- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Protoberberine alkaloids and benzophenanthridine alkaloids (BZDAs) are subgroups of benzylisoquinoline alkaloids (BIAs), which represent a diverse class of plant-specialized natural
metabolites with many pharmacological properties. Microbial biosynthesis has been allowed for accessibility and scalable production of high-value BIAs. Here, we engineer _Saccharomyces
cerevisiae_ to de novo produce a series of protoberberines and BZDAs, including palmatine, berberine, chelerythrine, sanguinarine and chelirubine. An ER compartmentalization strategy is
developed to improve vacuole protein berberine bridge enzyme (BBE) activity, resulting in >200% increase on the production of the key intermediate (_S_)-scoulerine. Another promiscuous
vacuole protein dihydrobenzophenanthridine oxidase (DBOX) has been identified to catalyze two-electron oxidation on various tetrahydroprotoberberines at N7-C8 position and
dihydrobenzophenanthridine alkaloids. Furthermore, cytosolically expressed DBOX can alleviate the limitation on BBE. This study highlights the potential of microbial cell factories for the
biosynthesis of a diverse group of BIAs through engineering of heterologous plant enzymes. SIMILAR CONTENT BEING VIEWED BY OTHERS DE NOVO BIOSYNTHESIS OF BERBERINE AND HALOGENATED
BENZYLISOQUINOLINE ALKALOIDS IN _SACCHAROMYCES CEREVISIAE_ Article Open access 09 February 2023 XYLOSE AND SHIKIMATE TRANSPORTERS FACILITATES MICROBIAL CONSORTIUM AS A CHASSIS FOR
BENZYLISOQUINOLINE ALKALOID PRODUCTION Article Open access 28 November 2023 ENGINEERING COFACTOR SUPPLY AND RECYCLING TO DRIVE PHENOLIC ACID BIOSYNTHESIS IN YEAST Article 28 April 2022
INTRODUCTION The misuse and abuse of antibiotics in human and livestock settings has led to the emergence of antimicrobial resistance, which poses a serious threat on global public health,
as highlighted by the World Health Organization in 20151. As an alternative approach, the exploration of phytogenic natural products with antimicrobial properties has gained much attention.
Protoberberine alkaloids and benzophenanthridine alkaloids (BZDAs) are two classes of such natural products that have attracted increasing interest due to their bioactivity and safety
against microorganisms and viruses2. Both protoberberines and BZDAs belong to the big family of benzylisoquinoline alkaloids (BIAs), which encompasses more than 2500 structurally diverse
compounds known for their pharmacological properties3. Within this family, berberine stands out as a prominent protoberberine alkaloid, demonstrating efficacy against numerous pathogenic
bacteria and viruses4. Furthermore, many clinical trials on berberine have been conducting to evaluate its pharmacological properties, including its anti-hyperlipidemic5, anti-diabetic5,
anti-inflammation6, and the potential to prevent atherosclerosis7. Another notable protoberberine alkaloid, palmatine exerts similar antioxidant and anti-inflammatory properties with
berberine and has been proposed as a promising DNA phototherapy drug8. Regarding BZDAs, one notable BZDA-containing product, Sangrovit®, mainly derived from bloodroot plants _Macleaya
cordata_ and _Sanguinaria canadensis_, has been developed as a safe natural feed additive9, exhibiting benefits in terms of improving growth performance and health in livestock farming and
aquaculture10,11. Beyond their antimicrobial and animal growth-promoting properties, many BZDAs have demonstrated great potential for medical importance. For instance, sanguinarine has been
identified as a potential anticancer drug due to its ability to stabilize human telomeric G-quadruplex DNA12,13. Chelerythrine, a well-known protein kinase C inhibitor, and macarpine, which
shows an anti-proliferative effect on several cancer cell lines, hold promise for various applications14,15. Notably, chelerythrine has been recently reported as a multi-purpose adjuvant for
the treatment of COVID-1916,17. Although isolation of these compounds from plants is possible, it is challenging to meet the increasing demand via large-scale plant cultivation and
therefore alternative methods, i.e., microbial biosynthesis for reliable and scalable supply of these products are urgently desired. The key to microbial-based production is to improve the
performance of enzymes thus ensuring an efficient biocatalytic process. In plants, the committed step for protoberberines and BZDAs biosynthesis is stereoselective conversion of
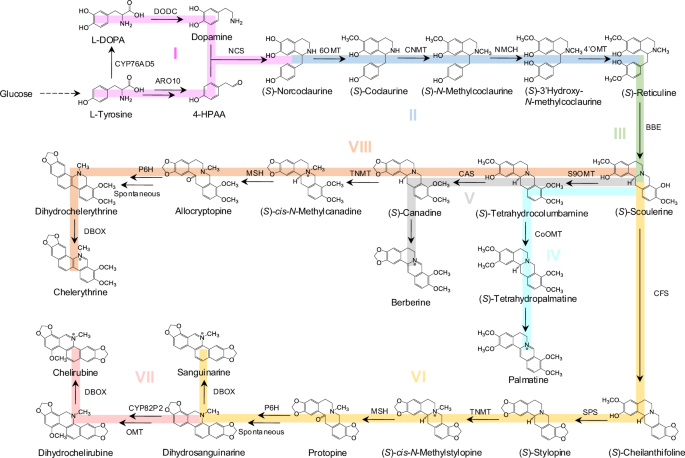
(_S_)-reticuline ((_S_)-RET) to (_S_)-scolerine ((_S_)-SCO), catalyzed by berberine bridge enzyme (BBE) (Fig. 1). This flavoprotein oxidase has been identified as a key rate-limiting enzyme
in the biosynthesis of protoberberines and BZDAs. However, enhancing the catalytic efficiency of BBE in heterologous microbial hosts is challenging, likely due to the differences in
microenvironments between plants and microbes. Previous attempts to increase BBE expression level via plasmid or multiple chromosomal integration did not significantly improve its conversion
efficiency in microbial hosts18,19,20. In vivo assays demonstrated that BBE is expressed via the secretory pathway and localized to the plant vacuole21,22, implying the importance of
post-translational modifications and trafficking processes for its activity. Since the N-terminal signal sequence is usually essential to direct the protein to undergo such processing steps,
the common strategies to improve the folding or stability of plant enzymes through N-terminus engineering would not be feasible for BBE optimization. Indeed, the N-terminal truncations of
BBE resulted into its decreased activity in yeast18,23. Therefore, improving BBE efficiency in heterologous microbial hosts such as _S. cerevisiae_ remains challenging. The final step for
protoberberines and BZDAs biosynthesis involves the conversion of tetrahydroprotoberberines and dihydrophenanthridines by additional flavoproteins, including (_S_)-tetrahydroprotoberine
oxidase (STOX) or dihydrobenzophenanthridine oxidase (DBOX). Although there were reports on the production of tetrahydroprotoberberines such as (_S_)-tetrahydropalmatine24 and
dihydrophenanthridines such as dihydrosanguinarine18,19, microbial de novo production of protoberberines and BZDAs such as palmatine, chelerythrine and sanguinarine in yeast have been
absent. One major challenge is the functional expression of the final catalytic flavoprotein in yeast. There have been several flavoenzymes reported to catalyze this type of reaction leading
to two-electron (one double bond formation) or four-electron oxidations (two double bonds formation) in vitro, such as AmSTOX25, CjTHBO26 and PsFADOX527. However, the in vivo activity of
the corresponding enzymes was neither confirmed in plants nor observed in yeast. Recently, the BZDAs biosynthetic pathways were characterized in _Macleaya cordata_28, which provides new
opportunities for microbial production of protoberberines and BZDAs. In this work, we develop a yeast-based platform for de novo production of a series of protoberberines and BZDAs. We first
construct platform strains for producing the key intermediate (_S_)-SCO, its titer reaching up to 113 mg/L through a series of modifications involving ER re-localization of vacuolar BBE, ER
expansion, and the alleviation of H2O2 toxicity in the ER. By leveraging this (_S_)-SCO platform yeast, we extend the pathway to produce a wide spectrum of protoberberines and BZDAs,
including dehydroscoulerine, columbamine, palmatine, berberine, chelerythrine, sanguinarine and chelirubine. Specifically, we functionally express additional vacuolar oxidase McDBOX2 from
_M. cordata_, capable of removing two electrons to form one N7 = C8 band in vivo. The introduction of this enzyme enables the complete reconstruction of heterologous BZDAs pathway in yeast.
However, its expression in vacuole causes some post-translational processing competitions with BBE, this issue can be alleviated to some extent by cytosolic expression of McDBOX2.
Furthermore, we demonstrate that McDBOX2 could catalyze many tetrahydroprotoberberines to produce corresponding 13,14-dihydroprotoberberines in yeast. Considering flavoenzyme-catalyzed
oxidation as a common step involved in BIAs biosynthesis, the strategies developed in this study provide insights towards generalizing the engineering of _S. cerevisiae_ for de novo
producing a broader class of medicinal BIAs. RESULTS CONSTRUCTING A PLATFORM STRAIN FOR (_S_)-NORCOCLAURINE PRODUCTION In this study, we constructed yeast strains for de novo production of
protoberberines and BZDAs, such as berberine, palmatine, chelerythrine, sanguinarine and chelirubine. To streamline the reconstruction process, the whole biosynthetic pathway was divided
into eight modules as depicted in Fig. 1 (An overview of constructed strains can be found in Supplementary Fig. 1 and Supplementary Data 1). To achieve efficient (_S_)-norcoclaurine
((_S_)-NOR) production, we first focused on selecting an optimal tyrosine hydroxylase for dopamine biosynthesis (Fig. 2a). Several candidate enzymes reported previously to catalyze the
hydroxylation of tyrosine were evaluated, including CYP76AD1* and CYP76AD5 from the sugar beet _Beta vulgaris_29,30, C3H from _Arabidopsis thaliana_ and _Zea mays_31, HpaB and HpaC from
_Pseudomonas aeruginosa_ and _Salmonella enterica_32. Among these, CYP76AD5 led to at least three-fold higher dopamine production than the other candidates (Supplementary Fig. 2a). Next, we
compared several candidates to identify a superior norcoclaurine synthase (NCS) enzyme for (_S_)-NOR biosynthesis: CyNCS12 from _Corydalis yanhusuo_, PsNCS3 from _Papaver somniferum_, PbNCS5
from _Papaver bracteatum_, StNCS2 and StNCS4 from _Stephania tetrandra_, BtNCS from _Berberis thunbergii_, CjNCS from _Coptis japonica_. N-terminal truncation of CjNCS has been reported to
improve its expression level33, we thus truncated 24, 29 and 35 amino acids on the N-terminus of CjNCS to generate CjNCS∆24, CjNCS∆29 and CjNCS∆35, respectively. All these NCS candidates
were compared in a dopamine producing strain XJ001, with CjNCS∆35 resulted in the highest (_S_)-NOR titer (Supplementary Fig. 2b). Subsequently, we constructed a reference strain XJ023 by
augmenting the flux towards tyrosine biosynthesis based on our previous study34, including overexpression of yeast native genes _ARO1_, _ARO2_, _ARO3_, and expression of EcaroL from _E.
coli_, MtPDH1 from _Medicago truncatula_, and two mutants Aro4K229L and Aro7G141S. By introducing the optimal enzyme CYP76AD5, DODC and CjNCS∆35 into strain XJ023, the resultant strain XJ053
produced 23.4 mg/L (_S_)-NOR (Fig. 2b). However, large amounts of dopamine (164.3 mg/L), 4-hydroxyphenylacetic acid (4-HPAC, 34.8 mg/L) and tyrosol (281.7 mg/L) were also detected in XJ053
(Supplementary Fig. 3), indicating that most 4-hydroxyphenylaldehyde (4-HPAA) flux flew towards by-product formation. To address this issue, three different strategies were tested. The first
strategy involved encapsulating CjNCS∆35 and Aro10 (encoding phenylpyruvate decarboxylase) or CjNCS∆35, Aro10, and DODC within a bacterial nanocompartment derived from _Myxococcus
xanthus_35. However, both attempts only showed a marginal increase on (_S_)-NOR production (6% _p_ = 0.0117 and 13% _p_ = 0.0002) compared with the control (Supplementary Fig. 4a–c). The
second strategy employed peroxisome-based compartmentalization. Unanticipatedly, targeting CjNCS∆35 or its combination with Aro10, DODC, and CYP76AD5 into the peroxisome resulted in a
significant decrease on (_S_)-NOR production in all cases (Supplementary Fig. 5). This contradicted a recent report but could be attributed to the differences on NCS expression levels,
precursor production, or strain backgrounds36. For the third strategy, we removed some competition from potential aldehyde reductases or dehydrogenases on the substrate 4-HPAA, such as
_ARI1_, _ADH6_, _YPR1_, _YDR541C_, _AAD3_, _GRE2_ and _HFD1_37. Although disruption of all seven genes slightly increased (_S_)-NOR production in strain XJ059, it also led to a 24% (_p_ =
0.0003) decrease on biomass yield compared with strain XJ058 without deletion of _YDR541C_ and _AAD3_ (Fig. 2b). To evaluate if the expression level of downstream enzyme CjNCS∆35 was
limited, strain XJ058 was selected to introduce more copies of CjNCS∆35. Two additional copies of CjNCS∆35 (strain XJ063) prompted (_S_)-NOR titer to 214.8 mg/L, a 2-fold increase compared
with strain XJ058 (Fig. 2c). Further integration of more copies did not increase (_S_)-NOR titers (Supplementary Fig. 6a). Intriguingly, dopamine, tyrosol and 4-HPAC titers remained at
around 100 mg/L between second and five copies integration (Supplementary Fig. 6b), indicating that there might be some uncharacterized aldehyde reductases or dehydrogenases in yeast, which
exhibits a relative higher affinity on 4-HPAA compared to CjNCS∆35. To further improve the availability of 4-HPAA, we employed aromatic amino acid decarboxylase (AAAD) to channel more flux
towards 4-HPAA (Fig. 2a). Apart from endogenous Aro10, three aromatic amino acid (AAS) homologies, including PcAAS from _Petroselinum crispum_38, RrAAS form _Rhodiola Salidroside_39, and a
mutated PsAAAD* (Y350F) from _P. somniferum_40, were compared in strain XJ063 expressing three copies of CjNCS∆35. All four resultant strains produced more (_S_)-NOR than the control strain
XJ063, with strain XJ0633 containing PsAAAD* yielding the highest titer (295.5 mg/L), a 38% (_p_ < 0.0001) increase relative with strain XJ063 (Fig. 2d and Supplementary Fig. 7).
Expanding the possibility of rewiring tyrosine biosynthesis, we further explored a plant-based tyrosine pathway in yeast including a PPA aminotransferase (PAT), and an arogenate
dehydrogenase (ADH) (Fig. 2a and Supplementary Fig. 8a). To do so, three PATs (AtPAT from _A. thaliana_, BvPAT1 and BvPAT2 from _B. vulgaris_)41,42 and four ADHs (BvADHα from _B. vulgaris_,
MjADHα from _Mirabilis jalapa_, MtncADH from _M. truncatula_, and GmncADH from _Glycine max_)43,44 were tested in a _TYR1_ deletion strain, which exhibited a tyrosine auxotrophic phenotype.
Strikingly, five combinations of PAT and ADH (AtPAT and BvADHα, AtPAT and MtncADH, BvPAT1, and BvADHα, BvPAT1 and MtncADH, BvPAT2, and MtncADH) restored the growth of _TYR1_-deficiency
strain, suggesting the function of the plant-based tyrosine pathway in yeast (Supplementary Fig. 8b). These five specific enzyme combinations were then evaluated in strain XJ0633 expressing
PsAAAD*. The BvPAT2 and MtncADH combination displayed a negative impact on (_S_)-NOR titer, whereas the other four led to the improved (_S_)-NOR titers, especially with AtPAT and MtncADH
combination exhibiting a 22% (_p_ = 0.0156) increase (XJ0636, 315.9 mg/L), compared to the control strain XJ0633 (Fig. 2e and Supplementary Fig. 8c). Therefore, XJ0636 was utilized as the
(_S_)-NOR-producing platform strain for subsequent experiments. CONSTRUCTING A PLATFORM STRAIN FOR PRODUCING (_S_)-RETICULINE Module II focused on modifying (_S_)-NOR to produce downstream
(_S_)-reticuline ((_S_)-RET), which sequentially catalyzed by 6-_O_-methyltransferase (6OMT), coclaurine _N_-methyltransferase (CNMT), _N_-methylcoclaurine 3’-hydroxylase (NMCH) and
4’-_O_-methyltransferase (4’OMT) (Fig. 3a). To identify the optimal enzyme combination of the first three steps, Ps6OMT, PsCNMT from _P. somniferum_, EcNMCH from _Eschscholzia californica_,
along with alternative Cy6OMT, CyCNMT and CyNMCH from _C. yanhusuo_ were tested in a simple background strain XJ040, harboring a cytochrome P450 reductase and a 4’OMT from _P. somniferum_
(PsCPR and Ps4’OMT) by measuring (_S_)-RET titers. The combination, consisting of Ps6OMT, PsCNMT and CyNMCH, led to the highest (_S_)-RET production and CyNMCH performed generally better
than EcNMCH whereas others did not display significant difference (Supplementary Fig. 9). When reverse-sequentially expressing the second copy of module II enzymes, only CyNMCH
overexpression displayed a significant increase on (_S_)-RET titer (Supplementary Fig. 10), indicating that the hydroxylation catalyzed by NMCH is a rate-limiting step. In eukaryotes, the
most ubiquitous P450s belong to class II of P450 superfamily45, which are localized to the membrane of ER and composed of two components, a P450 domain and a FAD/ FMN containing NAD(P)H CPR
domain, shuttling the electrons to P450. To improve the efficiency of CyNMCH, we subsequentially explored coupling different cytochrome P450 reductase (CPR) to transfer the electrons. Apart
from PsCPR, ATR1 and ATR2 from _A. thaliana_, were also compared in the (_S_)-NOR platform strain XJ0636 with other four optimal enzymes (Ps6OMT, PsCNMT, CyNMCH, and Ps4’OMT) for (_S_)-RET
biosynthesis. Both ATR1 (273.6 mg/L) and ATR2 (255.7 mg/L) led to (_S_)-RET titer improvement, compared to PsCPR (222.3 mg/L), indicating better pairing of ATR1/2 with CyNMCH (Fig. 3b).
However, significant accumulation of intermediates 3’hydroxy-_N_-methylcoclaurine and (_S_)-_N_-methylcoclaurine were observed in the best strain XJ0675 containing ATR1 (Supplementary Fig.
11). We thus increased the copy number of CyNMCH and Ps4’OMT in strain XJ0675 by using a landing pad system for precise multicopy gene integration46. Briefly, the landing pads, carrying
single nonnative gRNA cutting site, were inserted when knocking out five genes _ARI1_ (LP3.T7), _ADH6_ (LP3.T7), _YPR1_ (LP3.T7), _GRE2_ (LP1.T8) and _HFD1_ (LP4.T9). The resulting strain
XJ0691, harboring 2 copies of Ps4’OMT and 5 copies of CyNMCH, produced 425.2 mg/L (_S_)-RET, representing an 85% (_p_ < 0.0001) increase relative to the control strain XJ0675 after 72 h
in shake flask cultivation with 20 g/L glucose (Fig. 3c). FINE-TUNING BBE TO IMPROVE (_S_)-SCOULERINE TITER In module III, we focused on BBE which catalyzes the oxidative cyclization of the
_N_-methyl moiety of (_S_)-RET into the berberine bridge of (_S_)-scoulerine ((_S_)-SCO), a major branch intermediate leading to the biosynthesis of protoberberines and BZDAs47 (Fig. 4a). To
select an optimal enzyme, four BBEs including PsBBE from _P. somniferum_, CjBBE from _C. japonica_, CyBBE from _C. yanhusuo_, StBBE from _Stephania tetrandra S. Moore_ were compared in a
low (_S_)-RET producing strain XJ048 (Supplementary Fig. 12). Expressing all candidates except for StBBE led to (_S_)-SCO production, with CyBBE showing the highest activity. However, even
in the CyBBE expressing strain the remaining amounts of (_S_)-RET was approximately twice of the produced (_S_)-SCO in cells (Supplementary Fig. 12), indicating a potential for further
improvement on (_S_)-RET conversion efficiency. A previous in vitro study reported the optimum pH at 8.9 for BBE48, while in vivo experiment has revealed that BBE, containing an ER signal
peptide and a vacuolar sorting signal, was finally localized to the vacuole in plant, where the pH is supposed to be acidic22. We hypothesized this divergence resulted in the low activity of
BBE. To compromise such issue, we sought to retarget such enzyme into various subcompartments. In yeast, pH in the mitochondrial matrix was reported to be 7.0-7.5, cytosol and ER pH both
around 7.0, and pH in the Golgi network gradually decreases from _cis_-Golgi to _trans_-Golgi (from 6.5 to 6.0)49. To express CyBBE in the cytosol, we first attempted N-terminal truncations
to generate two variants CyBBE∆29 and CyBBE∆46, by removal of predicted ER targeting signal, or both ER targeting signal and vacuolar sorting signal, respectively. Besides, additional
truncation CyBBE29Δ46 was also constructed by deleting vacuole signal but retaining ER signal (Supplementary Fig. 13a and d). Unexpectedly, all three CyBBE variants resulted in undetectable
(_S_)-SCO production (Supplementary Fig. 13b), indicating that such truncations affected its proper expression or correct folding. Indeed, fluorescence analysis showed that CyBBE∆29 and
CyBBE∆46 cytosolically expressed but along with severe aggregate formation, while CyBBE29∆46 might enter the trafficking pathway due to the surviving ER signal peptide (Supplementary Note 1
and Supplementary Fig. 13f). Furthermore, western blot revealed that wild type CyBBE and CyBBE29Δ46 were blotted with larger size bands than that of CyBBEΔ29 and CyBBEΔ46, both of which
expressed similarly as expected (Supplementary Note 1 and Supplementary Fig. 13c). Interestingly, after the treatment of PNGaseF, capable of specifically removing the N-linked glycosylation,
the size shift was no longer observed for the wild type CyBBE and CyBBE29Δ46 (Supplementary Fig. 13e). These results clearly suggested that CyBBE underwent trafficking and
post-translational modifications, such as N-linked glycosylation. Given the importance of both signals for CyBBE, we turned to retrograde CyBBE from Golgi to ER or retain it in Golgi,
instead of trafficking the enzyme to the native destination vacuole. We tailored CyBBE with an ER C-terminal HDEL peptides (CyBBE_ERTS) to mimic ER lumen soluble protein50, or a N-terminal
118-amino acid Golgi targeting sequences (GOTS_CyBBE) to mimic type II Golgi integral membrane protein51, and tested two variants in the (_S_)-RET platform strain XJ0691. GOTS_CyBBE and
CyBBE_ERTS led to the improvement of (_S_)-SCO production by 33% (_p_ = 0.0001) and 206% (_p_ < 0.0001), respectively, compared to the wild type CyBBE (Fig. 4b and Supplementary Fig.
15a). To determine where both variants and wild type CyBBE localized in yeast, we performed colocalization microscopy analysis of green fluorescent protein (GFP)-tagged both variants and
wild type CyBBE, and red fluorescent protein mRuBy2-fused marker proteins for ER, vacuole, and Golgi (_Elo3_, _Vph1_ and _Chs5_, respectively52). The results confirmed that wild type CyBBE
was transported to the vacuole, GOTS_CyBBE enabled retention in the Golgi, while CyBBE_ERTS was redirected back to the ER (Fig. 4a and b). To better understand possible mechanisms of ER
retrograding strategy, we performed RT-qPCR, western blot, in vitro activity assays with various pH, as well as molecular dynamic simulations to compare the difference between CyBBE_ERTS and
wild type CyBBE (Supplementary Note 2). Although GOTS_CyBBE expression was significantly increased, CyBBE_ERTS expression exhibited a comparable level to the wild type CyBBE (Supplementary
Fig. 15b and c), indicating that over two-fold increase of product (_S_)-SCO was caused by increased enzyme activity rather than expression level of CyBBE_ERTS. Also, western blot with the
treatment of PNGaseF for CyBBE_ERTS showed a similar pattern as the wild type CyBBE (Supplementary Fig. 15d), suggesting that they have gone through similar post-modifications. Moreover, in
vitro assays confirmed that higher pH (as comparing the environment in ER to vacuole) indeed favored the efficiency of BBE-catalyzed reaction, which matched well with the proposed molecular
mechanisms from enzyme and substrate level (Supplementary Note 2 and Supplementary Fig. 16a–f). Molecular dynamic simulation inferred that the C-terminus HDEL was beneficial to the stability
of CyBBE (Supplementary Fig. 17a–f). Therefore, such ER targeting strategy guaranteed CyBBE going through secretory pathway for proper processing and post-modifications, while at the same
time the strategy could retrograde it to ER, which provides a more favorable microenvironment thereby leading to its increased activity. While CyBBE_ERTS promoted (_S_)-SCO titer up to 76.5
mg/L (strain XJ0695), more than 100 mg/L substrate (_S_)-RET remained (Supplementary Fig. 15a), suggesting limitations persisted for this step. We initially questioned if substrate
availability was the issue since (_S_)-RET was detected extracellularly. We thus expressed two transporters PsPUB1 and PsBUP6, derived from _P. somniferum_53, to enhance the re-uptake
efficiency of extracellular (_S_)-RET. However, no significant effects on (_S_)-SCO titers were observed (Supplementary Fig. 18a). Next, we attempted to spatially express Ps4’OMT in close
proximity to ER-targeted CyBBE_ERTS by localizing Ps4’OMT to ER membrane via fusion with a N-terminal ER membrane-bound _CYB5_ (CYB5-Ps4’OMT), or to the lumen with a N-terminal SPCyBBE and
C-terminal ERTS (SPCyBBE-Ps4’OMT_ERTS), or targeting the fusion of both enzymes to ER lumen (SPCyBBE-Ps4’OMT-CyBBE_ERTS, CyBBE-Ps4’OMT_ERTS). Direct fusions resulted in a dramatic decrease
on (_S_)-SCO production whereas CYB5-Ps4’OMT and SPCyBBE-Ps4’OMT_ERTS did not affect (_S_)-SCO production (Supplementary Fig. 18b). These results suggested that substrate availability was
not a limiting factor at the current stage. Secondly, we suspected that excess substrate (_S_)-RET remaining was likely due to the inefficiency of the CyBBE_ERTS variant. However, extra copy
introduction of wild type CyBBE, GOTS_CyBBE or CyBBE_ERTS in strain XJ0695 resulted in a varying decrease on the (_S_)-SCO titers (Supplementary Fig. 18c), indicating other factors limiting
this step. Thirdly, we considered the potential toxicity of the products, (_S_)-SCO and hydrogen peroxide (H2O2), as both have been shown to inhibit BBE activity48. Since (_S_)-SCO was
mainly detected extracellularly, our attention was turned to H2O2. We expressed ER-localized catalase _Ctt1_, _Cta1_ and one _Cta1_ mutant (removal of potential N-glycosylation N241),
through fusion with N-terminal signal peptide SPkar2 and C-terminal ERTS in strain XJ0695. All candidates caused a retarded growth and around 40% (_p_ < 0.0001) decrease on the (_S_)-SCO
titers (Supplementary Fig. 19a). Instead of troubleshooting on these constructs, we tested a mammalian based peroxiredoxin IV (PRDX4) with a N-terminal signal sequence and a C-terminal ERTS
(α-mPRDX4_ERTS)54, which could transfer the electrons derived from protein oxidative folding to H2O2, thereby producing H2O (Fig. 4a and Supplementary Fig. 19b). As a control, two nonsense
mutants α-mPRDX4C127S_ERTS and α-mPRDX4C248S_ERTS were also generated by mutating either of active-site cysteines. Intriguingly, α-mPRDX4_ERTS expression improved (_S_)-SCO titer up to 98.4
mg/L, 30% (_p_ = 0.0028) more than that in control strain XJ0695, whereas both mutants led to decrease on (_S_)-SCO titer (Supplementary Fig. 19b). These results confirmed the toxicity of
H2O2 was a potential issue for CyBBE_ERTS activity. Fourthly, since BBE is a FAD-dependent oxidase which undergoes post-translational modification through the trafficking pathway55, we
thought to engineer trafficking pathway as an alternative to optimize CyBBE expression through 1) ER expansion, by deletion of _PAH1_, encoding Mg2+ - dependent phosphatidate (PA)
phosphatase which could dephosphorylate PA to yield diacylglycerol, _OPI1_, encoding a transcriptional regulator which negatively regulates phospholipid biosynthesis, or overexpression of
_INO2_, encoding a transcription activator for phospholipid biosynthesis, INO2* (encoding an Opi1-insensitive Ino2L119A)56; 2) Protein folding, by overexpression of sliced _HAC1_, chaperone
_CPR5_ or deletion of kinase _GCN2_; 3) Impairing the ER associated degradation via _HRD1_ or _DER1_ deletion to minimize aberrant protein retro-translocation to the cytoplasm and offer
extra time for correctly folding, or disruption of the vacuolar proteinase _PEP4_ to reduce protein degradation; 4) Overexpression of HDEL receptors _ERD1_ or _ERD2_ to improve the retention
of HDEL-carrying protein in ER by alleviating the readily saturated receptor-mediated retrieval of luminal ER proteins from the secretory pathway. Among all tested targets, only
overexpression of _INO2_, INO2*, deletion of _OPI1_ improved (_S_)-SCO production by 17% (_p_ = 0.4225), 18% (_p_ = 0.1726), and 36% (_p_ = 0.0022), respectively, whereas overexpression of
_CPR5_ and deletion of _HRD1_, _DER1_ exhibited a severely opposite impact (Supplementary Fig. 19c). Taken together, we first improved BBE activity by ER compartmentalization strategy, and
observed that _OPI1_ deletion-mediated ER expansion caused extra 36% (_p_ < 0.0001) increase on (_S_)-RET conversion, and further alleviating the feedback inhibition of by-product H2O2
via expression of mammalian-derived PRDX4 resulted in additional 30% (_p_ = 0.0068) increase on (_S_)-RET conversion. With the combination of all these strategies, the final strain XJ0843
produced 113.1 mg/L (_S_)-SCO, representing the highest titer ever reported. (Fig. 4c). DE NOVO PRODUCTION OF PROTOBERBERINE ALKALOIDS IN YEAST In modules IV and V, we aimed to biosynthesize
two protoberberine alkaloids, palmatine and berberine (Fig. 1). Palmatine biosynthesis involved three steps from (_S_)-SCO, which are catalyzed by scoulerine 9-_O_-methyltransferase
(S9OMT), columbamine _O_-methyltransferase (CoOMT) and tetrahydroprotoberberine oxidase (Fig. 5a and Supplementary Fig. 20a). Initially, we sought to select the optimal methyltransferases
from TfS9OMT* (_Thalictrum flavum_)24, CjCoOMT (_C. japonica_), ThCoOMT (_Thalictrum thalictroides_) and CySOMT (_C. yanhusuo_) in genetic-background-simple strain XJ083. Apparently, a
bifunctional methyltransferase CySOMT, converting (_S_)-SCO to (_S_)-tetrahydropalmatine ((_S_)-THP) directly, exhibited a lower activity on converting (_S_)-SCO to
(_S_)-tetrahydrocolumbamine ((_S_)-THC) than TfS9OMT*, and a comparable activity on (_S_)-THP production to CjCoOMT (Supplementary Fig. 20b). We then transformed TfS9OMT* and CySOMT,
combining with GOTS_CyBBE, CyBBE_ERTS, or wild type CyBBE, respectively, into (_S_)-RET producing platform strain XJ0691. The derived strain XJ0839 containing CyBBE_ERTS, TfS9OMT*, CySOMT
and the deletion of _OPI1_ produced the highest (_S_)-THP with 68.6 mg/L (Fig. 5b and Supplementary Fig. 20c and f). Palmatine is biosynthesized from the oxidation of (_S_)-THP by losing
four electrons. Currently, there are a few plant-derived flavoprotein oxidases reported to catalyze this type of reactions in vitro, such as STOX and DBOX candidates, but their heterologous
function remained unexplored in yeast. Six codon-optimized STOX homologs, including AmSTOX from _Argemone Mexicana_25, BwSTOX from _Berberis wilsoniae_25, CjTHBO from _C. japonica_26,
PsFADOX5 from _P. somniferum_27, McDBOX1, McDBOX2 from _M. cordata_28 and four fusions, HPA2-AmSTOX (HPA2 encoding tetrameric histone acetyltransferase), CySOMT-AmSTOX, HPA2-PsFADOX5,
CySOMT-PsFADOX5, were transformed into strain XJ0700 (XJ0691+CyBBE_ERTS+TfS9OMT*+CySOMT). All resultant strains and the control strain XJ0700, produced comparable palmatine at low
quantities, which might be from spontaneous, instead of enzymatic reaction (Supplementary Fig. 20d and e). Nevertheless, we observed that the production levels of (_S_)-THC and (_S_)-THP in
strains XJ0727 (expressing McDBOX1), XJ0728 (expressing McDBOX2) and XJ0841 (expressing McDBOX2) significantly decreased compared to their control strains XJ0700 or XJ0839 (Supplementary
Fig. 20e and f), indicating that some unknown compounds were produced from (_S_)-THC and (_S_)-THP by the expression of McDBOX1 or McDBOX2. Intermediates analysis of stain XJ0841 expressing
McDBOX2 by LC-MS/MS showed that a significant peak of _m/z_ 354.16 [M]+, which exhibited different retention time (26.93 min) and tandem mass spectrum (MS2) with that of commercial standard
7,8-dihydropalmatine (27.32 min) bearing the same _m/z_ (Supplementary Fig. 21b–d). Moreover, we found that the standard 7,8-dihydropalmatine was highly unstable, which would be totally
transformed into palmatine even in minimal media during 24 h, whereas the produced compounds remained stable in shake flask fermentation even after 144 h (Supplementary Fig. 22a and b).
Previous studies have confirmed that the oxidation of tetrahydroprotoberberine catalyzed by STOX only occurred to its C-ring27, and the oxidative product with N7 = C14 iminium bond was
unstable, undergoing rapid disproportionation to form a mixture of the corresponding quaternary protoberberine and the tetrahydroprotoberberine57. Therefore, we inferred that (_S_)-THP was
oxidized to produce 13,14-dihydropalmatine harboring N7 = C8 bond by expressing McDBOX2 in strain XJ0841 (Supplementary Fig. 21a), as the production of quaternary palmatine was not increased
(Supplementary Fig. 20f). Similarly, (_S_)-THC was transformed into 13,14-dihydrocolumbamine by McDBOX2 oxidation in such strain (Supplementary Fig 21e and f), and (_S_)-SCO was transformed
into 13,14-dihydroscoulerine in strain XJ0842 with McDBOX2 expression (Supplementary Fig. 21g and h). Recently, one study reported that heating enhanced the transformation from
tetrahydroprotoberberine to the corresponding quaternary protoberberine via losing four electrons58. We therefore tested heating the cultures from shake flask fermentation of strain XJ0839
at 98 °C for varying durations. After heating for four hours, 72% (_p_ = 0.0034) (_S_)-THP (49.2 mg/L) disappeared, while 14.3 mg/L palmatine was produced (Supplementary Fig. 24a). By
increasing heating time to 36 hours, 91% (_p_ < 0.0001) (_S_)-THP was transformed into 54.0 mg/L palmatine with 86% (_p_ = 0.0008) conversion efficiency (Fig. 5c and Supplementary Fig.
24a). In parallel, berberine biosynthesis undergoes three steps from (_S_)-SCO, catalyzed by S9OMT, canadine synthase (CAS) and tetrahydroprotoberberine oxidase (Fig. 5a and Supplementary
Fig. 23a). With the introduction of TfS9OMT* and CjCAS (_C. japonica_) into strain XJ0796 (XJ0695 _ΔOPI1_), its resultant strain XJ0834 produced 23.2 mg/L (_S_)-canadine (Fig. 5b and
Supplementary Fig. 23c). We further attempted to express CjTHBO, ER-targeted CjTHBO_ERTS, Golgi-targeted GOTS_CjTHBO or McDBOX2 in strain XJ0834. Apparently, all four enzymes exhibited no
activity on berberine production improvement (Supplementary Figs. 23b and c). Only the expression of McDBOX2 in strain XJ0835 (XJ0834 + McDBOX2) allowed for the conversion of (_S_)-THC and
(_S_)-canadine to 13,14-dihydrocolumbamine and 13,14-dihydroberberine, respectively (Supplementary Fig. 23d–f). Furthermore, we sought to convert (_S_)-canadine to berberine through heating
for varying durations at 98 °C. Heating the cultures of strain XJ0834 by four hours led to that 87% (_p_ < 0.0001) (_S_)-canadine was transformed while berberine production was only 3.9
mg/L. Once prolonging the heating time until 36 hours, berberine production was gradually increased, reaching up to 16.9 mg/L finally (Fig. 5c and Supplementary Fig. 24c). It is worth noting
that 13,14-dihydropalmatine and 13,14-dihydroberberine from strains XJ0841 and XJ0835, respectively, were readily oxidized by heating (Supplementary Fig. 24b and d). In addition, another
two types of protoberberines, dehydroscoulerine and columbamine were also detected after four hours’ heating (Supplementary Fig. 25a–f). DE NOVO PRODUCTION OF BENZOPHENANTHRIDINE ALKALOIDS
IN YEAST The module VI was to extend the pathway for production of sanguinarine from (_S_)-SCO, sequentially catalyzed by cheilanthifoline synthase (CFS), stylopine synthase (SPS),
tetrahydroprotoberberine _N_-methyltransferase (TNMT), methylstylopine 14-hydroxylase (MSH), protopine 6-hydroxylase (P6H) and DBOX (Fig. 1). To reconstruct the module VI more accessible, we
first extended the downstream pathway to produce the intermediate protopine (Supplementary Fig. 28a). Two enzyme candidates from _E. californica_ and C. _yanhusuo_ for first two steps were
co-expressed with PsTNMT and PsMSH (both from _P. somniferum_) in (_S_)-SCO-producing strain XJ0695. Total ion chromatography (TIC) and MS2 analysis evidenced that the intermediate protopine
was produced, and the enzyme combination of EcCFS and EcSPS exhibited the optimal activity (Supplementary Fig. 28b). Prior studies have reported trace amounts of sanguinarine production
from dihydrosanguinarine in yeast, by spontaneous conversion or unknown yeast native enzymatic catalyzation18,19. As aforementioned, this kind of reaction can be catalyzed by flavoprotein
oxidases. Thus, six previously mentioned STOX homologs, AmSTOX, BwSTOX, CjTHBO, PsFADOX5, McDBOX1 and McDBOX2 were transformed together with EcP6H (from _E. californica_) into
protopine-producing strain XJ0743. Western blot analysis revealed that BwSTOX, McDBOX1 and McDBOX2 expressed well, PsFADOX5 smeared, whereas AmSTOX and CjTHBO were not properly expressed
(Supplementary Fig. 29a and b). However, we did observe that all transformants produced sanguinarine even in the control strain XJ07437 only expressing EcP6H, consistent with previous
observations of low sanguinarine production without functional oxidase catalyzation in yeast18,19 (Supplementary Fig. 28c and e). Surprisingly, the dihydrosanguinarine peak disappeared from
strain XJ0750 expressing McDBOX2 (Supplementary Fig. 28c), inferring that McDBOX2 was functional in conversion of dihydrosanguinarine to sanguinarine. With the introduction of McDBOX2,
combined with EcP6H, McP6H, or CyP6H, all resultant strains could produce sanguinarine with EcP6H exhibiting the optimal performance in strain XJ0750 (Supplementary Fig. 28d). Sanguinarine
downstream derivative chelirubine, exhibiting the anti-proliferative effects on several cancer cell lines can be used as a DNA fluorescent probe, which have been drawn increased
interest14,59. It requires three enzymes, containing P450 hydroxylase, _O_-methyltransferase and flavoprotein oxidase to fulfill chelirubine biosynthesis from dihydrosanguinarine as
illustrated in module VII (Fig. 1 and Supplementary Fig. 31a). We first expressed three P450s CYP82P2, CYP82P3 and CYP82P4 from _E. californica_ in sanguinarine producing strain XJ0822
containing McDBOX2. A peak corresponding to _m/z_ 348.09 [M]+ was observed only in strain XJ0851 expressing CYP82P2 (Supplementary Fig. 31b and c), presumably indicating that CYP82P2
catalyzed dihydrosanguinarine to generate 10-hydroxydihydrosanguinarine, which was then oxidized to generate 10-hydroxysanguinarine by McDBOX2 catalyzation. Subsequently, we characterized
two potential _O_-methyltransferases Ec2OMT and Ec11OMT from _E. californica_ together with CYP82P2 in strain XJ0832. A peak corresponding to _m/z_ 362.1 [M]+ was observed only in strain
expressing CYP82P2 and Ec11OMT, the MS2 spectrum of which peak was mostly identical to that of chelirubine standard reported in a previous study60 (Supplementary Fig. 31d and e). All these
results demonstrated that chelirubine was de novo biosynthesized in yeast. Likewise, we extended the pathway to generate chelerythrine from (_S_)-SCO in module VIII by further introduction
of TfS9OMT*, CAS, PsTNMT, PsMSH, EcP6H and McDBOX2 (Fig. 1). To produce intermediate allocryptopine, CjCAS from _C. japonica_ or CyCAS from _C. yanhusuo_, TfS9OMT*, PsTNMT and PsMSH were
chromosomally incorporated into (_S_)-SCO producing strain XJ0695 (Supplementary Fig. 32a). Both enzyme combinations enabled production of allocryptopine, while CjCAS in XJ0741 exhibited a
higher activity compared to CyCAS in strain XJ0742 (Supplementary Fig. 32b). With the introduction of McDBOX2, combined with EcP6H, McP6H, or CyP6H, all resultant strains produced
chelerythrine with EcP6H performing the best in strain XJ0747 (Supplementary Fig. 32c). These results indicated that McDBOX2 exhibited a broad substrate scope, leading to the biosynthesis of
chelerythrine, sanguinarine and chelirubine. OPTIMIZING DBOX EXPRESSION FOR THE IMPROVEMENT OF BZDAS PRODUCTION McDBOX2 could catalyze various substrates, playing a vital role in BZDAs
biosynthesis (Fig. 6a). However, we observed that McDBOX2 expression caused a decreased OD600 and an increased (_S_)-RET accumulation in both chelerythrine producing strain XJ07411 and
sanguinarine producing strain XJ07437 (Fig. 6b). Likewise, decreased (_S_)-SCO production and increased (_S_)-RET accumulation were also found in (_S_)-SCO producing strain XJ0695 when
expressing McDBOX2 (Fig. 6b). These results indicated that McDBOX2 expression displayed some limitations on BBE activity, which is unexpected as CyBBE was engineered to localize inside ER,
while wild type McDBOX2 was assumed to be in vacuole in our case (Fig. 4b, d). Although they are not spatially localized together, there are three shared traits between CyBBE_ERTS and
McDBOX2 expression in yeast, including being FAD-dependent oxidases, producing by-product H2O2, and undergoing trafficking pathway from ER to Golgi. H2O2 production was not considered since
H2O2 generated by McDBOX2 was in vacuole which might not affect ER-localized CyBBE. Then we considered the potential competition for the cofactor FAD. We sought to improve FAD availability
by transforming plasmids 1) bearing FAD ER-localized transporters _FLC1_, _FLC2_, _FLC3_ and _YPR365C_; 2) mitochondria FAD transporter _FLX1_; 3) plasma transporter _MCH5_; 4) FAD
biosynthesis-related genes _FMN1_ and _FAD1_; 5) BsRiba and BsRibc from _Bacillus subtilis_ into strain XJ0774 (Supplementary Fig. 34a). We observed that the derived strains expressing MCH5
or BsRibc exhibited 24% (_p_ = 0.0095) and 39% (_p_ = 0.0038) increase on chelerythrine production, respectively, compared to the control strain expressing empty plasmid, while others
displayed no significant impacts on chelerythrine production (Supplementary Fig. 34b). However, comparable (_S_)-RET titer remained in strains expressing MCH5, BsRibc or empty plasmid
(Supplementary Fig. 34b), which indicated that MCH5 or BsRibc expression probably improved McDBOX2 activity at this stage thereby leading to increased chelerythrine production, instead of
alleviating the limitation on CyBBE_ERTS for conversion of (_S_)-RET to (_S_)-SCO. Next, we engineered the coat protein II (COPII) vesicles for transporting from ER to Golgi in strain XJ0819
by plasmid-based overexpression of _SAR1_ or replacement of the promoter _SEC16_ with strong promoters _GPD1_p or _TEF1_p. _SAR1_ overexpression resulted in severe growth retardation and
decrease on chelerythrine titer, whereas promoter substitution of _SEC16_ showed no significant effect on chelerythrine production compared to the control strain (Supplementary Fig. 35).
Although the mechanism of such limitation is unknown, we thought to circumvent the issue via different compartmentalization of McDBOX2. Since most vacuole proteins suffer from degradation in
the vacuole, we first attempted to target McDBOX2 into the ER. Western blot confirmed that McDBOX2_ERTS expressed well in the ER, identical to the wild type enzyme (Supplementary Fig. 36a).
However, ER-targeted McDBOX2 didn’t alleviate the issue as (_S_)-RET titers and final OD600 were comparable with that of the strains expressing vacuolar McDBOX2, even slightly decreased
chelerythrine and sanguinarine production (Supplementary Fig. 36b). Next, cytosolic expression of McDBOX2 was tested by truncating its N-terminal 29 amino acids. Western blot analysis
suggested that one protein band appeared from the strain expressing McDBOX2 truncation, the size of which was close to the theoretical 56.8 kDa of McDBOX2∆29, whereas wild type enzyme was
blotted with several bands (Supplementary Fig. 36c). We observed that McDBOX2∆29, which was cytosolically expressed as demonstrated by fluorescent tagging, resulted in unarresting cell
growth and parallel (_S_)-RET titers, compared to those of strain XJ07411 without expressing McDBOX2 (Fig. 6c–e). Furthermore, such truncation in strain XJ07472 (XJ07411 + McDBOX2∆29) could
convert all the dihydrochelerythrine to chelerythrine, the production of which was, however, slightly less than that of strain XJ0747 (XJ07411 + McDBOX2) after 40 hours’ growth, probably due
to more accumulated intermediates, (_S_)-THC and (_S_)-_cis_-_N_-methylcanadine, instead of decreased activity of McDBOX2∆29 (Fig. 6c, e and Supplementary Fig. 37a). Likewise, McDBOX2∆29 in
strain XJ07502 (XJ07437 + McDBOX2∆29) resulted in recovering growth, accumulating less (_S_)-RET, converting all dihydrosanguinarine, and producing more sanguinarine, compared to those of
the control strain XJ0750 (Supplementary Fgs. 36d, e and b). It suggested that the functional McDBOX2∆29 to some extent alleviated the limitation on BBE. To improve chelerythrine and
sanguinarine production, we leveraged the aforementioned strategies to engineer the strain XJ07472 and XJ07502, both expressing McDBOX2∆29. In our final strains XJ07474 and XJ07504,
combination of riboflavin transporter _MCH5_, FAD synthase BsRibc and ER expansion mediated by _OPI1_ deletion resulted in 38.1 mg/L of chelerythrine, and 3.8 mg/L of sanguinarine,
respectively (Fig. 6f and Supplementary Fig. 36f). Moreover, intracellular product levels were measured in strains XJ0827 and XJ0832 by using ACN extraction. 9.6% (_p_ = 0.0008)
chelerythrine and 22% (_p_ < 0.0001) sanguinarine were accumulated in the cell pellet, while less than 6% (_p_ = 0.0002) of other intermediates were observed intracellularly, indicating
that compared to final product chelerythrine and sanguinarine, other intermediates were more readily secreted into medium (Supplementary Table 1). DISCUSSION In this study, we demonstrated
de novo biosynthesis of a series of protoberberines, including columbamine, palmatine, berberine, and BZDAs, including sanguinarine, chelerythrine and chelirubine in yeast. Initially, we
constructed the distinct platform strains for producing (_S_)-NOR (XJ0636 315.9 mg/L), (_S_)-RET (XJ0691 425.2 mg/L) and (_S_)-SCO (XJ0843 113.1 mg/L), respectively, through a combination of
various strategies, including enlarging the tyrosine pool, impairing the undesirable 4-HPAA scattering, increasing copies of rate-limiting enzymes, introducing plant-derived tyrosine
pathway, 4-HPAA biosynthesis pathway, CPR pairing, BBE relocalization, ER expansion and PRDX4-mediated H2O2 degradation. Subsequently, the (_S_)-SCO platform strain was engineered to produce
tetrahydroprotoberberines and dihydrobenzophenanthridine alkaloids, which can be converted into 13,14-dihydroprotoberberines and BZDAs by FAD-dependent oxidase McDBOX2. Meanwhile, we
observed that tetrahydroprotoberberines and 13,14-dihydroprotoberberines can be transformed into protoberberines by heating or spontaneously, and cytosolically expressed McDBOX2 could
alleviate some limitations on CyBBE_ERTS activity. These results highlight the potential of yeast cell factories to access and scall up the production of distinct valuable BIAs for drug
development. The nascent BBE protein matures via the secretory pathway and is ultimately localized to plant vacuole21,22. In this study, we have confirmed that CyBBE was functionally
expressed and finally targeted to the vacuole in yeast, the activity of which on (_S_)-RET, however, was quite low (Fig. 4b and supplementary Fig. 12a). Previous attempts to improve the
efficiency of BBE, such as multiple chromosomal integration and BBE truncations, didn’t yield significant improvement20,23. Recent studies have reported that replacing the N-terminal signal
peptides of THCAS and CBDAS with a vacuolar localization tag from yeast, and engineering AbLS by N-terminal fusions which may alter sorting from the _trans_-Golgi network TGN, enabled these
plant-based vacuolar proteins functionally expressed in yeast61,62. Our initial attempts, including signal peptides replacement of CyBBE with a signal sequence from vacuolar proteinase A and
engineering CyBBE by N-terminally fusing soluble domains with various oligomerization state, resulted in no significant impact on (_S_)-SCO titers (Supplementary Fig. 14a and b). Given that
each subcellular compartment in yeast offers a unique physiochemical environment, we questioned if the activity of CyBBE was improved when expressed in various compartments in yeast,
including peroxisome, ER, Golgi and cytosol. The peroxisomal variant CyBBE_ePTS1, led to comparable (_S_)-SCO production to wild type CyBBE (Supplementary Fig. 14a), while cytosolically
expressed truncations CyBBE∆29 and CyBBE∆46 almost completely lost their activity (Supplementary Fig. 13b), suggesting the importance of guiding CyBBE into secretory pathway for proper
expression or correct folding (Supplementary note 1). Regarding the secretory pathway in yeast, it shares most organelles and post-transcriptional modifications within plants, and is
responsible for the synthesis, folding and delivery of a diverse array of cellular proteins among these organelles. Guided by signals, these proteins first enter the ER, further transport to
the Golgi to ensure proper folding and modification. Afterwards, some proteins are retrograded to ER, while others reside in Golgi or are transported to later compartments, such as the
vacuole and extracellular space. For instance, the proteins with vacuole sorting sequences will go from _trans_-Golgi apparatus to the vacuole, including endogenous Pep4p and BBE in this
study. In addition, there are some proteins that first reach the _cis_-Golgi apparatus and then retrieve back to the ER for proper function. These protein includes 1) the soluble ER
proteins, carrying a C-terminal sequence HDEL50,63, such as Kar2p; 2) the type I integral membrane ER proteins, possessing an important C-terminal cytoplasmic motif64,65, such as Wbp1p; and
3) type II integral membrane ER proteins, bearing a N-terminal cytoplasmic ER retention signal66, such as Mns1p and Alg5p. Currently, the commonly used strategy of ER localization in
metabolic engineering was to mimic the type II integral membrane ER proteins by signal replacement, as demonstrated in one recent study of targeting CjSTOX into ER via Mns1 signal peptide
substitution58. In our case, we have mimicked both type II transmembrane ER protein SPALG5_CyBBE though N-terminal Alg5p signal peptide replacement and type I transmembrane ER protein
CyBBE_C36WBP1 though C-terminal fusion with the last 36 amino acids of Wbp1p, both of which, however, almost lost their activity on (_S_)-SCO production (Supplementary Fig. 15e and f).
Intriguingly, we verified that ER offered a more favorable environment for CyBBE activity by mimicking soluble ER protein CyBBE_ERTS though C-terminal HDEL fusion (Supplementary note 2).
This ER retrograding strategy would be generally applicable for heterologous expression of specific enzymes requiring post-translational processing. In addition, each compartment has their
unique physicochemical environment, such as pH and oxidative status, which may provide favorable conditions for the function of distinct enzymes. In yeast, it is known that vacuolar has a
more acidic environment while the pH in ER is close to neutral. We now have shown that pH is an important factor rendering ER-localized BBE outperforming the vacuolar targeted BBE
(Supplementary note 2 and Supplementary Fig. 16a–f). Therefore, this ER retrograding strategy provides a more favorable microenvironment (i.e., pH) for maximizing the catalytic activity of
BBE, which could be applicable for expression of enzymes requiring proper pH conditions. Moreover, the C-terminal cytosolic tail results in Golgi retention of type I integral membrane
proteins, Kex1p and Kex2p67,68, while the N-terminal cytoplasmic domain containing aromatic residues (FXFXD) is both necessary and sufficient for Golgi retention of type II integral membrane
protein Ste13p69. Likewise, we constructed an artificial type II transmembrane Golgi protein GOTS_CyBBE which led to the improvement of (_S_)-SCO production by 33% (_p_ = 0.0001). These
results highlighted that the ER and Golgi might be utilized as promising organelles for metabolic engineering of heterologous pathway. Due to the microenvironmental differences between
plants and yeast, it is important to consider different strategies when re-localizing specific plant-based enzymes to distinct organelles in yeast. We have confirmed that McDBOX2 catalyzed
the last step for BZDAs biosynthesis in yeast. Due to the substrate promiscuity of STOXs in vitro activity assays25, we also explored McDBOX2 function on other substrates, by searching
potential products in strains XJ07438 (XJ0743 + McDBOX2) with LC-MS/MS analysis. We observed two peaks, corresponding to _m/z_ 324.13 [M]+, and _m/z_ 322.11 [M]+, respectively, which might
be the products derived from (_S_)-cheilanthifoline and (_S_)-stylopine via two-electron oxidation to form one N7 = C8 band (Supplementary Fig. 30a–e). Combined with the results of
13,14-dihydropalmatine, 13,14-dihydrocolumbamine, 13,14-dihydroscoulerine and 13,14-dihydroberberine production catalyzed by McDBOX2 (Supplementary Fig. 21b, e, g and d), we assumed that
McDBOX2 has a very broad substrate scope of tetrahydroprotoberberine alkaloids to produce 13,14-dihydroprotoberberine alkaloids. As it is known, the fragmentation way of protoberberines was
not the retro-Diels-Alder cleavage, due to their unsaturated C-ring structure (Supplementary Fig. 26a)70,71, which was mostly consistent with MS2 spectrum of dihydroprotoberberine alkaloids
produced in this study (Supplementary Figs. 21c, f, h, 23e and 26b). Although we have identified that McDBOX2 could catalyze tetrahydroprotoberberine to produce corresponding
13,14-dihydroprotoberberine by forming one double band in yeast, we didn’t manage to express functional STOX catalyzing tetrahydroprotoberberine to protoberberine by forming two double bands
directly. Our results indicated that a possibility of catalyzation from tetrahydroprotoberberine to the corresponding protoberberine was consecutively accomplished by two enzymes
(Supplementary Fig. 27a and b). This may provide new insights for screening additional STOXs to complete the biotransformation, thus realizing biological production of quaternary
protoberberine. In addition, the intermediates profiling from strain XJ0743 showed that three additional peaks, corresponding to _m/z_ 342.18 [M]+, 340.16 [M]+ and _m/z_ 338.14 [M]+,
respectively, were assumed to be the products of (_S_)-SCO, (_S_)-cheilanthifoline and (_S_)-Stylopine _N_-methylation by PsTNMT catalyzation (Supplementary Fig. 33b–g). Likewise, two peaks
corresponding to _m/z_ 354.18 [M]+ and _m/z_ 356.19 [M]+ appeared in strain XJ0741, likely derived from (_S_)-canadine and (_S_)-THC undergoing _N_-methylation by PsTNMT catalyzation
(Supplementary Fig. 33h–k). These findings provided experimental evidence that PsTNMT could accept (_S_)-SCO, (_S_)-cheilanthifoline, (_S_)-Stylopine, (_S_)-canadine and (_S_)-THC as
substrates for _N_-methylation (Supplementary Fig. 33a), confirming a broad substrates scope of PsTNMT18,28. Our engineered yeast strains could biosynthesize a series of BIA compounds, some
of which might not be found in plants due to localization-specific enzymes or potential transporters involved in the biosynthetic processes. In contrast to several reports on the
difficulties to realize the final step of oxidation in protoberberine and BZDAs biosynthesis in yeast18,19,58,72, one recent study reported the expression of one oxidase FADOX5 from _P.
somniferum_ for sanguinarine production in _S. cerevisiae_73. However, the same enzyme exhibited smear expression in yeast in our study and resulted in comparable sanguinarine titer in
strain XJ07434 relative with the control strain XJ07437 lacking oxidase candidate (Supplementary Fig. 28c and e). Production of sanguinarine without the expression of DBOX enzyme has been
observed before, due to spontaneous and/or promiscuous enzyme activities in yeast18,19, however, the production level was significantly lower than that McDBOX2 catalyzation (Supplementary
Fig. 28e). In this study, our (_S_)-RET platform strain can be used for scale-up production of many other natural BIAs, including compounds of the morphine family. Compared to vacuolar
expression of CyBBE, ER lumenally expressed CyBBE_ERTS increased the intermediate (_S_)-SCO titer by more than 200% (_p_ < 0.0001) in strain XJ0695, demonstrating its potential as a
platform strain for highly producing the anticancer noscapine. The introduction of McDBOX2 completed the reconstruction of BZDAs pathway, allowing to biosynthesize chelerythrine,
sanguinarine and chelirubine, with chelerythrine reaching a titer of 38.1 mg/L in yeast. Due to wide substrates specificity of McDBOX2 and PsTNMT, we observed the production of several BIAs
in our engineered yeast, which can be used to explore their structure and medicinal activities. Furthermore, we envision that our final strains producing chelerythrine and chelirubine will
facilitate the screening of downstream pathway enzymes to biosynthesize other pharmacological BZDAs, such as chelilutine and macarpine. METHODS STRAINS AND REAGENTS All strains used in this
work are listed in Supplementary Data 1. _Escherichia coli_ DH5α strain was used for routine plasmid assembly. All yeast strains were derived from IMX581 (_MATa ura3-52 can1Δ:: cas9-natNT2
TRP1 LEU2 HIS3, CEN.PK113-5D_). YPD medium, consisting of 20 g L−1 peptone (Difco), 10 g L−1 yeast extract (Merck Millipore), and 20 g L−1 glucose (VWR), was used for routine yeast culture
and competent cells preparation. Synthetic dropout minus uracil medium (SD-Ura) (6.7 g L−1 yeast nitrogen base (YNB) without amino acids (Formedium), 0.77 g L−1 complete supplement mixture
without uracil (CSM-URA, Formedium) and 20 g L-1 glucose (VWR)) was used for screening positive colonies transformed with _URA3_ marker plasmid. CSM + 5-FOA medium, containing 6.7 g L−1 YNB
without amino acids, 0.79 g L−1 complete supplement mixture (CSM, Formedium) and 0.8 g L−1 5-fluoroorotic acid (5-FOA, Sigma) was used for recycling the _URA3_ marker. When necessary for
preparing plates, 20 g L−1 agar (Merck Millipore) was added. Delft media, containing 7.5 g L−1 (NH4)2SO4, 14.4 g L−1 KH2PO4, 0.5 g L−1 MgSO4 ⋅ 7H2O, 20 g L−1 glucose, 2 ml L−1 trace metal
solutions and 1 ml L−1 vitamin solutions74, supplemented with 60 mg L−1 uracil if required, was used for shake flask fermentation for producing BIAs. Gibson Assembly Kit and Golden Gate
Assembly Kit were supplied from NEB. PrimeSTAR HS polymerase and Phusion polymerase were bought from Takara and ThermoFisher, respectively. Authentic standards dopamine hydrochloride
(Sigma), (_S_)-reticuline (Cymit Pamplona, Spain), 4-hydroxyphenylacetic acid (4-HP acid) (TCI), 2-(4-hydroxyphenyl) ethanol (tyrosol) (TCI) were supplied. (S)-(-)-norcoclaurine
hydrobromide, (_S_)-scoulerine, (-)-tetrahydrocolumbamine, chelerythrine chloride and sanguinarium chloride were purchased from Toronto Research Chemicals (Toronto, Canada). (_S_)-canadine
and berbeine were supplied from Sigma. 7,8-dihydropalmatine and 7,8-dihydroberberine were bought from Yuanye (Shanghai China). Some intermediates of pathway were gifted from Guo Juan’ lab
(National Resource Center for Chinese Materia Medica, Beijing, China). GENETIC MANIPULATIONS All primers used in this work are listed in Supplementary Data 2. IMX581 carrying chromosomally
integrated Cas9 gene under TEF1 promoter, was employed as starting strain for all genetic manipulations. CRISPR-Cas9 based genome editing method was used for chromosomal gene deletion,
insertion, and promoter replacement75. Promoters and terminators were amplified from IMX581 genome DNA by using PrimeSTAR HS or Phusion polymerase. The heterologous genes listed in
Supplementary Data 3 were codon-optimized synthesized by GenScript. All primers were synthesized from Eurofins or Integrated DNA Technologies. For gene deletion, one 120 base pair
(bp)-length oligo was designed, with 60 bp homologous to promoter and terminator of target gene, respectively. For gene insertion, up-stream homologous arm, promoter, gene, terminator, and
down-stream homologous arm were first amplified with primers carrying 5’-terminal 30-40 bp overhangs. The entire repair fragment was assembled via overlapping PCR76. In cases where two or
more genes were inserted into the one locus and the whole fragment was too long to assemble, the repair fragment was divided into several parts for construction. For promoter replacement,
the candidate promoters with two-terminal 50 bp overhangs, identical to the upstream region of target promoter and target gene, respectively, were amplified from genome DNA. Specifically for
the cassette design of ER-targeted CyBBE, we fused C-terminal HDEL peptides to CyBBE with a GSGS linker. In detail, we first amplified up-stream homologous arm XII-4 us, promoter CCW12p,
fused gene sequence CyBBE_ERTS, terminator PGI1t, and down-stream homologous arm XII-4 ds by primer sets XII-4 us-F 571/ XII-4 us-CCW12p-R 572, CCW12p1-F/ CCW12p-DODC-R1, CCW12p-CyBBE-F 573/
BBE1-ERTS-PGI1t-R 920, BBE1-ERTS-PGI1t-F 921/ XII-5-PGI1t-r-F, and PGI1t-XII-4 ds-F 575/ XII-4 ds-R 576 (Supplementary Data 2) with yeast genome DNA or synthetized CyBBE sequence as
templates. The whole cassette was assembled by overlap PCR and then transformed into the corresponding yeast strains along with pMEL10-XII-4-g RNA plasmid. All plasmids used in this work are
listed in Supplementary Data 4. To ease the assessment experiments in some cases, P416 plasmids were constructed for gene expression. Briefly, the vector scaffold was PCR amplified with
p416_GPD plasmid as template, the candidate genes and vector scaffold were assembled into complete plasmids based on standard Gibson Assembly method. For gRNA plasmid construction, either
Gibson Assembly or Golden Gate Assembly was used. gRNAs were first predicted by using open-access tool (http://crispr.dbcls.jp/) for genes of target. pMEL10 was employed as background
plasmid carrying SNR52 promoter and gRNA scaffold. For the plasmid bearing one gRNA, we first linearized the starting vector by PCR amplification. Two reverse complementary 60 bp oligos,
each one consisting of 20 bp homologous segment with SNR52 promoter, 20 bp gRNA sequence and 20 bp homologous fragment with gRNA scaffold, were synthesized from Eurofins. Aliquot of oligos
were annealed for 10 min at 95 °C, then slowly cold down to room temperature. The annealed oligo mix and linearized vector were assembled to complete plasmid. For the plasmid harboring two
or more gRNAs, we first constructed host plasmid pMEL10-BsaI carrying only two BsaI cutting sites between SNR52 promoter and gRNA scaffold by Gibson Assemble. Briefly, we divided linearized
vector pMEL10 into two parts by PCR amplification with the aim of removal of one BsaI site located in the antibiotic marker ampicillin sequence. An unrelated sequence to yeast genome, with
one BsaI site adding to each terminus, was amplified. The three fragments were assembled into host plasmid pMEL10-BsaI. The commonly used template gRNA-SNR52p was obtained by overlap PCR.
Based on this common template, we then obtained insertion fragments BsaI-N20(1)-gRNA-SNR52p-N20(2)-BsaI, … BsaI-N20(n-1)-gRNA-SNR52p-N20(n)-BsaI by different primers amplification. These
insertion fragments and host plasmid pMEL10-BsaI were assembled into target plasmid with a certain number of gRNAs by Golden Gate Assembly. After sequencing all plasmids by Eurofins, the
positive one was selected for further work. High-efficiency yeast transformation was performed by using the LiAc/SS carrier DNA/PEG method77. STRAIN CULTIVATION AND METABOLITE QUANTIFICATION
Three or more biologically independent colonies were inoculated into 1 mL delft media supplemented with 60 mg L−1 uracil within 14 mL culture tubes for 18-24 h at 30 °C, 220 rpm. The
preculture was then transferred into 20 mL fresh delft media supplemented with 60 mg L−1 uracil within 125 mL shake flakes with the initial OD600 0.05. Unless otherwise specified, the
culture was kept for 72 h. For strains carrying P416 plasmid, the cultivation process was identical to strains without plasmid, except that the delft media without 60 mg L−1 uracil was used.
After fermentation, OD600 was measured, and metabolites were extracted from culture. Briefly, 500 µL of culture was mixed with 500 µL of 30% acetonitrile (ACN), vortexed thoroughly, and
then centrifuged for 5 min at 13000 _g_. If necessary, the supernatant was further diluted with 15% ACN to the suitable concentration for LC-MS/MS analysis. To perform the conversion of
tetrahydroprotoberberine to the corresponding quaternary protoberberine though heating, the culture was transferred to PCR tubes, and heated at 98 °C in PCR instrument. The samples were then
stored at -20 °C until analysis. One microliter of each sample was injected for analysis by LC-MS/MS system, consisting of a Shimadzu Nexera UHPLC system and a high-end hybrid triple
quadrupole ion trap instrument (Sciex QTRAP 6500 + ) with Luna Omega 1.6 μm Polar C18 100 Å column (Phenomenex). Analytes were eluted at a constant flow rate of 400 µL min−1 with 0.1% formic
acid as solvent A and ACN with 0.1% formic acid as solvent B. For analytes tyrosol, 4-HPAC, dopamine, (_S_)-NOR and (_S_)-RET, the following MS parameters were used: Curtain Gas (50);
Collision Gas (Medium); IonSpray Voltage: -4500 V (4-HP acid and Tyrosol), 4500 V (Dopamine, (_S_)-NOR and (_S_)-RET); Temperature (450 °C). The gradient method was used as follows: 2% B to
10% B from 0-4 min, 10% B to 85% B from 4-6 min, held at 85% B from 6-7 min, 85% B to 2% B from 7–7.1 min, and held at 2% B from 7.1–9 min. (_S_)-SCO, intermediates of module VI, VII and
VIII, chelerythrine and sanguinarine were separated with the following gradient method: 10% B from 0-0.1 min, 10% B to 45% B from 0.1-5 min, 45% B to 90% B from 5–5.5 min, held at 90% B from
5.5–7 min, 90% B to 10% B from 7–7.01 min, and held at 10% B from 7.01-9.5 min by using the identical column, flow rate and mobile phases. The source parameters were set as follows: Curtain
Gas (30); Collision Gas (High); IonSpray Voltage (4500 V); Temperature (600 °C); Ion Source Gas 1 and 2 (60). Compounds from module IV and V were separated with the following gradient
method: 10% B from 0–0.1 min, 10% B to 25% B from 0.1–6.0 min, 25% B to 70% B from 6.0–7.0 min, 70% B to 98% B from 7.0–7.1 min, held at 98% B from 7.1-8.5 min, 98% B to 10% B from 8.5–8.6
min, and held at 10% B from 8.6–10 min by using the identical column, flow rate and mobile phases. The source parameters were set as follows: Curtain Gas (40); Collision Gas (Medium);
IonSpray Voltage (3000 V); Temperature (500 °C); Ion Source Gas 1 (40) and 2 (50). For quantification of analytes, MRM mode was used to monitor the transitions based on authentic standards.
For qualification of analytes, Q3 MI mode or MS2 mode were used (Supplementary Table 2). Analyst and MultiQuant 3.0.3 software were used for data acquisition and processing. Unknown
compounds analysis was performed by using a Waters Xevo G2-X QTOF system with an electrospray ionization source operating in positive mode. Full scanning was conducted over an _m/z_ range of
100-800. The scanning time was 0.5 s, the detection time was 30 min, the low energy impact voltage was 6 V, and the high energy impact voltage was 15-40 V. Nitrogen gas was used as the
solvent gas, and leucine enkephalin was used for real-time correction. All the target compounds were eluted at a constant flow rate of 300 µL min−1 by using column HSS T3 Column, 100 Å, 1.8
µm, 2.1 mm×100 mm, with 0.04% formic acid as solvent A and methanol with 0.04% formic acid as solvent B. The gradient method was used as followed: 5% B to 10% B from 0-5.0 min, 10% B to 15%
B from 5.0-20.0 min, 15% B to 25% B from 20.0-23.0 min, 25% B to 40% B from 23.0-26.0 min, 40% B to 90% B from 26.0-27.0 min, held at 90% B from 27.0-28.5 min, 90% B to 5% B from 28.5-28.6
min, and held at 5% B from 28.6-30 min. MassLnx software was used for data acquisition and processing. WESTERN BLOT The strains were cultured in 1 mL delft media for overnight at 30 °C, 220
rpm. The preculture was then transferred with 1:50 dilution into 5 mL fresh delft media and grown until OD600 around 1. Then 3 OD cells were pelleted, washed twice with Phosphate buffered
saline (PBS). 200 μL of acid-washed glass beads and 300 μL of PBS with 1x protease inhibitor cocktail (Sigma) were added to resuspend the pellet, and then cells were broken by running
FastPrep according to manufacturer instructions. For the removal of potential N-linked oligosaccharide, cell lysates were first processed in denaturing buffer (0.5% SDS and 40 mM DTT) for 10
min at 98 °C, and subsequently treated with amidase PNGase F (ThermoFisher) for 1 h at 37 °C. Samples, added with 4 X NuPAGE™ LDS Sample Buffer (ThermoFisher), were boiled for 10 min at 95
°C. 10 μL of samples were loaded into 4–20% Mini-PROTEAN® TGX Stain-Free™ Protein Gels (Bio-Rad), and run for 1 h at 150 V. Proteins were transferred onto Trans-Blot® Turbo™ Mini PVDF
Transfer Packs (Bio-Rad) and blocked 2 h at room temperature in PBST (PBS + 0.1% Tween 20) with 5% milk. The membrane was washed three times with PBST for 5 min and incubated for 1 h at room
temperature with 6x-His Tag Monoclonal Antibody (HIS.H8) (ThermoFisher), or Anti-GAPDH Antibody (G-9) (Santa Cruz Biotechnology). The membrane was washed three times with PBST for 5 min and
incubated for 1 h at room temperature with HRP conjugated Anti-Mouse IgG (H + L) Secondary Antibodies (Invitrogen). The membrane was washed three times with PBST for 5 min and incubated for
5 min with West Pico plus HRP substrate (ThermoFisher), and then analyzed with a ChemiDoc XRS image analyzer (Bio-Rad). RT-QPCR To test the transcription level of CyBBE and its two
variants, we performed RT-qPCR. Briefly, around 1 OD600 cells were harvested by centrifugation at 12,000 × g for the RNA extraction using the RNeasy® kit from QIAGEN following the
manufacturer’s protocol. cDNA was synthesized by using QuantiTect Reverse Transcription kit (QIAGEN). The PCR reactions were done using the DyNAmo Flash SYBR® Green qPCR kit (Thermo Fisher
Scientific) in Mx3005P qPCR system (Agilent). Gene _ACT1_ acted as a reference to normalize RNA levels. IN VITRO ACTIVITY ASSAYS To test the activities of CyBBE and CyBBE_ERTS in distinct pH
conditions in vitro, we processed the yeast cells with the identical method for western blot. subsequently, the lysate was washed twice with 100 mM tris-HCl buffer (pH 7.5) at 20000 x g for
5 min under 4 °C, and then suspended in citrate-phosphate buffer79 or tris-HCl buffer with various pH. Adding commercial standard (_S_)-reticuline to keep the final concentration 5 mg/L,
and processing the mix for 2 h under 37 °C. The dilutions were analyzed by LC-MS/MS later. FLUORESCENCE MICROSCOPY ANALYSIS To investigate the localization of CyBBE and McDBOX2 variants,
green fluorescent protein (GFP) was fused to the C-terminus of each variant, and three marker genes, encoding ER protein Elo3, vacuole protein Vph1 and Golgi protein Chs5, respectively, were
fused with a C-terminal red fluorescent protein (mBuRy2). The corresponding plasmids (see Supplementary Data 1 for detailed information) were transformed into wild type IMX581. The
resultant strains were cultured in delft media for 18 h at 30 °C, 220 rpm. 2 μL of cells were spotted onto plain glass slides and visualized with a high-resolution confocal microscopy (The
Carl Zeiss LSM 980 with Airyscan 2). To examine the co-localization of multiple fluorescent proteins inside encapsulin compartments, two splitted venus fragments (VN and VC) and miRFP670
fused with a C-terminal targeting peptide were incorporated into strain expressing shell protein EncA. The resultant strains were cultured in delft media for 18 h at 30 °C, 220 rpm.
Subsequently, these yeast cells were visualized with a LEICA DM2000 microscope (Leica Microsystems CMS GmbH). MOLECULAR DYNAMIC SIMULATIONS Alphafold2.2.3 was first used to construct the
three-dimensional structures of CyBBE, CyBBEGSGSHDEL and CyBBEGSGSVIML, which were subsequently optimized by using UCSF Chimera. The atomic charges of the proteins were calculated under
AMBER14SB force field, and protonation states were assigned with the H + + 3 online tool. The structures of the small molecule (_S_)-reticuline and the cofactor FAD were obtained from the
PubChem database. The conformational sampling was performed with the open-source cheminformatics software package RDKit. The conformations were optimized in the MMFF94 force field, and the
AM1-BCC partial charges were assigned by using UCSF Chimera. Molecular docking experiments were performed by using AutoDock4.2 software. The optimal binding sites, predicted with SiteMap
software, were set as the docking center. The coordinates of the docking center were set as center x = 6.09, center y = −0.12, and center z = −3.63. The box size was set to a 22.5 Å cubic
box, and the spacing step size was set to 0.375. The maximum limitation for conformational search was set to 10,000. The conformational sampling and scoring were performed by using
Lamarckian Genetic Algorithm (LGA). The optimal complex of substrate, cofactor and protein were selected as initial models for subsequent dynamic simulation studies. The open-source software
package GROMACS5.1.5 was used to perform simulations. The simulation system was set in a closed environment with the temperature at 30 °C and pH to 5.5. The pressure in all systems was set
to 1 bar. The complexes were set at the center of the simulation system under periodic boundary conditions, and the minimum distance from the edge of the protein to the edge of the box was
set to 0.1 nm. The pdb2gmx was used to convert the receptor structure topology file into a recognized one by GROMACS under AMBEff14SB force field. The AmberTools was used to convert small
molecules into topology files recognized by GROMACS, and the GAFF force field was used to parse ligand atoms. TIP3P water molecules were added to simulate the water environment. The steepest
descent method was used to minimize the system energy for all atoms. In circumstance of protein position constrained, equilibrium simulations were carried out for 1000 ps under constant
values of particles, volume, and temperature (NVT) and particles, pressure, and temperature (NPT), respectively. After NVT and NPT equilibration, all systems were subjected to 50 ns of
production dynamics simulations, with simulations conducted every 2 fs. Covalent bond lengths were constrained by using the linear constraint solver algorithm, and long-range electrostatic
interactions were treated according to the Particle Mesh Ewald (PME) method. STATISTICAL ANALYSIS At least three biological replicates were performed for each quantification, and the
numerical values were depicted as means ± standard deviations. Statistical differences between control and derivative strains were assessed via unpaired _t_-tests using GraphPad Prism 8. In
all cases, _P_-values < 0.05 were considered significant. * _p_ < 0.05, ** _p_ < 0.01 and *** _p_ < 0.001. REPORTING SUMMARY Further information on research design is available
in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY Data supporting the findings of this work are available within the paper and its Supplementary Information
files. A reporting summary for this Article is available as a Supplementary Information file. Source data are provided with this paper. REFERENCES * World Health Organization. _Global
action plan on antimicrobial resistance_. (Jan 2016). * Schmeller, T., Latz-Brüning, B. & Wink, M. Biochemical activities of berberine, palmatine and sanguinarine mediating chemical
defence against microorganisms and herbivores. _Phytochemistry_ 44, 257–266 (1997). Article CAS PubMed Google Scholar * Liscombe, D. K. & Facchini, P. J. Evolutionary and cellular
webs in benzylisoquinoline alkaloid biosynthesis. _Curr. Opin. Biotechnol._ 19, 173–180 (2008). Article CAS PubMed Google Scholar * Imenshahidi, M. & Hosseinzadeh, H. Berberis
vulgaris and berberine: an update review. _Phytother. Res._ 30, 1745–1764 (2016). Article PubMed Google Scholar * Derosa, G., Maffioli, P. & Arrigo, F. G. C. Berberine on metabolic
and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. _Expert Opin. Biol. Ther._ 12, 8 (2012). Article Google Scholar * Reddi, K. K., Li, H., Li, W.
& Tetali, S. D. Berberine, A phytoalkaloid, inhibits inflammatory response induced by lps through nf-kappabeta pathway: possible involvement of the ikkalpha. _Molecules_ 26, 4733 (2021).
Article CAS PubMed PubMed Central Google Scholar * Yang, S., Li, D., Yu, Z., Li, Y. & Wu, M. Multi-pharmacology of berberine in atherosclerosis and metabolic diseases: potential
contribution of gut microbiota. _Front Pharm._ 12, 709629 (2021). Article CAS Google Scholar * Dumont, E. & Monari, A. Interaction of palmatine with DNA: an environmentally controlled
phototherapy drug. _J. Phys. Chem. B_ 119, 410–419 (2015). Article CAS PubMed Google Scholar * Stiborova, M. et al. Macleaya cordata extract and sangrovit genotoxicity. Assessment in
vivo. _Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc Czech Repub._ 152, 35–39 (2008). Article PubMed Google Scholar * Kantas, D., Papatsiros, V. G., Tassis, P. D., Athanasiou, L. V. &
Tzika, E. D. Effect of a natural feed additive (_Macleaya cordata_), containing sanguinarine, on the performance and health status of weaning pigs. _Anim. Sci. J._ 86, 92–98 (2015). Article
CAS PubMed Google Scholar * Rawling, M. D., Merrifield, D. L. & Davies, S. J. Preliminary assessment of dietary supplementation of Sangrovit® on red tilapia (_Oreochromis
niloticus_) growth performance and health. _Aquaculture_ 294, 118–122 (2009). Article CAS Google Scholar * Bessi, I. et al. Spectroscopic, molecular modeling, and NMR-spectroscopic
investigation of the binding mode of the natural alkaloids berberine and sanguinarine to human telomeric G-quadruplex DNA. _ACS Chem. Biol._ 7, 1109–1119 (2012). Article CAS PubMed Google
Scholar * Messeha, S. S., Zarmouh, N. O., Antonie, L. & Soliman, K. F. A. Sanguinarine inhibition of TNF-alpha-induced CCL2, IKBKE/NF-kappaB/ERK1/2 signaling pathway, and cell
migration in human triple-negative breast cancer cells. _Int. J. Mol. Sci._ 23, 8329 (2022). Article CAS PubMed PubMed Central Google Scholar * Slaninova, I., Slanina, J. &
Taborska, E. Quaternary benzo[c]phenanthridine alkaloids––novel cell permeant and red fluorescing DNA probes. _Cytom. A_ 71, 700–708 (2007). Article Google Scholar * Lin, W. et al. Protein
kinase C inhibitor chelerythrine selectively inhibits proliferation of triple-negative breast cancer cells. _Sci. Rep._ 7, 2022 (2017). Article ADS PubMed PubMed Central Google Scholar
* Valipour, M., Zarghi, A., Ebrahimzadeh, M. A. & Irannejad, H. Therapeutic potential of chelerythrine as a multi-purpose adjuvant for the treatment of COVID-19. _Cell Cycle_ 20,
2321–2336 (2021). Article CAS PubMed PubMed Central Google Scholar * Ghashghaeinia, M., Dreischer, P., Wieder, T. & Koberle, M. Coronavirus disease 2019 (COVID-19), human
erythrocytes and the PKC-alpha/-beta inhibitor chelerythrine-possible therapeutic implication. _Cell Cycle_ 19, 3399–3405 (2020). Article CAS PubMed PubMed Central Google Scholar *
Fossati, E. et al. Reconstitution of a 10-gene pathway for synthesis of the plant alkaloid dihydrosanguinarine in _Saccharomyces cerevisiae_. _Nat. Commun._ 5, 3283 (2014). Article ADS
PubMed Google Scholar * Trenchard, I. J. & Smolke, C. D. Engineering strategies for the fermentative production of plant alkaloids in yeast. _Metab. Eng._ 30, 96–104 (2015). Article
CAS PubMed PubMed Central Google Scholar * Xu, Z., Xia, L., Sun, M., Huang, P. & Zeng, J. Effects of codon optimization, N-terminal truncation and gene dose on the heterologous
expression of berberine bridge enzyme. _World J. Microbiol. Biotechnol._ 38, 77 (2022). Article CAS PubMed Google Scholar * Bock, A., Wanner, G. & Zenk, M. H. Immunocytological
localization of two enzymes involved in berberine biosynthesis. _Planta_ 216, 57–63 (2002). Article CAS PubMed Google Scholar * Bird, D. A. & Facchini, P. J. Berberine bridge enzyme,
a key branch-point enzyme in benzylisoquinoline alkaloid biosynthesis, contains a vacuolar sorting determinant. _Planta_ 213, 888–897 (2001). Article CAS PubMed Google Scholar *
Hawkins, K. M. & Smolke, C. D. Production of benzylisoquinoline alkaloids in _Saccharomyces cerevisiae_. _Nat. Chem. Biol._ 4, 564–573 (2008). Article CAS PubMed PubMed Central
Google Scholar * Valentic, T. R., Payne, J. T. & Smolke, C. D. Structure-guided engineering of a scoulerine 9-_O_-methyltransferase enables the biosynthesis of tetrahydropalmatrubine
and tetrahydropalmatine in yeast. _ACS Catal._ 10, 4497–4509 (2020). Article CAS Google Scholar * Gesell, A. et al. Heterologous expression of two FAD-dependent oxidases with
(S)-tetrahydroprotoberberine oxidase activity from _Argemone mexicana_ and _Berberis wilsoniae_ in insect cells. _Planta_ 233, 1185–1197 (2011). Article CAS PubMed Google Scholar *
Matsushima, Y., Minami, H., Hori, K. & Sato, F. Pathway engineering of benzylisoquinoline alkaloid biosynthesis in transgenic California poppy cells with ectopic expression of
tetrahydroberberine oxidase from _Coptis japonica_. _Plant Biotechnol._ 29, 473–481 (2012). Article CAS Google Scholar * Hagel, J. M. et al. Characterization of a flavoprotein oxidase
from opium poppy catalyzing the final steps in sanguinarine and papaverine biosynthesis. _J. Biol. Chem._ 287, 42972–42983 (2012). Article CAS PubMed PubMed Central Google Scholar *
Liu, X. et al. The genome of medicinal plant _Macleaya cordata_ provides new insights into benzylisoquinoline alkaloids metabolism. _Mol. Plant_ 10, 975–989 (2017). Article CAS PubMed
Google Scholar * Polturak, G. et al. Elucidation of the first committed step in betalain biosynthesis enables the heterologous engineering of betalain pigments in plants. _New Phytol._ 210,
269–283 (2016). Article CAS PubMed Google Scholar * DeLoache, W. C. et al. An enzyme-coupled biosensor enables (S)-reticuline production in yeast from glucose. _Nat. Chem. Biol._ 11,
465–471 (2015). Article CAS PubMed Google Scholar * Barros, J. et al. 4-Coumarate 3-hydroxylase in the lignin biosynthesis pathway is a cytosolic ascorbate peroxidase. _Nat. Commun._ 10,
1994 (2019). Article ADS PubMed PubMed Central Google Scholar * Liu, L. et al. Engineering the biosynthesis of caffeic acid in _Saccharomyces cerevisiae_ with heterologous enzyme
combinations. _Engineering_ 5, 287–295 (2019). Article CAS Google Scholar * Nishihachijo, M. et al. Asymmetric synthesis of tetrahydroisoquinolines by enzymatic pictet-spengler reaction.
_Biosci. Biotechnol. Biochem._ 78, 701–707 (2014). Article CAS PubMed Google Scholar * Liu, Q. et al. Rewiring carbon metabolism in yeast for high level production of aromatic chemicals.
_Nat. Commun._ 10, 4976 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Lau, Y. H., Giessen, T. W., Altenburg, W. J. & Silver, P. A. Prokaryotic nanocompartments
form synthetic organelles in a eukaryote. _Nat. Commun._ 9, 1311 (2018). Article ADS PubMed PubMed Central Google Scholar * Grewal, P. S., Samson, J. A., Baker, J. J., Choi, B. &
Dueber, J. E. Peroxisome compartmentalization of a toxic enzyme improves alkaloid production. _Nat. Chem. Biol._ 17, 96–103 (2020). Article PubMed Google Scholar * Pyne, M. E. et al. A
yeast platform for high-level synthesis of tetrahydroisoquinoline alkaloids. _Nat. Commun._ 11, 3337 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Torrens-Spence, M.
P. et al. Biochemical evaluation of a parsley tyrosine decarboxylase results in a novel 4-hydroxyphenylacetaldehyde synthase enzyme. _Biochem. Biophys. Res. Commun._ 418, 211–216 (2012).
Article CAS PubMed Google Scholar * Torrens-Spence, M. P., Pluskal, T., Li, F.-S., Carballo, V. & Weng, J.-K. Complete pathway elucidation and heterologous reconstitution of
_Rhodiola_ salidroside biosynthesis. _Mol. Plant_ 11, 205–217 (2018). Article CAS PubMed Google Scholar * Torrens-Spence, M. P. et al. Biochemical evaluation of the decarboxylation and
decarboxylation-deamination activities of plant aromatic amino acid decarboxylases. _J. Biol. Chem._ 288, 2376–2387 (2013). Article CAS PubMed Google Scholar * Holland, C. K., Berkovich,
D. A., Kohn, M. L., Maeda, H. & Jez, J. M. Structural basis for substrate recognition and inhibition of prephenate aminotransferase from _Arabidopsis_. _Plant J._ 94, 304–314 (2018).
Article CAS PubMed Google Scholar * Lopez-Nieves, S. et al. Relaxation of tyrosine pathway regulation underlies the evolution of betalain pigmentation in Caryophyllales. _New Phytol._
217, 896–908 (2018). Article CAS PubMed Google Scholar * Schenck, C. A., Chen, S., Siehl, D. L. & Maeda, H. A. Non-plastidic, tyrosine-insensitive prephenate dehydrogenases from
legumes. _Nat. Chem. Biol._ 11, 52–57 (2015). Article CAS PubMed Google Scholar * Schenck, C. A. et al. Molecular basis of the evolution of alternative tyrosine biosynthetic routes in
plants. _Nat. Chem. Biol._ 13, 1029–1035 (2017). Article CAS PubMed Google Scholar * Finnigan, J. D., Young, C., Cook, D. J., Charnock, S. J. & Black, G. W. Cytochromes P450 (P450s):
A review of the class system with a focus on prokaryotic P450s. _Adv. Protein Chem. Struct. Biol._ 122, 289–320 (2020). Article CAS PubMed Google Scholar * Bourgeois, L., Pyne, M. E.
& Martin, V. J. J. A highly characterized synthetic landing pad system for precise multicopy gene integration in yeast. _ACS Synth. Biol._ 7, 2675–2685 (2018). Article CAS PubMed
Google Scholar * Singh, A., Menéndez-Perdomo, I. M. & Facchini, P. J. Benzylisoquinoline alkaloid biosynthesis in opium poppy: an update. _Phytochem. Rev._ 18, 1457–1482 (2019). Article
Google Scholar * Steffens, P., Nagakura, N. & Zenk, M. H. Purification and characterization of the berberine bridge enzyme from _berberis beaniana_ cell cultures. _Phytochemistry_ 24,
2577–2583 (1985). Article CAS Google Scholar * Orij, R., Brul, S. & Smits, G. J. Intracellular pH is a tightly controlled signal in yeast. _Biochim. Biophys. Acta_ 1810, 933–944
(2011). Article CAS PubMed Google Scholar * Dean, N. & Pelham, H. R. Recycling of proteins from the Golgi compartment to the ER in yeast. _J. Cell Biol._ 111, 369–377 (1990). Article
CAS PubMed Google Scholar * Roberts, C. J., Nothwehr, S. E. & Stevens, T. H. Membrane protein sorting in the yeast secretory pathway: evidence that the vacuole may be the default
compartment. _J. Cell Biol._ 119, 69–83 (1992). Article CAS PubMed Google Scholar * Zhu, J. et al. A validated set of fluorescen-protein-based markers for major organelles in yeast
(_Saccharomyces cerevisiae_). _mBio_ 10, e01691–19 (2019). Article CAS PubMed PubMed Central Google Scholar * Dastmalchi, M. et al. Purine permease-type benzylisoquinoline alkaloid
transporters in Opium Poppy. _Plant Physiol._ 181, 916–933 (2019). Article CAS PubMed PubMed Central Google Scholar * Zito, E. et al. Oxidative protein folding by an endoplasmic
reticulum-localized peroxiredoxin. _Mol. Cell_ 40, 787–797 (2010). Article CAS PubMed PubMed Central Google Scholar * Winkler, A., Hartner, F., Kutchan, T. M., Glieder, A. &
Macheroux, P. Biochemical evidence that berberine bridge enzyme belongs to a novel family of flavoproteins containing a bi-covalently attached FAD cofactor. _J. Biol. Chem._ 281, 21276–21285
(2006). Article CAS PubMed Google Scholar * Kim, J. E. et al. Tailoring the _Saccharomyces cerevisiae_ endoplasmic reticulum for functional assembly of terpene synthesis pathway.
_Metab. Eng._ 56, 50–59 (2019). Article CAS PubMed Google Scholar * Grycova, L., Dostal, J. & Marek, R. Quaternary protoberberine alkaloids. _Phytochemistry_ 68, 150–175 (2007).
Article CAS PubMed Google Scholar * Han, J. & Li, S. De novo biosynthesis of berberine and halogenated benzylisoquinoline alkaloids in _Saccharomyces cerevisiae_. _Commun. Chem._ 6,
27 (2023). Article CAS PubMed PubMed Central Google Scholar * Rajecky, M. et al. Alkaloid chelirubine and DNA: blue and red luminescence. _Talanta_ 105, 317–319 (2013). Article CAS
PubMed Google Scholar * Son, S. Y., Rhee, H. S., Lee, M. W. & Park, J. M. Analysis of benzo[c]phenanthridine alkaloids in _Eschscholtzia californica_ cell culture using HPLC-DAD and
HPLC-ESI-MS/MS. _Biosci. Biotechnol. Biochem._ 78, 1103–1111 (2014). Article CAS PubMed Google Scholar * Luo, X. et al. Complete biosynthesis of cannabinoids and their unnatural
analogues in yeast. _Nature_ 567, 123–126 (2019). Article ADS CAS PubMed Google Scholar * Srinivasan, P. & Smolke, C. D. Biosynthesis of medicinal tropane alkaloids in yeast.
_Nature_ 585, 614–619 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Pelham, H. R., Hardwick, K. G. & Lewis, M. J. Sorting of soluble ER proteins in yeast. _EMBO
J._ 7, 1757–1762 (1988). Article CAS PubMed PubMed Central Google Scholar * Jackson, M. R., Nilsson, T. & Peterson, P. A. Identification of a consensus motif for retention of
transmembrane proteins in the endoplasmic reticulum. _EMBO J._ 9, 3153–3162 (1990). Article CAS PubMed PubMed Central Google Scholar * Nilsson, I., Whitley, P. & Heijne, G. V. The
COOH-terminal ends of internal signal and signal-anchor sequences are positioned differently in the er translocase. _J. Cell Biol._ 126, 1127–1132 (1994). Article CAS PubMed Google
Scholar * Schutze, M. P., Peterson, P. A. & Jackson, M. R. An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. _EMBO J._ 13, 1696–1705
(1994). Article CAS PubMed PubMed Central Google Scholar * Cooper, B. & Bussey, H. Yeast Kex1p is a Golgi-associated membrane protein: deletions in a cytoplasmic targeting domain
result in mislocalization to the vacuolar membrane. _J. Cell Biol._ 119, 1459–1468 (1992). Article CAS PubMed Google Scholar * Wilcox, C. A., Redding, K., Wright, R. & Fuller, R. S.
Mutation of a tyrosine localization signal in the cytosolic tail of yeast Kex2 protease disrupts golgi retention and results in default transport to the vacuole. _Mol. Biol. Cell._ 3,
1353–1371 (1992). Article CAS PubMed PubMed Central Google Scholar * Nothwehr, S. E., Roberts, C. J. & Stevens, T. H. Membrane protein retention in the yeast golgi apparatus:
dipeptidyl aminopeptidase a is retained by a cytoplasmic signal containing aromatic residues. _J. Cell Biol._ 121, 1197–1209 (1993). Article CAS PubMed Google Scholar * Sun, M., Liu, J.,
Lin, C., Miao, L. & Lin, L. Alkaloid profiling of the traditional Chinese medicine Rhizoma corydalis using high performance liquid chromatography-tandem quadrupole time-of-flight mass
spectrometry. _Acta Pharm. Sin. B_ 4, 208–216 (2014). Article PubMed PubMed Central Google Scholar * Wang, M. et al. Applying target data screening followed by characteristic fragment
filtering for the comprehensive screening and identification of alkaloids in _Corydalis yanhusuo_ W. T. Wang by UPLC-Q-TOF/MSE. _RSC Adv._ 7, 53545–53551 (2017). Article ADS CAS Google
Scholar * Galanie, S. & Smolke, C. D. Optimization of yeast-based production of medicinal protoberberine alkaloids. _Microb. Cell Fact._ 14, 144 (2015). Article PubMed PubMed Central
Google Scholar * Liu, T. et al. Construction of ajmalicine and sanguinarine de novo biosynthetic pathways using stable integration sites in yeast. _Biotechnol. Bioeng._ 119, 1314–1326
(2022). Article CAS PubMed Google Scholar * Verduyn, C., Postma, E., Scheffers, W. A. & Van Dijken, J. P. Effect of benzoic acid on metabolic fluxes in yeasts: a continuous-culture
study on the regulation of respiration and alcoholic fermentation. _Yeast_ 8, 501–517 (1992). Article CAS PubMed Google Scholar * Mikkelsen, M. D. et al. Microbial production of
indolylglucosinolate through engineering of a multi-gene pathway in a versatile yeast expression platform. _Metab. Eng._ 14, 104–111 (2012). Article CAS PubMed Google Scholar * Zhou, Y.
J. et al. Modular pathway engineering of diterpenoid synthases and the mevalonic acid pathway for miltiradiene production. _J. Am. Chem. Soc._ 134, 3234–3241 (2012). Article CAS PubMed
Google Scholar * Gietz, R. D. & Schiestl, R. H. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. _Nat. Protoc._ 2, 31–34 (2007). Article CAS PubMed
Google Scholar Download references ACKNOWLEDGEMENTS We thank Mikael Molin for kindly providing the mPRDX4 plasmid, Shan Jiang and Centre for Cellular Imaging from University of Gothenburg
for the help with fluorescent microscopes, and the Chalmers Mass Spectrometry Infrastructure for the assistance on metabolite analysis. This work was financially supported by Vetenskapsrådet
(2018-06003), Stiftelsen för internationalisering av högre utbildning och forskning, National Key R&D Program of China (2020YFA0908000) and National Natural Science Foundation of China
(82011530137 & 31961133007). FUNDING Open access funding provided by Chalmers University of Technology. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Life Sciences,
Chalmers University of Technology, Kemivägen 10, SE-412 96, Gothenburg, Sweden Xiang Jiao, Xiaozhi Fu, Otto Savolainen, Jens Nielsen & Yun Chen * State Key Laboratory for Quality
Ensurance and Sustainable Use of Dao-di Herbs, National Resource Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, 16 Neinanxiaojie, Dongcheng district, Beijing,
China Qishuang Li, Junling Bu, Xiuyu Liu, Luqi Huang & Juan Guo * Chalmers Mass Spectrometry Infrastructure, Chalmers University of Technology, Kemivägen 10, SE-412 96, Gothenburg,
Sweden Otto Savolainen * BioInnovation Institute, DK-2200, Copenhagen N, Denmark Jens Nielsen Authors * Xiang Jiao View author publications You can also search for this author inPubMed
Google Scholar * Xiaozhi Fu View author publications You can also search for this author inPubMed Google Scholar * Qishuang Li View author publications You can also search for this author
inPubMed Google Scholar * Junling Bu View author publications You can also search for this author inPubMed Google Scholar * Xiuyu Liu View author publications You can also search for this
author inPubMed Google Scholar * Otto Savolainen View author publications You can also search for this author inPubMed Google Scholar * Luqi Huang View author publications You can also
search for this author inPubMed Google Scholar * Juan Guo View author publications You can also search for this author inPubMed Google Scholar * Jens Nielsen View author publications You can
also search for this author inPubMed Google Scholar * Yun Chen View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.C. and X.J. conceived the
study. X.J. performed most of the experiments. X.J., Y.C. analyzed all the experimental data and drafted the manuscript. X.F., J.B. and X.L. performed part of plasmids and yeast strain
constructions, Q.L. and O.S. helped with LC-MS/MS analysis, J.G, L.H. and J.N. assisted with data analysis. All authors revised and approved the final version of the manuscript.
CORRESPONDING AUTHORS Correspondence to Luqi Huang, Juan Guo, Jens Nielsen or Yun Chen. ETHICS DECLARATIONS COMPETING INTERESTS Y.C. and X.J. are inventors of pending patent applications
(PCT/071270 and PCT/071276) arising from work on strategies for improved alkaloids production. Other authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature
Communications_ thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer
Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTION
OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN
ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format,
as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Jiao, X., Fu, X., Li, Q. _et al._ De
novo production of protoberberine and benzophenanthridine alkaloids through metabolic engineering of yeast. _Nat Commun_ 15, 8759 (2024). https://doi.org/10.1038/s41467-024-53045-3 Download
citation * Received: 17 July 2024 * Accepted: 27 September 2024 * Published: 09 October 2024 * DOI: https://doi.org/10.1038/s41467-024-53045-3 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative








