- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Many mammal species have declining populations, but the consequences of small population size on the genomic makeup of species remain largely unknown. We investigated the
evolutionary history, genetic load and adaptive potential of the Cat Ba langur (_Trachypithecus poliocephalus_), a primate species endemic to Vietnam’s famous Ha Long Bay and with less than
100 living individuals one of the most threatened primates in the world. Using high-coverage whole genome data of four wild individuals, we revealed the Cat Ba langur as sister species to
its conspecifics of the northern limestone langur clade and found no evidence for extensive secondary gene flow after their initial separation. Compared to other primates and mammals, the
Cat Ba langur showed low levels of genetic diversity, long runs of homozygosity, high levels of inbreeding and an excess of deleterious mutations in homozygous state. On the other hand,
genetic diversity has been maintained in protein-coding genes and on the gene-rich human chromosome 19 ortholog, suggesting that the Cat Ba langur retained most of its adaptive potential.
The Cat Ba langur also exhibits several unique non-synonymous variants that are related to calcium and sodium metabolism, which may have improved adaptation to high calcium intake and
saltwater consumption. SIMILAR CONTENT BEING VIEWED BY OTHERS GENOMIC BASIS OF INSULARITY AND ECOLOGICAL DIVERGENCE IN BARN OWLS (_TYTO ALBA_) OF THE CANARY ISLANDS Article Open access 29
September 2022 GHOST ADMIXTURE IN EASTERN GORILLAS Article Open access 27 July 2023 GENOME-SCALE EVOLUTION IN LOCAL POPULATIONS OF WILD CHIMPANZEES Article Open access 02 January 2025
INTRODUCTION As a result of human population expansion, habitat transformation, and other human activities including direct persecution, many species are on the brink of extinction or have
declining populations1,2,3,4,5,6. Non-human primates are no exception as 63% of the species are threatened with extinction and 93% have declining populations7,8. Threats to primates include,
among others, destruction and fragmentation of habitat, hunting, and poaching7. Small populations are vulnerable to a range of extrinsic and intrinsic processes, including habitat loss and
disease, demographic stochasticity as well as genetic effects9. In recent years, an increasing number of studies focused on the role of genetics on the long-term viability of small
populations and often revealed genomic erosion in such populations10,11,12,13,14,15,16,17,18,19,20,21. Small populations tend to have reduced viability due to a loss of genetic diversity and
increased inbreeding22,23,24,25. Moreover, in small, isolated, and inbred populations, genetic load, the presence of unfavorable genetic material, may accumulate and increase extinction
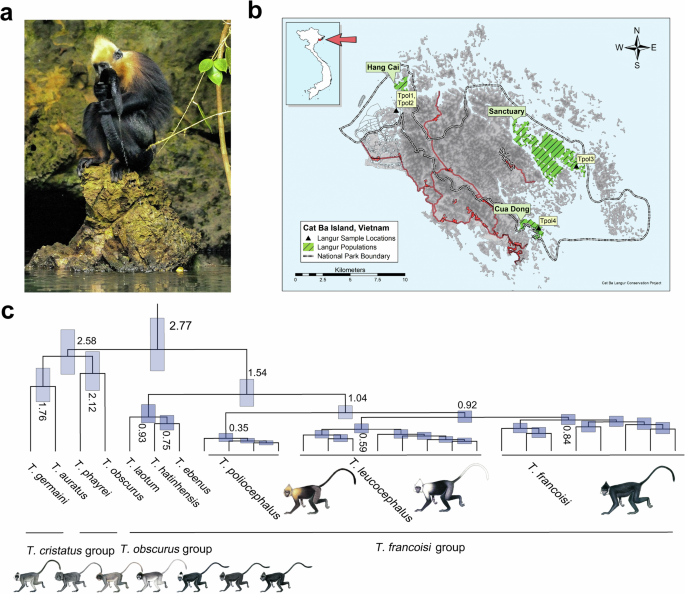
risk, although purging of deleterious mutations has been documented, too12,13,14,15,16,17,18,19,20,21,26,27,28. The critically endangered golden-headed or Cat Ba langur (_Trachypithecus
poliocephalus_), endemic to Cat Ba Island in Ha Long Bay, northeastern Vietnam (Fig. 1a, b) and one of the most threatened primates in the world29, is a good model to study the effects of
small population size and recent population declines on the genomic makeup of a species. The population may have contained 2400–2700 individuals in historical times30. However, the first
survey conducted in 1999 revealed only 104–135 individuals30, and in 2002–2004, the population declined to a minimum of 40 individuals29. Since then, the population increased to 74–79
individuals of which 38 are reproductively active (status: December 2023; NL pers. observation). Major reasons for the decline both in the past and until recently were hunting and poaching
for traditional medicine and sport, while today the species suffers mainly from disturbance and fragmentation of habitat, growing, but poorly managed tourism, and inbreeding29.
_Trachypithecus poliocephalus_ is a species of the colobine genus _Trachypithecus_ which contains a total of 22 species, grouped into four species groups31,32,33,34. _Trachypithecus
poliocephalus_ is one of the seven species of the _T. francoisi_ or limestone langur group31,32,33,34. Within this group, _T. poliocephalus_ forms the northern clade, together with
François’s langur (_T. francoisi_) and the white-headed langur (_T. leucocephalus_), but it remains unclear whether the species is basal within this clade as suggested by mitochondrial DNA34
or it is sister to _T. leucocephalus_ as indicated by similarities in fur coloration, which led Groves35 to treat them both as subspecies of a single species. As all species of the
limestone langur group, _T. poliocephalus_ is restricted to limestone karst habitats – in contrast to other species of _Trachypithecus_ that live in rainforest habitats31,32,33,34. Although
it has been suggested that limestone langurs originally may have also occurred in rainforests and survived only in karst habitats due to human pressure36, a recent genomic study revealed
evidence for genomic adaptation of this group to karst habitat, specifically to high calcium intake, since more than one million years37. However, even among limestone langurs and also
primates in general, _T. poliocephalus_ is probably unique as it seems to be able to cope with high salt concentrations in its diet. As the only limestone langur species living on a maritime
island, _T. poliocephalus_ is naturally exposed to high salt concentrations in the form of saline-rich drinking water and moisture on food plants, and animals are known to lick and even
drink sea water38 (Fig. 1a; Supplementary Movie 1). However, so far evidence for genomic adaptation to an increased saltwater tolerance in _T. poliocephalus_ is missing. In this work, we
examine the effects of small population size on the conservation status of _T. poliocephalus_ by analyzing whole-genome data of four wild individuals. We first investigate the phylogenetic
relationships among northern limestone langur species, estimate when they diverged, and if secondary gene flow occurred. Second, as a proxy for adaptive potential, we calculate the genomic
diversity and inbreeding level for _T. poliocephalus_ and compare them with data of other limestone langurs and further mammal species. Third, we examine the genetic load of _T.
poliocephalus_ in comparison to its conspecifics of the northern limestone langur clade. Finally, we investigate signatures of selection, which are potentially associated with adaptation to
the species’ unique environment. Our results provide insights into the evolutionary history of limestone langurs, how small population size affects the genomics of a critically endangered
primate, and how species adapt to challenging environmental conditions. RESULTS SAMPLING AND DATASETS We generated whole-genome sequencing data (range: 28.8–36.5×, mean: 32.7×; Supplementary
Table 1) from four wild _T. poliocephalus_ individuals representing all three extant sub-populations of the species (Hang Cai, Sanctuary, Cua Dong; Fig. 1b). Due to the expected high
inbreeding level in the population29, we tested for relatedness among the four _T. poliocephalus_ individuals, but found no evidence for any close relatedness among them (Supplementary Table
2). For comparative analyses, we added additional published genome data and mapped all samples to the _T. francoisi_ (Tfra_2.0) and rhesus macaque (_Macaca mulatta_; Mmul_10) reference
genomes (see “Methods” section). To avoid any bias arising from mapping to an ingroup and to take advantage of the high-quality chromosome-level annotation of Mmul_10, we performed most
analyses using the mapping data to Mmul_10 (for details see Supplementary Table 3). However, for genome-wide analyses of length and fraction of runs of homozygosity (ROHs), and signatures of
selection, we used the mapping data to the more closely related Tfra_2.0 reference genome. PHYLOGENY AND POPULATION STRUCTURE We explored the phylogenetic position of _T. poliocephalus_ by
reconstructing neighbor-joining (NJ) and maximum-likelihood (ML) trees based on autosomal nucleotide variants. Both trees supported _T. poliocephalus_ as the sister lineage to _T. francoisi_
and _T. leucocephalus_ (NJ and ML bootstrap values of 100% and >95%, respectively; Fig. 1c; Supplementary Figs. 1 and 2). Together, these three species formed the northern clade of
limestone langurs and represented the sister group to the southern clade consisting of _T. laotum_, _T. hatinhensis_ and _T. ebenus_. Using protein-coding sequences (CDS) on autosomes, we
estimated the initial split among the investigated _Trachypithecus_ species at 2.8 (95% confidence interval: 2.2–3.5) million years ago (Mya; Fig. 1c; Supplementary Fig. 3, Supplementary
Table 4), separating limestone langurs from two other species groups of the genus (_T. obscurus_ and _T. cristatus_ groups). Among limestone langurs, southern and northern clades diverged
1.5 (1.2–2.0) Mya, and in the northern clade, _T. poliocephalus_ separated from _T. francoisi_ and _T. leucocephalus_ 1.0 (0.8–1.3) Mya, while the latter two diverged 0.9 (0.8–1.1) Mya.
Admixture plots and a principal component analysis (PCA) supported the division of the three northern limestone langur species into three clusters and the basal position of _T.
poliocephalus_ among them (Supplementary Figs. 4 and 5; Supplementary Table 5). We further tested for gene flow events among these three species, but found no evidence for extensive,
post-divergence gene flow among them (_D_-statistics; no significant difference under the _X_2 test, _p_ = 0.740 > 0.05; Supplementary Tables 6 and 7). GENETIC DIVERSITY AND INBREEDING As
a proxy for their adaptive potential22, we inferred nucleotide diversity π of limestone langurs and compared it with those of other mammals. Results showed that nucleotide diversity of _T.
poliocephalus_ was with 0.033% one of the lowest among a set of 55 mammal species (Fig. 2a). Likewise, genome-wide autosomal heterozygosity _He_ of _T. poliocephalus_ was with 0.36
(0.35–0.38) heterozygous sites per 1000 bp the lowest among limestone langurs (Fig. 2b). However, although in all limestone langurs heterozygosity was generally lower in protein-coding
(exons) versus non-protein-coding regions, _T. poliocephalus_ exhibited in exons the highest heterozygosity rate among limestone langurs (One-way ANOVA test, _p_ < 0.001; Supplementary
Fig. 6; Supplementary Data 1). Also, the ratio of non-synonymous to synonymous variants was increased in _T. poliocephalus_ compared to the other two species, although not significant
compared to _T. francoisi_ (_p_ < 0.001 vs. _T. leucocephalus_; Supplementary Fig. 7; Supplementary Data 1). Next, we investigated ROHs, contiguous homozygous segments of the genome where
identical haplotypes are inherited from both parents and which give insights into the degree of inbreeding39,40. The longest ROHs were identified in _T. poliocephalus_ (25.48–40.16 Mb),
followed by _T. leucocephalus_ (10.31–22.81 Mb), _T. francoisi_ (7.68–16.59 Mb), _T. laotum_ (13.11 Mb), _T. hatinhensis_ (11.12 Mb), and _T. ebenus_ (7.09 Mb). Overall, the _T.
poliocephalus_ genome contained the lowest number of ROHs, but due to the comparatively large number of long ROHs (>1 Mb), the fraction of the genome in ROHs was with 88.98% the highest
among limestone langurs (Fig. 2c; Supplementary Figs. 8–10). Likewise, also the genomic inbreeding coefficient _F_, based on all ROHs, was highest for _T. poliocephalus_ (_F_ROH = 0.85; Fig.
2d). We further inspected nucleotide diversity and ROHs for individual chromosomes. We observed a significantly higher nucleotide diversity (π = 0.075%; One-way ANOVA test, _p_ < 0.001)
and a significantly smaller fraction of ROHs (_f_ROH = 0.42; _p_ < 0.001) for the human chromosome 19 ortholog compared to all other autosomes (Fig. 2e; Supplementary Fig. 11). Likewise,
on the human chromosome 19 ortholog the fraction of genes in ROHs was with 0.64 significantly smaller (_p_ < 0.001) than on any other autosome (range: 0.74–0.82; Supplementary Table 8).
DELETERIOUS MUTATIONS AND GENETIC LOAD We first estimated the individual masked and realized load using autosomal polymorphism data and genomic evolutionary rate profiling (GERP) scores.
_Trachypithecus poliocephalus_ with an average of 0.89 and _T. leucocephalus_ with an average of 0.94 showed a significantly lower (One-way ANOVA test, _p_ < 0.05) masked load than _T.
francoisi_ (1.01; Supplementary Fig. 12). However, _T. poliocephalus_ with an average of 1.85 had a significantly higher (_p_ < 0.001) realized load than the others (_T. leucocephalus_:
1.20; _T. francoisi_: 0.94). We then investigated the impact of deleterious mutations on protein function using four effect categories (modifier, low, moderate, high) which we obtained by
snpEFF annotation. In all three northern limestone langur species, the portion of homozygous and heterozygous deleterious mutations with high impact was less than 0.018% of all deleterious
mutations and not significantly different among species, but for those with moderate impact, _T. francoisi_ showed a significantly lower rate than the other two (One-way ANOVA test, _p_ <
0.05); no significant differences among the three species were found in categories low and modifier (Fig. 3a; Supplementary Data 2 and 3). However, when considering only homozygous
deleterious mutations with high impact, _T. poliocephalus_ exhibited a significantly (_p_ < 0.05) higher rate than _T. francoisi_ and _T. leucocephalus_ (Fig. 3b; Supplementary Data 2 and
3). Although not always significant, similar trends with an increased rate of homozygous deleterious mutations in _T. poliocephalus_ compared to the other two species were observed also in
effect categories moderate and low, while in the category modifier, a lower rate was found (Fig. 3b; Supplementary Data 2 and 3). Functional enrichment analysis revealed that genes
containing homozygous high-impact deleterious mutations in _T. poliocephalus_ are mainly related to the immune system, signal transduction, RNA metabolism, and gene expression (Supplementary
Data 4). POSITIVE SELECTION AND NON-SYNONYMOUS VARIANTS We identified putative targets of selection between _T_. _poliocephalus_ and _T. francoisi_ using strict scan methods (XP-EHH, θπ,
Ka/Ks) and revealed a total of 205 candidate genes under strong selection in _T. poliocephalus_ compared to _T. francoisi_ (Supplementary Fig. 13, Supplementary Data 5). Functional
classification and enrichment analyses showed that many of these genes are related to, among others, metabolism of proteins and RNA, the immune system, organic anion transporters,
transcriptional regulation by TP53, and diseases (Benjamini and Hochberg corrected test, _p_ < 0.05; Supplementary Data 6). We further performed whole-genome scans aiming to detect
non-synonymous variants that occur only in _T_. _poliocephalus_. Using this method, we revealed, among others, a total of 92 genes related to calcium pathways 16 genes in the KEGG “calcium
signaling pathway” (_p_ < 0.01), 71 genes in GO term “calcium ion binding” (corrected _p_ < 0.001) and 13 genes in GO term “calcium-mediated signaling” (corrected _p_ < 0.01)
(Supplementary Table 9, Supplementary Data 7; note that some genes are present in multiple pathways). Seventy of the calcium-related genes (highlighted in red in Supplementary Data 7)
contain at least one non-synonymous mutation private to _T. poliocephalus_. In the other 22 genes, we detected amino acid changes that were fixed in _T. poliocephalus_, but occurred with
some frequency (max. 28.95%) in other limestone langurs. In 19 (_ACKR3_, _ACKR4_, _ADCY1_, _ASPH_, _CCKAR_, _EDN1_, _EIF2AK3_, _FPR2_, _GRIN2D_, _LAT2_, _MCTP2_, _MYLK4_, _NTSR1_, _P2RX6_,
_PLA2G4B_, _PLCD3_, _SPHK1_, _TPCN1_, _TRDN_) of the 24 genes found in GO term “calcium-mediated signaling” and the KEGG “calcium signaling pathway” (some genes are present in both
pathways), homozygous non-synonymous mutations were private to _T. poliocephalus_ (Supplementary Fig. 14, Supplementary Data 7), while in five genes (_PLCG2_, _GRIN2C_, _LAP3_, _PDE1A_,
_RYR1_) these mutations occurred (max. frequency 10.53%) also in other _Trachypithecus_ species (Supplementary Fig. 14, Supplementary Data 7). We further identified 30 non-synonymous
mutations in 22 genes related to sodium transport and homeostasis, of which nine genes (_SLC4A4_, _SLC4A11_, _SLC38A7_, _SLC34A3_, _SLC5A1_, _SLC5A2_, _SCNN1D_, _SLC5A6_, _SCN5A_) are linked
to GO term “sodium ion transport” (corrected _p_ < 0.05; Supplementary Data 8, Supplementary Table 10). Among these 30 mutations, 21 were homozygous and occurred only in _T.
poliocephalus_, while the other nine mutations were either heterozygous in some other limestone langurs, homozygously present in the distantly related members of the _T. obscurus_ group or,
as in one case, heterozygous in one of the four _T. poliocephalus_ individuals (Supplementary Data 8). To further investigate the potential adaptation of _T. poliocephalus_ to saltwater
consumption, we examined genes known to be involved in adaptation to different salinities in other vertebrates41,42,43,44,45,46,47,48,49,50,51 and found in one gene (_CDH26_) a premature
stop codon and in ten genes (_ASH1L_, _DPP10_, _CFAP65_, _COL14A1_, _COL17A1_, _EPPK1_, _PKP1_, _SLC4A9_, _SLC22A18_, _STAC_) at least one non-synonymous variant that occurred only in _T.
poliocephalus_ and not in any of the other _Trachypithecus_ species (Supplementary Data 9). DISCUSSION PHYLOGENETIC RELATIONSHIPS AMONG NORTHERN LIMESTONE LANGURS _Trachypithecus
poliocephalus_ is a member of the northern limestone langur clade, but the phylogenetic relationships among the three species in this clade remained disputed. While similarities in fur
coloration suggest a sister group relationship between _T. poliocephalus_ and _T. leucocephalus_35, mitochondrial genome data supported a basal position of _T. poliocephalus_ among northern
limestone langurs34. However, mitochondrial DNA represents just a single locus and can result in branching patterns different from the species tree and nuclear phylogenies52. In our
nuclear-based phylogenies (Fig. 1c; Supplementary Figs. 1 and 2), underpinned by the results of Admixture plots and PCA (Supplementary Figs. 4 and 5, Supplementary Table 5), _T.
poliocephalus_ is revealed as sister species to _T. francoisi_ and _T. leucocephalus_. Overall, this supports the classification of the three northern limestone langur taxa as distinct
species31,32,33,34 and disagrees with the classification of these three taxa into only two species, _T. francoisi,_ and _T. poliocephalus_, with the latter containing _T. leucocephalus_ as a
subspecies35. Further support for the species-level classification of the three northern limestone langur taxa is provided by the fact that they diverged approximately 0.9–1.0 Mya (Fig. 1c;
Supplementary Fig. 3, Supplementary Table 4) and that no notable secondary gene flow among them was detected (Supplementary Tables 5 and 6). At least for _T. poliocephalus_, gene flow with
its conspecifics would have been surprising as this species is endemic to Cat Ba Island and geographically clearly isolated from the other two species. Other islands in Ha Long Bay are
either too small or do not contain suitable habitat, and on the mainland close to Cat Ba Island, limestone formations as potential habitat for limestone langurs are absent, suggesting that
_T. poliocephalus_ has been restricted to Cat Ba Island for an extended period of time30. GENETIC DIVERSITY AND INBREEDING With π = 0.033%, _T. poliocephalus_ showed one of the lowest
nucleotide diversities among a set of 55 mammal species (Fig. 2a). Similarly, genome-wide autosomal heterozygosity was with _He_ = 0.36 per 1000 bp (Fig. 2b) amongst the lowest documented
for primates so far (_He_ = 0.34–7.14)53,54. Although snub-nosed monkeys (_Rhinopithecus_ spp.) have similarly low heterozygosity values as _T. poliocephalus_ (_He_ = 0.34–0.42; exception
_R. brelichi: He_ = 0.69)53, other primates, such as the other limestone langur species (_He_ = 0.62–1.33; Fig. 2b) or great apes (_He_ = ∼0.6–2.4)28,55 showed a ∼2–6-fold higher
heterozygosity. Likewise, when compared to other mammals, for instance, some _Capra_ species18, Sumatran rhinoceros20, mainland gray fox13, European gray wolf56, giant panda57 or European
brown bear58 (_He_ values of ∼0.77 to ∼1.90; for details see Supplementary Data 10), _T. poliocephalus_ exhibits a comparatively low heterozygosity. However, values were similar or higher
than in Alpine ibex18, moose (Minnesota population)59, beluga60, narwhal60, vaquita61, polar bear62, brown hyena63, snow leopard64, cheetah65, Iberian lynx66, Eurasian lynx66, and the San
Nicolas population of the Channel Island fox13 (_He_ values of 0.01 to 0.34; Supplementary Data 10). Many of these species have larger populations and/or distributions. Thus, considering
that _T. poliocpehalus_ is endemic to the relatively small island of Cat Ba (∼140 km2) and that the current population traces back to only 40 individuals in 2002–2004, the observed
heterozygosity of _He_ = 0.36 is comparatively high. This suggests that the long-term population size was likely larger and only recently dropped to the low number of around 100 individuals
estimated in the first survey, conducted in 199930. In all six investigated limestone langur species, we observed a lower heterozygosity in protein-coding compared to non-protein-coding
regions, which is in agreement with the general expectation that heterozygosity is lower in functionally important regions (Supplementary Fig. 6; Supplementary Data 1). However, in _T.
poliocephalus_ heterozygosity in protein-coding regions and the ratio of non-synonymous to synonymous variants were both higher (1.1–1.3-fold and 1.3–1.8-fold, respectively) than in its
conspecifics (Supplementary Figs. 6 and 7; Supplementary Data 1). This is in line with predictions that in small populations, the proportion of non-synonymous variants can increase due to
weakened selection which was previously shown for non-African humans67,68 and the San Nicolas population of the Channel Island fox13. A similar result was found for the narwhal and beluga
with overall lower heterozygosity in coding versus non-coding regions, a pattern which was also less prominent in the narwhal, i.e., again in the species with overall lower genetic
diversity. This finding of overall low levels of heterozygosity combined with little difference in diversity levels between coding and non-coding regions across the narwhal genome was
interpreted as evidence that heterozygosity levels have reached a diversity stasis across the genome and that any decreases in genetic diversity might be problematic for the longer-term
survival of the species60. The same may apply to the Cat Ba langur, suggesting that efforts should be undertaken to ensure no further erosion of genetic diversity in this species. To
estimate the degree of inbreeding, we analyzed ROHs. In _T. poliocephalus_, the fraction of the genome in ROHs >100 kb was with 88.98% (Fig. 2c) the highest among limestone langurs, and
the species exhibited also the longest ROHs (25.48–40.16 Mb) and the largest number of long ROHs (>1 Mb; Supplementary Figs. 8–10). Such comparatively long ROHs are indicative for recent
mating between closely related individuals, most likely during the past few generations69. Similarly, the genomic inbreeding coefficient _F_ based on ROHs was with _F_ROH = 0.85 (Fig. 2d)
the highest among limestone langurs and higher than in other vertebrates known to be also affected by high levels of inbreeding (_F_ROH = 0.05–0.79)15,21,28,53,61. Population structure as a
potential explanation for the high _F_ is unlikely as males are known to regularly move between sub-populations. Interestingly, _T. poliocephalus_ showed a significantly higher nucleotide
diversity while the fraction of ROHs was significantly smaller on the human chromosome 19 ortholog compared to other autosomes (Fig. 2e; Supplementary Fig. 11). The same pattern was observed
for _T. leucocephalus_, a species with also a relatively small population size. Some of the other _Trachypithecus_ species with generally larger population sizes showed the same trend,
especially _T. francoisi_, but for none of them, the differences between the human chromosome 19 ortholog and the remaining autosomes were significant. The human chromosome 19 is well known
for its unusually high gene density with more than double the number of genes compared to the genome-wide average and 20 tandemly clustered gene families70. This pattern seems to be
conserved among primates71, indicating the biological and evolutionary significance of chromosome 19 and its orthologs in other primates. Overall, our findings suggest that _T.
poliocephalus_, despite low genome-wide diversity and high inbreeding level, maintained relatively high genetic diversity in functionally important regions such as protein-coding genes and
the generally gene-rich human chromosome 19 ortholog. DELETERIOUS MUTATIONS AND GENETIC LOAD Genetic load refers to the reduction in individual and mean population fitness due to the
accumulation of deleterious mutations72,73. Genetic load can be divided into realized load (all sites where a deleterious allele is expressed, mainly sites that are homozygous for recessive
deleterious alleles) and masked load (sites that are heterozygous where a recessive deleterious allele does not contribute to loss of fitness)72. We observed in _T. poliocephalus_ a
significantly higher realized and a (significantly) lower masked load compared to the other two northern limestone langur species (Supplementary Fig. 12), implying an accumulation of
deleterious alleles in homozygous state in _T. poliocephalus_. Similar patterns were also found in the pink pigeon74, the Scandinavian wolf population75,76, the Florida panther77, and the
Scandinavian Arctic fox population78, all of which represent populations that have suffered from severe bottlenecks followed by inbreeding in the relatively recent past. Likewise, when
investigating the impact of deleterious mutations, we observed among northern limestone langur species no significant differences in the proportion of all (homozygous and heterozygous)
high-impact deleterious mutations (Fig. 3a). However, when considering only homozygous deleterious mutations, the proportion in categories high, moderate and low was significantly higher in
_T. poliocephalus_ compared to the other two species (Fig. 3b). Our findings are in agreement with predictions that high levels of inbreeding can lead to increased homozygosity of recessive
deleterious mutations, an effect observed in a number of small and isolated populations19,72,79,80. ADAPTATION TO HIGH CALCIUM INTAKE Limestone langurs, including _T. poliocephalus_, live in
karst habitats, land formations with steep and tall cliffs formed by highly soluble and porous bedrock such as limestone and characterized by alkaline soil with poor nutrient content except
minerals81,82. Because food plants and drinking water in karst habitats contain high concentrations of calcium and other minerals, limestone langurs have a naturally high calcium
intake37,83,84,85,86,87. It was previously shown that limestone langurs (five species have been genomically investigated so far: _T. francoisi_, _T. leucocephalus_, _T. hatinhensis_, _T.
laotum,_ and _T. ebenus_) are adapted to high calcium intake in that they downregulate the calcium entry into the cell37. Most likely responsible for this adaptation are amino acid changes
in seven positively selected genes (_CACNA1B, CACNA1C, CD38, EGFR, HTR2B, ITPKB, MYLK_) of the KEGG “calcium signaling pathway” and “oxytocin signaling pathway”37. We found the same amino
acid changes in _T. poliocephalus_, but also additional variants that may have further increased the species’ tolerance to high calcium intake. Using whole-genome scans, we identified
various genes related to calcium metabolism that contain non-synonymous variants largely private to _T. poliocephalus_ (Supplementary Fig. 14, Supplementary Table 9, Supplementary Data 7).
Most of these genes encode proteins located on the cell membrane and are involved in ion binding and transfer. For instance, _MCTP2_ (multiple C2 and transmembrane domain-containing protein
2), an integral component of the membrane, enables calcium ion and lipid binding88,89. Some of the other genes such as _ADCY1_ (adenylate cyclase 1), _ASPH_ (aspartate ß-hydroxylase),
_P2RX6_ (purinergic receptor P2X 6), _PLCD3_ (phospholipase C delta 3), _RYR1_ (ryanodine receptor 1), and _MYLK4_ (myosin light chain kinase family member 4) are also known to play
important roles in calcium homeostasis37,90,91,92,93,94,95,96, and amino acid changes in these and other genes may have improved the adaptability of _T. poliocephalus_ to high calcium intake
even further. POTENTIAL ADAPTATION TO SALTWATER CONSUMPTION For most vertebrates, high external salt concentrations can lead to toxic levels of ion accumulation and water loss within
cells97. Vertebrates living in or close to the sea are exposed to high salt concentrations and hence, salinity is an important extrinsic factor affecting their ecology, evolution, and
distribution98,99,100. For some species, it is known that they have evolved genetic mechanisms to persist under salinized or brackish conditions101. _Trachypithecus poliocephalus_ as a
species living on a maritime island is exposed to saltwater in the form of moisture on food plants and animals are known to lick and drink brackish water38, a behavior unique among primates.
Thus, we hypothesized that _T. poliocephalus_ may has also evolved mechanisms to cope with high salt concentrations. We identified in _T. poliocephalus_ 30 largely species-specific
non-synonymous variants in 22 genes related to sodium transport and homeostasis (Supplementary Data 8, Supplementary Table 10). Previous studies showed that transmembrane transporters
encoded by genes of the solute carrier family (SLC) have been linked to osmoregulation and salinity adaptation45,46,50,51. For instance, _SLC4A11_ (solute carrier family 4 member 11) encodes
a voltage-regulated, electrogenic sodium-coupled borate cotransporter, which mediates transcellular chloride ion reabsorption via SLC4A11 anion exchangers102,103. Zhu et al.104 reported
that salt-sensitivity of mice is associated with increased renal protein expressions of _SLC4A4_ and common variants in _SLC4A4_ contribute to variation in blood pressure responses to
dietary sodium intake in Han Chinese105. In both genes, _T. poliocephalus_ exhibits unique amino acid changes, and other genes with unique or largely unique amino acid changes in _T.
poliocephalus_, such as _SLC5A1_, _SLC5A2_, _SCNN1D,_ and _SCN5A_, are also known to play important roles in sodium ion transportation106,107,108. Using a list of genes potentially related
to adaptation to different salinities in vertebrates41,42,43,44,45,46,47,48,49,50,51, we identified another ten genes (_ASH1L_, _DPP10_, _CFAP65_, _COL14A1_, _COL17A1_, _EPPK1_, _PKP1_,
_SLC4A9_, _SLC22A18_, _STAC_) which contained species-specific non-synonymous variants in _T. poliocephalus_ (Supplementary Data 9). All these genes are involved in ion transportation,
osmoregulation, homeostasis, and cell-cell adhesion41,42,43,44,45,46,47,48,49,50,51. Another gene, potentially related to adaptation to different salinities, is _CDH26_ (cadherin
26)42,43,48. All four _T. poliocephalus_ individuals exhibited in _CDH26_ a premature stop codon resulting in the loss of the complete cytoplasmatic component (Supplementary Figs. 15–17).
CDH26 is a member of the cadherin protein family, which are calcium-dependent adhesion molecules that mediate cell-cell adhesion in all solid tissues and modulate a wide variety of processes
including cell polarization, migration, and differentiation109,110,111,112. In humans, _CDH26_ is known to exhibit high expression levels in prostate and urinary bladder113. Moreover,
over-expression of _CDH26_ might be related to myocardial infarction and progression of atherosclerosis114,115, and high salt diet is known as high-risk factor for cardiovascular disease
which creates a substrate for arrhythmias, myocardial infarction, and atherosclerosis116,117,118,119. In the American green treefrog, lower expression of _CDH26_ in coastal versus inland
populations was suggested as adaptation to higher salinity42,43 and a genomic study of vendace showed that outlier single nucleotide polymorphisms (SNPs) in _CDH26_ may be associated with
divergent selection related to environments exhibiting different salinities48. Thus, down-regulated expression of _CDH26_ might be an important factor for high salt adaptation in vertebrates
and we speculate that the disrupted protein function (loss of the intracellular catenin-interacting domain) may have contributed, in combination with amino acid changes in genes related to
sodium metabolism, to genomic adaptation to increased saltwater tolerance in _T. poliocephalus_. Our whole-genome analysis of the Cat Ba langur illustrates the competing effects, small
population size has on genetic diversity. While our data reveal low genetic diversity across the genome as well as long runs of homozygosity and an accumulation of deleterious mutations,
genetic diversity has been partially preserved in functionally important regions. Our study also revealed the potential genetic basis of adaptations of this species to its unusual insular
habitat, particularly to high calcium intake and saltwater consumption. However, these results need to be treated as preliminary because only four Cat Ba langur individuals have been
investigated in this study, albeit this refers to approximately 5% of the species’ global population. Undoubtedly, the Cat Ba langur is unique among primates and even among limestone
langurs, further emphasizing the importance to protect this critically endangered species. METHODS SAMPLE COLLECTION We obtained blood samples from two translocated females and tissue
samples from two deceased infants from the Cat Ba Langur Conservation Project. The blood samples were taken by experienced veterinarians in 2012 during a wild-to-wild translocation aimed at
reintroducing two isolated females to the larger of the two breeding sub-populations. The translocation was initially proposed prior to 2008, but the master plan was submitted to relevant
Vietnamese authorities and the international community in 2010. Approval to carry out the translocation was granted by HPPC (No: 4398/UBND-NN) and MARD (No: 245/TCLN-BTTN) in early 2010. The
two females were immobilized by the delivery of chemical agents (ketamine and medetomidine) via a blow dart after being trapped in a sleeping cave. After initial immobilization, they were
lowered one by one to the ground by a basket and there intubated and maintained on gaseous anesthesia. Immobilized animals were continuously observed for vital parameters such as
respiration, pulse frequency, and internal body temperature. Whole blood samples (5 ml) were collected from the femoral vein, placed in EDTA tubes, and kept frozen at –80 °C until DNA
extraction. Tissue samples from the two deceased infants found in the wild were collected in 2015 and 2018 and stored in 80% ethanol until further processing. All research complied with
protocols approved by the Animal Welfare Body of the German Primate Center and adhered to the legal requirements of Vietnam. We conducted the study in compliance with the Convention on
International Trade in Endangered Species of Wild Fauna and Flora (CITES; export nr. 18VN0331N/CT-KL, import nr. DE-E-02092/18) and the principles of the American Society of Primatologists
for the ethical treatment of non-human primates. DNA EXTRACTION AND SEQUENCING DNA was extracted with the Gentra Puregene Blood & Tissue Kit (Qiagen) following the manufacturer’s
instructions. DNA quality was checked with pulsed-field gel electrophoresis and concentration was measured with a NanoDrop Microvolume Spectrophotometer (ThermoFisher). 200 ng of DNA was
subjected to whole-genome sequencing following the Illumina DNA prep workflow. Sequencing was done on Illumina’s HiSeq 4000 (151 bp paired-end) to a mean coverage of 32.7× (Supplementary
Table 1) at Novogene China. Short-read sequencing data of the four _T. poliocephalus_ individuals are available on NCBI under BioProject PRJNA949813. ADDITIONAL SEQUENCE DATA For comparative
reasons, we added sequence data of another 23 individuals representing nine _Trachypithecus_ species (each eight individuals of _T. francoisi_ and _T. leucocephalus_, and each one
individual of _T. laotum_, _T. hatinhensis_, _T. ebenus_, _T. germaini_, _T. auratus_, _T. obscurus_ and _T. phayrei_) which were obtained from a recently published study (BioProject
PRJNA488530)37. Additional outgroup taxa were download from NCBI’s Sequence Read Archive (SRA): _Rhinopithecus roxellana_ (SRR8718596, SRR8718597), _Colobus angolensis_ (SRR1687497), _Papio
anubis_ (SRR8762000) _Macaca mulatta_ (SRR16119994), _Chlorocebus sabaeus_ (SRR6196475), _Pongo abelii_ (SRR11032814), _Gorilla gorilla_ (SRR9703449), _Pan troglodytes_ (SRR11892898) and
_Homo sapiens_ (SRR11075380). Published paired-end SRA data were split by sratoolkit v2.9.6 (https://trace.ncbi.nlm.nih.gov/Traces/sra/sra.cgi?view=software) using “fastq-dump --split-3”
parameters following the NCBI protocol and then compressed with pigz v2.7 (http://zlib.net/pigz/) using multiple default threads. MAPPING AND GENOTYPE CALLING Raw sequence reads were
adapter-trimmed and quality-filtered with fastp v0.23.1120 with 1. reads with unidentified nucleotides (_N_) > 10% discarded, and 2. reads with the proportion of low-quality base (phred
quality < = 5) > 50% discarded. We then mapped high-quality reads to the reference genome of either _M. mulatta_ (Mmul_10) or _T. francoisi_ (Tfra_2.0,
https://www.ncbi.nlm.nih.gov/genome/31690?genome_assembly_id=749809, GCF_009764315.1) using the Burrows-Wheeler Aligner v0.7.12121 with MEM algorithms. SAM format files were imported to
samtools v1.9122 for sorting with recommend parameters and then imported to Picard v2.20.2 (http://broadinstitute.github.io/picard/) to remove duplicates and build indexed BAM files. Mapping
results for the four _T. poliocephalus_ samples to Tfra_2.0 are shown in Supplementary Tables 1 and 11. We genotyped reads using a pipeline implemented in GATK v4.2.2123. SNP calling was
performed following GATK’s best practice. This included realignment of insertion/deletion (indel) polymorphisms with the “RealignerTargetCreator” and “IndelRealigner” functions, which were
used to re-calibrate quality scores, excluding from the error model variant positions that were pre-called using HaplotypeCaller. For the genotype calling, we obtained the GVCF file for each
individual using the “HaplotypeCaller” method in GATK and then, using the GenotypeGVCFs based method with the “includeNonVariantSites” flag, to get the population VCF file, including all
confident sites. We then applied the “SelectVariants” to exclude indels and split the data into variant and nonvariant sites. The hard filter command “VariantFiltration” was applied to
exclude potential false positive variant calls with the following criteria: “filterExpression QD < 2.0 | | FS > 60.0 | | MQ < 40.0 || ReadPosRankSum < --8.0 || MQRankSum
<12.5” and “genotypeFilterExpression DP < 4.0”. Additionally, sites were removed if there was an “N” in the reference sequence or the site spanned an indel plus a buffer of 3 bp in
both directions and the site included >10% missing genotypes. To obtain the genotype file for subsequent analyses, a PERL script was used to transfer the VCF format to genotype format
(e.g., AA, AT) and degenerate bases format (e.g., ‘M’ = ‘AC’) for all langurs and then again to generate final genotypes in VCF format. Our final VCF files contained 89,241,059 and
53,321,193 variants derived from the mapping to Mmul_10 and Tfra_2.0, respectively. Following the snpEFF v4.3124 best-practice pipeline for annotation, all individual genotype files were
annotated using an own-build Tfra_2.0 dataset (both gtf and gff3 files were download from NCBI; Supplementary Table 1). RELATEDNESS AMONG INDIVIDUALS To investigate the kinship coefficient
among the four _T. poliocephalus_ individuals, we used King v2.3.2125 to check family relationship and flag pedigree errors (Supplementary Table 2). An estimated kinship coefficient range
>0.354, [0.177, 0.354], [0.0884, 0.177] and [0.0442, 0.0884] corresponds to duplicate sample/monozygotic twin, 1st-degree, 2nd-degree, and 3rd-degree relationship, respectively125.
PHYLOGENY, POPULATION STRUCTURE, AND GENE FLOW Phylogenetic trees based on autosomal SNPs were calculated with NJ and ML algorithms using _R. roxellana_ as outgroup. The NJ tree was
generated with TreeBest software (http://treesoft.sourceforge.net/treebest.shtml) with 1000 bootstrap replicates and the HKY model as it was determined as the most appropriate model by
MrModeltest126. The ML tree was reconstructed in IQ-tree v2.1.3127 with 1000 ultrafast bootstrap replicates128,129 and the ‘TVM + F + R2’ model as automatically determined by IQ-tree.
FigTree v1.4.0 (http://tree.bio.ed.ac.uk/software/figtree/) was used to visualize phylogenetic trees. Divergence times were calculated in IQ-tree based on coding regions on autosomes (CDS
regions were selected using Mmul_10 annotation gtf files) using the phylogenetic dating option130 in IQ-tree and applying a relaxed clock model. For this analysis, the ‘TVM + F + I + G4’
model was selected as best-fit model and again 1000 ultrafast bootstrap replicates were performed. To obtain 95% confidence intervals we resampled branch lengths 100 times and used the
default setting of 0.2 for the standard deviation of the lognormal distribution130. The final alignment with a length of 36,811,173 bp contained 36 sequences (27 _Trachypithecus_
individuals, _R. roxellana_, _C. angolensis_, _M. mulatta_, _P. anubis_, _C. sabaeus_, _P. abelii_, _G. gorilla_, _P. troglodytes_, _H. sapiens_). To calibrate the molecular clock, we
constrained eight nodes based on fossil evidence131. As IQ-tree requires at least one fixed node age and allows only to set hard minimum, hard maximum, or both, we constrained the eight
nodes as follows: 1. most recent common ancestor (MRCA) _Homo_ and _Pan_ 4.631–15.0 Mya; 2. MRCA Hominidae 12.3–25.235 Mya; 3. MRCA _Papio_ and _Macaca_ 5.33–12.51 Mya; 4. MRCA
Cercopithecinae 6.5–15.0 Mya; 5. MRCA Colobinae 8.1.25–15.0 Mya; 6. MRCA Cercopithecidae 12.47–25.235 Mya, and 7. MRCA Catarrhini 35.102 Mya131. The genetic population structure of _T.
poliocephalus_ and its closest relatives, _T. francoisi_ and _T. leucocephalus_, was first inferred with _frappe_ v1.1132. Admixture v1.3.0133 was used to estimate individual ancestry
proportions. We predefined the number of genetic clusters _K_ from 2 to 5 and used cross-validation error methods to choose the best _K_ value. The maximum iteration of the
expectation-maximization algorithm was set to 10,000. The PCA was conducted with EIGENSOFT v7.2.1134 and the significance of eigenvectors was determined with the Tracy-Widom test in
EIGENSOFT. We analyzed allele sharing using _D_-statistics, with qpDstat of the Admixtools package135,136 and Dtrios & Dinvestigate programs in Dsuite137 to explore potential gene flow
events between _T. poliocephalus_, _T. francoisi_ and _T. leucocephalus_. Using quartets of populations with the topology (((P1, P2), P3), O), where O represents the outgroup (_R. roxellana_
in our study), we computed the normalized product of the allele differences for population 1 (P1) and 2 (P2), and population 3 (P3) and outgroup, averaged over all SNPs. By a whole-genome
scan using qpDstat and Dtrios, we obtained the best model among these three species. GENETIC DIVERSITY, RUNS OF HOMOZYGOSITY AND INBREEDING To infer the nucleotide diversity π of _T.
poliocephalus_ and compare it with that of other mammal species, we measured differences between chromosome pairs in the wild-born limestone langurs (rainforest langurs were excluded as
three of them, _T. obscurus_, _T. germaini_ and _T. auratus_, were captive born and thus may not reflect the nucleotide diversity characteristic for wild-born animals) with vcftools
v0.1.14138 (genome-wide-site methods, --site-pi and non-overlapping-50kb-window methods, --window-pi 50000). Data from other mammal species were obtained from previous
publications18,139,140. We counted the genome-wide autosomal heterozygosity and heterozygosity in protein-coding (including non-synonymous and synonymous variants) versus non-protein-coding
regions for all individuals using genotype call files from merged VCF files and the “.gtf” annotation files from NCBI. We inferred ROHs across the limestone langur genomes using PLINK
v1.9141 and bcftools v1.7121. To this end, we ran sliding windows of 20 SNPs on the VCF files of included genomes, requiring at least one SNP per 50 kb (parameters of PLINK: --homozyg
--homozyg-density 50 --homozyg-gap 100 --homozyg-kb 100 --homozyg-snp 50 --homozyg-window-het 1 --homozyg-window-snp 20 --homozyg-window-threshold 0.05). In each individual genome, we
allowed for a maximum of one heterozygous and 50 missing calls per window before we considered the ROH to be broken. As only for _T. poliocephalus_, _T. francoisi_ and _T. leucocephalus_
more than three individuals were available, we performed comparative ROH analyses on population level only for these three species, using One-way ANOVA test to check for significant
differences. The inbreeding coefficient _F_ for each species was calculated with PLINK v1.9 as (observed homozygous SNPs-expected homozygous SNPs)/(total called SNPs-expected homozygous
SNPs) for each sample and then plotted separately for each species. DELETERIOUS MUTATIONS AND GENETIC LOAD We downloaded a multiple alignment with 100 vertebrate species (“100way alignment”)
including Mmul_10 from the UCSC genome browser (http://hgdownload.soe.ucsc.edu/goldenPath/hg38/multiz100way/maf/). The GERP scores were subsequently transferred from hg38 to Mmul_10 using
LiftOver142. The vcfR package was used to read the vcf files into r v4.1.1143 and all subsequent calculations were performed in R using the package tidyverse144. After removing identical
positions shared by all three limestone langur species, we used autosomal polymorphism data to estimate the individual masked load as the sum of the GERP scores of all deleterious derived
alleles in heterozygous genotypes, divided by the number of called genotypes per individual to account for differences in callability between individuals. The realized load was calculated as
the sum of GERP scores of deleterious derived alleles in homozygous genotypes divided by all called sites in the genome. Based on the distribution of synonymous and deleterious
non-synonymous mutations, we set a GERP score threshold of 4 to define a mutation as potentially deleterious18,76,140,145. To calculate the proportion of heterozygous versus homozygous
derived genotypes in an individual, we divided the number of sites of each genotype by the total number of called genotypes for that individual (including sites homozygous for the ancestral
allele, genotyped because they were polymorphic in other individuals in our dataset)76. Obtained SNPs were then grouped into four effect categories (modifier, low, moderate, high) based on
snpEFF annotation (a simple estimation of putative impact/deleteriousness) and compared among the three northern limestone langur species. For genes containing homozygous deleterious
mutations of high impact we performed functional enrichment analysis with KOBAS 3.0146,147; corrected _p_ values were calculated using Benjamini and Hochberg corrected test. POSITIVE
SELECTION AND NON-SYNONYMOUS VARIANTS To identify genomic regions that may have been subject to selection in _T. poliocephalus_, we investigated pair-wise comparisons between _T.
poliocephalus_ and _T. francoisi_. We calculated average nucleotide diversity (θπ), using the genome-wide sliding windows. As the variance of θπ depends on the number of SNPs used for each
calculation, spurious selection signals will be more likely in windows with few variable sites. To reduce the number of false positives we used only windows with a minimum of 20 variable
sites. We tested a range of different window sizes (20, 40, and 100 kb) and note that a window size of 40 kb (20 kb steps) resulted in a low number of windows with few SNPs. Consequently,
and also according to linkage distance analysis, a window size of 40 kb allowed us to screen a large fraction of the genome at a false positive rate that likely is lower than if a smaller
window size would have been used. Then, we estimated the cross-population extended haplotype homozygosity (XP-EHH)148 statistic with R package rehh149. The XP-EHH scores for all variants
were also Z-transformed using the math module in python v2.7. The threshold for identifying candidate selective regions in the XP-EHH was set to the top 5% outliers. Furthermore, we used
KaKs_calculator v2.0150 to identify positively selected sites based on sliding windows across the full CDS sequence with “-YN” parameters. As our selection methods are pruned to small sample
size and to avoid any bias, we recognized only genes under strong selective sweep when they were supported by at least two selection methods. The functional classification and enrichment
analyses of candidate genes under selective sweep were performed with KOBAS v3.0146,147; corrected _p_ values were calculated using the Benjamini and Hochberg corrected test. We used snpEFF
annotated VCF files and identified non-synonymous variants in _T. poliocephalus_ following two criteria: 1. mutations must be classified as non-synonymous variants, and 2. these variants
should be homozygous in all four _T. poliocephalus_ individuals. Next, we investigated whether genes with these non-synonymous variants were supported by any of the three selection methods
based on the top 5% outlier results (see above). We further screened genes known to be responsible for adaptation to high calcium intake in other limestone langur species37 and performed
literature searches to identify candidate genes potentially related to differential salinity adaptation in vertebrates41,42,43,44,45,46,47,48,49,50,51. Genes reported in these studies were
then checked for the presence of non-synonymous mutations unique to _T. poliocephalus_ using snpEFF. REPORTING SUMMARY Further information on research design is available in the Nature
Portfolio Reporting Summary linked to this article. DATA AVAILABILITY Newly generated short-read sequencing data are available on NCBI under BioProject PRJNA949813
(https://www.ncbi.nlm.nih.gov/bioproject/949813). Publicly available genome data used in this study can be found under BioProject PRJNA488530 (https://www.ncbi.nlm.nih.gov/bioproject/488530)
and in Sequence Read Archive SRR8718596, SRR8718597, SRR1687497, SRR8762000, SRR6196475, SRR16119994, SRR11032814, SRR9703449, SRR11892898, and SRR11075380. Samples of the two deceased Cat
Ba langur individuals and the two translocated females are stored at the Cat Ba Langur Conservation Project, Cat Ba Island, Vietnam, and the Wildlife Conservation Society, Hanoi, Vietnam,
respectively. Source data are provided with this paper. CODE AVAILABILITY We have described all the tools and methods used for the analyses in the “Methods” section. REFERENCES * Diamond, J.
M. The present, past and future of human–caused extinctions. _Philos. Trans. R. Soc. B_ 325, 469–476 (1989). ADS CAS Google Scholar * Barnosky, A. D. et al. Has the Earth’s sixth mass
extinction already arrived? _Nature_ 471, 51–57 (2011). Article ADS CAS PubMed Google Scholar * Mawdsley, J., Midgley, G. & Hannah, L. Climate change, extinction risk, and public
policy. In _Saving a Million Species_ (ed. Hannah, L.) (Island Press/Center for Resource Economics, 2012). * Pimm, S. L. et al. The biodiversity of species and their rates of extinction,
distribution, and protection. _Science_ 344, 1246752 (2014). Article CAS PubMed Google Scholar * Ceballos, G. et al. Accelerated modern human–induced species losses, entering the sixth
mass extinction. _Sci. Adv._ 1, e1400253 (2015). Article ADS PubMed PubMed Central Google Scholar * Ceballos, G. & Ehrlich, P. R. The misunderstood sixth mass extinction. _Science_
360, 1080–1081 (2018). Article ADS PubMed Google Scholar * Estrada, A. et al. Impending extinction crisis of the world’s primates, why primates matter. _Sci. Adv._ 3, e1600946 (2017).
Article ADS PubMed PubMed Central Google Scholar * Estrada, A. & Garber, P. A. Principal drivers and conservation solutions to the impending primate extinction crisis, introduction
to the special issue. _Int. J. Primatol._ 43, 1–14 (2022). Article PubMed PubMed Central Google Scholar * Caughley, G. Directions in conservation biology. _J. Anim. Ecol._ 63, 215
(1994). Article Google Scholar * Frankham, R. Genetics and extinction. _Biol. Conserv._ 126, 131–140 (2005). Article Google Scholar * Kohn, M. H., Murphy, W. J., Ostrander, E. A. &
Wayne, R. K. Genomics and conservation genetics. _Trends Ecol. Evol._ 21, 629–637 (2006). Article PubMed Google Scholar * Xue, Y. et al. Mountain gorilla genomes reveal the impact of
long–term population decline and inbreeding. _Science_ 348, 242–245 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Robinson, J. A. et al. Genomic flatlining in the
endangered island fox. _Curr. Biol._ 26, 1183–1189 (2016). Article CAS PubMed Google Scholar * Robinson, J. A., Brown, C., Kim, B. Y., Lohmueller, K. E. & Wayne, R. K. Purging of
strongly deleterious mutations explains long–term persistence and absence of inbreeding depression in island foxes. _Curr. Biol._ 28, 3487–3494 (2018). Article CAS PubMed PubMed Central
Google Scholar * Feng, S. et al. The genomic footprints of the fall and recovery of the crested ibis. _Curr. Biol._ 29, 340–349 (2019). Article CAS PubMed PubMed Central Google Scholar
* van der Valk, T., Díez-Del-Molino, D., Marques-Bonet, T., Guschanski, K. & Dalén, L. Historical genomes reveal the genomic consequences of recent population decline in eastern
gorillas. _Curr. Biol._ 29, 165–170 (2019). Article PubMed Google Scholar * van der Valk, T., Manuel, M. D., Marques-Bonet, T. & Guschanski, K. Estimates of genetic load in small
populations suggest extensive purging of deleterious alleles. Preprint at _boiRxiv_ https://www.biorxiv.org/content/10.1101/696831v2 (2019). * Grossen, C., Guillaume, F., Keller, L. F. &
Croll, D. Purging of highly deleterious mutations through severe bottlenecks in Alpine ibex. _Nat. Commun._ 11, 1001 (2020). Article ADS CAS PubMed PubMed Central Google Scholar *
Dussex, N. et al. Population genomics of the critically endangered kākāpō. _Cell Genom._ 1, 100002 (2021). Article CAS PubMed PubMed Central Google Scholar * von Seth, J. et al. Genomic
insights into the conservation status of the world’s last remaining Sumatran rhinoceros populations. _Nat. Commun._ 12, 2393 (2021). Article ADS Google Scholar * Wang, P. et al. Genomic
consequences of long–term population decline in brown eared pheasant. _Mol. Biol. Evol._ 38, 263–273 (2021). Article CAS PubMed Google Scholar * Lande, R. & Shannon, S. The role of
genetic variation in adaptation and population persistence in a changing environment. _Evolution_ 50, 434–437 (1996). Article PubMed Google Scholar * Keller, L. F. & Waller, D. M.
Inbreeding effects in wild populations. _Trends Ecol. Evol._ 17, 230–241 (2002). Article Google Scholar * Charlesworth, B. Fundamental concepts in genetics, effective population size and
patterns of molecular evolution and variation. _Nat. Rev. Genet._ 10, 195–205 (2009). Article CAS PubMed Google Scholar * Bijlsma, R. & Loeschcke, V. Genetic erosion impedes adaptive
responses to stressful environments. _Evol. Appl._ 5, 117–129 (2012). Article CAS PubMed Google Scholar * Lynch, M., Conery, J. & Burger, R. Mutation accumulation and the extinction
of small populations. _Am. Nat._ 146, 489–518 (1995). Article Google Scholar * Caballero, A., Bravo, I. & Wang, J. Inbreeding load and purging: implications for the short-term
survival and the conservation management of small populations. _Heredity_ 118, 177–185 (2017). Article CAS PubMed Google Scholar * van der Valk, T., Jensen, A., Caillaud, D. &
Guschanski, K. Comparative genomic analyses provide new insights into the evolutionary history and conservation genomics of gorillas. _BMC Evol. Biol._ 24, 14 (2024). Article Google Scholar
* Rawson, B. M., Leonard, N., Covert, H. & Nadler, T. _Trachypithecus poliocephalus_. The IUCN Red List of Threatened Species 2020: e.T39871A17959804 (accessed on 10 February 2023)
https://doi.org/10.2305/IUCN.UK.2020-2.RLTS.T39871A17959804.en (2020). * Nadler, T. & Ha, T. L. _The Cat Ba Langur: Past, Present and Future, the Definitive Report on Trachypithecus
Poliocephalus, the World’s Rarest Primate_ (Hanoi, Frankfurt Zoological Society 2000). * Mittermeier, R. A., Rylands, A. B. & Wilson, D. E. _Handbook of the Mammals of the World_, Vol.
3, Primates (Lynx Editions, Barcelona, 2013). * Roos, C. et al. An updated taxonomy and conservation status review of Asian primates. _Asian Primates J._ 4, 2–38 (2014). Google Scholar *
Roos, C., Liedigk, R., Thinh, V. N., Nadler, T. & Zinner, D. The hybrid origin of the Indochinese gray langur _Trachypithecus crepusculus_. _Int. J. Primatol._ 40, 9–27 (2019). Article
Google Scholar * Roos, C. et al. Mitogenomic phylogeny of the Asian colobine genus _Trachypithecus_ with special focus on _Trachypithecus phayrei_ (Blyth, 1847) and description of a new
species. _Zool. Res._ 41, 656–669 (2020). Article PubMed PubMed Central Google Scholar * Groves, C. _Primate Taxonomy_ (Smithsonian Institution Press, Washington, DC, 2001). * Li, Z.
& Rogers, M. E. Are limestone hills a refuge or essential habitat for white–headed langurs in Fusui, China? _Int. J. Primatol._ 26, 437–452 (2005). Article Google Scholar * Liu, Z. et
al. Genomic mechanisms of physiological and morphological adaptations of limestone langurs to karst habitats. _Mol. Biol. Evol._ 37, 952–968 (2020). Article CAS PubMed Google Scholar *
Hendershott, R., Rawson, B. M. & Behie, A. Home range size and habitat use by Cat Ba Langurs (_Trachypithecus poliocephalus_) in a disturbed and fragmented habitat. _Int. J. Primatol._
39, 547–566 (2018). Article Google Scholar * Gibson, J., Morton, N. E. & Collins, A. Extended tracts of homozygosity in outbred human populations. _Hum. Mol. Genet._ 15, 789–795
(2006). Article CAS PubMed Google Scholar * Keller, M. C., Visscher, P. M. & Goddard, M. E. Quantification of inbreeding due to distant ancestors and its detection using dense single
nucleotide polymorphism data. _Genetics_ 189, 237–249 (2011). Article PubMed PubMed Central Google Scholar * Dalongeville, A., Benestan, L., Mouillot, D., Lobreaux, S. & Manel, S.
Combining six genome scan methods to detect candidate genes to salinity in the Mediterranean striped red mullet (_Mullus surmuletus_). _BMC Genom._ 19, 217 (2018). Article Google Scholar *
Albecker, M. A. & McCoy, M. W. Local adaptation for enhanced salt tolerance reduces non–adaptive plasticity caused by osmotic stress. _Evolution_ 73, 1941–1957 (2019). Article CAS
PubMed Google Scholar * Albecker, M. A., Stuckert, A. M. M., Balakrishnan, C. N. & McCoy, M. W. Molecular mechanisms of local adaptation for salt–tolerance in a treefrog. _Mol. Ecol._
30, 2065–2086 (2021). Article CAS PubMed Google Scholar * Beichman, A. C. et al. Aquatic adaptation and depleted diversity: a deep dive into the genomes of the sea otter and giant otter.
_Mol. Biol. Evol._ 36, 2631–2655 (2019). Article CAS PubMed PubMed Central Google Scholar * Huelsmann, M. et al. Genes lost during the transition from land to water in cetaceans
highlight gnomic changes associated with aquatic adaptations. _Sci. Adv._ 5, eaaw6671 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Walsh, J. et al. Genomics of rapid
ecological divergence and parallel adaptation in four tidal marsh sparrows. _Evol. Lett._ 3, 324–338 (2019). Article PubMed PubMed Central Google Scholar * Rautsaw, R. M. et al. Genomic
adaptations to salinity resist gene flow in the evolution of Floridian watersnakes. _Mol. Biol. Evol._ 38, 745–760 (2021). Article CAS PubMed Google Scholar * Lopez, M. E. et al. Lack of
panmixia of Bothnian Bay vendace–Implications for fisheries management. _Front. Mar. Sci._ 9, 1028863 (2022). Article Google Scholar * Pratt, E. A. L. et al. Seascape genomics of coastal
bottlenose dolphins along strong gradients of temperature and salinity. _Mol. Ecol._ 31, 2223–2241 (2022). Article PubMed Google Scholar * Tong, C. & Li, M. Convergent genomic
signatures of adaptation to an extreme environment in cyprinoid fishes. Preprint at _bioRxiv_ https://www.biorxiv.org/content/10.1101/2022.03.30.486405v2 (2022). * Yu, X. et al. Genomic
analysis of a Nile tilapia strain selected for salinity tolerance shows signatures of selection and hybridization with blue tilapia (_Oreochromis aureus_). _Aquaculture_ 560, 738527 (2022).
Article CAS Google Scholar * Sørensen, E. F. et al. Genome-wide coancestry reveals details of ancient and recent male-driven reticulation in baboons. _Science_ 380, eabn8153 (2023).
Article PubMed Google Scholar * Kuang, W. et al. Genetic diversity, inbreeding level, and genetic load in endangered snub-nosed monkeys (_Rhinopithecus_). _Front. Genet._ 11, 615926
(2020). Article CAS PubMed PubMed Central Google Scholar * Kuderna, L. F. K. et al. A global catalog of whole-genome diversity from 233 primate species. _Science_ 380, 906–916 (2023).
Article ADS CAS PubMed Google Scholar * Prado-Martinez, J. et al. Great ape genetic diversity and population history. _Nature_ 499, 471–475 (2013). Article ADS CAS PubMed PubMed
Central Google Scholar * Fan, Z. et al. Worldwide patterns of genomic variation and admixture in gray wolves. _Genome Res._ 26, 163–173 (2016). Article CAS PubMed PubMed Central Google
Scholar * Li, R. et al. The sequence and de novo assembly of the giant panda genome. _Nature_ 463, 311–317 (2010). Article ADS CAS PubMed Google Scholar * de Jong, M. J. et al.
Range-wide whole-genome resequencing of the brown bear reveals drivers of intraspecies divergence. _Commun. Biol._ 6, 153 (2023). Article PubMed PubMed Central Google Scholar * Kyriazis,
C. C. et al. Genomic underpinnings of population persistence in Isle Royale moose. _Mol. Biol. Evol._ 40, msad021 (2023). Article CAS PubMed PubMed Central Google Scholar * Westbury,
M. V. et al. Narwhal genome reveals long-term low genetic diversity despite current large abundance size. _IScience_ 15, 592–599 (2019). Article ADS CAS PubMed PubMed Central Google
Scholar * Robinson, J. A. et al. The critically endangered vaquita is not doomed to extinction by inbreeding depression. _Science_ 376, 635–639 (2022). Article ADS CAS PubMed PubMed
Central Google Scholar * Lan, T. et al. Insights into bear evolution from a Pleistocene polar bear genome. _Proc. Natl Acad. Sci. USA_ 119, e2200016119 (2022). Article CAS PubMed PubMed
Central Google Scholar * Westbury, M. V. et al. Extended and continuous decline in effective population size results in low genomic diversity in the world’s rarest hyena species, the
brown hyena. _Mol. Biol. Evol._ 35, 1225–1237 (2018). Article CAS PubMed PubMed Central Google Scholar * Cho, Y. S. et al. The tiger genome and comparative analysis with lion and snow
leopard genomes. _Nat. Commun._ 4, 2433 (2013). Article ADS PubMed Google Scholar * Dobrynin, P. et al. Genomic legacy of the African cheetah, _Acinonyx jubatus_. _Genome Biol._ 16, 277
(2015). Article PubMed PubMed Central Google Scholar * Abascal, F. et al. Extreme genomic erosion after recurrent demographic bottlenecks in the highly endangered Iberian lynx. _Genome
Biol._ 17, 251 (2016). Article PubMed PubMed Central Google Scholar * Sousa, V., Peischl, S. & Excoffier, L. Impact of range expansions on current human genomic diversity. _Curr.
Opin. Genet. Dev._ 29, 22–30 (2014). Article CAS PubMed Google Scholar * Li, Y. et al. Resequencing of 200 human exomes identifies an excess of low-frequency non-synonymous coding
variants. _Nat. Genet._ 42, 969–972 (2010). Article CAS PubMed Google Scholar * Thompson, E. A. Identity by descent: variation in meiosis, across genomes, and in populations. _Genetics_
194, 301–326 (2013). Article CAS PubMed PubMed Central Google Scholar * Grimwood, J. et al. The DNA sequence and biology of human chromosome 19. _Nature_ 428, 529–535 (2004). Article
ADS CAS PubMed Google Scholar * Harris, R. A., Raveendran, M., Worley, K. C. & Rogers, J. Unusual sequence characteristics of human chromosome 19 are conserved across 11 nonhuman
primates. _BMC Evol. Biol._ 20, 33 (2020). Article CAS PubMed PubMed Central Google Scholar * Bertorelle, G. et al. Genetic load: genomic estimates and applications in non-model
animals. _Nat. Rev. Genet._ 23, 492–503 (2022). Article CAS PubMed Google Scholar * Mochales-Riaño, G. et al. Genomics reveals introgression and purging of deleterious mutations in the
Arabian leopard (_Panthera pardus nimr_). _iScience_ 26, 107481 (2023). Article ADS PubMed PubMed Central Google Scholar * Jackson, H. A. et al. Genomic erosion in a demographically
recovered bird species during conservation rescue. _Conserv. Biol._ 36, e13918 (2022). Article PubMed PubMed Central Google Scholar * Liberg, O. et al. Severe inbreeding depression in a
wild wolf (_Canis lupus_) population. _Biol. Lett._ 1, 17–20 (2005). Article CAS PubMed PubMed Central Google Scholar * Smeds, L. & Ellegren, H. From high masked to high realized
genetic load in inbred Scandinavian wolves. _Mol. Ecol._ 32, 1567–1580 (2023). Article PubMed Google Scholar * Johnson, W. E. et al. Genetic restoration of the Florida panther. _Science_
329, 1641–1645 (2010). Article ADS CAS PubMed PubMed Central Google Scholar * Hasselgren, M. et al. Genomic and fitness consequences of inbreeding in an endangered carnivore. _Mol.
Ecol._ 30, 2790–2799 (2021). Article PubMed Google Scholar * Charlesworth, D. & Willis, J. H. The genetics of inbreeding depression. _Nat. Rev. Genet._ 10, 783–796 (2009). Article
CAS PubMed Google Scholar * Kleinman-Ruiz, D. et al. Purging of deleterious burden in the endangered Iberian lynx. _Proc. Natl Acad. Sci. USA_ 119, e2110614119 (2022). Article CAS
PubMed PubMed Central Google Scholar * Clements, R., Sodhi, N. S., Schilthuizen, M. & Ng, P. Limestone karsts of Southeast Asia: imperiled arks of biodiversity. _BioScience_ 56,
733–742 (2006). Article Google Scholar * Ford, D. & Williamsk, P. W. _Karst Hydrogeology and Geomorphology_ (Wiley, New York, 2007). * Huang, C. & Li, Y. How does the white–headed
langur (_Trachypithecus leucocephalus_) adapt locomotor behavior to its unique limestone hill habitat? _Primates_ 46, 261–267 (2005). Article PubMed Google Scholar * Ji, F. T., Li, N.
& Deng, X. Calcium contents and high calcium adaptation of plants in karst areas of China. _Chin. J. Plant. Ecol._ 33, 926–935 (2009). CAS Google Scholar * Luo, X. Q., Wang, C. Y.,
Yang, H. Y. & Liao, X. R. Studies on adaptive mechanisms of karst dominant plant species to drought and high calcium stress. _Chin. Agric. Sci. Bull._ 28, 1–5 (2012). Google Scholar *
Hao, Z., Kuang, Y. & Kang, M. Untangling the influence of phylogeny, soil and climate on leaf element concentrations in a biodiversity hotspot. _Funct. Ecol._ 29, 165–176 (2015). Article
Google Scholar * Liu, X., Wu, Q., Huang, Z., Huang, C. & Zhou, Q. Nutritional content of dry season foods and its influences on food choice of Francois’ langurs at Nonggang. _Acta
Ther. Sin._ 36, 241–247 (2016). Google Scholar * Lalani, S. R. et al. _MCTP2_ is a dosage–sensitive gene required for cardiac outflow tract development. _Hum. Mol. Genet._ 22, 4339–4348
(2013). Article CAS PubMed PubMed Central Google Scholar * Téllez-Arreola, J. L., Martínez-Torres, A., Flores-Moran, A. E., Lazaro-Guevara, J. M. & Estrada-Mondragón, A. Analysis of
the MCTP amino acid sequence reveals the conservation of putative calcium- and lipid-binding pockets within the C2 domains in silico. _J. Mol. Evol._ 90, 271–282 (2022). Article ADS
PubMed Google Scholar * Samsó, M., Wagenknecht, T. & Allen, P. D. Internal structure and visualization of transmembrane domains of the RyR1 calcium release channel by cryo–EM. _Nat.
Struct. Mol. Biol._ 12, 539–544 (2005). Article PubMed PubMed Central Google Scholar * Hasdemir, C. et al. Transcriptional profiling of septal wall of the right ventricular outflow tract
in patients with idiopathic ventricular arrhythmias. _Pacing Clin. Electrophysiol._ 33, 159–167 (2010). Article PubMed Google Scholar * Hernández-Ochoa, E. O., Pratt, S. J. P., Lovering,
R. M. & Schneider, M. F. Critical role of intracellular RyR1 calcium release channels in skeletal muscle function and disease. _Front. Physiol._ 6, 420 (2016). Article PubMed PubMed
Central Google Scholar * Sethna, F. et al. Enhanced expression of _ADCY1_ underlies aberrant neuronal signalling and behaviour in a syndromic autism model. _Nat. Commun._ 8, 14359 (2017).
Article ADS CAS PubMed PubMed Central Google Scholar * Gong, D. et al. The m6A–suppressed P2RX6 activation promotes renal cancer cells migration and invasion through ATP–induced Ca2+
influx modulating ERK1/2 phosphorylation and MMP9 signaling pathway. _J. Exp. Clin. Cancer Res._ 38, 233 (2019). Article PubMed PubMed Central Google Scholar * Greve, J. M., Pinkham, A.
M., Thompson, Z. & Cowan, J. A. Active site characterization and activity of the human aspartyl (asparaginyl) β–hydroxylase. _Metallomics_ 13, mfab056 (2021). Article PubMed Google
Scholar * Sakakibara, I. et al. Myofiber androgen receptor increases muscle strength mediated by a skeletal muscle splicing variant of Mylk4. _iScience_ 24, 102303 (2021). Article ADS CAS
PubMed PubMed Central Google Scholar * Willmer, P., Stone, G. & Johnston, I. _Environmental Physiology of Animals_ (Blackwell Publishing, Oxford, 2005). * Pinder, A. M., Halse, S.
A., McRae, J. M. & Shiel, R. J. Aquatic invertebrate assemblages of wetlands and rivers in the wheatbelt region of Western Australia. _Rec. West. Aust. Mus. Suppl._ 67, 7–37 (2004).
Article Google Scholar * Lorenz, J. J. A review of the effects of altered hydrology and salinity on vertebrate fauna and their habitats in northeastern Florida Bay. _Wetlands_ 1, 189–200
(2014). Article Google Scholar * Castillo, A. M., Sharpe, D. M. T., Ghalambor, C. K. & de León, L. F. Exploring the effects of salinization on trophic diversity in freshwater
ecosystems: a quantitative review. _Hydrobiologia_ 807, 1–17 (2018). Article CAS Google Scholar * Babonis, L. S. & Brischoux, F. Perspectives on the convergent evolution of tetrapod
salt glands. _Integr. Comp. Biol._ 52, 245–256 (2012). Article PubMed Google Scholar * Xu, J. et al. Slc26a11, a chloride transporter, localizes with the vacuolar H(+)–ATPase of
A–intercalated cells of the kidney. _Kidney Int._ 80, 926–937 (2011). Article CAS PubMed Google Scholar * Zaika, O., Tomilin, V., Mamenko, M., Bhalla, V. & Pochynyuk, O. New
perspective of ClC–Kb/2 Cl– channel physiology in the distal renal tubule. _Am. J. Physiol. Ren. Physiol._ 310, F923–F930 (2016). Article CAS Google Scholar * Zhu, G. et al. The
salt‐sensitivity in C57Bl/6J mice is linked to increased renal protein expressions of SLC4A4 and SLC4A5. _FASEB J._ 33, 533.11 (2019). Article Google Scholar * Guo, L. et al. Common
variants in the Na(+)–coupled bicarbonate transporter genes and salt sensitivity of blood pressure: the GenSalt study. _J. Hum. Hypertens._ 30, 543–548 (2016). Article CAS PubMed Google
Scholar * Sabino-Silva, R. et al. The Na(+)/glucose cotransporters: from genes to therapy. _Braz. J. Med. Biol. Res._ 43, 1019–1026 (2010). Article CAS PubMed Google Scholar * Dahlberg,
J., Sjögren, M., Hedblad, B., Engström, G. & Melander, O. Genetic variation in NEDD4L, an epithelial sodium channel regulator, is associated with cardiovascular disease and
cardiovascular death. _J. Hypertens._ 32, 294–299 (2014). Article CAS PubMed Google Scholar * Yan, Y. et al. ANKRD36 Is involved in hypertension by altering expression of ENaC genes.
_Circ. Res._ 129, 1067–1081 (2021). Article CAS PubMed Google Scholar * Ferreira, M. A. et al. Association and interaction analyses of eight genes under asthma linkage peaks. _Allergy_
64, 1623–1628 (2009). Article CAS PubMed Google Scholar * Caldwell, J. M. et al. Cadherin 26 is an alpha integrin–binding epithelial receptor regulated during allergic inflammation.
_Mucosal Immunol._ 10, 1190–1201 (2017). Article CAS PubMed PubMed Central Google Scholar * Lachowicz-Scroggins, M. E. et al. Cadherin–26 (CDH26) regulates airway epithelial cell
cytoskeletal structure and polarity. _Cell Discov._ 4, 7 (2018). Article PubMed PubMed Central Google Scholar * Wagner, R. et al. Epithelial cell–adhesion protein cadherin 26 is
dysregulated in congenital diaphragmatic hernia and congenital pulmonary airway malformation. _Pediatr. Surg. Int._ 37, 49–57 (2021). Article PubMed Google Scholar * Fagerberg, L. et al.
Analysis of the human tissue–specific expression by genome–wide integration of transcriptomics and antibody–based proteomics. _Mol. Cell Proteom._ 13, 397–406 (2014). Article CAS Google
Scholar * Derda, A. A. et al. Gene expression profile analysis of aortic vascular smooth muscle cells reveals upregulation of cadherin genes in myocardial infarction patients. _Physiol.
Genom._ 50, 648–657 (2018). Article CAS Google Scholar * He, J. et al. Genome–wide associated variants of subclinical atherosclerosis among young people with HIV and gene–environment
interactions. _J. Transl. Med._ 20, 609 (2022). Article CAS PubMed PubMed Central Google Scholar * Ketonen, J., Merasto, S., Paakkari, I. & Mervaala, E. M. High sodium intake
increases vascular superoxide formation and promotes atherosclerosis in apolipoprotein E–deficient mice. _Blood Press._ 14, 373–382 (2005). Article CAS PubMed Google Scholar * Baldo, M.
P., Teixeira, A. K., Rodrigues, S. L. & Mill, J. G. Acute arrhythmogenesis after myocardial infarction in normotensive rats, influence of high salt intake. _Food Chem. Toxicol._ 50,
473–477 (2012). Article CAS PubMed Google Scholar * Zhao, X. et al. Dietary salt intake and coronary atherosclerosis in patients with prehypertension. _J. Clin. Hypertens._ 16, 575–580
(2014). Article CAS Google Scholar * Forechi, L., Baldo, M. P., Araujo, I. B., Nogueira, B. V. & Mill, J. G. Effects of high and low salt intake on left ventricular remodeling after
myocardial infarction in normotensive rats. _J. Am. Soc. Hypertens._ 9, 77–85 (2015). Article CAS PubMed Google Scholar * Chen, S., Zhou, Y., Chen, Y. & Gu, J. fastp: an ultra–fast
all–in–one FASTQ preprocessor. _Bioinformatics_ 34, i884–i890 (2018). Article PubMed PubMed Central Google Scholar * Li, H. & Durbin, R. Fast and accurate short read alignment with
Burrows–Wheeler transform. _Bioinformatics_ 25, 1754–1760 (2009). Article CAS PubMed PubMed Central Google Scholar * Li, H. et al. The Sequence Alignment/Map format and SAMtools.
_Bioinformatics_ 25, 2078–2079 (2009). Article PubMed PubMed Central Google Scholar * McKenna, A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation
DNA sequencing data. _Genome Res._ 20, 1297–1303 (2010). Article CAS PubMed PubMed Central Google Scholar * Cingolani, P. et al. A program for annotating and predicting the effects of
single nucleotide polymorphisms, SnpEff: SNPs in the genome of _Drosophila melanogaster_ strain w1118; iso–2; iso–3. _Fly_ 6, 80–92 (2012). Article CAS PubMed PubMed Central Google
Scholar * Manichaikul, A. et al. Robust relationship inference in genome-wide association studies. _Bioinformatics_ 26, 2867–2873 (2010). Article CAS PubMed PubMed Central Google
Scholar * Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: more models, new heuristics and parallel computing. _Nat. Methods_ 9, 772 (2012). Article CAS PubMed
PubMed Central Google Scholar * Minh, B. Q. et al. IQ–TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. _Mol. Biol. Evol._ 37, 1530–1534 (2020).
Article CAS PubMed PubMed Central Google Scholar * Minh, B. Q., Nguyen, M. A. & von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. _Mol. Biol. Evol._ 30, 1188–1195
(2013). Article CAS PubMed PubMed Central Google Scholar * Hoang, D. T., Chernomor, O., von Haeseler, A., Minh, B. Q. & Vinh, L. S. UFBoot2: improving the ultrafast bootstrap
approximation. _Mol. Biol. Evol._ 35, 518–522 (2018). Article CAS PubMed Google Scholar * To, T. H., Jung, M., Lycett, S. & Gascuel, O. Fast dating using least–squares criteria and
algorithms. _Syst. Biol._ 65, 82–97 (2016). Article CAS PubMed Google Scholar * de Vries, D. & Beck, R. M. D. Twenty–five well–justified fossil calibrations for primate divergences.
_Palaeontol. Electron._ 26, 1–52 (2023). Google Scholar * Tang, H., Peng, J., Wang, P. & Risch, N. J. Estimation of individual admixture: analytical and study design considerations.
_Genet. Epidemiol._ 28, 289–301 (2005). Article PubMed Google Scholar * Alexander, D. H., Novembre, J. & Lange, K. Fast model–based estimation of ancestry in unrelated individuals.
_Genome Res._ 19, 1655–1664 (2009). Article CAS PubMed PubMed Central Google Scholar * Price, A. L. et al. Principal components analysis corrects for stratification in genome-wide
association studies. _Nat. Genet._ 38, 904–909 (2006). Article CAS PubMed Google Scholar * Patterson, N., Price, A. L. & Reich, D. Population structure and eigenanalysis. _PLoS
Genet._ 2, e190 (2006). Article PubMed PubMed Central Google Scholar * Patterson, N. et al. Ancient admixture in human history. _Genetics_ 192, 1065–1093 (2012). Article PubMed PubMed
Central Google Scholar * Malinsky, M., Matschiner, M. & Svardal, H. Dsuite-fast D-statistics and related admixture evidence from VCF files. _Mol. Ecol. Resour._ 21, 584–595 (2021).
Article PubMed Google Scholar * Danecek, P. et al. The variant call format and VCFtools. _Bioinformatics_ 27, 2156–2158 (2011). Article CAS PubMed PubMed Central Google Scholar *
Leffler, E. M. et al. Revisiting an old riddle, what determines genetic diversity levels within species? _PLoS Biol._ 10, e1001388 (2012). Article CAS PubMed PubMed Central Google
Scholar * Mooney, J. A., Marsden, C. D., Yohannes, A., Wayne, R. K. & Lohmueller, K. E. Long–term small population size, deleterious variation, and altitude adaptation in the Ethiopian
wolf, a severely endangered canid. _Mol. Biol. Evol._ 40, msac277 (2023). Article CAS PubMed Google Scholar * Purcell, S. et al. PLINK: a tool set for whole–genome association and
population–based linkage analyses. _Am. J. Hum. Genet._ 81, 559–575 (2007). Article CAS PubMed PubMed Central Google Scholar * Kuhn, R. M., Haussler, D. & Kent, W. J. The UCSC
genome browser and associated tools. _Brief. Bioinform._ 14, 144–161 (2013). Article CAS PubMed Google Scholar * R. Core Team. _R: A Language and Environment for Statistical Computing_
(R Foundation for Statistical Computing, Vienna, 2021). * Wickham, H. et al. Welcome to the Tidyverse. _J. Open Source Softw._ 4, 1686 (2019). Article ADS Google Scholar * Huber, C. D.,
Kim, B. Y. & Lohmueller, K. E. Population genetic models of GERP scores suggest pervasive turnover of constrained sites across mammalian evolution. _PLoS Genet._ 16, e1008827 (2020).
Article CAS PubMed PubMed Central Google Scholar * Xie, C. et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. _Nucleic Acids Res._ 39,
W316–W322 (2011). Article CAS PubMed PubMed Central Google Scholar * Bu, D. et al. KOBAS–i: intelligent prioritization and exploratory visualization of biological functions for gene
enrichment analysis. _Nucleic Acids Res._ 49, W317–W325 (2021). Article CAS PubMed PubMed Central Google Scholar * Sabeti, P. C. et al. Genome–wide detection and characterization of
positive selection in human populations. _Nature_ 449, 913–918 (2007). Article ADS CAS PubMed PubMed Central Google Scholar * Gautier, M., Klassmann, A. & Vitalis, R. rehh 2.0: a
reimplementation of the R package rehh to detect positive selection from haplotype structure. _Mol. Ecol. Resour._ 17, 78–90 (2017). Article CAS PubMed Google Scholar * Wang, D., Zhang,
Y., Zhang, Z., Zhu, J. & Yu, J. KaKs_Calculator 2.0: a toolkit incorporating gamma-series methods and sliding window strategies. _Genom. Proteom. Bioinform._ 8, 77–80 (2010). Article
CAS Google Scholar Download references ACKNOWLEDGEMENTS We thank Tran Van Lan, Nguyen Ba Tiep, Hoang Van Thap, Nguyen Van Phien, Vu Hong Van, Alexandra Kolodyazhnaya, and Michael Meyerhoff
for logistic support and help with data analysis, and Stephan D. Nash for permission to use his langur illustrations. We are grateful to the Cat Ba langur Conservation Project, Zoo Leipzig,
Allwetterzoo Münster, the Zoologische Gesellschaft für Arten- und Populationsschutz, the Endangered Primate Rescue Center, Cat Ba National Park, Cuc Phuong National Park and the Ministry
for Agricultural and Rural Development for continuous support. Financial support for this research was provided by grants from the German Research Foundation (HO 3492/9-1 to M.H. and RO
3055/7-1 to C.R.), Sino-German Mobility Programme (M-0084 to M.Li and C.R.), Chinese Academy of Sciences (CAS XDB31000000 to M.Li) and National Natural Science Foundation of China (NSFC
31821001 to M.Li) and Vietnamese Ministry of Science and Technology’s Program 562 (ĐTĐL.CN-64/19 to M.D.L.). T.M.-B. was supported by funding from the European Research Council (ERC) under
the European Union’s Horizon 2020 research and innovation program (grant agreement No. 864203), PID2021-126004NB-100 (MICIIN/FEDER, UE), “Unidad de Excelencia María de Maeztu”, funded by the
AEI (CEX2018-000792-M), NIH 1R01HG010898-01A1 and Secretaria d’Universitats i Recerca and CERCA Programme del Departament d’Economia i Coneixement de la Generalitat de Catalunya (GRC 2021
SGR 00177). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Primate Genetics Laboratory, German Primate Center, Leibniz Institute for Primate Research, Göttingen, Germany Liye Zhang, Truong
Van Nguyen, Lutz Walter & Christian Roos * International Max Planck Research School for Genome Science (IMPRS-GS), University of Göttingen, Göttingen, Germany Liye Zhang * CAS Key
Laboratory of Animal Ecology and Conservation Biology, Institute of Zoology, Chinese Academy of Sciences, Beijing, China Liye Zhang & Ming Li * Cat Ba Langur Conservation Project
(CBLCP), Cat Ba National Park, Cat Ba Island, Cat Hai District, Hai Phong Province, Vietnam Neahga Leonard, Rick Passaro, Mai Sy Luan, Pham Van Tuyen, Le Thi Ngoc Han & Nguyen Huy Cam *
Taronga Conservation Society Australia, Mosman, NSW, Australia Larry Vogelnest * Melbourne Zoo, Zoos Victoria, Parkville, VIC, Australia Michael Lynch * Wildlife Conservation Society (WCS),
Health Program, New York, NY, USA Amanda E. Fine * Wildlife Conservation Society (WCS), Vietnam Country Program, Hanoi, Vietnam Nguyen Thi Thanh Nga & Nguyen Van Long * World Wildlife
Fund for Nature (WWF) International, Gland, Switzerland Benjamin M. Rawson * School of Archaeology and Anthropology, The Australian National University, Canberra, ACT, Australia Alison Behie
* Evolutionary Adaptive Genomics, Institute of Biochemistry and Biology, Department of Science, University of Potsdam, Potsdam, Germany Truong Van Nguyen & Michael Hofreiter * Central
Institute for Natural Resources and Environmental Studies, Vietnam National University, Hanoi, Vietnam Truong Van Nguyen & Minh D. Le * Faculty of Environmental Sciences, University of
Science, Vietnam National University, Hanoi, Vietnam Minh D. Le * Three Monkeys Wildlife Conservancy, Nho Quan District, Ninh Binh Province, Ninh Binh, Vietnam Tilo Nadler * Institute of
Evolutionary Biology (UPF-CSIC), PRBB, Barcelona, Spain Tomas Marques-Bonet * Catalan Institution of Research and Advanced Studies (ICREA), Barcelona, Spain Tomas Marques-Bonet * CNAG-CRG,
Centre for Genomic Regulation (CRG), Barcelona Institute of Science and Technology (BIST), Barcelona, Spain Tomas Marques-Bonet * Institut Català de Paleontologia Miquel Crusafont,
Universitat Autònoma de Barcelona, Edifici ICTA-ICP, Cerdanyola del Vallès, Spain Tomas Marques-Bonet * College of Life Sciences, Capital Normal University, Beijing, China Zhijin Liu * Gene
Bank of Primates, German Primate Center, Leibniz Institute for Primate Research, Göttingen, Germany Christian Roos Authors * Liye Zhang View author publications You can also search for this
author inPubMed Google Scholar * Neahga Leonard View author publications You can also search for this author inPubMed Google Scholar * Rick Passaro View author publications You can also
search for this author inPubMed Google Scholar * Mai Sy Luan View author publications You can also search for this author inPubMed Google Scholar * Pham Van Tuyen View author publications
You can also search for this author inPubMed Google Scholar * Le Thi Ngoc Han View author publications You can also search for this author inPubMed Google Scholar * Nguyen Huy Cam View
author publications You can also search for this author inPubMed Google Scholar * Larry Vogelnest View author publications You can also search for this author inPubMed Google Scholar *
Michael Lynch View author publications You can also search for this author inPubMed Google Scholar * Amanda E. Fine View author publications You can also search for this author inPubMed
Google Scholar * Nguyen Thi Thanh Nga View author publications You can also search for this author inPubMed Google Scholar * Nguyen Van Long View author publications You can also search for
this author inPubMed Google Scholar * Benjamin M. Rawson View author publications You can also search for this author inPubMed Google Scholar * Alison Behie View author publications You can
also search for this author inPubMed Google Scholar * Truong Van Nguyen View author publications You can also search for this author inPubMed Google Scholar * Minh D. Le View author
publications You can also search for this author inPubMed Google Scholar * Tilo Nadler View author publications You can also search for this author inPubMed Google Scholar * Lutz Walter View
author publications You can also search for this author inPubMed Google Scholar * Tomas Marques-Bonet View author publications You can also search for this author inPubMed Google Scholar *
Michael Hofreiter View author publications You can also search for this author inPubMed Google Scholar * Ming Li View author publications You can also search for this author inPubMed Google
Scholar * Zhijin Liu View author publications You can also search for this author inPubMed Google Scholar * Christian Roos View author publications You can also search for this author
inPubMed Google Scholar CONTRIBUTIONS L.Z., T.M.-B., M.H., M.Li, Z.L., and C.R. designed the research; L.Z., L.W., M.H., and C.R. analyzed the data; N.L., R.P., M.S.L., P.V.T., L.T.N.H.,
N.H.C., L.V., M.Ly., A.E.F., N.T.T.N., N.V.L., B.M.R., A.B., N.V.T., M.D.L., and T.N. collected samples; L.Z., N.L., M.H., and C.R. wrote and revised the paper with contributions from all
authors. CORRESPONDING AUTHORS Correspondence to Liye Zhang, Michael Hofreiter, Ming Li, Zhijin Liu or Christian Roos. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no
competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is
available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY
INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4
SUPPLEMENTARY DATA 5 SUPPLEMENTARY DATA 6 SUPPLEMENTARY DATA 7 SUPPLEMENTARY DATA 8 SUPPLEMENTARY DATA 9 SUPPLEMENTARY DATA 10 SUPPLEMENTARY MOVIE 1 REPORTING SUMMARY SOURCE DATA SOURCE DATA
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Zhang, L., Leonard, N., Passaro, R. _et al._ Genomic adaptation to small population size and saltwater consumption in the critically endangered Cat Ba langur. _Nat Commun_ 15, 8531
(2024). https://doi.org/10.1038/s41467-024-52811-7 Download citation * Received: 26 April 2023 * Accepted: 23 September 2024 * Published: 02 October 2024 * DOI:
https://doi.org/10.1038/s41467-024-52811-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








