- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Two-dimensional carbides and nitrides, known as MXenes, are promising for water-processable coatings due to their excellent electrical, thermal, and optical properties. However,
depositing hydrophilic MXene nanosheets onto inert or hydrophobic polymer surfaces requires plasma treatment or chemical modification. This study demonstrates a universal salt-assisted
assembly method that produces ultra-thin, uniform MXene coatings with exceptional mechanical stability and washability on various polymers, including high-performance polymers for extreme
temperatures. The salt in the Ti3C2T_x_ colloidal suspension reduces surface charges, enabling electrostatically hydrophobized MXene deposition on polymers. A library of salts was used to
optimize assembly kinetics and coating morphology. A 170 nm MXene coating can reduce radiation temperature by ~200 °C on a 300 °C PEEK substrate, while the coating on Kevlar fabric provides
comfort in extreme conditions, including outer space and polar regions. SIMILAR CONTENT BEING VIEWED BY OTHERS NANOSCALE CONTROL BY CHEMICALLY VAPOUR-DEPOSITED POLYMERS Article 19 June 2020
ULTRA-THIN SELF-HEALING VITRIMER COATINGS FOR DURABLE HYDROPHOBICITY Article Open access 01 September 2021 RECASTABLE ASSEMBLIES OF CARBON DOTS INTO MECHANICALLY ROBUST MACROSCOPIC MATERIALS
Article Open access 25 October 2023 INTRODUCTION MXenes have emerged as a large family of conductive two-dimensional (2D) materials in the past decade1. Recent findings have shown that
MXene nanosheet films have outstanding thermal properties, including a wide range of emissivity in the mid-infrared (IR) spectrum, from very low to very high, along with low thermal
conductivity in the out-of-plane direction2. As a result, MXene nanosheet films can provide thermal shielding or insulation at submicrometer thickness, having weight orders of magnitude
smaller than conventional insulating materials, and work at elevated temperatures. With the IR emissivity at the level of polished metal and at least two orders of magnitude lower thermal
conductivity, Ti3C2T_x_ nanosheet film offers exceptional infrared radiation screening capability2,3, which can save large amounts of energy if MXene coatings are applied to thermal
equipment. In combination with small thickness, negligible weight per unit of area, and high flexibility, MXene coatings can provide an unprecedented level of thermal management in wearable
or aerospace applications, where low weight of thermal protection is critical. For example, a 200-nm-thick Ti3C2T_x_ coating reaches an average IR emissivity of 0.06, comparable to polished
metal2. Moreover, high electrical conductivity combined with the controlled IR emissivity allows the use of MXenes films, fibers or coatings as heaters for thermal management at low
temperatures in space, at high altitudes, or in arctic climates4,5. MXene coating on polymers from aqueous suspension at room temperature can not only replace metallization performed by
evaporating metal in vacuum, but also add numerous other functionalities. When integrated with a flexible polymer substrate, MXene is an excellent candidate for the thermal management of
individuals and equipment with both Joule heating and thermal camouflage (minimal heat loss) capabilities. However, achieving a uniform assembly of MXene nanosheets to produce smooth
coatings on many synthetic polymers from an aqueous suspension is challenging because of the hydrophobic and/or chemically inert nature of these polymers. These include many of the most
important polymers such as polyethylene (PE), polyetheretherketone (PEEK), poly(tetrafluoroethylene) (PTFE), and poly-paraphenylene terephthalamide (branded Kevlar) that have the best
mechanical and thermal properties and are widely used in aerospace, high altitude, polar regions, and other extreme environments6,7,8,9. MXene nanosheets produced by wet chemical etching
come with oxygen-based terminations and are hydrophilic in nature10. Take the most widely used MXene, Ti3C2T_x_, as an example. T_x_ represents surface terminations, mainly -OH, =O, and a
small amount of -Cl and -F, providing Ti3C2T_x_ nanosheets with a zeta potential below −30 mV and a pH of 6-7, and allowing them to form stable aqueous colloidal suspensions11. Assembly
mechanisms of MXene nanosheets on polymers in aqueous suspension can be classified as forced deposition (driven by water evaporation) and self-assembly. In forced deposition, uniform coating
usually requires wetting the polymer substrate with the MXene nanosheet colloidal suspension during fabrication, such as dip coating12 or spray coating13. In self-assembly, chemical and/or
physical interactions ensure the effective attraction between polymers and MXene nanosheets3,14,15,16. For example, polyelectrolyte can be introduced to create electrostatic attraction
between the polymer and MXene nanosheets and among MXene nanosheets. Then, a layer-by-layer deposition of MXene nanosheets can be achieved3,17. Sometimes, the two mechanisms can be
integrated to enhance the assembly effectiveness18. However, hydrophobic polymers (e.g., PE) cannot be wetted by MXene nanosheet aqueous suspension, and chemically inert polymers (e.g.,
Kevlar) do not bond to MXene nanosheets strongly enough to ensure adhesion of the MXene nanosheet. Establishing chemical bonds (ionic, hydrogen, and covalent bonds) between MXene nanosheets
and polymers by adding adhesive polymer binders (e.g., polydopamine15,16), activating the polymer substrate by oxygen plasma or acid/base treatment16, and creating electrostatic or
hydrophobic interaction via the addition of surfactants (e.g., polyelectrolyte3) has been used to coat these polymers with MXene nanosheets. However, these approaches may jeopardize the
performance of MXene coatings and/or the structural integrity of the polymer substrates. For example, the addition of poly(diallyldimethylammonium chloride) decreases the electrical
conductivity of the resulting Ti3C2T_x_ nanosheet film3. Plasma treatment may damage the surfaces of polymer substrates14 and can be difficult to apply to some structures. Chemical
treatments can be time-consuming and environmentally harmful. To address these challenges and include a wider range of polymers, a new MXene coating strategy is desirable. In this study, we
report a non-destructive, efficient, and universal salt-assisted assembly (SAA) of MXene nanosheets from aqueous suspension on various polymer substrates by adding water-soluble salts into
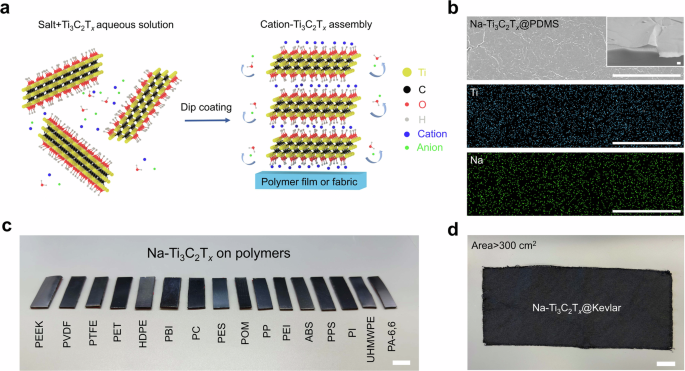
MXene nanosheet colloids (Fig. 1a). SALT-ASSISTED ASSEMBLY OF MXENE ON POLYMERS We obtained hydrophilic Ti3C2T_x_ nanosheets by etching Ti3AlC2 MAX phase and subsequent lithium-ion
intercalation of the produced multilayer MXene (Supplementary Fig. 1)19. We chose hydrophobic polydimethylsiloxane (PDMS) as the substrate for demonstration because its molecular-level flat
surface facilitates structural characterization (e.g., thickness and roughness) of the MXene coating. In an aqueous suspension, hydrophilic single- and few-layer Ti3C2T_x_ nanosheets are
stably dispersed as their negatively charged surface prevents aggregation of the nanosheets20. The SAA process includes adding salt (e.g., NaCl) to a 10 mg mL−1 (or 1 wt. %) Ti3C2T_x_
aqueous suspension, redispersion of the salt-added MXene suspension in an ultrasound bath (40 kHz, 60 W) for 15 min to prevent the aggregation of MXene nanosheets, and dipping a PDMS
substrate into the redispersed suspension using a customized dip coater (Supplementary Fig. 2). The salt concentration can be tailored to control the assembly process. In this study, we kept
the salt concentration at 0.01 mol L−1 (0.058 wt.%) in the MXene suspension unless noted otherwise. A uniform coating of Ti3C2T_x_ nanosheets on PDMS was produced (Fig. 1b). In contrast,
dipping a PDMS substrate into pristine MXene suspension (without salt) using the same dipping parameters resulted in trace amounts of MXene on PDMS (Supplementary Fig. 3). The dip-coating
process of SAA is much faster compared to conventional dip coating because of the differences in assembly mechanisms. In conventional dip coating, a thin layer of suspension containing
particles wets the substrate withdrawn from the suspension, and the evaporation at the solid-liquid-vapor interface forces the deposition of particles onto the substrate. On the contrary,
the SAA process is not evaporation-driven. The energetically favorable assembly happens at the MXene-polymer interface in the suspension. In the SAA process, the dipping speed reaches 1.5 m
min−1, 1–3 orders of magnitude higher than conventional dip coating, which is limited by slow evaporation. Herein, for chloride salt-assisted assembly of Ti3C2T_x_, the cation was used to
denote the samples. For example, Na-Ti3C2T_x_ represents Ti3C2T_x_ coating produced with NaCl. Salt ions are embedded in the assembled structures, as shown in the element mapping where both
Na from salt and Ti from MXene are uniformly distributed across the entire surface (Fig. 1b). This low concentration of NaCl (0.01 mol L−1) allows deposition of Ti3C2T_x_ (after a 15-min dip
coating and 132 ± 40 nm in thickness) with an electrical conductivity of ~20,500 S cm−1, which is comparable to the best-reported values of Ti3C2T_x_ films (Supplementary Table 2). Salt
solutions with a higher concentration (up to saturated solution) can also be used, providing a process variable that can be used to control the assembly kinetics and the resultant MXene
coating architecture (Supplementary Fig. 4). SAA is a universal assembly method for hydrophobic and hydrophilic polymers. In Fig. 1c, we show Ti3C2T_x_ coatings on 16 polymers, including
those with the highest mechanical strength and thermal resistance, such as hydrophilic Kevlar and polyimide, and hydrophobic PE and PEEK (Supplementary Fig. 5). Before the adoption of SAA,
many of these polymers needed complicated chemical modifications to be coated from aqueous MXene dispersions (Supplementary Table 3). However, with the utilization of SAA, all of them can be
uniformly coated with Ti3C2T_x_, as confirmed by SEM images (Supplementary Fig. 6). Furthermore, the thicknesses and electrical conductivities of the MXene coatings (Supplementary Table 4)
are comparable to the those on PDMS. This suggests that the substrate chemistry does not affect the morphology and properties of the coating. Moreover, the SAA strategy is feasible for both
flat and structured substrates. We have shown the MXene assembly on polymer fibers (Supplementary Fig. 7), curved surfaces, and 3D printed structures (Supplementary Fig. 8). Further, we
prepared a large-scale (>300 cm2) Kevlar fabric coated with Ti3C2T_x_ nanosheets (Fig. 1d), demonstrating the scalability of the SAA strategy. MECHANISM OF SAA STRATEGY The mechanism of
SAA can be understood by analyzing the evolution of interactions between MXene, the substrate, and solution upon adding salt through both experiment and molecular dynamics (MD) simulation.
In Fig. 2a, we have demonstrated increased contact angles (CA) of NaCl solution (i.e., from 0.01 mol L−1 to 3 mol L−1) on Na-Ti3C2T_x_ thin film assembled on PDMS substrate using the SAA
method. Similar trends are identified in a collection of polymer substrates (Supplementary Fig. 9). These results suggest that the salt solution repels both pristine MXene and polymer
substrates, enabling energetically favorable adhesion of MXene on polymers. We have also found the CA of water on Na-Ti3C2T_x_ thin film (74.1°) is higher than that on pristine Ti3C2T_x_
thin film obtained by vacuum-assisted filtration (57.5°) suggesting salt treatment increases the hydrophobicity of MXene (Supplementary Fig. 9). In summary, both the dehydration effect of
salt in the suspension and increased hydrophobicity of NaCl salt treated MXene promote the assembly of MXene. For NaCl concentration of 0.01 mol L−1, the MD simulation yields a CA of 86.24 ±
1.35°, while the experimental measurement reaches 81.89 ± 3.42°. Upon increasing concentration to 3 mol L−1, the MD-predicted CA increases to 95.81 ± 1.51°, while the experiment yields
92.48 ± 6.30°. The reasonably consistent results not only demonstrate the reliability of computation compared to the experiments but also collectively suggest a transition of MXene from
being hydrophilic to more hydrophobic with the addition of salt. Together with the hydrophobic nature of PDMS, the ion-driven hydrophobicity increase assists the adherence of MXene to the
PDMS substrate and subsequent MXene coating assembly. The SAA was further studied computationally in two consecutive steps, i.e., the MXene-PDMS assembly and the MXene-MXene assembly (Fig.
2b). Figure 2c shows that, for MXene and PDMS, the system potential energy drops noticeably at a gap distance of approximately 12 Å, in both pure water and salty suspension. The presence of
ions decreases the potential energy of the MXene surface, making assembly energetically more favored. These findings echo our experimental observations (Supplementary Fig. 3c) that while
only a small number of MXene nanosheets adhere to PDMS in pure water, upon introducing salts, MXene nanosheets promptly adhere to and cover the entire PDMS surface. After the initial layer
of MXene nanosheets has formed, ions continue to enable assemblies of multilayer MXene coatings. This phenomenon could be described by the extended DLVO (xDLVO) theory21,22,23,24. However,
many system and material parameters must be determined before applying the xDLVO theory, which often requires extensive experiments and/or simulations. Alternatively, the MD simulation
incorporates all significant physical factors, most of which were considered in the DLVO and xDLVO theories. The MD simulation is employed to compute the variations of the system potential
as a metric to delineate the stability of the MXene coatings. In pure water, the system potential energy slightly increases when two MXene nanosheets approach each other, suggesting an
energetically unfavored process that is unlikely to occur. However, in the NaCl solution (3 mol L−1), the energy drops at a gap of approximately 15 Å, making the assembly energetically
favored. Overall, ions assist assembly as they tune the hydrophobicity and mitigate the electronegativity and repulsion between MXene nanosheets. During assembly, MXene nanosheets with
adsorbed cations that deplete the negative surface charges undergo the expulsion of water molecules and anions, while some cations remain trapped within the assembled layers. The discharge
process is depicted in Fig. 2d, which shows the evolution of electrical double layers (EDLs) between two MXene nanosheets that approach each other to mimic an assembly process. Molar
densities of water molecules, cations, and anions are plotted in the MXene nanosheets of four different gap distances in the presence of a 3 mol L−1 NaCl solution. At the gap distance of 16
Å, interfacial attraction leads to a peak water density of about 120 mol L−1 in the first solvation shell (FSS), which doubles the density of bulk water (55.5 mol L−1) (see Supplementary
Fig. 10 for EDL on a MXene surface in bulk solution). As the assembly proceeds and the gap closes, water density in the confined FSS continues to drop. A similar reduction also occurs in the
anions but not in the cations. A high density of cations exceeding three times the bulk concentration is found inside the nanosheets due to the electronegativity of MXene surfaces, and they
remain trapped as water is depleted. By comparison, anions initially show a minor peak about 7 Å away from the MXene surface, gradually fading as the expulsion occurs. It is important to
note that adding salt to MXene suspension and MXene-polymer suspension can lead to flocculation or gelation of MXene (and polymer)25,26,27, which may affect the assembly uniformity. In SAA,
bath sonication is applied to redisperse MXene suspension after adding salt. The redispersed MXene nanosheets in the salt solution are stable during the assembly process, as demonstrated by
their stable size distribution (Supplementary Table 5). The redispersed MXene nanosheets in their high energy states assemble on the polymer after the insertion of the polymer substrate to
reduce the system’s energy. EFFECTS OF THE SALT COMPOSITION The salt ions that adhere to the surface of MXene can affect the structures and properties of the final MXene assembly. In
addition, the attached metal ions may enable new or enhanced functionalities, e.g., Ag+ for antibacterial function28, Al3+ for water treatment29, Sn4+ for Li-ion batteries30, and Pt4+ for
catalysis and electrocatalysis31. To fully explore the potential of the SAA method, we examined 49 salts with different combinations of cations and anions (Fig. 3a, b). The concentrations of
the salts and Ti3C2T_x_ nanosheets in the mixed suspension were kept constant at 0.01 mol L−1 and 5 mg mL−1, respectively. The assembly time was 15 minutes, and the substrate used was PDMS.
The detailed morphologies of some assemblies can be found in Supplementary Fig. 11. Element mappings of these assemblies confirm that ions from the salt are attached to the surface of
Ti3C2T_x_ nanosheets (Supplementary Figs. 12 and 13). Mostly cations of the salt were found on the surface of Ti3C2T_x_ nanosheets with a small number of anions. To prevent the interference
of Cl on the surface of Ti3C2T_x_ from the HF/HCl etching process, we used KBr as the salt, and EDS from scanning transmission electron microscope suggests the coexistence of K and Br
(Supplementary Fig. 14). The same evidence can also be found in X-ray photoelectron spectra (XPS) of pristine Ti3C2T_x_ and Cs-Ti3C2T_x_ (Supplementary Fig. 15). Salt species actively affect
the assembly kinetics. For example, by fixing the anion (i.e., Cl−) and changing the cations (i.e., Li+, Na+, K+, Cs+, Mg2+, and Al3+), we investigated the thickness and sheet resistance
evolution of Ti3C2T_x_ coatings on PDMS substrate with respect to assembly time and salt species (Fig. 3c, d). While similar trends of increased thickness and decreased sheet resistance with
respect to assembly time were observed for all salts, under the same conditions, the Cs-Ti3C2T_x_ coating was 10 times thicker than Na-Ti3C2T_x_. The deposition speed can be tailored by the
type of cations used, following the sequence of Cs+ > Al3+ > Mg2+ > K+ > Li+ > Na+. This trend can be attributed to the different dehydration capabilities of cations upon
confinement in Ti3C2T_x_ nanosheets32, as well as the charge of the ion. Cosmotropic Al3+ and Mg2+ produce stronger electrostatic attraction when intercalated between MXene nanosheets33. It
should be noted that though ions with higher dehydration capabilities, such as chaotropic Cs+ and K+, facilitate MXene assembly and lead to higher assembly speed, they result in increased
coating roughness (Supplementary Fig. 16). These results revealed the ion-specific interactions (i.e., Hofmeister effect34) in salt-assisted assembly of MXene. Moreover, the addition of salt
changes the spacing among MXene nanosheets. For example, by fixing the anion (i.e., Cl−) and changing the cations (i.e., Li+, Na+), the spacing of stacked Ti3C2T_x_ nanosheets changes from
14.1 (with Li+) to 13.2 Å (with Na+) (Supplementary Fig. 17) which can be used for tunable piezoresistive sensors, as previous studies have shown35. The Raman peak positions remain almost
unchanged, independent of the ion used, indicative of no detectable chemical changes in Ti3C2T_x_ coatings with metal ions compared to the pristine ones (Supplementary Fig. 18). THERMAL
MANAGEMENT USING MXENE-COATED POLYMERS To enable thermal management at high and low temperatures, Na-Ti3C2T_x_ nanosheets were assembled on two of the most temperature-resistant polymers:
PEEK film (Na-Ti3C2T_x_@PEEK, coating thickness: ~170 nm) and Kevlar fabric (Na-Ti3C2T_x_@Kevlar, coating thickness: ~870 nm) (Supplementary Fig. 19). The thermal management mechanism is
shown in Fig. 4a. When a MXene coating is applied to the polymer sample placed on a hot plate, the low-emissivity MXene leads to the measured by the IR camera temperature (_T_reduction) on
the surface being much lower than the hot plate temperature (_T_radiation). Figure 4b shows that when the hot plate was heated up to 300 °C for Na-Ti3C2T_x_@PEEK and 400 °C for
Na-Ti3C2T_x_@Kevlar, the temperature difference (_T_radiation - _T_reduction) reached ~200 °C for Na-Ti3C2T_x_@PEEK and ~250 °C for Na-Ti3C2T_x_@Kevlar. In comparison, the _T_radiation -
_T_reduction of pure PEEK and Kevlar was only 14 °C and 58 °C, respectively (Supplementary Fig. 20). The stability of the thermal camouflage properties was examined over 50 heating and
cooling cycles and in a long-term heating test for 48 hours, as shown in Figs. 4b and 4c (see detailed data in Supplementary Figs. 21–23 and Supplementary Table 6). The overlapping data at
the 1st, 25th, and 50th cycles of both sets of samples demonstrate excellent thermal stability and repeatability. After holding the sample at the highest _T_radiation for 48 h, _T_reduction
reaches 117.2 °C for Na-Ti3C2T_x_@PEEK and 158 °C for Na-Ti3C2T_x_@Kevlar with only a small increase of 2.8 °C and 4.9 °C compared to their initial values, respectively. While the thermal
camouflage capability can be mainly attributed to the Na-Ti3C2T_x_ coating, the outstanding cyclic and long-term thermal camouflage stability is a result of the stable polymer substrate and
the stable interface between the MXene and the polymer (Supplementary Fig. 24). In addition to the IR camera, we also used a thermocouple to probe the real surface temperature (_T_real) of
Na-Ti3C2T_x_@Kevlar on the 400 °C hot plate (Supplementary Fig. 23). The real surface temperature can still reach 334.6 °C after 120 s under _T_radiation of 400 °C. We further demonstrated
the Joule heating performance of Na-Ti3C2T_x_@Kevlar, as shown in Fig. 4d–f. By regulating the applied voltages, different heating temperatures (75.2 °C at 4 V and 192.9 °C at 8 V by IR
camera) were rapidly achieved, where the _T_real measured by thermocouple showed similar values (71.7 °C at 4 V and 206.3 °C at 8 V) (Fig. 4e). Then, a long-term Joule heating test was
performed at 4 V for 4 h (Fig. 4f), showing excellent stability. The stable performance can be attributed to the robustness of Na-Ti3C2T_x_@Kevlar (Supplementary Fig. 24). In
wearable/flexible applications, both the flexibility and washing stability of MXene-coated polymer films and textiles are essential. We tested the bending durability of Na-Ti3C2T_x_@Kevlar
by comparing thermal camouflage performance and sheet resistance evolution before and after 2000 bending cycles (Fig. 4g). Both _T_reduction (from 151.0 °C to 157.3 °C at _T_radiation = 400
°C) and sheet resistance (from 2.7 Ohm sq−1 to 8.3 Ohm sq−1 at room temperature) experience a small increase. We also examined the washing stability through a stirring washing test. The
solution was stirred using a magnetic stir bar at 1000 rpm in a 1 L beaker to mimic the real washing conditions. Three types of solutions were tested: deionized water (DI) water, isopropanol
(IPA) solution, and an industrial strength washing agent Synthrapol (10%, v/v). The sheet resistances of Na-Ti3C2T_x_@PEEK and Na-Ti3C2T_x_@Kevlar before and after washing were compared
(Fig. 4h). After 168 hours of continuous washing in the harshest Synthrapol solution, sheet resistance increased from 3.4 Ohm sq−1 to 82 Ohm sq−1 for Na-Ti3C2T_x_@PEEK and from 2.6 Ohm sq−1
to 86.5 Ohm sq−1 for Na-Ti3C2T_x_@Kevlar. Considering a washing frequency of once a week for 1 h, such wearables can last for at least 3 years. The strong interface between Na-Ti3C2T_x_
nanosheets and the polymer substrates can explain this excellent performance (Supplementary Figs. 25 and 26). The combination of outstanding mid-IR reflectivity, low thermal conductivity,
Joule heating capability, and bending and washing stability of MXene-coated high-performance polymers can be used in protective gear for individuals and equipment operating in
extreme-temperature environments. As shown in Fig. 4i, we compared the performance range of the Na-Ti3C2T_x_@Kevlar system (red region) with the state-of-the-art values from MXene@polymer
systems (gray region) in terms of the highest _T_radiation, the highest temperature difference in thermal camouflage, the highest Joule heating temperature, bending durability, and washing
durability. Compared with other MXene composite structures with similar bending and washing durability, Na-Ti3C2T_x_@Kevlar significantly outperforms them thermally (Supplementary Table 7).
To demonstrate the potential applications, we designed a three-layer heat-management gear that can be used, e.g., in Mars exploration, to overcome the ultralow temperature (mean temperature:
−65 °C). As shown in Fig. 4j, from top to bottom, a Na-Ti3C2T_x_@Kevlar layer was connected to an external power source and used as the heating layer (H-layer), an insulating Kevlar layer
was used as a separator (S-layer) to prevent short circuits, and another Na-Ti3C2T_x_@Kevlar layer was used to reflect mid-IR from the heating layer (R-layer) and prevent radiative loss to
the dry ice (about −80 °C) environment (Fig. 4k). Such gear can be called H-S-R gear. An IR camera was used to monitor the temperature of the H-layer. With a 4 V bias, the H-layer reached
51.1 °C. For comparison, if the R-layer is substituted with an S-layer to form an H-S-S gear, the H-layer can only reach 41.7 °C. This ~10 °C difference is significant considering that the
Na-Ti3C2T_x_ layer on the R-layer is only 872 nm thick. Note that it is not necessary to build H-S-R gear all over the body. As hands and feet are the most vulnerable body parts at low
temperatures, we suggest building H-S-R into mittens and boots to reduce energy consumption. Of course, there is much room for optimization of this design. For example, other MXenes with
higher emissivity can be used for the heating layer, and a smooth Ti3C2T_x_ coating on a polymer film can be used for the reflective layer. We developed a universal salt-assisted assembly
protocol for fast large-scale assembly of MXene coatings on polymer substrates. By adding NaCl (or dozens of other salts) to the MXene colloidal suspension in water, we increased the
hydrophobicity of both MXene and the polymer, promoting substrate-independent MXene deposition. Furthermore, the assembly kinetics, overall coating thickness, and architecture can be
tailored by altering the salt ions and concentration. Coating high-performance polymers, such as Kevlar and PEEK, with MXenes holds the potential for significant advancements in thermal
management under extremely low and high temperatures, preventing heat loss and protecting equipment and personnel. There are numerous applications for polymers coated with conductive MXene
films, which possess a variety of optical and electronic properties. Incorporating catalytic metals like platinum or bactericidal silver ions further expands the range of potential
applications of these materials. METHODS MATERIALS All the materials used in this work are summarized in Supplementary Table S1. SYNTHESIS OF TI3C2T_X_ NANOSHEET SUSPENSION Ti3C2T_x_ was
synthesized by the selective etching of Ti3AlC2 MAX phase powder (<40 μm particle size, Carbon-Ukraine) with a mixture of hydrofluoric (HF) (29 M, Acros Organics) and hydrochloric (HCl)
(12 M, Fisher Chemical) acids36. First, 2 mL of HF, 12 mL of HCl, and 6 mL of de-ionized (DI) water were combined. After that, 1 g of MAX phase powder was added to the solution and stirred
for 24 h at 35 °C. After etching, the reaction product was washed with DI water through 5-min centrifugation cycles at 2054 × _g_ until pH exceeded 6. The obtained sediment was dispersed in
20 mL of 1 mol L−1 LiCl solution for Li+ intercalation, and the reaction was allowed to proceed for 12–24 h at 300 rpm and 35 °C. The mixture was then washed with DI water to remove excess
LiCl using 10-min centrifugation cycles at 2054 × _g_ until the supernatant darkened and the sediment swelled. Then a final washing cycle was performed at 2054 × _g_ for 1 h. The resulting
clear supernatant was decanted and exchanged with DI water to redisperse the sediment with agitation. The mixture was centrifuged at 2054 × _g_ rpm for 10 min, with the dark supernatant
being collected as a single layer Ti3C2T_x_ dispersion. Sediment redispersion, 10-minute centrifugation at 2054 × _g_, and supernatant (Ti3C2T_x_) collection were repeated till the
supernatant became clear. ASSEMBLY OF TI3C2T_X_ NANOSHEETS ON POLYMER SUBSTRATES A Ti3C2T_x_ nanosheet colloidal suspension (10 mg L−1, 10 mL) was diluted by adding a prepared salt solution
(0.02 mol L−1, 10 mL). In this way, the salt-Ti3C2T_x_ suspension was fabricated, where the resultant salt and Ti3C2T_x_ nanosheet concentrations were 0.01 mol L−1 and 5 mg mL−1,
respectively. Because the Ti3C2T_x_ nanosheets aggregation occurs when mixing salt and Ti3C2T_x_ nanosheet suspension, the salt-Ti3C2T_x_ suspension was sonicated for 15 min in a sonication
bath (40 kHz, 60 W) to disperse Ti3C2T_x_ nanosheet. After that, we used a customized dip coater (average dipping speed = 1.5 m min−1) to coat various polymer substrates. It is worth noting
that the polymer substrates were submerged in the suspension during the whole assembly process. The assembled Ti3C2T_x_ coatings on polymer substrates were dried with flowing compressed
nitrogen gas to remove the excess suspension. To prevent salt crystals from precipitating from the suspension during drying, a DI water rinsing step was applied to the dried surface,
followed by another round of nitrogen gas drying. MODELS AND COMPUTATIONAL METHODS Figure 2b illustrates the molecular model for energetically analyzing the assembly process. The model
consisted of a polydimethylsiloxane (PDMS) layer serving as the substrate and MXene monolayers arranged parallel to the PDMS surface. The computational study involved two steps, i.e., the
PDMS-MXene assembly, followed by the MXene-MXene assembly. The simulation box had the dimensions of 66 × 66 × 95 Å3. Periodic boundary conditions were applied to all three dimensions. The
amorphous PDMS comprised 20 chains, each with 50 repeating units. The PDMS model was initialized by energy minimization and relaxation to achieve the desired density with atomistic surface
roughness. Each MXene monolayer consisted of 255-unit cells in the 17 × 15 pattern, resulting in a membrane of 45.63 Å × 44.78 Å positioned at the center of the simulation box. The Ti3C2O2
MXene unit cell structure was derived from crystallographic experimental results37, and the surface was negatively charged to reflect MXene’s electronegativity. The system was filled with
7338 water molecules and 3 mol L−1 Na+ and Cl− ions to study ionic effects. For comparison, another system with pure water was built, which had 7739 water molecules. In studying the
PDMS-MXene assembly, the distance between the top surface of PDMS and the bottom surface of MXene varied from 15 Å to 9 Å. In studying the MXene-MXene assembly, the distance between the two
MXene surfaces varied from 17 Å to 10 Å. Molecular dynamics (MD) simulations were carried out using the large-scale atomic/ molecular massively parallel simulator (LAMMPS)38. Force field
parameters for MXene were obtained from the work by Xu et al.39,40, which is widely used to simulate the behavior of MXene in suspension environments41,42. Water molecules were described by
the SPC/E model43. Ions were modeled by using the parameters proposed by Loche et al.44. PDMS was also described by the LJ potential and partial charges45,46,47. Detailed LJ parameters and
charges are shown in Supplementary Table 8. Interatomic LJ interactions were described by the Lorentz-Berthelot combining rule. A cut-off distance of 10 Å was employed for both the LJ and
Coulombic terms. Long-range interactions were handled by the particle-particle particle-mesh (PPPM) algorithm with a 10−5 precision48. MD simulation was carried out under the NVT ensemble
with a temperature of 293.15 K maintained using the Nose-Hoover thermostat and the atmospheric pressure. The time step was 2 fs. At each gap distance during the assembly analysis, the system
was relaxed for 10 ns to reach equilibrium; subsequently, a production run of 15 ns was performed to obtain the number density distribution and averaged system potential energy.
CHARACTERIZATION The X-ray diffraction (XRD) analyses of Ti3AlC2 MAX phase powder, pristine Ti3C2T_x_ film made by drop-casting on a glass slide, and Ti3C2T_x_ assemblies on PDMS substrates
obtained by SAA method were performed on a Rigaku Miniflex X-ray Diffractometer (40 kV and 15 mA) with Cu Kα radiation and a scanning speed of 10° min−1. The Ti3C2T_x_ nanosheet size
distribution was measured by the dynamic light scattering (DLS) (Malvern Zetasizer Nano ZS) using a suspension diluted to 0.01 mg mL−1. The monolayer Ti3C2T_x_ nanosheet thickness on Si/SiO2
wafer was determined by atomic force microscopy (AFM) (Park Systems NX10) in a noncontact mode. The contact angle of water and salt solutions (~5 μL) on polymer substrates and Ti3C2T_x_
nanosheet films was measured using a lab-made contact angle tester. The scanning electron microscope (SEM) images and x-ray energy dispersive spectrum (EDS) mapping of Ti3C2T_x_ assemblies
on different polymer films and fibers were acquired using a field emission scanning electron microscope (FE-SEM) (Hitachi S-4800 SEM) at 20 kV and 20 mA without sputtering. For the tilted
angle view SEM images of the samples, we coated a 6 nm gold layer on both, the top surface and the side of the samples. The high angle annular dark field (HAADF) images, electron diffraction
spectroscopy (EDS), and elemental mapping measurements were performed with double-corrected Titan cubed Themis G2 operated at 300 kV in the Electron Microscopy Center (EMC) of Shared
Equipment Authority (SEA) at Rice University. The microscope is equipped with a Ceta camera, Gatan Quantum 966 energy filter, and an electron monochromator. X-ray photoelectron spectra (XPS)
were obtained using a PHI VersaProbe 5000 spectrometer (Physical Electronics, U.S.) with a monochromatic Al Kα X-ray source (1486.6 eV) at a 200 µm spot size and 50 W power. The spectra
were collected with a 23.5 eV pass energy and an increment of 0.05 eV. All samples were mounted on conductive carbon tapes and electrically grounded via copper tape. High-resolution XPS data
were fitted using the CasaXPS software package, employing a Tougaard background for transition metal-based species. The chemical states of Ti3C2T_x_ MXene and the cations were deduced from
core-level spectral fits. Raman spectra of Ti3C2T_x_ and SAS Ti3C2T_x_ coatings on polymer substrates were obtained using a WITec alpha300 confocal Raman microscope at an excitation laser
wavelength of 785 nm with an 20× objective. The integration time was fixed to 2 s. The thickness and roughness of salt-treated Ti3C2T_x_ assemblies on PDMS were measured by a Keyence
VK-X1000 optical profilometer. The sample was placed under a 50× magnification lens of a Keyence VK-X1000 optical profilometer and evaluated using laser confocal scanning. Plane correction
was performed on the scan using the accompanying software before three areas (with slight overlap and together covering the entire scan) were arbitrarily selected for roughness calculations.
The areal average roughness (Sa) was chosen as the representative roughness parameter, which is calculated as the mean of the average height difference for the average surface. The sheet
resistance of salt-treated Ti3C2T_x_ assemblies was determined by four-point probe measurements (Jandel ResTest). For each sample, 10 points were measured, the average value was presented,
and the standard deviation was calculated as the error. The surface temperature of Na-Ti3C2T_x_ assemblies on PEEK film and Kevlar fabrics is recorded by an IR camera (HIKMICRO B20). The
distance between the sample and the IR camera lens is fixed at 0.3 m, and the detected wavelength ranges from 8 to 14 μm. The absorbance/emissivity of salt-treated Ti3C2T_x_ assemblies at
different temperatures was tested using an FTIR spectrometer (Invenio-X, Bruker, Germany). An emission adapter (A540/3) was used to heat the samples and the black body reference (a soot
layer on the metal sheet). The emissivity in the 5–25 μm range is given by the ratio of sample emission (_ν_) and the reference emission at the same temperature (_T_). REPORTING SUMMARY
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All data are available in the main text or the
supplementary information. Source data are provided with this paper. REFERENCES * Naguib, M. et al. Two‐dimensional nanocrystals produced by exfoliation of Ti3AlC2. _Adv. Mater._ 23,
4248–4253 (2011). Article CAS PubMed Google Scholar * Han, M. et al. Versatility of infrared properties of MXenes. _Mater. Today_ 64, 31–39 (2023). Article CAS Google Scholar * Wang,
J., Shen, M., Liu, Z. & Wang, W. MXene materials for advanced thermal management and thermal energy utilization. _Nano Energy_ 97, 107177 (2022). Article CAS Google Scholar * Guo, Z.,
Sun, C., Wang, J., Cai, Z. & Ge, F. High-performance laminated fabric with enhanced photothermal conversion and joule heating effect for personal thermal management. _ACS Appl. Mater.
Interfaces_ 13, 8851–8862 (2021). Article CAS PubMed Google Scholar * Peng, Y. & Cui, Y. Advanced textiles for personal thermal management and energy. _Joule_ 4, 724–742 (2020).
Article CAS Google Scholar * Li, Q. et al. Flexible high-temperature dielectric materials from polymer nanocomposites. _Nature_ 523, 576–579 (2015). Article ADS CAS PubMed Google
Scholar * Luo, N. et al. Ice-based triboelectric nanogenerator with low friction and self-healing properties for energy harvesting and ice broken warning. _Nano Energy_ 97, 107144 (2022).
Article CAS Google Scholar * Gonzalez, G. M. et al. Para-aramid fiber sheets for simultaneous mechanical and thermal protection in extreme environments. _Matter_ 3, 742–758 (2020).
Article Google Scholar * Zhang, T. G., Satapathy, S. S., Vargas-Gonzalez, L. R. & Walsh, S. M. Ballistic impact response of ultra-high-molecular-weight polyethylene (UHMWPE). _Compos.
Struct._ 133, 191–201 (2015). Article Google Scholar * Lim, K. R. G. et al. Fundamentals of MXene synthesis. _Nat. Synth._ 1, 601–614 (2022). Article ADS Google Scholar * Naguib, M.,
Barsoum, M. W. & Gogotsi, Y. Ten years of progress in the synthesis and development of MXenes. _Adv. Mater._ 33, 2103393 (2021). Article CAS Google Scholar * Salles, P. et al.
Electrochromic effect in titanium carbide MXene thin films produced by dip‐coating. _Adv. Funct. Mater._ 29, 1809223 (2019). Article Google Scholar * Sarycheva, A. et al. 2D titanium
carbide (MXene) for wireless communication. _Sci. Adv._ 4, eaau0920 (2018). Article ADS PubMed PubMed Central Google Scholar * Park, T. H. et al. Shape-adaptable 2D titanium carbide
(MXene) heater. _ACS Nano_ 13, 6835–6844 (2019). Article CAS PubMed Google Scholar * Luo, J. et al. Superhydrophobic and breathable smart MXene-based textile for multifunctional wearable
sensing electronics. _Chem. Eng. J._ 406, 126898 (2021). Article CAS Google Scholar * Shi, M. et al. Ti3C2T_x_ MXene-decorated nanoporous polyethylene textile for passive and active
personal precision heating. _ACS Nano_ 15, 11396–11405 (2021). Article CAS PubMed Google Scholar * Bury, D. et al. Photocatalytic activity of the oxidation stabilized Ti3C2T_x_ MXene in
decomposing methylene blue, bromocresol green and commercial textile dye. _Small Methods_ 7, 2201252 (2023). Article CAS Google Scholar * Uzun, S. et al. Knittable and washable
multifunctional MXene‐coated cellulose yarns. _Adv. Funct. Mater._ 29, 1905015 (2019). Article CAS Google Scholar * Alhabeb, M. et al. Guidelines for synthesis and processing of
two-dimensional titanium carbide (Ti3C2T_x_ MXene). _Chem. Mater._ 29, 7633–7644 (2017). Article CAS Google Scholar * Maleski, K., Mochalin, V. N. & Gogotsi, Y. Dispersions of
two-dimensional titanium carbide MXene in organic solvents. _Chem. Mater._ 29, 1632–1640 (2017). Article CAS Google Scholar * Zachariah, Z., Heuberger, M. P. & Espinosa-Marzal, R. M.
in _One Hundred Years of Colloid Symposia: Looking Back and Looking Forward_ 31–47 (ACS Publications, 2023). * van Oss, C. J. in _Interface Science and Technology_ 16, 31–48 (Elsevier,
2008). * Shams, M., Alam, I. & Chowdhury, I. Aggregation and stability of nanoscale plastics in aquatic environment. _Water Res._ 171, 115401 (2020). Article CAS PubMed Google Scholar
* Mustin, B. & Stoeber, B. Single layer deposition of polystyrene particles onto planar polydimethylsiloxane substrates. _Langmuir_ 32, 88–101 (2016). Article CAS PubMed Google
Scholar * Cao, H. et al. Synthesis and Electronic Applications of particle-templated Ti3C2T_z_ MXene-polymer films via Pickering emulsion polymerization. _ACS Appl. Mater. Interfaces_ 13,
51556–51566 (2021). Article CAS PubMed Google Scholar * Cao, H. et al. Flocculation of MXenes and their use as 2D particle surfactants for capsule formation. _Langmuir_ 37, 2649–2657
(2021). Article CAS PubMed Google Scholar * Cao, H. et al. Electrically conductive porous Ti3C2T_x_ MXene-polymer composites from high internal phase emulsions (HIPEs). _2D Materials_ 9,
044004 (2022). Article CAS Google Scholar * Pandey, R. P. et al. Ultrahigh-flux and fouling-resistant membranes based on layered silver/MXene (Ti3C2T_x_) nanosheets. _J. Mater. Chem. A_
6, 3522–3533 (2018). Article CAS Google Scholar * Ding, L. et al. Effective ion sieving with Ti3C2T_x_ MXene membranes for production of drinking water from seawater. _Nat. Sustain._ 3,
296–302 (2020). Article Google Scholar * Luo, J. et al. Sn4+ ion decorated highly conductive Ti3C2 MXene: promising lithium-ion anodes with enhanced volumetric capacity and cyclic
performance. _ACS Nano_ 10, 2491–2499 (2016). Article CAS PubMed Google Scholar * Zhao, D. et al. MXene (Ti3C2) vacancy-confined single-atom catalyst for efficient functionalization of
CO2. _J. Am. Chem. Soc._ 141, 4086–4093 (2019). Article CAS PubMed Google Scholar * Gao, Q. et al. Tracking ion intercalation into layered Ti3C2 MXene films across length scales. _Energy
Environ. Sci._ 13, 2549–2558 (2020). Article CAS Google Scholar * Shpigel, N. et al. Direct assessment of nanoconfined water in 2D Ti3C2 electrode interspaces by a surface acoustic
technique. _J. Am. Chem. Soc._ 140, 8910–8917 (2018). Article CAS PubMed Google Scholar * Parsons, D. F. & Salis, A. Hofmeister effects at low salt concentration due to surface
charge transfer. _Curr. Opin. Colloid Interf. Sci._ 23, 41–49 (2016). Article CAS Google Scholar * Ma, Y. et al. A highly flexible and sensitive piezoresistive sensor based on MXene with
greatly changed interlayer distances. _Nat. Commun._ 8, 1207 (2017). Article ADS PubMed PubMed Central Google Scholar * Anayee, M. et al. Role of acid mixtures etching on the surface
chemistry and sodium ion storage in Ti3C2T_x_ MXene. _Chem. Commun._ 56, 6090–6093 (2020). Article CAS Google Scholar * Khazaei, M. et al. Novel electronic and magnetic properties of
two‐dimensional transition metal carbides and nitrides. _Adv. Funct. Mater._ 23, 2185–2192 (2013). Article CAS Google Scholar * Plimpton, S. Fast parallel algorithms for short-range
molecular dynamics. _J. Comput. Phys._ 117, 1–19 (1995). Article ADS CAS Google Scholar * Xu, K. et al. Effects of functional groups and anion size on the charging mechanisms in layered
electrode materials. _Energy Storage Mater_ 33, 460–469 (2020). Article Google Scholar * Xu, K. et al. Charging/discharging dynamics in two-dimensional titanium carbide (MXene) slit
nanopore: Insights from molecular dynamic study. _Electrochim. Acta_ 196, 75–83 (2016). Article CAS Google Scholar * Ma, X., Zhu, X., Huang, C. & Fan, J. Revealing the effects of
terminal groups of MXene on the water desalination performance. _J. Membrane Sci._ 647, 120334 (2022). Article CAS Google Scholar * Xu, K. et al. Tracking ionic rearrangements and
interpreting dynamic volumetric changes in two‐dimensional metal carbide supercapacitors: A molecular dynamics simulation study. _ChemSusChem_ 11, 1892–1899 (2018). Article CAS PubMed
Google Scholar * Berendsen, H. J., Postma, J. V., Van Gunsteren, W. F., DiNola, A. & Haak, J. R. Molecular dynamics with coupling to an external bath. _J. Chem. Phys._ 81, 3684–3690
(1984). Article ADS CAS Google Scholar * Loche, P., Steinbrunner, P., Friedowitz, S., Netz, R. R. & Bonthuis, D. J. Transferable ion force fields in water from a simultaneous
optimization of ion solvation and ion-ion interaction. _J. Phys. Chem. B_ 125, 8581–8587 (2021). Article CAS PubMed PubMed Central Google Scholar * Chen, S. et al. Multiscale modeling
to predict the hydrophobicity of an experimentally designed coating. _J. Phys. Chem. C_ 124, 9866–9875 (2020). Article ADS CAS Google Scholar * Argyris, D., Tummala, N. R., Striolo, A.
& Cole, D. R. Molecular structure and dynamics in thin water films at the silica and graphite surfaces. _J. Phys. Chem. C_ 112, 13587–13599 (2008). Article CAS Google Scholar *
Ismail, A. E., Grest, G. S., Heine, D. R., Stevens, M. J. & Tsige, M. Interfacial structure and dynamics of siloxane systems: PDMS-vapor and PDMS-water. _Macromolecules_ 42, 3186–3194
(2009). Article ADS CAS Google Scholar * Shi, B., Sinha, S. & Dhir, V. K. Molecular dynamics simulation of the density and surface tension of water by particle-particle particle-mesh
method. _J. Chem. Phys._ 124, 204715 (2006). Article ADS PubMed Google Scholar * Ma, H. et al. Blade-coated Ti3C2T_x_ MXene films for pseudocapacitive energy storage and infrared
stealth. _Diam. Relat. Mater._ 131, 109587 (2023). Article CAS Google Scholar * Li, X. et al. Wearable janus‐type film with integrated all‐season active/passive thermal management,
thermal camouflage, and ultra‐high electromagnetic shielding efficiency tunable by origami process. _Adv. Funct. Mater._ 33, 2212776 (2023). Article CAS Google Scholar * Yu, Q. et al.
Ti3C2T_x_@nonwoven fabric composite: Promising MXene-coated fabric for wearable piezoresistive pressure sensors. _ACS Appl. Mater. Interfaces_ 14, 9632–9643 (2022). Article CAS PubMed
Google Scholar * Zhou, Z., Song, Q., Huang, B., Feng, S. & Lu, C. Facile fabrication of densely packed Ti3C2 MXene/nanocellulose composite films for enhancing electromagnetic
interference shielding and electro-/photothermal performance. _ACS Nano_ 15, 12405–12417 (2021). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr. A.
Clark (Bryn Mawr College) for assistance with the XRD study, Dr. S. Dietrich (Villanova University) for the assistance with AFM, Drs. A. Jester and R. Lee (Villanova University) for the
electrical conductivity measurements, and B. Hammill (Drexel University) for supporting MXene synthesis. L.Z., Y.L, A.F., and B.L. were supported in part by the U.S. National Science
Foundation (Grants # AM-2003077, NRI-2221102, and MRI-2018852, B.L.), PA Manufacturing Fellows Initiative, Sport & Performance Engineering Seed Grant of College of Engineering, Villanova
University. MXene synthesis by L.B., Y.G. and D.Z. at Drexel University was supported in part by the U.S. National Science Foundation (Grant # DMR-2041050, Y.G.). MD simulation by L.L. and
J.H. was supported in part by the U.S. National Science Foundation (Grant # CBET-1751610, L.L.). The characterization of MXene films by T.Z. was supported by the U.S. Department of Energy,
Office of Science, Office of Basic Energy Sciences (Grant # DE-SC0018618, Y.G.). Work at Bryn Mawr College was supported by the U.S. National Science Foundation (Grants # DMR-2242796,
X.M.C.). The article processing charge is funded by Villanova University’s College of Engineering and Falvey Memorial Library Scholarship Open Access Reserve Fund. AUTHOR INFORMATION Author
notes * These authors contributed equally: Liang Zhao, Lingyi Bi, Jiayue Hu. AUTHORS AND AFFILIATIONS * Hybrid Nano-Architectures and Advanced Manufacturing Laboratory, Department of
Mechanical Engineering, Villanova University, Villanova, PA, USA Liang Zhao, Yun Li, Aidan Flynn & Bo Li * A. J. Drexel Nanomaterials Institute and Department of Materials Science and
Engineering, Drexel University, Philadelphia, PA, USA Lingyi Bi, Danzhen Zhang, Teng Zhang, Ruocun Wang & Yury Gogotsi * Department of Mechanical Engineering, Temple University,
Philadelphia, PA, USA Jiayue Hu & Ling Liu * Electron Microscopy Center, Shared Equipment Authority, Rice University, Houston, TX, USA Guanhui Gao * Department of Physics, Bryn Mawr
College, Bryn Mawr, PA, USA Xuemei M. Cheng Authors * Liang Zhao View author publications You can also search for this author inPubMed Google Scholar * Lingyi Bi View author publications You
can also search for this author inPubMed Google Scholar * Jiayue Hu View author publications You can also search for this author inPubMed Google Scholar * Guanhui Gao View author
publications You can also search for this author inPubMed Google Scholar * Danzhen Zhang View author publications You can also search for this author inPubMed Google Scholar * Yun Li View
author publications You can also search for this author inPubMed Google Scholar * Aidan Flynn View author publications You can also search for this author inPubMed Google Scholar * Teng
Zhang View author publications You can also search for this author inPubMed Google Scholar * Ruocun Wang View author publications You can also search for this author inPubMed Google Scholar
* Xuemei M. Cheng View author publications You can also search for this author inPubMed Google Scholar * Ling Liu View author publications You can also search for this author inPubMed Google
Scholar * Yury Gogotsi View author publications You can also search for this author inPubMed Google Scholar * Bo Li View author publications You can also search for this author inPubMed
Google Scholar CONTRIBUTIONS L.Z. and B.L. conceived the concept of the assembly method and designed the experiment. Y.G. directed the MXene synthesis, experiment design, and data analysis.
L.L. directed MD simulation. L.Z. performed the assembly process and sample preparation, SEM and EDS images, electrical conductivity test, FTIR, X-ray diffraction, contact angle
measurements, thermal camouflage, and Joule heating test. L.B. synthesized MXene suspension and performed characterizations of MXene nanosheets and coating morphology. J.H. performed the MD
simulation. D.Z. performed the high-temperature FTIR measurement. L.Z. and R.W. contributed to the Raman spectra measurement. G.G. performed the TEM imaging. Y.L. fabricated the 3D-printed
PDMS substrates. A.F. collected and summarized the Joule heating data. T.Z. contributed to the XPS measurement. X.M.C. contributed to the XRD characterization of MXene coatings. L.Z., L.B.,
J.H., L.L., Y.G. and B.L. co-wrote the manuscript. All authors discussed the results and commented on the manuscript. L.Z., L.B. and J.H. contributed equally to this work. CORRESPONDING
AUTHORS Correspondence to Ling Liu, Yury Gogotsi or Bo Li. ETHICS DECLARATIONS COMPETING INTERESTS L.Z. and B.L. filed a U.S. Patent (Applicant: Villanova University; Application No.
2023/0286015 A1) based on the salt-assisted assembly method. L.Z., L.B., Y.G., and B.L. filed a U.S. Provisional patent (Applicant: Villanova University; Application No. 63/623,093) based on
thermal management applications. The remaining authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks Agnieszka Jastrzębska, and the
other, anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION REPORTING SUMMARY TRANSPARENT PEER REVIEW FILE
SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Zhao, L., Bi, L., Hu, J. _et al._ Universal salt-assisted assembly of MXene from suspension on polymer substrates. _Nat Commun_ 15, 10027 (2024).
https://doi.org/10.1038/s41467-024-53840-y Download citation * Received: 18 February 2024 * Accepted: 21 October 2024 * Published: 25 November 2024 * DOI:
https://doi.org/10.1038/s41467-024-53840-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




