrophic common ancestor48. Similar to many other _Pseudomonas_ species, the nitrogen regulatory cascade in A1501 comprises the
AmtB–GlnK–NtrBC-RpoN global nitrogen regulation proteins and a set of regulatory ncRNAs that control the expression of _nif_ genes and the consequent optimal nitrogen fixation in response to
nutrient stress49,50,51,52. Comparative genomics analysis showed that A1501 does not possess the well-known QS systems and does not produce alginate, but it contains genes possibly involved
in cellulose biosynthesis and an incomplete _psl_ operon4,48. It was previously shown that a nonpolar mutation of the _fleQ_ gene, encoding FleQ (the main regulator of flagella synthesis),
impaired motility and root colonisation but enhanced biofilm formation by _P. stutzeri_ A150153. Additionally, Wang et al. investigated the effect of physiological conditions on the
formation and architecture of nitrogen-fixing biofilms by _P. stutzeri_ A150141. However, the composition of the polysaccharide matrix remains unknown. To date, studies on biofilm formation
by nitrogen-fixing rhizobacteria have focused on ecological, physiological and architectural analyses. Despite its importance to microbial adaptation and survival, there is surprisingly
little information about the genetics of nitrogen-fixing biofilm formation. In this work, physiological conditions leading to nitrogen-fixing biofilm formation by the root-associated
bacterium _P. stutzeri_ A1501 were further investigated. We found that conditions favouring biofilm formation differ between diazotrophic and non-diazotrophic _P. stutzeri_ strains, although
both strains contain the same set of regulatory genes involved in biofilm formation in other systems. Thus, we systematically characterised genetic elements and molecular mechanisms
involved in nitrogen-fixing biofilm formation. Genome-wide identification of putative genes involved in biofilm formation and mutant construction led to the identification of a complex
regulatory circuitry involving the alternative sigma factors RpoN and RpoS and the Gac/Rsm regulators, and to the proposal of a model that integrates multiple levels of positive and negative
regulation. RESULTS EFFECT OF CARBON AND NITROGEN SOURCES ON BIOFILM FORMATION AND BIOFILM-BASED NITROGENASE ACTIVITY It was previously shown that when lactate was the sole carbon source,
_P. stutzeri_ A1501 tended to form biofilms rather than maintain a planktonic state under nitrogen-deficient conditions41. To further examine this behaviour, the ability of A1501 to form
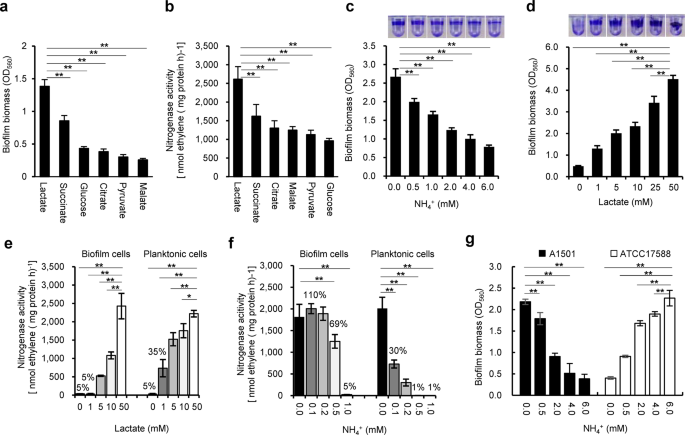
mature biofilms and fix nitrogen was assayed 48 h after inoculation using carbon sources other than lactate and different concentrations of NH4Cl. Among the carbon substrates tested at 50
mM, lactate was the best for both biofilm formation and nitrogen fixation (Fig. 1a, b). The ability of A1501 to form biofilms gradually decreased with increasing NH4+ concentration (Fig. 1c)
but was enhanced with increasing lactate concentration (Fig. 1d). In addition, ~35% of the maximum nitrogenase activity was observed in planktonic growth at a low lactate concentration (1.0
mM), but very low nitrogenase activity was detected in biofilm growth (Fig. 1e), indicating that biofilm cells were incapable of fixing nitrogen unless supplied with an adequately available
carbon source. These results indicated that nitrogen-fixing biofilm growth requires a sufficient supply of carbon sources, as both biofilm formation and nitrogen fixation are energetically
expensive and highly regulated processes16,54. In general, the expression of nitrogenase genes can be completely inhibited by small extracellular concentrations of NH4Cl49,54. The
nitrogenase activity of mature biofilms incubated without NH4+ was determined after the addition of different concentrations of NH4Cl (0.1 to 0.5 mM). As shown in Fig. 1f, the detectable
nitrogenase activity of the planktonic cells was limited in response to the addition of 0.1 to 0.2 mM NH4+. The nitrogenase activity of biofilm cells was much higher than that of planktonic
cells at low NH4+ concentrations (0.1 to 0.2 mM NH4+). In addition, 0.5 mM NH4+ caused a small reduction (31%) in nitrogenase activity of biofilm cells but almost total loss of that of
planktonic cells. This result is in good agreement with the gene expression data obtained from planktonic and biofilm cells treated with different concentrations of NH4+ (Supplementary Fig.
1). A1501 biofilms were previously reported to fix nitrogen in the presence of oxygen, suggesting that the matrix could be a barrier for oxygen diffusion41. It also appears that the matrix
could limit the inhibitory effect of NH4+ on enzyme synthesis and activity. FUNCTIONAL IDENTIFICATION OF GENETIC ELEMENTS GOVERNING BIOFILM FORMATION IN A1501 The A1501 genome contains a set
of nine genes (namely, _gacA_, _rsmA_, _rsmY_, _rsmZ_, _sadC_, _bifA_, _pslA_, _rpoN,_ and _rpoS_ (Fig. 2a)), which are present in other _Pseudomonas_ species and encode proteins and ncRNAs
known to play roles in biofilm formation16 (Fig. 2b). As a working hypothesis, we suggest that 1501 may use regulatory mechanisms such as the c-di-GMP signalling and Gac/Rsm pathways common
to other _Pseudomonas_ species for biofilm formation. To gain insights into the potential roles of the nine genes in nitrogen-fixing biofilm formation, we monitored the gene transcription
levels under nitrogen fixation conditions. In nitrogen-fixing biofilm cells, the expression of _rsmZ_ showed the most dramatic increase (>120-fold), followed by that of _rpoS_ (~5.0-fold)
compared with their expression in planktonic state cells (Fig. 2c). In particular, the relative expression of most of these genes was superior in nitrogen-fixing biofilms than in planktonic
biofilms (Fig. 2c). Indeed, the expression of genes specific for nitrogen fixation that were used as controls, such as _nifH_ and _nifA_, was increased by ~35-fold and ~4.0-fold,
respectively, in nitrogen-fixing biofilm cells compared to non-nitrogen-fixing biofilm cells (Fig. 2d). To establish whether these genes play a role in biofilm formation, we constructed a
set of mutant strains and corresponding strains containing complementing plasmids or overexpressing the gene. We found that mutations in most of the selected genes affect biofilm formation
by either decreasing or increasing biofilm formation (Table 1). These effects were not due to differences in planktonic growth, as the corresponding mutant strains displayed similar growth
as wild-type (WT) A1501 in minimal medium K containing 20 mM NH4+ and 50 mM lactate (Table 1). It should be noted that mutation of the Gac/Rsm pathway genes, such as _gacA_, _rsmZ_ and
_RsmY_, resulted in a partial but not total loss of nitrogen-fixing biofilm production (Table 1), suggesting the involvement of additional regulatory pathways. This assumption was further
confirmed by measuring the biofilm phenotypes of the strains lacking either _rpoN_ or _pslA_. As shown in Supplementary Table 1, an _rpoN_ mutation caused an almost total loss of biofilm
production under nitrogen fixation conditions; a similar phenotype was observed in a mutant lacking the _pslA_ gene, indicating that both RpoN and PslA are essential for nitrogen-fixing
biofilm formation in A1501. Interestingly, the nine genes cited above (shown in Fig. 2b) are present in _P. stutzeri_ ATCC17588, a non-diazotrophic strain isolated from a clinical specimen.
However, when we examined the effect of nitrogen availability on biofilm formation by this strain, we found that its physiological conditions favouring biofilm formation differ from those
found in A1501. Under nitrogen-sufficient conditions, the ability of this strain to form biofilms gradually increased with increasing NH4+ concentration, in contrast to the diazotrophic
strain A1501, which tended to form biofilms under nitrogen-deficient conditions (Fig. 1g). A reasonable explanation for this finding is that the mechanisms underlying biofilm formation
differ between diazotrophic and non-diazotrophic _P. stutzeri_ strains. RPON GOVERNS NITROGEN-FIXING BIOFILM FORMATION VIA TRANSCRIPTIONAL ACTIVATION OF _PSLA_ AND _NIFA_ Mutation of _rpoN_
resulted in a dramatic decrease in biofilm formation (Fig. 3a), consistent with the observation that the _rpoN_ mutant was severely impaired in exopolysaccharide production (Fig. 3b).
Furthermore, _rpoN_ mutation led to a total loss of nitrogenase activity (Fig. 3c), suggesting that _rpoN_ has a major role by controlling both the nitrogen fixation ability and the biofilm
polysaccharides. Furthermore, qRT-PCR analysis provided additional evidence showing that the _rpoN_ mutation affected the expression of GacA/Rsm pathway genes to different extents (Fig. 3d).
In nitrogen-fixing biofilm cells, the _rpoN_ mutation led to a significant decrease in the expression of _rsmA_ but an increase in the expression of _gacA_ and _rsmZ_, suggesting that RpoN
can exert a negative effect on the expression of GacA/Rsm pathway genes. A similar phenomenon has also been reported in the _P. aeruginosa rpoN_ mutant, where the expression of the _gacA_
gene was significantly increased55. Moreover, _pslA_ is the only gene among the nine genes studied (listed in Fig. 2b) to have an RpoN box-like element upstream of its transcription start
site, suggesting RpoN-dependent expression, which is also the case for the _nifLA_ promoter (Supplementary Fig. 2a, b). Indeed, it was determined by DNase I footprinting assays. As shown in
Fig. 3e, f, RpoN protects a 22 bp DNA region (CGACGGCACGCGGTTTGCAAAA) of the _nifLA_ promoter and a 27 bp DNA region (CCGGAGAGGCACGGTCGGAGCAGGAGT) of the _pslA_ promoter. Two regions overlap
with the putative RpoN-binding site located at positions −12 to −24 from the transcription start. Taken together, these data suggest that the expression of both _nifA_ and _pslA_ genes is
dependent on RpoN. Psl is the major exopolysaccharide of the _P. aeruginosa_ biofilm matrix encoded by the _psl_ gene cluster, but a disruption of the first _pslA_ gene of the cluster
resulted in severe attenuation of Psl production56. The _pslA_ gene found in A1501 encodes a UDP-glucose lipid carrier sharing 64% amino acid sequence identity with that of _P. aeruginosa_
PAO1 (Fig. 2b). Carbohydrate monomer composition analysis of the polysaccharides from the A1501 culture showed that they were composed of glucose, mannose, galactose, ribose and rhamnose
(Table 2), while those of the _pslA_ mutant culture were composed mainly of glucose. Furthermore, the total amount of exopolysaccharides from the _pslA_ mutant culture was 33% of the amount
observed for the WT strain, whereas the exopolysaccharide-producing ability of the _pslA_-overexpressing strain was ~2.0-fold greater than that of the WT strain (Fig. 3b), indicating that
PslA is a key player in the production of Psl-like exopolysaccharides. Most interestingly, the _pslA_ mutant completely failed to form biofilms under nitrogen-deficient conditions but
produced 64% of the biofilm that WT produced under nitrogen-rich conditions (Table 1). These results, together with the observation that _pslA_ expression was significantly upregulated in
nitrogen-fixing biofilm growth (Fig. 2d), favour an important role of _pslA_ in Psl-like exopolysaccharide production and consequently in nitrogen-fixing biofilm formation. In addition,
mutation of the _nifA_ gene, encoding an activator of all _nif_ genes, led to a complete loss of nitrogenase activity but had no effect on biofilm formation (Table 1). Our results indicate
that RpoN-driven positive regulation at the transcriptional level is one of the key mechanisms used in diazotrophic _P. stutzeri_ to govern nitrogen-fixing biofilm formation. RSMA
POSTTRANSCRIPTIONALLY REPRESSES BIOFILM FORMATION BY BINDING _PSLA_ AND _SADC_ MRNAS The presence of two RNA-binding proteins belonging to the CsrA family appears to be common in
pseudomonads, e.g., RsmA and RsmE of _P. fluorescens_ or RsmA and RsmF of _P. aeruginosa_57; however, only one gene encoding RsmA was found in the A1501 genome. In the biofilm model
bacterium _P. aeruginosa_, RsmA exerts a negative effect on biofilm formation through posttranscriptional repression of the _sadC_ and _pslA_ genes22,23. As shown in Fig. 2b, the _P.
stutzeri rsmA_, _pslA_, and _sadC_ gene products share a high identity with orthologous proteins in _P. aeruginosa_. Analysis of mutant strains showed that deletions of each of the three
genes affected the biofilm-forming ability of _P. stutzeri_ A1501 to different extents (Table 1). The deletion of _rsmA_ significantly enhanced biofilm formation compared with WT, whereas
overexpression of this gene reduced biofilm formation (Fig. 4a), suggesting that RsmA may negatively regulate biofilm formation in A1501. Interestingly, both deletion and overexpression
strains were more affected in biofilm formation under nitrogen-rich conditions than under nitrogen-deficient conditions (Table 1). As noted before, biofilm formation was strongly decreased
in the _pslA_ mutant under N limitation, which was correlated with a strong decrease in exopolysaccharide content (Fig. 3b). In addition, the _sadC_ mutant displayed a moderate decrease in
biofilm production (Fig. 4a), and its intracellular c-di-GMP level was reduced by approximately 50% compared with that of WT (Fig. 4b). In contrast, the intracellular c-di-GMP level was
significantly increased in a mutant lacking _bifA_, which encodes a c-di-GMP-degrading phosphodiesterase but was decreased by _bifA_ overexpression (Fig. 4b). Similar effects of _bifA_
mutation and overexpression on biofilm formation were observed (Table 1). _P. stutzeri_ A1501 RsmA is predicted to be a protein of 61 amino acids, sharing ~ 99% sequence identity with the
_P. aeruginosa_ PAO1 RsmA (Fig. 2b). Indeed, this high similarity is reflected at the level of the L and R regions in A1501 and _P. aeruginosa_ RmsA as well as other Rsm homologue proteins,
including _Escherichia coli_ CsrA (Csr/Rsm family), and has been reported to be involved in mRNA binding58. Furthermore, the 5′-UTR regions of both _pslA_ (Supplementary Fig. 2a) and _sadC_
(Fig. 4d) mRNAs were predicted to contain one GGA motif overlapping with the ribosome-binding site (RBS), which is generally the target of RsmA. RsmA typically represses gene expression by
binding to the GGA motif and directly blocking access of the ribosome to RBS. To obtain experimental evidence for the predicted interaction, ssRNA oligonucleotides containing the GGA motifs
present in the 5′-UTR of the _pslA_ and _sadC_ mRNAs were synthesised and incubated with increasing concentrations of fluorescently labelled RsmA. Specific interactions were quantified by
microscale thermophoresis (MST). The data indicated significant binding of RsmA to the _pslA_ and _sadC_ mRNAs with calculated Kd values of 19 ± 4 and 55 ± 8 nM, respectively (Fig. 4e, f),
whereas mutation in the GGA motifs of the _pslA_ and _sadC_ mRNAs completely inhibited binding with WT RsmA (Fig. 4g, h). Our results indicate that the influence on biofilm formation
observed for RsmA takes place through direct repression of _pslA_ and _sadC_ at the posttranscriptional level. THE RSMY AND RSMZ NCRNAS COMPETITIVELY SEQUESTER RSMA AWAY FROM ITS MRNA
TARGETS Among the ncRNAs identified in the A1501 genome, two sharing identity with RsmY and RsmZ are predicted to have multiple GGA motifs located on the single-stranded outer stem loops
(Supplementary Fig. 3), which is characteristic of Rsm ncRNAs able to bind the RsmA protein19. As expected from MST experiments, both RsmY and RsmZ bind to RsmA, exhibiting Kd values of 55 ±
6 and 320 ± 51 nM, respectively (Fig. 4i, j), and binding is abolished if the GGA motif is mutated (Fig. 4k, l). Furthermore, single deletion of either _rsmY_ or _rsmZ_ caused a limited
reduction in biofilm production (Fig. 4m), whereas deletion of both _rsmY_ and _rsmZ_ resulted in a significantly decreased biofilm biomass (~76%), which was similar to the effect (~79%)
observed in a _gacA_ mutant (Fig. 4m and Supplementary Table 1). Consistent with the report that both Rsm ncRNAs are known to be under the control of GacA in _P. aeruginosa_17, the
expression of both _rsmY_ and _rsmZ_ was strongly decreased in the A1501 _gacA_ mutant (Fig. 4n). Moreover, both _rsm_ genes possess a sequence corresponding to a conserved GacA-binding site
in their promoters (Supplementary Fig. 3), suggesting GacA-dependent activation of the two genes. The transcription pattern of _rsmZ_ greatly differed from that of _rsmY_. For example, RsmZ
expression in biofilm cells was upregulated more than 120-fold over that in the planktonic state, in contrast to RsmY, whose expression was downregulated 2.0-fold (Fig. 2c). Furthermore,
the expression level of RsmZ with different carbon sources showed no significant differences, whereas the RsmY level increased ∼6.8-fold in biofilm cells grown on glucose compared to those
grown on lactate, suggesting a significant induction by glucose (Fig. 4o). Overexpression of either _rsmY_ or _rsmZ_ led to increased biofilm production to different extents, while single
mutations of _rsmY_ and _rsmZ_ resulted in decreased and increased levels of nitrogenase activities, respectively (Supplementary Table 1). These results suggest that RsmY and RsmZ have
overlapping and different functions in A1501. Using a highly precise and absolute nucleic acid quantification technique59, termed droplet digital PCR (ddPCR), we further assessed the
absolute copy number of the RsmA/Z/Y pool during nitrogen-fixing biofilm development. At the time of inoculation, RsmZ was present at up to ~55,000 copies per ng total RNA, much higher than
RsmY and RsmA (Table 3). Most strikingly, the expression of RsmZ was upregulated rapidly (up to ~150,000) at the early stage of biofilm formation and then downregulated remarkably during
biofilm maturation, suggesting that RsmZ functions as a potent trigger for the initiation of biofilm formation. In contrast, RsmY expression showed only a small upregulation. This suggests
that in A1501, RsmZ but not RsmY antagonizes the posttranscriptional repression exerted by RsmA during biofilm development. RPOS NEGATIVELY REGULATES BIOFILM FORMATION VIA RSMZ UNDER
NITROGEN-SUFFICIENT CONDITIONS The involvement of the stress and stationary-phase sigma factor RpoS in biofilm formation led to conflicting data in different bacteria31,32. In _P. stutzeri_
A1501, the _rpoS_ gene is immediately downstream of _rsmZ_ (Fig. 2a), and it was shown to be involved in the regulation of two ncRNAs, _nfiS_ and _nfiR_, specifically induced under nitrogen
fixation conditions; therefore, the expression of both genes was downregulated in an _rpoS_ mutant. In the present work, we found that the _rpoS_ mutant exhibited increased biofilm
production compared to that of A1501 under nitrogen-sufficient conditions (Table 1), consistent with results previously described for the _P. aeruginosa rpoS_ mutant32 but different from the
_E. coli rpoS_ mutant showing decreased biofilm production31. Furthermore, ddPCR was used to measure the absolute transcription levels of the Gac/Rsm pathway genes during biofilm formation.
As shown in Supplementary Table 4, the levels of GacA, RsmA, and RsmY showed no significant differences between the WT and _rpoS_ mutant strains during biofilm development, whereas the
expression of RsmZ in the _rpoS_ mutant increased ~5.0-fold at the mature stage of biofilm development relative to that of the WT strain. This effect is probably indirect, as no RpoS-binding
site was identified in the promoter region of the _rsmZ_ gene (Supplementary Fig. 3b). These results, together with the fact that the level of _gacA_ mRNA did not vary significantly in the
_rpoS_ mutant (Table 4), suggest that RpoS exerts an inhibitory effect on RsmZ expression in a GacA-independent manner. The ddPCR results also showed that the mRNA level of _rpoS_ was very
low in early-stage biofilm cells but was enhanced ~7.0-fold in mature-stage biofilm cells (Fig. 5a). In addition, the _rsmA_ mutation remarkably increased the expression of _rpoS_ in
early-stage biofilm cells, indicating a negative effect of RsmA on _rpoS_ expression (Fig. 5a). Further analysis revealed the conserved RsmA-binding GGA motif in the 5′-UTR of _rpoS_ mRNA,
implying a direct interaction between _rpoS_ mRNA and RsmA (Fig. 5b). This possibility was checked by MST measurements, which showed that RsmA directly binds to the 5′-UTR containing the GGA
motif and the ribosome-binding site of _rpoS_ mRNA (Fig. 5c, d). These findings suggest a novel repression circuitry that fine-tunes biofilm development by modulating the timing and
intensity of both RsmZ and RpoS expression. DISCUSSION Numerous studies have established that regulatory circuits governing the transition from planktonic to biofilm lifestyles are very
complex and differ between _Pseudomonas_ species, although common regulatory mechanisms such as the c-di-GMP signalling and Gac/Rsm pathways exist. On the other hand, the available
literature on the regulatory mechanisms underlying biofilm formation by nitrogen-fixing bacteria is still very scarce. Here, we aim to fill this knowledge gap by elucidating the complex
mechanisms for fine-tuning nitrogen-fixing biofilm formation. In view of the data reported, we propose that multiple signalling systems regulate nitrogen-fixing biofilm formation in the
rhizosphere bacterium _P. stutzeri_ A1501 (as depicted in Fig. 6), including the well-studied Gac/Rsm pathway at the posttranscriptional level, RpoN-driven positive regulation at the
transcriptional level, and a RpoS-mediated repression circuit at both levels. The Gac/Rsm pathway is generally considered the main mechanism controlling biofilm formation in non-diazotrophic
_Pseudomonas_15,17. Indeed, we have shown that deletions of each of the A1501 _gac/rsm_ genes can positively or negatively affect biofilm formation, but _rpoN_, by controlling the
transcription of _nifA_ and _plsA_, is the only gene whose inactivation resulted in the poorest biofilm and a Nif-minus phenotype. These results suggest that RpoN-driven positive regulation
at the transcriptional level is one of the key mechanisms underlying nitrogen-fixing biofilm formation, which may override the effect of the Gac/Rsm pathway in diazotrophic _P. stutzeri_. An
additional level of complexity is added to this regulatory system by the presence of two structurally and functionally similar ncRNAs, RsmY and RsmZ. The presence of multiple ncRNAs with
structural similarity was reported in other systems, e.g., RsmX, RsmY, and RsmZ in _P. fluorescens_60 and RsmW, RsmY, and RsmZ in _P. aeruginosa_58. These regulatory ncRNAs show similar
secondary structures with numerous unpaired GGA motifs that act to sequester RsmA proteins from their targets, suggesting possible functional redundancy20. Since the effectiveness of ncRNA
regulation is directly related to ncRNA abundance relative to their mRNA targets, this redundancy has been proposed to permit a more efficient and precise regulatory response by providing
additional possibilities for integrating various signals into complex networks18. In the case of _P. stutzeri_ A1501, a double _rsmY/rsmZ_ mutation caused the same phenotypic effects on
biofilm formation as those observed in the _gacA_ mutant (Supplementary Table 1), suggesting that no additional Rsm ncRNAs participate in the activation of biofilm formation via the Gac/Rsm
cascade in A1501. Moreover, the transcription rates of the _rsmY_ and _rsmZ_ genes in A1501 are clearly distinct; _rsmZ_ is expressed at ~100-fold higher levels than _rsmY_ under biofilm
growth conditions, and the level of RsmZ is very high during biofilm growth compared to planktonic growth. In addition, a quantitative assessment by ddPCR demonstrated that RsmZ showed a
biofilm stage-dependent pattern of expression with a significant increase during early stages of biofilm formation caused by transcriptional activation by GacA, which was followed by a
decrease in mature biofilms. We thus propose that RsmZ rather than RsmY acts as a signal amplifier to trigger the phenotypic switch from the planktonic mode to the biofilm mode of growth.
Although the biological role of RsmY is unclear at this stage, a very strong induction of _rsmY_ expression by glucose was observed (Fig. 4o), implying that this ncRNA may be required for
glucose-related metabolism in A1501. We also observed that single mutations of _rsmY_ and _rsmZ_ limit biofilm formation by A1501 but decrease and increase nitrogenase activities,
respectively. These results suggest that both ncRNAs have overlapping functions in the regulation of biofilm formation but distinctive roles in the regulation of nitrogenase activity. Our
results from ddPCR experiments quantitatively show that RpoS is a mature stage-induced protein whose expression is downregulated by RsmA at the early stage of nitrogen-fixing biofilm
formation. Similarly, Huertas-Rosales et al. identified _rpoS_ as a target of Rsm proteins in RIP-seq experiments as an indication that RpoS regulation by Rsm proteins is direct61. In
addition, RpoS was also found to be negatively regulated by RsmA in _P. protegens_ CHA062. This led us to speculate that RpoS contributes to the significant reduction in RsmZ levels when
RsmZ is not needed at a high level in mature biofilm cells, while RsmA posttranscriptionally decreases RpoS expression and prevents the repression of RsmZ exerted by RpoS when RsmZ is most
needed at a high level in early-stage biofilm cells. In addition to RpoS, we also found that RpoN monitors global changes in gene expression that may lead to more complex effects on
nitrogen-fixing biofilm formation. For example, _rpoN_ mutation significantly increased the expression of _rsmZ_ in nitrogen-fixing biofilm cells, implying that RpoN likely acts as a
repressor in the regulation of the RsmZ level. At least part of this effect might be mediated by GacA, as described previously in _P. aeruginosa_55. This appears to contradict the enhanced
expression of RsmZ in nitrogen-fixing biofilm cells. However, the stronger effect of the RsmZ mutation on biofilm formation under NH4+-rich conditions than under NH4+-deficient conditions
suggests that additional repression of RpoN ensures accurate and economical but not consistently high expression of RsmZ since the Gac/Rsm pathway is not the dominant player in
nitrogen-fixing biofilm formation. The initiation of biofilm formation in _P. aeruginosa_ has been correlated with high intracellular levels of c-di-GMP16. In general, high internal levels
of c-di-GMP induce the production of adhesins and extracellular matrix components, which enable bacteria to form biofilms, whereas low c-di-GMP levels lead biofilm bacteria into dispersal to
shift to a planktonic mode of growth24. The Gac/Rsm cascade in _P. aeruginosa_ is genetically linked to c-di-GMP through SadC, whose production is repressed by RsmA. We also observed a
similar connection, but deletion of the _sadC_ gene resulted in a strain that is partially defective in biofilm formation and c-di-GMP synthesis. This means that SadC likely contributes some
but not all of the c-di-GMP under the conditions tested. Therefore, we can further infer that at least one other DGC in the A1501 genome can produce c-di-GMP. Indeed, the exact mechanism
underlying c-di-GMP synthesis and biofilm formation in A1501 remains to be elucidated. Phylogenetically close members of the _Pseudomonas_ genus produce a wide diversity of
exopolysaccharides, such as cellulose, Psl, and Pel3. The Psl polysaccharide, which is composed of mannose, glucose and rhamnose, was first described in _P. aeruginosa_63. Although research
on Psl polysaccharides has been mostly conducted in _P. aeruginosa_, a number of _psl_ gene clusters have been identified in several _Pseudomonas_ strains4 and recently the existence of a
_psl_-like gene cluster has been reported in some environmental non-aeruginosa _Pseudomonas_ species64. Furthermore, two _P. fluorescens_ strains isolated from rotted bell pepper, were
previously described to produce an exopolysaccharide composed of mannose, rhamnose, and glucose substituted with pyruvate and acetate65. Although the exact composition of the PlsA-dependent
polysaccharide, tentatively referred to as the Psl-like exopolysaccharide, is not yet established, the analysis of the glycosyl residues present in a _pslA_ mutant suggests that the A1501
Psl-like exopolysaccharide contains mannose and galactose since both sugars were not found in the mutant (Table 2). From this analysis, it can be deduced that A1501 Psl differs from _P.
aeruginosa_ Psl, which does not contain galactose28. As glucose is the main sugar produced by the _pslA_ mutant, it is likely that A1501 produces cellulose, in agreement with the presence of
a cluster of genes in its genome that are similar to the cellulose biosynthesis genes of _P. putida_ KT244048. In the most recent review, Herredia-Ponce et al.63 stress the fact that the
differences in polysaccharide composition depending on growth conditions may reflect better adaptation to specific environments due to the differential evolution that occurs in different
niches. In the case of _P. aeruginosa_, the Psl exopolysaccharide is known to be a key element at the early stage of biofilm formation and is regulated transcriptionally by RpoS22,30. Unlike
what was observed in _P. aeruginosa_, we found that in A1501, the PlsA-dependent exopolysaccharide is essential for biofilm formation under nitrogen fixation conditions but not under
nitrogen-sufficient conditions, in agreement with the fact that _pslA_ transcription is RpoN-dependent. In addition to playing a major structural role in biofilms, Psl was further shown to
have a signalling role in stimulating two DGCs, SiaD and SadC, to produce more of the intracellular second messenger molecule c-di-GMP66. A Psl-mediated increase in c-di-GMP was observed to
result in two- to threefold higher levels of _pslA_ transcripts, ultimately increasing the production of Psl itself and forming a unique positive feedback regulatory circuit66. These
observations led us to speculate that PslA may be a rate-limiting enzyme of Psl synthesis. To experimentally address this possibility, pL_pslA_ was introduced into A1501, generating a strain
overexpressing PslA. As predicted, this overexpression strain produces much more Psl than the wild-type strain (Fig. 3c). Bacteria in biofilms are surrounded by an extracellular matrix,
which can account for up to 90% of the biofilm biomass and create a microenvironment favourable for protecting cells against various stresses3,67. Biofilms may provide especially suitable
conditions for nitrogen fixation, as this process is extremely sensitive to oxygen and rapidly inhibited by ammonia. An early study reported that the production of exopolysaccharides under
N-limiting conditions may be a survival mechanism favouring the exclusion of oxygen and increasing nitrogenase activity68. In addition, biofilm formation enables A1501 to fix nitrogen under
aerobic conditions by forming EPS-encased cysts to protect nitrogenase from oxygen41. In accordance with these previous results, we found that biofilm formation was enhanced under
nitrogen-deficient and carbon-sufficient conditions, which favour nitrogen fixation. Interestingly, we also observed that biofilms displayed significant nitrogenase activity at a
concentration of NH4+ that completely abolished the nitrogenase activity of planktonic cells. Nitrogen-fixing bacteria occur predominately in the rhizosphere, where carbon-rich root exudates
can support the energy demands of the nitrogen fixation process, while microbial cell densities and microbial activities are the greatest, making nitrogen a key modulator of survival and
competitiveness. The colonisation of the root rhizosphere is an essential step in the establishment of efficient nitrogen-fixing associations, and thus, understanding the mechanism of
biofilm formation is of major interest. In the present work, we found that conditions favouring biofilm formation differ between the diazotrophic and non-diazotrophic _P. stutzeri_ strains,
although both strains contain the same set of Gac/Rsm and c-di-GMP signalling pathway genes, reflecting the differential evolution of their regulatory networks due to different physiologies
and niches. We hypothesised that variations in biofilm phenotypes could be due to differences in transcriptional regulation. However, we found no significant differences in the putative
promoter sequences of the genes listed in Fig. 2b between the two strains, suggesting that the mechanism that causes the biofilm phenotypes of the two strains to differ is much more complex
than we initially believed. In addition, it is not surprising that with evolutionary optimisation in the rice rhizosphere, A1501 has evolved sophisticated regulatory networks to respond to
multiple environmental cues and adapt to the environmental conditions of the rhizosphere. Of particular note is that RpoN, an alternative sigma factor typically associated with general
nitrogen responses in bacteria, was found to act as a critical regulatory hub to activate the transcription of _pslA_ and _nifA_, consequently forming a novel regulatory link between
nitrogen fixation and biofilm formation. This regulation is probably more direct and efficient than the Gac/Rsm regulatory cascades widely found in _Pseudomonas_, and is likely advantageous,
especially when diazotrophs face competition from other species in nitrogen-limited environments, such as the rhizosphere. To our knowledge, this is the unique example of multiple
regulatory networks governing the transition from the planktonic mode to the nitrogen-fixing biofilm mode, which may contribute to diazotrophic _P. stutzeri_ being highly adaptable to
nitrogen-poor environments and have implications for the control of biofilm-related interactions between diazotrophs and host plants. Our results provide a basis for understanding a
regulatory mechanism including RpoN, RpoS, Gac and Rsm regulators that underlies nitrogen-fixing biofilm development and may be applicable to various diazotrophic species. As nitrogen-fixing
bacteria are found ubiquitously in most ecosystems and widely used as biofertilizers worldwide, our systematic study of nitrogen-fixing biofilms will be of both ecological and
biotechnological importance. METHODS BACTERIAL STRAINS, PLASMIDS AND GROWTH CONDITIONS The bacterial strains and plasmids used in this study are listed in Supplementary Table 1. _P.
stutzeri_ A1501 and its derivatives were grown on LB medium or minimal medium K (containing 0.4 g l−1 KH2PO4, 0.1 g l−1 K2HPO4, 0.1 g l−1 NaCl, 0.2 g l−1 MgSO4·7H2O, 0.01 g l−1 MnSO4·H2O,
0.01 g l−1 Fe2(SO4)3·H2O, and 0.01 g l−1 Na2MoO4·H2O, pH 6.8) supplemented by the desired carbon and nitrogen sources at concentrations indicated in the text. Unless stated otherwise, growth
experiments were conducted using medium K containing NH4Cl (20 mM) and sodium lactate (50 mM) as the sole nitrogen and carbon sources at 30 °C under vigorous shaking in a water-bath shaker.
For measurements of biofilm formation, nitrogenase activity and gene expression, the concentrations of carbon substrates were adjusted to 50 mM. Antibiotics were used at the following
concentrations: 50 μg ml−1 ampicillin (Amp), 50 μg ml−1 kanamycin (Km), 10 μg ml−1 tetracycline (Tc), 34 μg ml−1 chloromycetin (Cm), and 20 μg ml−1 gentamicin sulfate (Gm). CONSTRUCTIONS OF
MUTANTS, COMPLEMENTING PLASMIDS AND OVEREXPRESSION STRAINS The plasmids and oligonucleotide primers used in this study are listed in Supplementary Tables 1 and 2, respectively. Strains and
plasmids were constructed using conventional techniques. Nonpolar insertion mutant strains (e.g., _rsmA_) were generated by homologous suicide plasmid integration as described previously50.
Appropriate oligonucleotide primers were designed to generate amplicons that were cloned into pK18mob as a vector69, and the resulting plasmids were introduced into A1501 by triparental
mating using pRK201370, generating the mutant strains. Correct recombination was confirmed by PCR followed by nucleotide sequencing of the amplicons obtained. To generate nonpolar deletion
mutant strains, amplification of DNA fragments located upstream and downstream of the target gene was performed using the appropriate primer sets upF/upR and downF/downR (Supplementary Table
2). Then, both amplicons and a DNA fragment containing the chosen resistance cassette gene were fused, and the resulting fragment was cloned into the pK18mob_sacB_ vector, as depicted in
Supplementary Fig. 4. The resulting plasmid was then introduced into A1501 by triparental mating as described above, and double recombination was selected on the basis of sucrose resistance.
Correct recombination was validated by PCR and sequencing using primers testF and testR. The complemented and overexpression strains were constructed using the broad host plasmid pLAFR3. A
DNA fragment containing a WT gene (e.g., _gacA_) with its promoter and terminator was amplified from genomic DNA of A1501 and cloned into pLAFR3. The resulting complementing plasmid was then
introduced into the WT or mutant strain by triparental mating, generating overexpression and complemented strains, respectively (Supplementary Table 1). The gene expression levels of the
overexpression strains were confirmed to be higher than those of the WT using qRT-PCR. The mutant with both _rsmZ_ and _rsmY_ deleted was constructed using _ΔrsmZ_ as the starting strain.
Briefly, a 1154 bp fragment containing the Gm resistance cassette located between the upstream and downstream DNA fragments of _rsmY_ was generated by overlap extension PCR, double-digested
with _Bam_HI/_Hin_dIII, and then cloned into the _Bam_HI/_Hin_dIII site of pK18mob_sacB_. The resulting plasmid, pK18_rsmY_, was introduced into the genome of _ΔrsmZ_ by triparental mating
and double recombination was selected on the basis of sucrose resistance. Correct recombination in the resulting _ΔrsmZ ΔrsmY_ double mutant was checked by PCR using the primers
M-_rsmY_(up)-F and M-_rsmY_(down)-R (Supplementary Table 2), followed by nucleotide sequencing of the obtained PCR products. The resulting double deletion mutant (Supplementary Table 1) was
used for further study. BIOFILM FORMATION ASSAYS Surface-adhered biofilm formation was assayed using the crystal violet (CV) method and performed in 96-well microtiter plates. Strains used
for biofilm experiments were grown overnight in LB at 30 °C. Cultures were centrifuged and diluted to a final OD600 of 0.2 in fresh minimal medium K containing different carbon sources with
or without 6 mM NH4Cl. Two hundred μl of each culture was aliquoted into separate wells in a 96-well PVC plate. Microtiter plates were carefully wrapped using parafilm and placed in a 30 °C
incubator without agitation for 12 or 48 h. In this study, the so-called early- and mature-stage biofilms were defined as biofilms formed 12 and 48 h after inoculation, respectively. At the
indicated points, nonadhered planktonic cells were removed using a multichannel pipette without disturbing the biofilm area, and individual wells were washed twice with 160 μl of sterile
double-distilled H2O. Then, 160 μl of 0.1% CV solution in ethanol was added to each well for 10 min and washed four times with 200 μl of ddH2O. Photos were taken, and the cell-associated CV
was solubilized with 30% acetic acid and quantified by measuring the OD560 of the resulting solution using a spectrophotometer (Thermo Scientific). NITROGENASE ACTIVITY ASSAYS Nitrogenase
activity was determined according to a previous protocol with modifications71. To examine the nitrogenase activity of cells grown planktonically, cells from an overnight culture in LB medium
were centrifuged and resuspended in a 60 ml flask containing 10 ml of minimal N-free and lactate-containing medium at an OD600 of 0.1. The suspension was incubated for 4 h at 30 °C with
vigorous shaking under an argon atmosphere containing 0.5% oxygen, and then 10% acetylene was added. Gas samples (0.25 ml) were taken at regular intervals (4, 6, 8, and 10 h) to determine
the amount of ethylene produced. Samples were analysed on a polydivinylbenzene porous bead GDX-502 column using an SP-2100 gas chromatograph fitted with a flame ionisation detector (Beijing
Beifen-Ruili Analytical Instrument Co., Ltd.). The ethylene content in the gas samples was determined by reference to an ethylene standard. To determine the biofilm-based nitrogenase
activity, strains used were grown overnight in LB at 30 °C. Cultures were centrifuged and diluted to a final OD600 of 0.2 in a 60 ml flask containing 10 ml of minimal NH4+-free and
lactate-containing medium. The suspension was incubated for 48 h at 30 °C under static conditions in air, and then 10% acetylene was added. Gas samples (0.25 ml) were taken at regular
intervals (4, 6, 8, and 10 h) to determine the amount of ethylene produced. A 4 h incubation time was chosen for qRT-PCR or ddPCR assays of biofilm-related gene expression. To examine the
effect of NH4+ on nitrogenase activity, both 10% acetylene and ammonium at different concentrations were added to 60 ml flasks containing suspensions of either biofilm or planktonic cells,
and then gas samples (0.25 ml) were taken at regular intervals (4, 6, 8, and 10 h) to determine the amount of ethylene produced using the same method as described above. The nitrogenase
activity was expressed as nmol ethylene min−1 mg−1 protein. Protein concentrations were determined using the Bio-Rad protein assay reagent kit (Bradford, Bio-Rad). RNA ISOLATION AND QRT-PCR
ASSAYS Total RNA was isolated with an innuPREP RNA Mini Kit (Analytik Jena) according to the manufacturer’s instructions. For quantification of gene expression, total RNA was reverse
transcribed using random primers and the High Capacity cDNA Transcription Kit (Applied Biosystems) according to the manufacturer’s instructions. PCR was carried out with Power SYBR Green PCR
Master Mix on an ABI Prism 7500 Sequence Detection System (Applied Biosystems) according to the manufacturer’s recommendations. The 16S rRNA gene was used as the endogenous reference
control, and relative gene expression was determined using the comparative threshold cycle 2−ΔΔCT method. Data were analysed using ABI PRISM 7500 Sequence Detection System Software (Applied
Biosystems). Primers were designed based on the full genome sequence of _P. stutzeri_ A1501, and they are listed in Supplementary Table 2. ABSOLUTE QUANTIFICATION OF RNA COPY NUMBER BY
DROPLET DIGITAL PCR (DDPCR) Total RNA isolation and reverse transcription were performed as described above for qRT-PCR. Quantification by ddPCR was carried out in 20 μl reactions containing
10 μl of QX200 ddPCR EvaGreen SuperMix, 250 nM each commercial probe, 900 nM specific commercial primers, and 1 μl of cDNA according to the manufacturer’s recommendations. A negative
control contained sterile double-distilled water only. Emulsified 1 nl reaction droplets were generated using a QX100 droplet generator (Bio-Rad) and a droplet generator DG8 cartridge
(Bio-Rad) containing 20 μl of reaction mixture and 70 μl of ddPCR droplet generation oil (Bio-Rad) per well. Thirty-five μl of the generated droplet emulsions was transferred to 96-well PCR
plates that were then heat-sealed using foil sheets. Target DNA amplification was performed by thermal cycling of the droplet emulsions as follows: initial denaturation at 95 °C for 10 min;
40 cycles of 94 °C for 30 s and 60 °C for 1 min; and then 98 °C for 10 min. The fluorescence of each thermal cycled droplet was measured using a QX100 droplet reader (Bio-Rad). Data were
analysed using QuantaSoft software (Bio-Rad) after setting a threshold using the fluorescence of negative controls. 5′ RAPID AMPLIFICATION OF CDNA ENDS TO DETERMINE TRANSCRIPTIONAL START
SITES The transcriptional start site of the six target genes (_pslA_, _nifLA_, _rpoS_, _sadC_, _rsmZ_, and _rsmY_) was determined using the rapid amplification of cDNA ends (5′ RACE) method
(Invitrogen) following the manufacturer’s instructions. Briefly, the first-strand cDNA was synthesised using the primer GSP1, which was specific for the target gene sequence. The purified
cDNA was tailed with dCTP by terminal deoxynucleotidyl transferase. PCR amplification was performed using the sequence-specific primer GSP2 and the anchor primer AAP. Primers GSP1 and GSP2,
specific for the target gene tested here, are listed in Supplementary Table 2. The 5′ RACE products were cloned into the pGEM-T Easy vector (Promega) and sequenced to map the 5′ end of the
transcript. EXPRESSION AND PURIFICATION OF RSMA FOR MICROSCALE THERMOPHORESIS (MST) MEASUREMENTS The RsmA protein was expressed and purified using the IMPACTTM (Intein Mediated Purification
with an Affinity Chitin-binding Tag) system according to the manufacturer’s instructions (New England Biolabs). To this end, a fragment of _rsmA_ was amplified by PCR using the
pTWIN1-rsmA-F/R primers (Supplementary Table 2). The PCR product was digested with _Nde_I and _Eco_RI and ligated into the protein expression vector pTWIN1, which had been digested with the
same enzymes. The resulting plasmid (named pTWIN1-RsmA) was introduced into the _E. coli_ BL21 (DE3) strain. Overproduction of the RsmA-intein fusion was induced by the addition of 0.5 mM
isopropyl β-d-1-thiogalactopyranoside (IPTG) to cells grown to mid-log phase (OD600 of 0.6). Cleavage of the RsmA-intein fusion was induced by equilibrating the chitin beads in buffer B3 (1
mM EDTA, 40 mM DTT, 20 mM Tris-HCl, 500 mM NaCl, pH 8.5) overnight. The untagged RsmA was eluted from the column and dialysed against 20 mM Tris-HCl and 500 mM NaCl, pH 8.0. The purity of
the protein was as high as 90%, as judged by SDS-Tris-glycine PAGE. RsmA was quantitated using the Bio-Rad protein assay reagent kit (Quick Start Bradford, Bio-Rad) and stored at −80 °C for
further use. MST MEASUREMENTS TO DETERMINE INTERACTIONS BETWEEN THE RSMA PROTEIN AND ITS TARGET RNAS MST experiments were performed as previously described72. DNA templates carrying _rsmY_,
_rsmZ_, _sadC_, _pslA_, and _rpoS_ bearing point mutations within the GGA sequences were amplified using the WT or mutagenic primers (Supplementary Table 2). The following transcripts were
synthesised from PCR-generated templates by GenePharma using a MAXIscript kit (Thermo Fisher): the full-length WT or mutated ncRNAs (RsmY and RsmZ) and the WT or mutated oligonucleotides
containing the 5′-UTR and the first 50 nucleotides of the coding sequences of _sadC_, _pslA_, and _rpoS_ mRNAs. The RsmA protein was labelled with NT-647-NHS dye using the Monolith Labelling
Kit RED-NHS (no. MO-L011, NanoTemper Technologies) according to the manufacturer’s instructions. For the measurements, the concentration of the labelled RsmA protein was kept constant (20
nM), while the concentrations of non-labelled ssRNA oligonucleotides varied from 0.3 nM to 10 μM. The binding reactions were carried out in MST buffer (10 mM HEPES pH 7.4, 150 mM NaCl, 10 mM
MgCl2) supplemented with 0.1% Tween. The reactants were initially incubated at 37 °C for 30 min to enable ssRNA binding with RsmA. The samples were then loaded onto NT.115 standard
capillaries (no. MO-K002, NanoTemper Technologies). The measurements were carried out at 25 °C with 40% excitation power and medium MST power. Data analyses were performed with NanoTemper
Analysis software (NanoTemper Technologies). PURIFICATION OF HIS-TAGGED RPON FOR DNASE I FOOTPRINTING ASSAYS The pET-28a expression system (Novagen) was used to produce C-terminally
His-tagged RpoN within host _E. coli_ BL21 (DE3) cells. A fragment of _rpoN_ was amplified by PCR using the pET28a-_rpoN_-F/R primers (Supplementary Table 2). The PCR product was digested
with _Nde_I and _Hin_dIII and ligated into the protein expression vector pET28a, which had been digested with the same enzymes. The resulting plasmid (pET28a-RpoN) was introduced into the
_E. coli_ BL21 (DE3) strain. An overnight culture of BL21 (DE3) harbouring the expression plasmid was used to inoculate LB medium containing the appropriate antibiotics. This cell culture
was incubated with shaking at 37 °C until the OD600 was 0.6–0.9, at which point production of His-tagged RpoN was induced by the addition of IPTG to a final concentration of 0.1 mM.
His-tagged RpoN was purified using a Ni-NTA Fast Start Kit (Qiagen, Venlo, Netherlands) according to the manufacturer’s instructions. The purity of the protein was as high as 90%, as judged
by SDS-Tris-glycine PAGE. His-tagged RpoN was quantitated using the Bio-Rad protein assay reagent kit (Quick Start™ Bradford, Bio-Rad) and stored at −80 °C for further use. DNASE I
FOOTPRINTING ASSAYS DNase I footprinting assays were performed by Tolo Biotech according to a method previously described73. The DNA probe was prepared by PCR-amplifying a 296-bp _nifA_
promoter region using the primers FP-_nifA_-F/FP-_nifA_-R and a 279-bp _pslA_ promoter region using the primers FP-_pslA_-F/FP-_pslA_-R (Supplementary Table 2). For each assay, 300 ng probes
were incubated with 0.2 μg purified protein RpoN in a total volume of 40 µl. After incubation for 30 min at 30 °C, 10 µl solution containing ~0.015 units DNase I (Promega) and 100 nmol
freshly prepared CaCl2 was added and further incubation was performed at 37 °C for 1 min. The reaction was stopped by adding 140 µl DNase I stop solution (200 mM unbuffered sodium acetate,
30 mM EDTA and 0.15% SDS). Samples were first extracted with phenol/chloroform, and then precipitated with ethanol. Pellets were dissolved in 30 µl MiniQ water. The preparation of the DNA
ladder, electrophoresis and data analysis were the same as previously described73, except that the GeneScan-LIZ600 size standard (Applied Biosystems) was used. EXOPOLYSACCHARIDE ISOLATION
AND CARBOHYDRATE MONOMER COMPOSITION ANALYSIS Bacterial aerated cultures were grown in minimal medium K containing 50 mM lactate and 6.0 mM NH4Cl. Then, the cultures were centrifuged. The
supernatants were collected and concentrated to 50 ml with a CentriVap concentrator (Labconco, USA). Exopolysaccharides were isolated from culture supernatants by the addition of two volumes
of chilled absolute ethanol, and then proteins were removed from the exopolysaccharides by protease hydrolysis. After further precipitation by cold absolute ethanol and lyophilization,
purified exopolysaccharides were obtained. The exopolysaccharide content of samples was monitored quantitatively by using the phenol-sulfuric acid method and was further normalised to the
total cell protein remaining after extraction, as determined using the Bio-Rad protein assay reagent kit (Quick Start™ Bradford, Bio-Rad). Final concentrations were expressed as mg
exopolysaccharide per g bacterial protein. Carbohydrate monomer composition analysis was conducted on the exopolysaccharide samples from the A1501 and _∆pslA_ mutant strains at Beijing
Ketian Technology Co., Ltd. Briefly, an exopolysaccharide sample (2.0 mg) was dissolved in 5 ml of trifluoroacetic acid (2 M) and subsequently hydrolysed at 120 °C for 4 h. After repeated
rotary evaporations to completely remove the trifluoroacetic acid, the sample was dissolved in 2 ml of deionized water. The hydrolysate (100 µl) was placed in a separate tube with 10 µl of
deuterium-labelled succinic acid (1.5 mg ml−1) as the internal standard and then lyophilised. Methyl glycosides were prepared from the dry sample by suspension in 50 µl of methoxyammonium
hydrochloride/pyridine solution (20 mg ml−1) at 40 °C for 80 min. The resulting sample was then per-_O_-trimethylsilylated with _N_-methyl-_N_-(trimethylsilyl) trifluoroacetamide (80 µl) in
a water-bath pot at 40 °C for 80 min. The sample was centrifuged at 12,000 r.p.m. for 5 min. The supernatant fraction was filtered through a 0.22 µm vacuum filter and collected for glycosyl
composition analysis. Samples were analysed using a gas chromatograph coupled to a mass selective detector (7890 A/5975 C MSD; Agilent Technologies, Inc.) equipped with an HP-5 (30 m by 0.32
mm, 0.25-µm film thickness; SGE Analytical Science) capillary column. The injector and detector temperatures were 250 °C and 240 °C, respectively. The column pressure was kept at 0.10 MPa,
with a 1.0 ml/min carrier gas (N2) flow rate. The chemical compounds were identified using a mass spectral library (NIST 08) and Agilent GC-MS Workstation software. Data from three
biological replicates were analysed for each strain. DETERMINATION OF THE INTRACELLULAR C-DI-GMP CONCENTRATIONS Intracellular c-di-GMP was extracted from the indicated strains, and then the
samples were analysed using reversed-phase-coupled HPLC-tandem mass spectrometry (MS/MS) as previously described74. Briefly, the number of cells for initial extraction was adjusted to the
equivalent of 10 ml of culture with an OD600 of 1.0. Following three cycles of heat and ethanol extractions, the supernatants were combined, dried using a Speed-Vac, and resuspended in 200
μl of water. Samples were analysed by reversed-phase-coupled HPLC-MS/MS (Agilent). Chromatographic separation was performed on an Agilent 1260 Series HPLC system with a reverse-phase C18
column (Eclipse plus C18 RRHD, 1.8 µm). Samples (2 µl) were injected and analysed at a flow rate of 0.2 ml/min with the following gradient: 0–5 min 95% A to 70% A, 5–7 min 70% A to 5% A, and
7–24 min 95% A [buffer A: 10 mM ammonium acetate and 0.1% (v/v) acetic acid; buffer B, methanol]. Analyte detection was performed on an Agilent G6400 series triple quadrupole mass
spectrometer. Commercially available c-di-GMP (InvivoGen) was used as a reference for the identification and quantification of c-di-GMP in cell extracts. Intracellular c-di-GMP content was
further normalised to the total cell protein remaining after extraction, as determined using the Bio-Rad protein assay reagent kit (Quick Start™ Bradford, Bio-Rad). Final concentrations were
expressed as pmol c-di-GMP per mg bacterial protein. Data from three biological replicates were analysed for each strain. REPORTING SUMMARY Further information on research design is
available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The data that support the findings of this study are available in the Supplementary Material of
this article. REFERENCES * Stoodley, P., Sauer, K., Davies, D. G. & Costerton, J. W. Biofilms as complex differentiated communities. _Annu. Rev. Microbiol._ 56, 187–209 (2002). Article
CAS PubMed Google Scholar * Morris, C. E. & Monier, J. M. The ecological significance of biofilm formation by plant-associated bacteria. _Annu. Rev. Phytopathol._ 41, 429–453 (2003).
Article CAS PubMed Google Scholar * Flemming, H. C. & Wingender, J. The biofilm matrix. _Nat. Rev. Microbiol._ 8, 623–633 (2010). Article CAS PubMed Google Scholar * Mann, E. E.
& Wozniak, D. J. _Pseudomonas_ biofilm matrix composition and niche biology. _FEMS Microbiol. Rev._ 36, 893–916 (2012). Article CAS PubMed Google Scholar * Oliveira, N. M. et al.
Biofilm formation as a response to ecological competition. _PLoS Biol._ 13, e1002191 (2015). Article PubMed PubMed Central CAS Google Scholar * Flemming, H. C. et al. Biofilms: an
emergent form of bacterial life. _Nat. Rev. Microbiol._ 14, 563–575 (2016). Article CAS PubMed Google Scholar * Hall-Stoodley, L., Costerton, J. W. & Stoodley, P. Bacterial biofilms:
from the natural environment to infectious diseases. _Nat. Rev. Microbiol._ 2, 95–108 (2004). Article CAS PubMed Google Scholar * Kassinger, S. J. & van Hoek, M. L. Biofilm
architecture: an emerging synthetic biology target. _Synth. Syst. Biotechnol._ 5, 1–10 (2020). Article PubMed PubMed Central Google Scholar * Brandwein, M., Steinberg, D. & Meshner,
S. Microbial biofilms and the human skin microbiome. _npj Biofilms Microbiomes_ 2, 3 (2016). Article PubMed PubMed Central Google Scholar * Lee, K. & Yoon, S. S. _Pseudomonas
aeruginosa_ biofilm, a programmed bacterial life for fitness. _J. Microbiol. Biotechnol._ 27, 1053–1064 (2017). Article CAS PubMed Google Scholar * Shirtliff, M. E., Mader, J. T. &
Camper, A. K. Molecular interactions in biofilms. _Chem. Biol._ 9, 859–871 (2002). Article CAS PubMed Google Scholar * Southey-Pillig, C. J., Davies, D. G. & Sauer, K.
Characterization of temporal protein production in _Pseudomonas aeruginosa_ biofilms. _J. Bacteriol._ 187, 8114–8126 (2005). Article CAS PubMed PubMed Central Google Scholar *
Heacock-Kang, Y. et al. Spatial transcriptomes within the _Pseudomonas aeruginosa_ biofilm architecture. _Mol. Microbiol._ 106, 976–985 (2017). Article CAS PubMed PubMed Central Google
Scholar * Varadarajan, A. R. et al. An integrated model system to gain mechanistic insights into biofilm-associated antimicrobial resistance in _Pseudomonas aeruginosa_ MPAO1. _npj Biofilms
Microbiomes_ 6, 46 (2020). Article CAS PubMed PubMed Central Google Scholar * Mikkelsen, H., Sivaneson, M. & Filloux, A. Key two-component regulatory systems that control biofilm
formation in _Pseudomonas aeruginosa_. _Environ. Microbiol._ 13, 1666–1681 (2011). Article CAS PubMed Google Scholar * Fazli, M. et al. Regulation of biofilm formation in _Pseudomonas_
and _Burkholderia_ species. _Environ. Microbiol._ 16, 1961–1981 (2014). Article CAS PubMed Google Scholar * Brencic, A. & Lory, S. Determination of the regulon and identification of
novel mRNA targets of _Pseudomonas aeruginosa_ RsmA. _Mol. Microbiol._ 72, 612–632 (2009). Article CAS PubMed PubMed Central Google Scholar * Chambers, J. R. & Sauer, K. Small RNAs
and their role in biofilm formation. _Trends Microbiol._ 21, 39–49 (2013). Article CAS PubMed Google Scholar * Lapouge, K. et al. RNA pentaloop structures as effective targets of
regulators belonging to the RsmA/CsrA protein family. _RNA Biol._ 10, 1031–1041 (2013). Article PubMed CAS Google Scholar * Janssen, K. H. et al. Functional analyses of the RsmY and RsmZ
Small Noncoding regulatory RNAs in _Pseudomonas aeruginosa_. _J. Bacteriol._ 200, e00736–17 (2018). CAS PubMed PubMed Central Google Scholar * Dubey, A. K., Baker, C. S., Romeo, T.
& Babitzke, P. RNA sequence and secondary structure participate in high-affinity CsrA-RNA interaction. _RNA_ 11, 1579–1587 (2005). Article CAS PubMed PubMed Central Google Scholar *
Irie, Y. et al. _Pseudomonas aeruginosa_ biofilm matrix polysaccharide Psl is regulated transcriptionally by RpoS and post-transcriptionally by RsmA. _Mol. Microbiol._ 78, 158–172 (2010).
CAS PubMed PubMed Central Google Scholar * Moscoso, J. A. et al. The diguanylate cyclase SadC is a central player in Gac/Rsm-mediated biofilm formation in _Pseudomonas aeruginosa_. _J.
Bacteriol._ 196, 4081–4088 (2014). Article PubMed PubMed Central CAS Google Scholar * Valentini, M. & Filloux, A. Biofilms and cyclic di-GMP (c-di-GMP) signaling: lessons from
_Pseudomonas aeruginosa_ and other bacteria. _J. Biol. Chem._ 291, 12547–12555 (2016). Article CAS PubMed PubMed Central Google Scholar * Merritt, J. H., Brothers, K. M., Kuchma, S. L.
& O’Toole, G. A. SadC reciprocally influences biofilm formation and swarming motility via modulation of exopolysaccharide production and flagellar function. _J. Bacteriol._ 189,
8154–8164 (2007). Article CAS PubMed PubMed Central Google Scholar * Rodesney, C. A. et al. Mechanosensing of shear by _Pseudomonas aeruginosa_ leads to increased levels of the
cyclic-di-GMP signal initiating biofilm development. _Proc. Natl Acad. Sci. USA_ 114, 5906–5911 (2017). Article CAS PubMed PubMed Central Google Scholar * Borlee, B. R. et al.
_Pseudomonas aeruginosa_ uses a cyclic-di-GMP-regulated adhesin to reinforce the biofilm extracellular matrix. _Mol. Microbiol._ 75, 827–842 (2010). Article CAS PubMed PubMed Central
Google Scholar * Franklin, M. J., Nivens, D. E., Weadge, J. T. & Howell, P. L. Biosynthesis of the _Pseudomonas aeruginosa_ extracellular polysaccharides, alginate, Pel, and Psl.
_Front. Microbiol._ 2, 167 (2011). Article PubMed PubMed Central Google Scholar * Jackson, K. D., Starkey, M., Kremer, S., Parsek, M. R. & Wozniak, D. J. Identification of _psl_, a
locus encoding a potential exopolysaccharide that is essential for _Pseudomonas aeruginosa_ PAO1 biofilm formation. _J. Bacteriol._ 186, 4466–4475 (2004). Article CAS PubMed PubMed
Central Google Scholar * Overhage, J., Schemionek, M., Webb, J. S. & Rehm, B. H. A. Expression of the _psl_ operon in _Pseudomonas aeruginosa_ PAO1 biofilms: PslA performs an essential
function in biofilm formation. _Appl. Environ. Microbiol._ 71, 4407–4413 (2005). Article CAS PubMed PubMed Central Google Scholar * Adams, J. L. & McLean, R. J. C. Impact of _rpoS_
deletion on _Escherichia coli_ biofilms. _Appl. Environ. Microbiol_ 65, 4285–4287 (1999). Article CAS PubMed PubMed Central Google Scholar * Xu, K. D., Franklin, M. J., Park, C. H.,
McFeters, G. A. & Stewart, P. S. Gene expression and protein levels of the stationary phase sigma factor, RpoS, in continuously-fed _Pseudomonas aeruginosa_ biofilms. _FEMS Microbiol.
Lett._ 199, 67–71 (2001). Article CAS PubMed Google Scholar * Corona-Izquierdo, F. P. & Membrillo-Hernandez, J. A mutation in _rpoS_ enhances biofilm formation in _Escherichia coli_
during exponential phase of growth. _FEMS Microbiol. Lett._ 211, 105–110 (2002). Article CAS PubMed Google Scholar * Schuster, M., Hawkins, A. C., Harwood, C. S. & Greenberg, E. P.
The _Pseudomonas aeruginosa_ RpoS regulon and its relationship to quorum sensing. _Mol. Microbiol._ 51, 973–985 (2004). Article CAS PubMed Google Scholar * Sapi, E., Theophilus, P. A.,
Pham, T. V., Burugu, D. & Luecke, D. F. Effect of RpoN, RpoS and LuxS pathways on the biofilm formation and antibiotic sensitivity of _Borrelia Burgdorferi_. _Eur. J. Microbiol.
Immunol._ 6, 272–286 (2016). Article CAS Google Scholar * Danhorn, T. & Fuqua, C. Biofilm formation by plant-associated bacteria. _Annu. Rev. Microbiol._ 61, 401–422 (2007). Article
CAS PubMed Google Scholar * Rinaudi, L. V. & Giordano, W. An integrated view of biofilm formation in rhizobia. _FEMS Microbiol. Lett._ 304, 1–11 (2010). Article CAS PubMed Google
Scholar * Jones, K. & Bradshaw, S. B. Biofilm formation by the enterobacteriaceae: A comparison between _Salmonella enteritidis_, _Escherichia coli_ and a nitrogen-fixing strain of
_Klebsiella pneumoniae_. _J. Appl. Bacteriol._ 80, 458–464 (1996). Article CAS PubMed Google Scholar * Rinaudi, L. et al. Effects of nutritional and environmental conditions on
_Sinorhizobium meliloti_ biofilm formation. _Res. Microbiol._ 157, 867–875 (2006). Article CAS PubMed Google Scholar * Meneses, C. H. S. G., Rouws, L. F. M., Simoes-Araujo, J. L., Vidal,
M. S. & Baldani, J. I. Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte _Gluconacetobacter diazotrophicus_. _Mol.
Plant Microbe Interact._ 24, 1448–1458 (2011). Article CAS PubMed Google Scholar * Wang, D., Xu, A. M., Elmerich, C. & Ma, L. Y. Z. Biofilm formation enables free-living
nitrogen-fixing rhizobacteria to fix nitrogen under aerobic conditions. _ISME J._ 11, 1602–1613 (2017). Article CAS PubMed PubMed Central Google Scholar * Pereg-Gerk, L., Paquelin, A.,
Gounon, P., Kennedy, I. R. & Elmerich, C. A transcriptional regulator of the LuxR-UhpA family, FlcA, controls flocculation and wheat root surface colonization by _Azospirillum
brasilense_ Sp7. _Mol. Plant Microbe Interact._ 11, 177–187 (1998). Article CAS PubMed Google Scholar * Fujishige, N. A., Kapadia, N. N., De Hoff, P. L. & Hirsch, A. M.
Investigations of _Rhizobium_ biofilm formation. _FEMS Microbiol. Ecol._ 56, 195–206 (2006). Article CAS PubMed Google Scholar * Janczarek, M. Environmental signals and regulatory
pathways that influence exopolysaccharide production in _Rhizobia_. _Int. J. Mol. Sci._ 12, 7898–7933 (2011). Article CAS PubMed PubMed Central Google Scholar * Nocelli, N., Bogino, P.
C., Banchio, E. & Giordano, W. Roles of extracellular polysaccharides and biofilm formation in heavy metal resistance of _Rhizobia_. _Materials_ 9, 418 (2016). Article PubMed Central
CAS Google Scholar * Ramirez-Mata, A., Pacheco, M. R., Moreno, S. J., Xiqui-Vazquez, M. L. & Baca, B. E. Versatile use of _Azospirillum brasilense_ strains tagged with _egfp_ and
mCherry genes for the visualization of biofilms associated with wheat roots. _Microbiol. Res._ 215, 155–163 (2018). Article CAS PubMed Google Scholar * Jijon-Moreno, S., Baca, B. E.,
Castro-Fernandez, D. C. & Ramirez-Mata, A. TyrR is involved in the transcriptional regulation of biofilm formation and D-alanine catabolism in _Azospirillum brasilense_ Sp7. _PLoS ONE_
14, e0211904 (2019). Article CAS PubMed PubMed Central Google Scholar * Yan, Y. L. et al. Nitrogen fixation island and rhizosphere competence traits in the genome of root-associated
_Pseudomonas stutzeri_ A1501. _Proc. Natl Acad. Sci. USA_ 105, 7564–7569 (2008). Article CAS PubMed PubMed Central Google Scholar * Yan, Y. L. et al. Global transcriptional analysis of
nitrogen fixation and ammonium repression in root-associated _Pseudomonas stutzeri_ A1501. _BMC Genomics_ 11, 11 (2010). Article PubMed PubMed Central CAS Google Scholar * Zhan, Y. H.
et al. The novel regulatory ncRNA, NfiS, optimizes nitrogen fixation via base pairing with the nitrogenase gene _nifK_ mRNA in _Pseudomonas stutzeri_ A1501. _Proc. Natl Acad. Sci. USA_ 113,
E4348–E4356 (2016). Article CAS PubMed PubMed Central Google Scholar * Zhan, Y. H. et al. NfiR, a new regulatory noncoding RNA (ncRNA), is required in concert with the NfiS ncRNA for
optimal expression of nitrogenase genes in _Pseudomonas stutzeri_ A1501. _Appl. Environ. Microbiol._ 85, e00762–19 (2019). Article CAS PubMed PubMed Central Google Scholar * Zhang, H.
Y. et al. The _Pseudomonas stutzeri_-specific regulatory noncoding RNA NfiS targets _katB_ mRNA encoding a catalase essential for optimal oxidative resistance and nitrogenase activity. _J.
Bacteriol._ 201, e00334–19 (2019). Article CAS PubMed PubMed Central Google Scholar * Ma, Y. et al. Identification of the nitrogen-fixing _Pseudomonas stutzeri_ major flagellar gene
regulator FleQ and its role in biofilm formation and root colonization. _J. Integr. Agr._ 15, 339–348 (2016). Article CAS Google Scholar * Dixon, R. & Kahn, D. Genetic regulation of
biological nitrogen fixation. _Nat. Rev. Microbiol._ 2, 621–631 (2004). Article CAS PubMed Google Scholar * Heurlier, K., Denervaud, V., Pessi, G., Reimmann, C. & Haas, D. Negative
control of quorum sensing by RpoN (sigma54) in _Pseudomonas aeruginosa_ PAO1. _J. Bacteriol._ 185, 2227–2235 (2003). Article CAS PubMed PubMed Central Google Scholar * Byrd, M. S. et
al. Genetic and biochemical analyses of the _Pseudomonas aeruginosa_ Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. _Mol.
Microbiol._ 73, 622–638 (2009). Article CAS PubMed PubMed Central Google Scholar * Schulmeyer, K. H. et al. Primary and secondary sequence structure requirements for recognition and
discrimination of target RNAs by _Pseudomonas aeruginosa_ RsmA and RsmF. _J. Bacteriol._ 198, 2458–2469 (2016). Article CAS PubMed PubMed Central Google Scholar * Miller, C. L. et al.
RsmW, _Pseudomonas aeruginosa_ small non-coding RsmA-binding RNA upregulated in biofilm versus planktonic growth conditions. _BMC Microbiol._ 16, 155 (2016). Article PubMed PubMed Central
CAS Google Scholar * Hindson, C. M. et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. _Nat. Methods_ 10, 1003–1005 (2013). Article CAS PubMed PubMed
Central Google Scholar * Kay, E., Dubuis, C. & Haas, D. Three small RNAs jointly ensure secondary metabolism and biocontrol in _Pseudomonas fluorescens_ CHA0. _Proc. Natl Acad. Sci.
USA_ 102, 17136–17141 (2005). Article CAS PubMed PubMed Central Google Scholar * Huertas-Rosales, O. et al. The _Pseudomonas putida_ CsrA/RsmA homologues negatively affect c-di-GMP
pools and biofilm formation through the GGDEF/EAL response regulator CfcR. _Environ. Microbiol._ 19, 3551–3566 (2017). Article CAS PubMed PubMed Central Google Scholar * Heeb, S.,
Valverde, C., Gigot-Bonnefoy, C. & Haas, D. Role of the stress sigma factor RpoS in GacA/RsmA-controlled secondary metabolism and resistance to oxidative stress in _Pseudomonas
fluorescens_ CHA0. _FEMS Microbiol. Lett._ 243, 251–258 (2005). Article CAS PubMed Google Scholar * Heredia-Ponce, Z., de Vicente, A., Cazorla, F. M. & Gutierrez-Barranquero, J. A.
Beyond the wall: exopolysaccharides in the biofilm lifestyle of pathogenic and beneficial plant-associated. _Pseudomonas Microorg._ 9, 445 (2021). Article Google Scholar * Heredia-Ponce,
Z. et al. Biological role of EPS from _Pseudomonas syringaepv. syringae_ UMAF0158 extracellular matrix, focusing on a Psl-like polysaccharide. _npj Biofilms Microbiomes_ 6, 37 (2020).
Article CAS PubMed PubMed Central Google Scholar * Fett, W. F., Osman, S. F. & Dunn, M. F. Characterization of exopolysaccharides produced by plant-associated fluorescent
pseudomonads. _Appl. Environ. Microbiol._ 55, 579–583 (1989). Article CAS PubMed PubMed Central Google Scholar * Irie, Y. et al. Self-produced exopolysaccharide is a signal that
stimulates biofilm formation in _Pseudomonas aeruginosa_. _Proc. Natl Acad. Sci. USA_ 109, 20632–20636 (2012). Article CAS PubMed PubMed Central Google Scholar * Tseng, B. S. et al. The
extracellular matrix protects _Pseudomonas aeruginosa_ biofilms by limiting the penetration of tobramycin. _Environ. Microbiol._ 15, 2865–2878 (2013). CAS PubMed PubMed Central Google
Scholar * Postgate, J. Trends and perspectives in nitrogen fixation research. _Adv. Microb. Physiol._ 30, 1–22 (1989). CAS PubMed Google Scholar * Schafer, A. et al. Small mobilizable
multi-purpose cloning vectors derived from the _Escherichia coli_ plasmids pK18 and pK19: selection of defined deletions in the chromosome of _Corynebacterium glutamicum_. _Gene_ 145, 69–73
(1994). Article CAS PubMed Google Scholar * Figurski, D. H. & Helinski, D. R. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided
in trans. _Proc. Natl Acad. Sci. USA_ 76, 1648–1652 (1979). Article CAS PubMed PubMed Central Google Scholar * Desnoues, N. et al. Nitrogen fixation genetics and regulation in a
_Pseudomonas stutzeri_ strain associated with rice. _Microbiol. SGM_ 149, 2251–2262 (2003). Article CAS Google Scholar * Jerabek-Willemsen, M., Wienken, C. J., Braun, D., Baaske, P. &
Duhr, S. Molecular interaction studies using microscale thermophoresis. _Assay. Drug Dev. Technol._ 9, 342–353 (2011). Article CAS PubMed PubMed Central Google Scholar * Wang, Y., Cen,
X. F., Zhao, G. P. & Wang, J. Characterization of a new GlnR binding box in the promoter of _amtB_ in _Streptomyces coelicolor_ inferred a PhoP/GlnR competitive binding mechanism for
transcriptional regulation of _amtB_. _J. Bacteriol._ 194, 5237–5244 (2012). Article CAS PubMed PubMed Central Google Scholar * Spangler, C., Bohm, A., Jenal, U., Seifert, R. &
Kaever, V. A liquid chromatography-coupled tandem mass spectrometry method for quantitation of cyclic di-guanosine monophosphate. _J. Microbiol. Methods_ 81, 226–231 (2010). Article CAS
PubMed Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Key R&D Program of China (2019YFA0904700), National Natural Science Foundation of
China (31930004, 31230004, and 31770067), National Research Project of Transgenic Crops of China (2019ZX08010-002 and 2016ZX08009003-002), and Agricultural Science and Technology Innovation
Program (ASTIP-CAASZDRW202009). We also appreciate the support of the Institut Pasteur and the Guangdong Innovative and Entrepreneurial Research Team Program. AUTHOR INFORMATION Author notes
* *These authors contributed equally: Liguo Shang, Yongliang Yan. AUTHORS AND AFFILIATIONS * Biotechnology Research Institute/Key Laboratory of Agricultural Genomics (MOA), Chinese Academy
of Agricultural Sciences, Beijing, China Liguo Shang, Yongliang Yan, Yuhua Zhan, Xiubin Ke, Yahui Shao, Hua Yang, Shanshan Wang, Shuling Dai, Jiasi Lu, Ning Yan, Zhimin Yang, Wei Lu &
Min Lin * School of Basic Medicine, Guangxi University of Chinese Medicine, Nanning, China Liguo Shang * Key Laboratory of Tropical Biological Resources of Ministry of Education, School of
Life and Pharmaceutical Sciences, Hainan University, Haikou, China Yaqun Liu & Zhu Liu * Citrus Research Institute, Southwest University/Chinese Academy of Agricultural Sciences,
Chongqing, China Shanchun Chen & Min Lin * Institut Pasteur, Paris, France Claudine Elmerich Authors * Liguo Shang View author publications You can also search for this author inPubMed
Google Scholar * Yongliang Yan View author publications You can also search for this author inPubMed Google Scholar * Yuhua Zhan View author publications You can also search for this author
inPubMed Google Scholar * Xiubin Ke View author publications You can also search for this author inPubMed Google Scholar * Yahui Shao View author publications You can also search for this
author inPubMed Google Scholar * Yaqun Liu View author publications You can also search for this author inPubMed Google Scholar * Hua Yang View author publications You can also search for
this author inPubMed Google Scholar * Shanshan Wang View author publications You can also search for this author inPubMed Google Scholar * Shuling Dai View author publications You can also
search for this author inPubMed Google Scholar * Jiasi Lu View author publications You can also search for this author inPubMed Google Scholar * Ning Yan View author publications You can
also search for this author inPubMed Google Scholar * Zhimin Yang View author publications You can also search for this author inPubMed Google Scholar * Wei Lu View author publications You
can also search for this author inPubMed Google Scholar * Zhu Liu View author publications You can also search for this author inPubMed Google Scholar * Shanchun Chen View author
publications You can also search for this author inPubMed Google Scholar * Claudine Elmerich View author publications You can also search for this author inPubMed Google Scholar * Min Lin
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.Y. and M.L. designed the experiments; L.S. performed most of the experiments. Y.Y., Y.S.
and W.L. performed RT-PCR and ddPCR experiments; Y.Y., Y.Z. and X.K. performed MST experiments; Y. L. and Z.L. performed carbohydrate monomer composition analysis. H.Y., S.W., S.D., J.L.,
N.Y. and Z.Y. helped construct the _P. stutzeri_ A1501 mutant strains, purified proteins and conducted the biofilm formation and nitrogenase activity assays. L.S., Y.Y., S.C., C.E. and M.L.
analysed data and prepared figures; Y.Y., C.E. and M.L. wrote the manuscript, with input from all authors. CORRESPONDING AUTHOR Correspondence to Min Lin. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION REPORTING SUMMARY RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shang, L., Yan, Y., Zhan, Y. _et al._ A regulatory network involving Rpo, Gac and
Rsm for nitrogen-fixing biofilm formation by _Pseudomonas stutzeri_. _npj Biofilms Microbiomes_ 7, 54 (2021). https://doi.org/10.1038/s41522-021-00230-7 Download citation * Received: 21
January 2021 * Accepted: 16 June 2021 * Published: 01 July 2021 * DOI: https://doi.org/10.1038/s41522-021-00230-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative