- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Olfaction may play a restricted role in human behavior, yet paradoxically, its absence in anosmia is associated with diverse deleterious outcomes, culminating in reduced life
expectancy. The mammalian nose serves two purposes: olfaction and breathing. Because respiratory patterns are impacted by odors, we hypothesized that nasal respiratory airflow may be altered
in anosmia. We apply a wearable device that precisely logs nasal airflow for 24-hour-long sessions in participants with isolated congenital anosmia and controls. We observe significantly
altered patterns of respiratory nasal airflow in anosmia in wake and in sleep. These differences allow classification of anosmia at 83% accuracy using the respiratory trace alone. Patterns
of respiratory airflow have pronounced impact on health, emotion and cognition. We therefore suggest that a portion of the deleterious outcomes associated with anosmia may be attributed to
altered patterns of respiratory nasal airflow rather than a direct result of lost odor perception per se. SIMILAR CONTENT BEING VIEWED BY OTHERS ODOR IDENTIFICATION PERFORMANCE IN CHILDREN
AGED 3–6 YEARS Article Open access 26 July 2020 ODOURS COUNT: HUMAN OLFACTORY ECOLOGY APPEARS TO BE HELPFUL IN THE IMPROVEMENT OF THE SENSE OF SMELL Article Open access 19 August 2021
OLFACTORY DISTORTIONS IN THE GENERAL POPULATION Article Open access 13 June 2022 INTRODUCTION There is a common notion that olfaction is an “unimportant” sensory system in humans. This
notion was promoted by perhaps the most influential observer of human behavior to date, namely Sigmund Freud1. Freud2, together with the culturally influential Havelock Ellis3, denigrated
the behavioral significance of human olfaction, arguing that olfaction reflected merely “animalistic behavior”, and was linked to human behavioral pathology2. This 20th-century line of
thought remains well-established in 21st-century culture where a poll found that 53% of respondents aged 16–22 would rather give up their sense of smell than give up technology such as
cell-phone or laptop4. Yet in the face of this notion on an “unimportant sense” stands the painful outcome of lost olfaction, a condition known as anosmia. The prevalence of anosmia is
poorly documented, such that reports range between 1.4% to 15% of the population5,6,7 (and 24% above age 538). The reported prevalence for the causes of anosmia also vary widely, but aside
of neurodegeneration9, mostly rank-order as: viral infection (most notably COVID-1910), followed by nasal/sinus disease, head trauma, toxins, and congenital anosmias11 (CA). CA alone likely
accounts for ~4% of anosmia cases12, making for a populational prevalence of about 1 in 10,00013. Congenital anosmia can be either syndromic, such as in Kallmann syndrome14, or isolated
(ICA) i.e., of unknown cause. Although there are some known genetic predispositions to ICA15, and most (but not all16) individuals with ICA have significantly reduced or absent olfactory
bulbs17, the functional reason for lost olfaction in ICA remains mostly unknown. In contrast to the notion of olfaction as an unimportant sense, anosmia is associated with assorted
deleterious outcomes, and significantly reduced quality of life11,18,19,20. The negative impact most commonly associated with anosmia, particularly acquired anosmia, is dulled affect and
depression21. Sufferers often report feelings of personal isolation and emotional blunting22. This aspect of life with anosmia garnered significant attention in COVID-19, which is associated
with anosmia that may linger far past the viral attack23. Sufferers often describe the anhedonia associated with olfactory loss as life-altering23. In addition to this primary impact of
anosmia, additional consequences include dietary complications11,18,24, social difficulties11,18,25, and loss of an important signal for danger, particularly smoke11,18,26. This long list
culminates in an outcome in acquired anosmia where severity is related to 5-year mortality such that (acquired) anosmic older adults have over three times the odds of death compared to
normosmic individuals27. How does all of the above deleterious cascade occur following the loss of an unimportant sense? One answer is that the notion of olfaction being an unimportant sense
for humans is simply completely misguided and wrong28. Humans display extensive odor-guided behavior29, including social behavior30, and these topics have been reviewed extensively31,32.
That said, it is still not immediately evident how an acquired-anosmia-derived impairment would relate to a 5-year three-fold mortality rate. With this in mind, a second possible answer is
that when loosing olfaction, humans may lose more than odor perception alone. Humans use their nose for two tasks, smelling and breathing, and these two tasks are interconnected, i.e.,
smelling effects breathing. More specifically, nasal inhalations are shaped by odorant properties in what we refer to as the _sniff-response_, where nasal inhalation magnitude is inversely
proportional to odorant intensity and pleasantness33. This phenomenon persists even when we are unaware of the odorant in sleep34 and wake35, and even in significantly disordered states of
consciousness36. The impact of odors on respiration is not only event-related as in the sniff-response, it is also ongoing. For example, various concentrations of the odor propionic acid
lead to reductions in ongoing inhalation volumes37, and similarly, different malodors presented during tasks resulted in reduced ongoing inhalation flow38. Given the effect of odors on
ongoing respiratory patterns, we hypothesized that ongoing respiration may be altered in anosmia. Whereas it is not completely clear how loss of odor perception per se may lead to outcomes
such as reduced life expectancy, there are several paths by which altered respiratory patterns may have deleterious physiological outcomes. With this in mind, we set out to compare ongoing
nasal airflow in anosmic participants and normosmic controls, with special attention to respiratory patterns that may reflect olfactory exploration. We apply a wearable device that measures
nasal airflow for 24-h periods and find significantly altered patterns of nasal airflow in anosmia. Differences include an overwhelming reduction in respiratory peaks (sniffs) during wake,
which we attribute to the absence of olfactory exploration in anosmia. Moreover, we observe significant reshaping of the overall anosmic nasal respiratory waveform in wake and sleep. The
shift in sleep implies that this alteration goes beyond odor-driven responses alone, and reflects that humans without a sense of smell breathe differently. RESULTS ANOSMICS AND NORMOSMICS
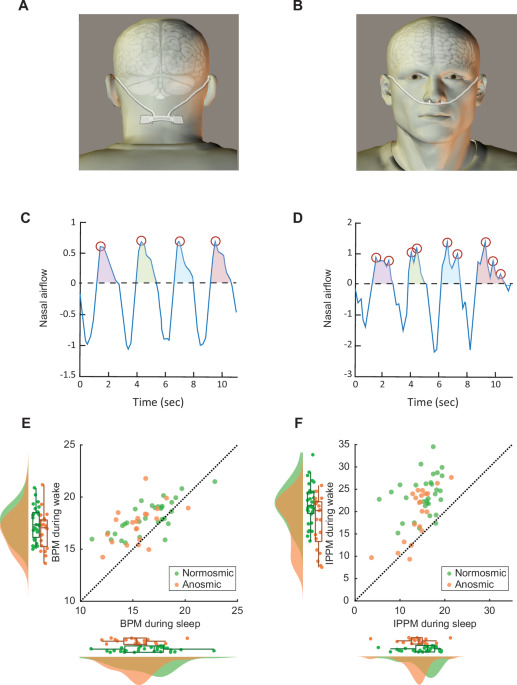
BREATHE AT THE SAME OVERALL RATE We applied a wearable device (Fig. 1A, B) that precisely measures and logs nasal airflow in each nostril separately39 at 6 Hz (Methods online), in 21
participants with isolated congenital anosmia (ICA) (8 women, 13 men, mean age = 32 ± 7 years) and 31 normosmic controls (19 women, 12 men, mean age = 28 ± 4 years). Participants went about
their daily living routine unaltered and returned to the lab 24 hours later to have the device removed and the data downloaded (all the raw data is available online, see “Methods” section).
Participants also maintained a daily activity diary, with special attention to registering sleep and wake times. Normal resting respiratory frequency is about 0.25 Hz. Because we measure
nasal airflow at about 20 times this rate and use highly sensitive pressure sensors (SDP3x from Sensirion, Stäfa, Switzerland), we can observe local minute within-breath variations in the
airflow trace. Thus, we make a clear distinction between respiratory rate and respiratory nasal airflow patterns. This is clarified in Fig. 1, which contains four consecutive breaths from an
anosmic participant, which contain 4 respiratory peaks (Fig. 1C), and four consecutive breaths from a normosmic participant, which contain 9 respiratory peaks (Fig. 1D). The added peaks
seen in normosmia may reflect exploratory sniffing that can ride on the respiratory wave. With this distinction between breathing rate and pattern in mind, we first asked whether anosmics
breathe at the same overall pace as normosmics. We applied analyses of variance (both Bayesian and parametric) with conditions of Arousal (sleep/wake), and Sense of Smell (normosmic/anosmic)
to the automatically identified breaths per minute (BPM) in the respiratory trace (Methods). Bayesian analysis provided strong evidence for an effect of Arousal (BF10 > 150), no evidence
for an effect of Sense of Smell (BF10 = 0.797), and moderate evidence for an interaction (BF01 = 3.09, comparing to using arousal only). A parametric analysis of variance similarly
uncovered a significant effect for Arousal (_F_1,50 = 54.8, _p_ < 0.001, _η_2 = 0.16), but no effect for Sense of Smell (_F_1,50 = 2.5, _p_ = 0.12), nor interaction (_F_1,50 = 0.49, _p_ =
0.49). This significant effect for Arousal reflected the well-known phenomena of slower respiration during sleep vs. wake (Normosmic sleep: 16.5\(\pm\)2.5 BPM (Median: 17.1 BPM), wake:
18.1\(\pm\)1.6 BPM (Median: 17.9 BPM), _t_30 = 5.48, _d_’ = 0.99, _p_ < 0.001, Bayesian BF10 = 3391. Anosmic sleep: 15.5\(\pm\)1.9 BPM (Median: 15.5 BPM), wake: 17.4\(\pm\)1.8 BPM
(Median: 17.7 BPM), _t_20 = 4.98, _d_’ = 1.1, _p_ < 0.001, Bayesian BF10 = 369) (Fig. 1E). ANOSMICS HAVE SIGNIFICANTLY LESS INHALATION PEAKS THAN NORMOSMICS Having found that normosmics
and anosmics breathe at the same overall rate, we next set out to directly test our hypothesis of increased, possibly olfaction-related, exploratory nasal inhalations in normosmia. We
observe that the unfiltered unsmoothed respiratory trace often contains several peaks within a single inhalation (Fig. 1D). We therefore applied the same analysis scheme as above to the
number of inhalation peaks per minute (IPPM) rather than to the overall respiratory pace. Bayesian analysis provided strong evidence for an effect of Arousal (BF10 > 150), no evidence for
an effect of Sense of Smell (BF10 = 1.459), yet strong evidence for an interaction (BF01 = 6.641). A parametric analysis of variance similarly uncovered a significant effect for Arousal
(_F_1,50 = 118, _p_ < 0.001, _η_2 = 0.37), a significant effect for Sense of Smell (_F_1,50 = 6.3, _p_ = 0.016, _η_2 = 0.05), and a significant interaction (_F_1,50 = 4.7, _p_ = 0.03,
_η_2 = 0.015). This reflected a significantly increased frequency of nasal inhalation peaks in normosmics during wake alone. Specifically, during sleep normosmics nasally inhale at
15.1\(\pm\)3.5 IPPM (Median: 16.3 IPPM) and anosmics nasally inhale at 13.9\(\pm\)3.5 IPPM (Median: 14.4 IPPM) (_t_50 = 1.2, _p_ = 0.23, Bayesian BF10 = 0.52), yet during wake normosmics
increase nasal inhalation peaks to 23.8\(\pm\)4.5 IPPM (Median: 23.1 IPPM) but anosmics nasally peak at only 19.5\(\pm\)5.8 IPPM (Median: 22 IPPM) (_t_50 = 3, _d_’ = 0.84, _p_ = 0.004,
Bayesian BF10 = 6.4) (Fig. 1F). To further ask whether the difference between anosmics and normosmics during wake was specific to a particular time of day, we ran through the data with a
moving average over 1-h blocks. We observed that the difference was consistently significant in all waking hours (Supplementary Fig. 2). This added four IPPM in controls amounts to a
remarkable added ~240 inhalation peaks per hour in normosmia over anosmia. In other words, consistent with our hypothesis, ongoing daily patterns of respiratory nasal airflow are profoundly
altered in anosmia. This difference is pervasive, and its depiction in Fig. 1 was consistent across the entire cohort (Supplementary Fig. 1). One may ask whether the increased IPPM in
normosmia reflected a response to a fluctuating olfactory environment in this naturalistic experiment or, in turn, whether it reflected a fixed shift in normosmic vs. ansomic nasal
inhalation patterns, regardless of odors. To address this, we tested an additional cohort of 32 normosmic participants in an odorant-free room. This is an experimental room coated in odorant
non-adherent materials, and subserved by high throughput HEPA and carbon filtration. We observe that under these odorant-free conditions, the rate of IPPM in normosmia was 17.2\(\pm\)3.3
(Median: 17.6 IPPM), a value not significantly different from that previously observed in anosmia (_t_51 = −1.91, _p_ = 0.06). We acknowledge that the information in this control is limited
by the fact that, unlike the naturalistic main experiment where participants were also moving out and about, here they were seated in a room, and this difference between moving about vs
seated will obviously reflect in respiratory patterns, regardless of smells. With this limitation in mind, this control nevertheless implies that the added IPPM in normosmia likely reflects
interaction with an odor environment. THE ANOSMIC BREATHING WAVEFORM SIGNIFICANTLY DIFFERS FROM THE NORMOSMIC WAVEFORM To this point, we first analyzed the overall respiratory rate, where we
saw no difference in anosmics. We next analyzed the rate of inhalation peaks, which we associate with olfactory exploration. Here we saw a significant decrease in anosmics, which behaved
throughout the active day like normosmics seated in an odorless room. To now further gauge whether nasal respiratory flow is altered in anosmia beyond IPPM alone we took advantage of a
recently developed toolbox for parametrization of nasal airflow, which defined 24 time-domain parameters40. To this set of parameters, we now also add the above-defined IPPM, and derive each
parameter for sleep and wake separately, culminating in 50 parameters (25 for wake and 25 for sleep). We then culled 23 parameters with particularly high intercorrelation (\(\left|r\right|
\, > \, 0.7,{p} \, < \, 1\times {10}^{-7}\)) as they will lack added information, resulting in a list of 27 informative parameters (Supplementary Table 1). We then compared anosmics
and normosmics on each of these measures (Fig. 2A). We observed significant differences in 8 of these parameters, with four parameters surviving additional cutoff following
Benjamini–Hochberg correction for multiple comparisons (corrected _p_ < 0.0074). At this cutoff, we observe significant differences in “Percent of breaths with inhale pause in wake” (mean
anosmic: 81%\(\pm\)7%, mean normosmic: 75%\(\pm\)7%, _t_50 = 3, _p_ = 0.004, _d_’ = 0.85) (Fig. 2B), “IPPM in wake” (mean anosmic: 19.5\(\pm\)5.8, mean normosmic: 23.8\(\pm\)4.5, _t_50 =
−3, _p_ = 0.004, _d_’ = −0.84) (Fig. 1F), “CoV of inhale volume in sleep” (mean anosmic: 0.34\(\pm\)0.08, mean normosmic: 0.29\(\pm\)0.04, _t_50 = 2.98, _p_ = 0.004, _d_’ = 0.84) (Fig. 2C),
and “Exhale peak value in wake” (mean anosmic: 1.57\(\pm\)0.37, mean normosmic: 1.81\(\pm\)0.24, _t_50 = −2.82, _p_ = 0.007, _d_’ = −0.8) (Fig. 2D). Put in words, in addition to the
previously noted reduction in IPPM, ansomic respiration is characterized by added inhalation pauses and reduced exhalation peak flow in wake, and increased covariance of inhale volume in
sleep. WE CAN CLASSIFY ANOSMIA FROM BREATHING ALONE WITHOUT THE USE OF ODORS Given these differences that all had meaningful effect sizes (all |_d_’| > 0.8), we set out to ask if we could
classify anosmia without odors, based on respiratory patterns alone. We entered the 4 parameters that survived correction into a KNN classifier and tested using a leave-one-out scheme such
that testing was performed on participants, not in the learning set. We obtained a receiver operating curve (ROC) with the area under curve of 79% (Fig. 2E), providing for 83% classification
accuracy, with 67% true positive rate (anosmics classified as anosmics) and 94% true negative rate (normosmics classified as normosmics). To estimate the significance of this
classification, we repeated the process 10,000 times, each time randomly shuffling the “anosmic” and “normosmic” labels. This provided for a chance distribution of classification accuracies.
We observed no better classification in any of these iterations, such that the significance of our classification is _p_ = 0.0001 (Fig. 2F). In other words, we can determine congenital
anosmia at 83% accuracy without using any odorant in our test. We note that this result was not dependent on the previously identified measure of IPPM, as rerunning the classifier without
IPPM still achieved 81% accuracy, _p_ = 0.0001 (Supplementary Fig. 3), yet the classifier was highly dependent on “CoV of inhale volume in sleep”, and rerunning the classifier without this
parameter reduced classification to 62%, _p_ = 0.06 (Supplementary Fig. 3). The fact that anosmic respiration differed from normosmic respiration in sleep in five respiratory parameters at
_p_ < 0.05 (and in one following correction), implies that anosmic respiration is also altered regardless of odorant sampling. We state this as olfactory sleep responses were evident only
when high-concentration odorants were pumped by olfactometer to the nose of sleeping participants34, yet here the natural sleeping olfactory environment remains largely constant. Thus, the
differences we observe between anosmics and normosimics in sleep are unlikely to reflect responses to odors. Finally, by measuring airflow in each nostril independently, we could also
address the possibility of an altered nasal cycle39 in anosmia, yet we found no evidence for this (Supplementary Fig. 4). DISCUSSION Because odors influence respiration37,38, we hypothesized
altered nasal airflow in anosmia. We observed two types of differences: During the wake, normosmia was associated with a pronounced increase in the rate of respiratory peaks. This effect
disappeared when normosmics were seated in an odorless room. Despite the unavoidable limitations of the odorless room control, it implies that the increased peaks in normosmia may reflect
odor-driven exploration. In turn, we observed additional differences in nasal airflow parameters, many of them persistent or, in fact, increased during sleep. We therefore speculate that
through life development without olfaction, the respiratory pattern is shifted in congenital anosmia. Such shifted respiratory patterns, and particularly nasal airflow patterns, may have an
impact on physiological and mental health. A dramatic example of the possible impact of respiratory airflow patterns on health is the importance of _sighing_, which was uncovered following
the advent of steel lungs to treat polio. Despite full-volume artificial respiration, many early patients receiving artificial ventilation were dying. It quickly became apparent that to
maintain life, patients need not only to breathe rhythmically, but also sigh every 5 min or so, as this is critical for preventing collapse of alveoli in the lungs41. In other words, beyond
respiratory rate alone, the intricate dynamic patterns of respiratory airflow can be highly consequential for health. Beyond the general physiological state, respiratory patterns have
significant implications particularly on neural state42,43. Nasal airflow orchestrates volleys of neural activity throughout the brain, such that every sniff is associated with a cascade of
activity spreading from the olfactory bulb and throughout the cortex44. The current study implies that normosmics experience an added ~240 such neural waves per active waking hour over
anosmics. This is potentially a profound difference in brain activity. Consistent with this, resting-state brain activity is indeed altered in acquired anosmia45,46, yet inconsistent with
our hypothesis; functional connectivity was not altered in congenital anosmia47. Consequent to nasal-airflow-induced patterns of brain activity, patterns of airflow have been linked to
emotional48 and cognitive49,50,51,52,53 states. More specifically, patterns and particularly phase (inhale vs. exhale) of nasal airflow have been linked to performance in memory
consolidation49 and retrieval50, quality of mental imagery51,52, discrimination of facial fear50, and visuospatial processing53. Given all of these implications, although we acknowledge the
many other well-recognized potential explanations for the untoward morbidities and mortality of individuals with olfactory loss, we submit that it is now plausible that a portion of the
deleterious outcomes associated with anosmia may be related to altered respiratory patterns. This study and our method have several limitations we would like to acknowledge. First, we would
have preferred to sample at a higher frequency, e.g., 25 Hz, which is recommended for extracting respiratory nuances54. Our lower sampling rate reflected a power-consumption constraint, as
had we sampled any faster, we could not have maintained a 24-h recording. Thus, there may be additional differences we failed to capture. Second, our method is oblivious to oral airflow.
Although we think our particular interest in nasal airflow is justified, it is very possible that measures of oral airflow may have added to this picture. Third, an oversight of our study
was not to formally verify normal olfaction in the control participants. Although they all self-reported an intact sense of smell, this does not assure healthy olfaction20,55. However, we
note in this respect that if there were participants with impaired olfaction in the control group, this could only reduce the reported effects, not drive them. Finally, it will be
interesting to add a future comparison to acquired anosmia as well. Are these respiratory changes immediate after olfactory loss? Do they develop over time? Or do they develop at all in
acquired anosmia? We have no answers to these questions in the current study. Despite these limitations, we think we provide convincing evidence for the main claim of this study, namely that
people with congenital anosmia breathe differently. One may ask how was this difference not observed previously. We note that long-term respiration is typically measured with piezoelectric
respiratory belts or derived from plethysmography (RRp), and these almost always contain an inherent 3 Hz low-pass filter. Critically, we further observe that if we apply a 3 Hz low-pass
filter to our data, the difference between anosmics and normosmics completely disappears (shift from _t_50 = 3, _d_’ = 0.84, _p_ = 0.004, Bayesian BF10 = 6.4 to _t_50 = 0.2, _d_’ = 0.08, _p_
= 0.85, Bayesian BF10 = 0.37). In other words, long-term measurement of respiratory airflow without low-pass temporal filtration can uncover meaningful information. In this study, it
uncovered altered breathing patterns in anosmia. METHODS PARTICIPANTS All participants provided written informed consent to procedures approved by the Weizmann Institute IRB committee. To
estimate the necessary sample size, we applied a power analysis56 assuming a large effect size (_η_2 = 0.14)57. We find that at an alpha level of 0.05 and 90% power we need to study at least
20 participants per group. With this in mind, we recruited 21 anosmics (8 women, 13 men, mean age = 32 ± 7 years) and 31 normosmics (19 women, 12 men, mean age = 28 ± 4 years). Gender was
self-reported. With the limited availability of participants with congenital anosmia in mind, we made an effort to equate the number of men and women, and we did not enter gender as a factor
in the analysis, as the group size would be too small. All participants with anosmia underwent nasal endoscopy and medical history review by an ENT physician (co-author SS) to rule out
non-congenital causes of anosmia. All anosmics reported a lifelong absence of olfactory perception without any memory of odors and scored as “Anosmic” on the University of Pennsylvania Smell
Identification Test (UPSIT)58 (mean score = 11.1 ± 3, highest score = 17). Anatomical MRI scans revealed no or neglected olfactory bulbs in all anosmics. Normosmic participants were all in
general good health, with no reported history of neurological or mental illness, and had neither olfactory deficits nor chronic or acute conditions that involved the respiratory tracts. All
participants were asked to rate their own sense of smell on a scale from 1 (poor) to 9 (excellent). Anosmic self-ratings were mean = 1.1 ± 0.4, yet normosmic self-ratings were 7.5 ± 1.2. The
lowest self-rating in normosmia was 5. All participants were compensated for their participation at a rate of 400 NIS (equivalent to ~100 USD). DATA ACQUISITION Nasal airflow was acquired
using a device we previously described in detail39. For the final 11 participants, we used a further miniaturized version of the same device that we call the Nasal Holter, as seen in Fig. 1.
In brief, the device is a wearable logger that uses a “stereo” nasal cannula to measure pressure in each nostril separately and converts the pressure time-series into a flow time-series.
The data is acquired at 6 Hz (part of the data was acquired at 5.5 Hz, specified in Supplementary Data File 1), and stored within the device for later download. Additionally, all
participants maintained a daily diary of activity, with special emphasis on logging sleep and wake times. PARAMETERIZATION All data were analyzed using MATLAB R2020a (MathWorks) and
customized code. In order to derive respiratory parameters, we split the nasal airflow trace into 5-min blocks and used the activity diary to label each block as “Sleep” or “Wake”. We then
used a standard respiratory signal processing toolbox, BreathMetrics40, to extract respiratory features. Additionally, we applied a standard peak-finding algorithm to extract the number of
inhalation peaks per minute (https://www.mathworks.com/help/signal/ref/findpeaks.html). Subsequently, we calculated the average of each parameter from all 5-min blocks with the same label
(Sleep/Wake). STATISTICAL ANALYSIS All statistical analysis was done using JASP (version 0.14.1)59. All airflow properties were analyzed using a repeated-measures analysis of variance
(rmANOVA), with a sense of smell (normosmic/anosmic) as a between-subjects parameter and arousal (Sleep/Wake) as a within-subject parameter. For statistical comparison between normosmic and
anosmic individuals, independent-sample _t_-tests were used. For statistical analysis within each group, matched-sample t-tests were used. The power of the effects was estimated by
calculating Cohen’s _D_ (_d_’). The significance of the classifier was assessed using bootstrapping with 10,000 iterations. Additional estimation of the strengths of the effects was assessed
by Bayes factors BF10 when comparing to the null model and BF01 when comparing to the best model60 with uniform priors. REPORTING SUMMARY Further information on research design is available
in the Nature Portfolio Reporting Summary linked to this article. DATA AVAILABILITY All the raw data for this manuscript is available at
https://gitlab.com/liorg/anosmics-breathe-differently/-/tree/main/Data. Source data are provided with this paper. CODE AVAILABILITY The custom code used to process the data collected in this
study is available at https://gitlab.com/liorg/anosmics-breathe-differently. REFERENCES * Haggbloom, S. J. et al. The 100 most eminent psychologists of the 20th century. _Rev. Gen.
Psychol._ 6, 139–152 (2002). Article Google Scholar * Harrington, A. & Rosario, V. in _Science of Olfaction_ (eds Serby, M. J. & Chobor, K. L.) 3–27 (Springer, New York, 1992). *
Kalogerakis, M. G. The role of olfaction in sexual development. _Psychosom. Med._ 25, 420–432 (1963). Article CAS PubMed Google Scholar * McCann Worldgroup. The Truth About Youth.
https://idoc.pub/queue/mccannworldgroup-truth-about-youth-vylyzxw81d4m (2011). * Hoffman, H. J., Ishii, E. K. & Macturk, R. H. Age‐related changes in the prevalence of smell/taste
problems among the United States adult population: results of the 1994 Disability Supplement to the National Health Interview Survey (NHIS). _Ann. N. Y. Acad. Sci._ 855, 716–722 (1998). *
Nordin, S., Brämerson, A. & Bende, M. Prevalence of self-reported poor odor detection sensitivity: the Skövde population-based study. _Acta Oto-laryngol._ 124, 1171–1173 (2004). Article
Google Scholar * Landis, B. N., Konnerth, C. G. & Hummel, T. A study on the frequency of olfactory dysfunction. _Laryngoscope_ 114, 1764–1769 (2004). Article CAS PubMed Google
Scholar * Murphy, C. et al. Prevalence of olfactory impairment in older adults. _JAMA_ 288, 2307–2312 (2002). Article PubMed Google Scholar * Doty, R. L. Olfactory dysfunction in
neurodegenerative diseases: is there a common pathological substrate? _Lancet Neurol._ 16, 478–488 (2017). Article PubMed Google Scholar * Han, A. Y., Mukdad, L., Long, J. L. & Lopez,
I. A. Anosmia in COVID-19: mechanisms and significance. _Chem. Senses_ 45, 423–428 (2020). Article CAS Google Scholar * Croy, I., Nordin, S. & Hummel, T. Olfactory disorders and
quality of life—an updated review. _Chem. Senses_ 39, 185–194 (2014). Article PubMed Google Scholar * Nordin, S. & Brämerson, A. Complaints of olfactory disorders: epidemiology,
assessment and clinical implications. _Curr. Opin. Allergy Clin. Immunol._ 8, 10–15 (2008). Article PubMed Google Scholar * Croy, I., Negoias, S., Novakova, L., Landis, B. N. &
Hummel, T. Learning about the functions of the olfactory system from people without a sense of smell. _PLoS ONE_ 7, e33365 (2012). Article ADS CAS PubMed PubMed Central Google Scholar
* Karstensen, H. & Tommerup, N. Isolated and syndromic forms of congenital anosmia. _Clin. Genet._ 81, 210–215 (2012). Article CAS PubMed Google Scholar * Kamarck, M. L. et al.
Identifying candidate genes underlying isolated congenital anosmia. _Clin. Genet._ 105, 376–385 (2024). Article CAS PubMed Google Scholar * Ghadami, M. et al. Isolated congenital anosmia
with morphologically normal olfactory bulb in two Iranian families: a new clinical entity? _Am. J. Med. Genet. Part A_ 127, 307–309 (2004). Article Google Scholar * Manan, H. A., Yahya,
N., Han, P. & Hummel, T. A systematic review of olfactory-related brain structural changes in patients with congenital or acquired anosmia. _Brain Struct. Funct._ 1–26 (2022). * Keller,
A. & Malaspina, D. Hidden consequences of olfactory dysfunction: a patient report series. _BMC Ear Nose Throat Disord._ 13, 1–20 (2013). Article Google Scholar * Patel, Z. M. et al.
International consensus statement on allergy and rhinology: Olfaction. _Int. Forum Allergy Rhinol._ 12, 327–680 (2022) (Wiley Online Library). * Whitcroft, K. et al. Position paper on
olfactory dysfunction: 2023. _Rhinology_ 61, 1–108 (2023). * Kohli, P., Soler, Z. M., Nguyen, S. A., Muus, J. S. & Schlosser, R. J. The association between olfaction and depression: a
systematic review. _Chem. Senses_ 41, 479–486 (2016). Article PubMed PubMed Central Google Scholar * Toller, S. V. Assessing the impact of anosmia: review of a questionnaire’s findings.
_Chem. Senses_ 24, 705–712 (1999). Article CAS PubMed Google Scholar * Yom-Tov, E., Lekkas, D. & Jacobson, N. C. Association of COVID19-induced anosmia and ageusia with depression
and suicidal ideation. _J. Affect. Disord. Rep._ 5, 100156 (2021). Article PubMed PubMed Central Google Scholar * Aschenbrenner, K. et al. The influence of olfactory loss on dietary
behaviors. _Laryngoscope_ 118, 135–144 (2008). Article PubMed Google Scholar * Blomkvist, A. & Hofer, M. Olfactory impairment and close social relationships. A narrative review.
_Chem. Senses_ 46, bjab037 (2021). Article PubMed PubMed Central Google Scholar * Santos, D. V., Reiter, E. R., DiNardo, L. J. & Costanzo, R. M. Hazardous events associated with
impaired olfactory function. _Arch. Otolaryngol.–Head Neck Surg._ 130, 317–319 (2004). Article PubMed Google Scholar * Pinto, J. M., Wroblewski, K. E., Kern, D. W., Schumm, L. P. &
McClintock, M. K. Olfactory dysfunction predicts 5-year mortality in older adults. _PLoS ONE_ 9, e107541 (2014). Article ADS PubMed PubMed Central Google Scholar * McGann, J. P. Poor
human olfaction is a 19th-century myth. _Science_ 356, eaam7263 (2017). Article PubMed PubMed Central Google Scholar * Stevenson, R. J. An initial evaluation of the functions of human
olfaction. _Chem. Senses_ 35, 3–20 (2010). Article PubMed Google Scholar * Chen, D. & Haviland-Jones, J. Human olfactory communication of emotion. _Percept. Mot. Skills_ 91, 771–781
(2000). Article CAS PubMed Google Scholar * Yeshurun, Y. & Sobel, N. An odor is not worth a thousand words: from multidimensional odors to unidimensional odor objects. _Annu. Rev.
Psychol._ 61, 219–241 (2010). Article PubMed Google Scholar * Dikeçligil, G. N. & Gottfried, J. A. What does the human olfactory system do, and how does it do it? _Annu. Rev.
Psychol._ 75, 155–181 (2024). Article PubMed Google Scholar * Mainland, J. & Sobel, N. The sniff is part of the olfactory percept. _Chem. Senses_ 31, 181–196 (2006). Article PubMed
Google Scholar * Arzi, A. et al. Humans can learn new information during sleep. _Nat. Neurosci._ 15, 1460–1465 (2012). Article CAS PubMed Google Scholar * Arzi, A., Rozenkrantz, L.,
Holtzman, Y., Secundo, L. & Sobel, N. Sniffing patterns uncover implicit memory for undetected odors. _Curr. Biol._ 24, R263–R264 (2014). Article CAS PubMed Google Scholar * Arzi, A.
et al. Olfactory sniffing signals consciousness in unresponsive patients with brain injuries. _Nature_ 581, 428–433 (2020). Article ADS CAS PubMed Google Scholar * Walker, J. C. et al.
Human responses to propionic acid. II. Quantification of breathing responses and their relationship to perception. _Chem. Senses_ 26, 351–358 (2001). Article CAS PubMed Google Scholar *
Danuser, B., Moser, D., Vitale-Sethre, T., Hirsig, R. & Krueger, H. Performance in a complex task and breathing under odor exposure. _Hum. Factors_ 45, 549–562 (2003). Article PubMed
Google Scholar * Kahana-Zweig, R. et al. Measuring and characterizing the human nasal cycle. _PLoS ONE_ 11, e0162918 (2016). Article PubMed PubMed Central Google Scholar * Noto, T.,
Zhou, G., Schuele, S., Templer, J. & Zelano, C. Automated analysis of breathing waveforms using BreathMetrics: a respiratory signal processing toolbox. _Chem. Senses_ 43, 583–597 (2018).
Article PubMed PubMed Central Google Scholar * Del Negro, C. A., Funk, G. D. & Feldman, J. L. Breathing matters. _Nat. Rev. Neurosci._ 19, 351–367 (2018). Article PubMed PubMed
Central Google Scholar * Herrero, J. L., Khuvis, S., Yeagle, E., Cerf, M. & Mehta, A. D. Breathing above the brain stem: volitional control and attentional modulation in humans. _J.
Neurophysiol._ 119, 145–159 (2018). * Kluger, D. S., Balestrieri, E., Busch, N. A. & Gross, J. Respiration aligns perception with neural excitability. _elife_ 10, e70907 (2021). Article
CAS PubMed PubMed Central Google Scholar * Sobel, N. et al. Sniffing and smelling: separate subsystems in the human olfactory cortex. _Nature_ 392, 282–286 (1998). Article ADS CAS
PubMed Google Scholar * Park, M., Chung, J., Kim, J. K., Jeong, Y. & Moon, W.-J. Altered functional brain networks in patients with traumatic anosmia: resting-state functional MRI
based on graph theoretical analysis. _Korean J. Radiol._ 20, 1536–1545 (2019). Article PubMed PubMed Central Google Scholar * Esposito, F. et al. Olfactory loss and brain connectivity
after COVID‐19. _Hum. Brain Mapp._ 43, 1548–1560 (2022). Article PubMed PubMed Central Google Scholar * Peter, M. G. et al. Normal olfactory functional connectivity despite lifelong
absence of olfactory experiences. _Cereb. Cortex_ 31, 159–168 (2021). Article PubMed Google Scholar * Ashhad, S., Kam, K., Del Negro, C. A. & Feldman, J. L. Breathing rhythm and
pattern and their influence on emotion. _Annu. Rev. Neurosci._ 45, 223–247 (2022). Article CAS PubMed PubMed Central Google Scholar * Arshamian, A., Iravani, B., Majid, A. &
Lundström, J. N. Respiration modulates olfactory memory consolidation in humans. _J. Neurosci._ 38, 3360–3317 (2018). Article Google Scholar * Zelano, C. et al. Nasal respiration entrains
human limbic oscillations and modulates cognitive function. _J. Neurosci._ 36, 12448–12467 (2016). Article CAS PubMed PubMed Central Google Scholar * Bensafi, M. et al. Olfactomotor
activity during imagery mimics that during perception. _Nat. Neurosci._ 6, 1142 (2003). Article CAS PubMed Google Scholar * Park, H.-D. et al. Breathing is coupled with voluntary
initiation of mental imagery. _NeuroImage_ 264, 119685 (2022). Article PubMed Google Scholar * Perl, O. et al. Human non-olfactory cognition phase-locked with inhalation. _Nat. Hum.
Behav._ 3, 501 (2019). Article PubMed Google Scholar * Berry, R. B. _The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications.
Version 2.1_ (American Academy of Sleep Medicine, Darien, IL, 2014). * Mady, L. J. et al. Exploring olfactory dysfunction as a marker of frailty and postoperative outcomes in head and neck
cancer. _JAMA Otolaryngol.–Head Neck Surg._ 149, 828–836 (2023). Article PubMed PubMed Central Google Scholar * Faul, F., Erdfelder, E., Lang, A.-G. & Buchner, A. G.* Power 3: a
flexible statistical power analysis program for the social, behavioral, and biomedical sciences. _Behav. Res. Methods_ 39, 175–191 (2007). Article PubMed Google Scholar * Cohen, J.
_Statistical Power Analysis for the Behavioral Sciences_ (Routledge, 2013). * Doty, R. L., Shaman, P., Kimmelman, C. P. & Dann, M. S. University of Pennsylvania Smell Identification
Test: a rapid quantitative olfactory function test for the clinic. _Laryngoscope_ 94, 176–178 (1984). Article CAS PubMed Google Scholar * Team, J. _JASP (Version 0.10. 1)[Computer
Software]_ (JASP, 2019). * Kass, R. E. & Raftery, A. E. Bayes factors. _J. Am. Stat. Assoc._ 90, 773–795 (1995). Article MathSciNet Google Scholar Download references ACKNOWLEDGEMENTS
This work was funded by grants from the Sagol Weizmann—MIT Bridge Program (2021/134368) (to author N.S.), The Minerva Foundation (714146) (to author N.S.), and ERA PerMed JTC2019-101
(project PerBrain) (to author N.S.), and an ISF BRG grant (2751/23) (to author N.S.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * The Azrieli National Institute for Human Brain Imaging and
Research, Weizmann Institute of Science, Rehovot, Israel Lior Gorodisky, Danielle Honigstein, Aharon Weissbrod, Reut Weissgross, Timna Soroka, Sagit Shushan & Noam Sobel * Department of
Brain Sciences, Weizmann Institute of Science, Rehovot, Israel Lior Gorodisky, Danielle Honigstein, Aharon Weissbrod, Reut Weissgross, Timna Soroka, Sagit Shushan & Noam Sobel * The
Institute of Nose and Sinus Therapy and Clinical Investigations, The Edith Wolfson Medical Center, Holon, Israel Sagit Shushan * Department of Otolaryngology—Head & Neck Surgery, The
Edith Wolfson Medical Center, Holon, Israel Sagit Shushan * Faculty of Medical & Health Sciences, Tel-Aviv University, Tel Aviv, Israel Sagit Shushan Authors * Lior Gorodisky View author
publications You can also search for this author inPubMed Google Scholar * Danielle Honigstein View author publications You can also search for this author inPubMed Google Scholar * Aharon
Weissbrod View author publications You can also search for this author inPubMed Google Scholar * Reut Weissgross View author publications You can also search for this author inPubMed Google
Scholar * Timna Soroka View author publications You can also search for this author inPubMed Google Scholar * Sagit Shushan View author publications You can also search for this author
inPubMed Google Scholar * Noam Sobel View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Developed the concepts: L.G. and N.S. Built device
hardware: A.W. Wrote device software: D.H. Examined anosmic participants: S.S. Designed experiments: L.G., R.W., and N.S. Ran experiments: L.G. and R.W. Analyzed data: L.G., T.S., and N.S.
Wrote first draft of paper: L.G. Edited final draft of paper: L.G., A.W., D.H., R.W., T.S., S.S., and N.S. CORRESPONDING AUTHORS Correspondence to Lior Gorodisky or Noam Sobel. ETHICS
DECLARATIONS COMPETING INTERESTS All authors (L.G., D.H., A.W., R.W., T.S., S.S., and N.S.) have co-authored a patent application by The Weizmann Institute of Science for classifying anosmia
by nasal airflow. Authors D.H., A.W., and N.S. have applied for a patent on the device used to measure nasal airflow, and have financial interests in a startup company developing this
device (although not for anosmia). The startup company had no link to the current study. PEER REVIEW PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewer(s) for
their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional
claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SUPPLEMENTARY DATA SOURCE DATA SOURCE DATA
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use,
sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or
other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Gorodisky, L.,
Honigstein, D., Weissbrod, A. _et al._ Humans without a sense of smell breathe differently. _Nat Commun_ 15, 8809 (2024). https://doi.org/10.1038/s41467-024-52650-6 Download citation *
Received: 10 July 2023 * Accepted: 18 September 2024 * Published: 22 October 2024 * DOI: https://doi.org/10.1038/s41467-024-52650-6 SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative





