- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT A key open question in the study of layered superconducting nickelate films is the role that hydrogen incorporation into the lattice plays in the appearance of the superconducting
state. Due to the challenges of stabilizing highly crystalline square planar nickelate films, films are prepared by the deposition of a more stable parent compound which is then transformed
into the target phase _via_ a topotactic reaction with a strongly reducing agent such as CaH2. Recent studies, both experimental and theoretical, have introduced the possibility that the
incorporation of hydrogen from the reducing agent into the nickelate lattice may be critical for the superconductivity. In this work, we use secondary ion mass spectrometry to examine
superconducting La1−_x__X__x_NiO2 / SrTiO3 (_X_ = Ca and Sr) and Nd6Ni5O12 / NdGaO3 films, along with non-superconducting NdNiO2 / SrTiO3 and (Nd,Sr)NiO2 / SrTiO3. We find no evidence for
extensive hydrogen incorporation across a broad range of samples, including both superconducting and non-superconducting films. Theoretical calculations indicate that hydrogen incorporation
is broadly energetically unfavorable in these systems, supporting our conclusion that extensive hydrogen incorporation is not generally required to achieve a superconducting state in layered
square-planar nickelates. SIMILAR CONTENT BEING VIEWED BY OTHERS CRITICAL ROLE OF HYDROGEN FOR SUPERCONDUCTIVITY IN NICKELATES Article 01 March 2023 ATOMIC SCALE DISORDER AND RECONSTRUCTION
IN BULK INFINITE-LAYER NICKELATES LACKING SUPERCONDUCTIVITY Article Open access 14 June 2024 SUPERCONDUCTIVITY IN PRESSURIZED TRILAYER LA4NI3O10−_Δ_ SINGLE CRYSTALS Article 17 July 2024
INTRODUCTION Superconductivity in nickelates has been pursued ever since the discovery of the cuprates1,2,3,4,5, but it was not until 2019 that it was demonstrated in thin films of the
infinite-layer compound NdNiO2 via hole doping with Sr6. This discovery introduced a novel family of layered nickelate superconductors that has now been extended to include the Pr- and La-
analogs of the infinite-layer compound as well as the five-layer material Nd6Ni5O127,8,9,10,11,12. While superconducting nickelates exhibit many interesting phenomena13,14,15,16,17, they
also represent a unique materials synthesis challenge18,19,20,21,22. In general, layered square-planar nickelates cannot be synthesized directly, instead requiring a two-step fabrication
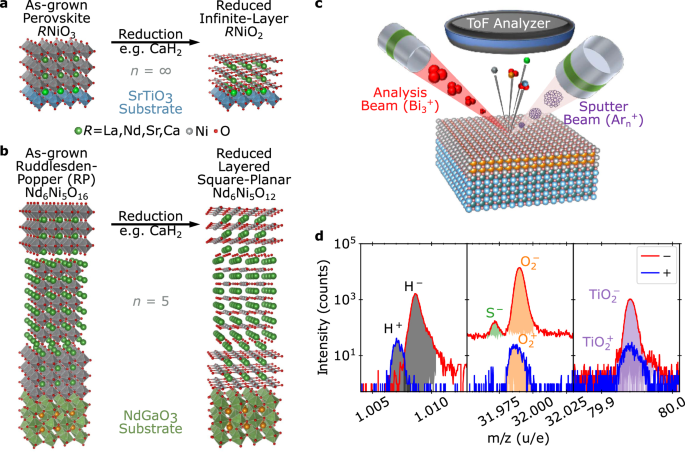
method wherein an oxygen-rich precursor material is grown by traditional thin film deposition methods and then topotactically reduced, as illustrated in Fig. 1a, b23. Typically, the
reduction is performed _via_ a thermal anneal employing a chemical reducing agent and oxygen sink, such as H2, NaH, or CaH26,24,25,26. One of the most pressing open questions is the degree
to which the reduction process incorporates hydrogen into the nickelate film, and whether hydrogen is important in stabilizing superconductivity. A notable recent study by Ding et al.
reported that hydrogen is critical for the emergence of superconductivity, requiring a stoichiometry around Nd0.8Sr0.2NiO2H0.2527. However, in this study, hydrogen and oxygen stoichiometry
are highly correlated through reduction. Furthermore, recent results using alternative reduction processes with no hydrogen source have cast doubt on the role of incorporated hydrogen on the
electronic state9,25. Previous theoretical works have argued that _R_NiO2 (_R_ = La, Nd) could be energetically unstable with respect to topotactic hydrogen, significantly altering the
electronic structure28,29,30. In light of Ref. 27, some calculations have shown that an optimal H concentration may be beneficial to promote superconductivity31, while others have indicated
that the electron phonon-coupling in hydrogen-intercalated nickelates is not strong enough to drive electron pairing and thus cannot be responsible for the superconductivity32. Given the
differing conclusions in the literature, a comprehensive examination of the role of hydrogen incorporation in superconducting nickelates is urgently needed. To understand more broadly
applicable trends rather than the specifics of one sample type or fabrication protocol, we used time-of-flight secondary ion mass spectrometry (ToF-SIMS) to study the relationship between
hydrogen incorporation and superconductivity in a broad range of nickelate films grown and reduced by three different research groups. The films used in this study were grown _via_ either
molecular beam epitaxy (MBE) or pulsed laser deposition (PLD), in a variety of geometries, and reduced with CaH2 at different conditions. As the energetics of incorporating hydrogen may vary
greatly depending on stoichiometry and structure28, we compared multiple nickelate systems, including superconducting examples of La1−_x_Ca_x_NiO2, La1−_x_Sr_x_NiO2, and Nd6Ni5O12. We also
examined non-superconducting NdNiO2 and Nd1−_x_Sr_x_NiO2. We find no evidence that a large concentration of incorporated hydrogen is necessary to observe superconductivity. Instead, a wide
range of films, superconducting and non-superconducting, exhibit H− intensities that are similar to the substrate background. Theoretical calculations support this picture, revealing that
hydrogen incorporation is energetically unfavorable across all materials studied in this work. RESULTS As illustrated in Fig. 1c, ToF-SIMS is a destructive technique in which an ion beam
sputters through the film, and ejected molecular ions are analyzed using a mass spectrometer to provide a depth- and element-resolved picture of the ejected species and, thereby, chemical
composition. ToF-SIMS allows the isolation and identification of elemental H±, and O± as well as larger ejected molecules such as OH±, \({{{{\rm{O}}}}}_{2}^{\pm }\), and
\({{{{\rm{TiO}}}}}_{2}^{\pm }\), as shown in Fig. 1d. The change in molecular species intensity over time as the sample is sputtered results in a depth-resolved understanding of the chemical
composition with depth, in which layers only a few nm thick may be readily separated. However, the measured intensity depends significantly on the sputtering conditions, chemical
environment, film composition, density, electronic state, and prevalence of structural defects. Absolute scaling of stoichiometry and depth, therefore, requires calibration standards with
known stoichiometry and a similar chemical environment to the films of interest. Since the defect levels and chemistry in superconducting nickelates evolve extensively during the reduction
process, such standards are nearly impossible to obtain, and we instead adopt the convention of Ding et al., in which the hydrogen level observed in the SrTiO3 or NdGaO3 substrate is
considered to be the background level representing minimal hydrogen27. SUPERCONDUCTING LA1−_X_(SR,CA)_X_NIO2 We first present results from two doped superconducting infinite-layer systems:
La0.78Ca0.22NiO2 and La0.8Sr0.2NiO2 grown by pulsed laser deposition on SrTiO3 substrates, as described in the Methods section. The quality of representative samples has been previously
demonstrated through X-ray diffraction (XRD), cross-sectional scanning transmission electron microscopy (STEM), and electron energy loss spectroscopy (EELS) analysis33,34. To ensure
depth-wise uniformity, the film thickness was limited to below 6 nm. Figure 2a shows the superconducting transitions for these samples, which have a residual resistivity ratio ≈4.1,
comparable to the highest reported values so far21,35. After reduction, an amorphous SrTiO3 cap is deposited to act as an oxidation barrier, with varying thickness due to the challenges
associated with room-temperature growth. Film and cap thicknesses were verified using X-ray reflectometry (XRR), shown in Fig. 2b, which reveals that the initial perovskite phases are
uniform with the expected scattering length densities. After reduction, the sharp interfaces slightly roughen, likely linked to the energetic deposition of the caps. While here we focus on
superconducting films, we also measured the as-grown perovskite film from the same growth, the details of which can be found in the Supplementary Information. To understand the sensitivity
of this experiment to hydrogen, we first compare the overall hydrogen content of as-grown La0.78Ca0.22NiO3 and superconducting La0.78Ca0.22NiO2 samples in Fig. 2c. Here, we show the H− peak
for both samples integrated across all sputtering times. The as-grown sample contains negligible hydrogen within either the film or substrate, indicating an extremely clean growth and
handling process. The superconducting sample, in contrast, exhibits a clearly-resolved H− peak much larger than the measurement background. The additional hydrogen introduced into the
reduced superconducting sample is easily detectable. We next compare the integrated peak intensity, indicated by the shaded region, over sputter time (depth). For this same pair of samples,
Fig. 2d shows the H− intensity in just the SrTiO3 substrate. Interestingly, while the substrate intensity of the as-grown sample is less than 40 counts per frame, the SrTiO3 substrate
associated with the superconducting La0.78Ca0.22NiO2 is an excellent match for the samples in Ding et al.27, with approximately 200 counts per frame of H−. Thus we may be confident that the
observed hydrogen levels in the substrates are above the instrumental detection limit and closely match previous observations. Having firmly established that the hydrogen levels reported
previously in superconducting films are readily detectable with the instrument used in this study, we show SIMS data from superconducting La0.78Ca0.22NiO2 and La0.8Sr0.2NiO2 in Fig. 2e, f.
Here the \({{{{\rm{NiO}}}}}_{2}^{-}\) and \({{{{\rm{TiO}}}}}_{2}^{-}\) intensities are normalized to their maximum values while the H− and OH− intensities are normalized to the steady-state
value within the substrate; alternative normalizations and raw counts are shown in Supplementary Note 5. The film and substrate positions are indicated by the peak and dip in
\({{{{\rm{NiO}}}}}_{2}^{-}\) and \({{{{\rm{TiO}}}}}_{2}^{-}\) intensities, respectively. The trends in H− and OH− intensities clearly disagree with prior reports: the superconducting
La0.78Ca0.22NiO2 and La0.8Sr0.2NiO2 films do not exhibit the large 1–3 order of magnitude increases in H− or OH− signal which would be expected for extensive, multiple-percent hydrogen
incorporation27,36,37,38. Instead, apart from the quickly decaying signal associated with surface adsorbates, the H− and OH− signals within the La0.78Ca0.22NiO2 film are invariant within a
factor of two of the signals within the substrate. Interestingly, the La0.78Ca0.22NiO2 sample, with a thicker amorphous SrTiO3 cap (29 nm), exhibits higher H− intensity within the cap than
within the nickelate film, concentrated near the interface. In the La0.8Sr0.2NiO2 sample with a thinner SrTiO3 cap (approximately 6 nm), H− is much lower in the nickelate film than either
the SrTiO3 substrate or the other superconducting film. We speculate that the SrTiO3 cap may play a role in hydrogen capture or transport39. Most importantly, the coexistence of different
low hydrogen concentrations with superconductivity definitively demonstrates that extensive hydrogen doping is not required for superconductivity in the infinite-layer nickelates.
SUPERCONDUCTING ND6NI5O12 To test whether our findings are applicable more broadly within the square-planar nickelate family, beyond the infinite-layer structure, we examine the
superconducting quintuple-layer nickelate Nd6Ni5O12. This film consists of 23 nm Nd6Ni5O12 synthesized on NdGaO3 (110) (see Synthesis Section 2 for details), with 10 nm titanium followed by
100 nm platinum patterned on the film surface as electrodes. Figure 3a shows the zero-field superconducting transition of this sample from Ref. 10, with a residual resistivity ratio of 3.8.
Further characterization of this sample can be found in Ref. 10. Figure 3b shows a representative STEM image of this sample, revealing the five-layer square-planar structure. Figure 3c plots
the SIMS depth profile of this superconducting sample. \({{{{\rm{NiO}}}}}_{2}^{-}\) and \({{{{\rm{GaO}}}}}_{2}^{-}\) peaks are normalized to their maximum values, and clearly identify the
electrode, film, and substrate regions. As before, we obtain information regarding the hydrogen concentration by examining the relative intensity of the H− and OH− peaks in the film and
substrate. An advantage of the relatively thick electrode is the removal of surface contaminant effects from the measurement. Both the H− and OH− intensities are low in the platinum and
titanium, increase slowly in the Nd6Ni5O12 film, and further increase deeper into the NdGaO3 substrate. Similar to the superconducting La0.8Sr0.2NiO2 sample, we find that the hydrogen
content appears to be highest in the substrate, although again the nickelate film and substrate intensities are very similar. Once again there is no evidence of an order of magnitude
increase in H− intensity in the film. NON-SUPERCONDUCTING FILMS With little evidence of extensive hydrogen incorporation in high-quality superconducting samples, the question remains whether
some structures or processes are more susceptible to hydrogen. We speculate that films with increased defect densities, whether due to growth conditions or from long or overly aggressive
reduction treatments, may incorporate additional hydrogen as a defect compensation mechanism. These films do not exhibit superconductivity, but do provide a mechanism for understanding the
extent to which hydrogen can be incorporated during reduction and whether it might inhibit the fabrication of superconducting films. We first consider 17 nm NdNiO3/SrTiO3 (001) films grown
by MBE and subjected to an incomplete reduction, at a lower temperature but for longer times compared to the optimized treatment for achieving high-quality NdNiO2. XRD scans shown in Fig. 4a
indicate a reduction toward the infinite-layer NdNiO2 phase, but with a modest decrease in crystallinity. Electron microscopy measurements on an equivalent sister sample, shown in Fig. 4b,
reveal the presence of defects and phase boundaries, as expected. The film was cut in half before reduction, and both as-grown and reduced samples were measured using ToF-SIMS, yielding the
intensity depth profiles in Fig. 4c, d. As before, the NiO−, \({{{{\rm{NiO}}}}}_{2}^{-}\), and \({{{{\rm{TiO}}}}}_{2}^{-}\) peaks are normalized to their maximum value while the H− and OH−
are normalized to the steady-state values in the substrate. The H− and OH− signals are slightly higher in the SrTiO3 substrate than in the as-grown NdNiO3 film. Upon reduction, H− and OH−
increase at the surface of the films, and the lineshape of this increase only partially matches that of various peaks including \({{{{\rm{C}}}}}_{2}^{-}\) and Ca+ (see Supplementary Note 3),
indicating that they do not solely originate from surface adsorbates introduced during the reduction process. Near the substrate interface, which has previously been shown to be the
highest-quality region of the film20,33, the H− intensity remains lower than in the SrTiO3 substrate. Thus, while some insignificant hydrogen content may be introduced during the reduction
process, it seems to be limited near the surface of these uncapped films. We next present our findings on non-superconducting infinite-layer Nd0.8Sr0.2NiO2/SrTiO3 films, which are
appropriately doped to result in superconductivity but were reduced aggressively at high temperatures (600 °C compared to ~300 °C); this reduction is enough to significantly hydrogen-dope a
similar perovskite material, BaZrO338. Furthermore, a common practice is to cap perovskite nickelate films with SrTiO3 prior to reduction to provide balanced strain for structural stability
of the film throughout its entire thickness6,20. We, therefore, compare samples with and without a SrTiO3 capping layer grown in situ on the 10 nm Nd0.8Sr0.2NiO3 before reduction. XRD
measurements, shown in Fig. 5a, reveal that the crystalline quality of both capped and uncapped films prior to reduction is lower, with broader, lower-intensity film peaks. Importantly,
while the (002) Nd0.8Sr0.2NiO3 peak is sharp, the expected perovskite (001) film peak is suppressed; further higher-resolution measurements, shown in Supplementary Fig. 12, resolve the
presence of potential Ruddlesden–Popper phase. As before, the topotactic reduction process reduces the _c_-axis lattice parameter, but the peak intensity drops dramatically. Transmission
electron micrographs of these samples, such as that shown in Fig. 5b, reveal the segregation of the film into multiple crystalline phases and amorphous-like regions. Thus, unlike the uniform
superconducting samples, the aggressive reduction of these films is non-uniform and disordered, resulting in increased mosaicity and a loss of crystalline quality. This is corroborated by
electronic transport, as discussed further in the Supplementary Information, which indicates that capped and uncapped films exhibit sharply different resistivities. As shown in Fig. 5c,
which plots SIMS measurements from the as-grown, uncapped Nd0.8Sr0.2NO3 film, the initial transient region shows much higher yields of all ions which may indicate differences in
crystallinity near the surface, potentially from the emergence of a polycrystalline scale layer in uncapped samples over time21. In the bulk region, the H− and OH− intensities are similar to
the substrate. Despite the significant difference in crystalline quality and reduction conditions, the effects of reduction are similar to our other observations. Figure 5d shows the
integrated peak intensities in the reduced SrTiO3/Nd0.8Sr0.2NiO2 bilayer. Once again, H− and OH− intensities are elevated at the cap/film interface—though over a broader spatial extent—with
almost a 50% increase over the baseline in the substrate. Thus, while the SrTiO3 cap appears to trap hydrogen, this enhancement is again far below the orders of magnitude that would be
expected for significant hydrogen incorporation, remaining within a factor of two of the substrate values. THEORETICAL CALCULATIONS To further understand the lack of hydrogen incorporation
in the nickelates analyzed above via SIMS (both superconducting and non-superconducting), density-functional theory (DFT)-based calculations were performed to explore the energetics of
topotactic hydrogen in infinite-layer _R_NiO2 (_R_ = rare-earth, both doped and undoped) as well as in the quintuple-layer nickelate Nd6Ni5O12. To investigate whether it is energetically
favorable to intercalate hydrogen, we compute the hydrogen binding energy (_E__b_) for the topotactic process as done in previous work28: $${E}_{b}=\{E[R{{{{\rm{NiO}}}}}_{2}]+n\times \mu
[H]-E[R{{{{\rm{NiO}}}}}_{2}{{{\rm{H}}}}]\}/n,$$ (1) where _E_[_R_NiO2] and _E_[_R_NiO2H] are the total energies for the infinite-layer _R_NiO2 and hydride-oxide _R_NiO2H compounds, _μ_[_H_]
= _E_[H2]/2 is the chemical potential of H, and _n_ represents the number of H atoms in the (super)cell. Analogous expressions are used for _R_0.75(Sr,Ca)0.25NiO2 and Nd6Ni5O12. A positive
(negative) _E__b_ indicates that the topotatic hydrogen intercalation is favorable (unfavorable). The calculated binding energies are summarized in Fig. 6. We find that the incorporation of
H into _R_NiO2, _R_0.75(Sr,Ca)0.25NiO2, Nd6Ni5O12 is systematically unfavorable, in agreement with experiments (only for LaNiO2 a very small positive E_b_ value of approximately 10 meV/H is
obtained). DISCUSSION In summary, we searched for hydrogen across a wide range of superconducting and non-superconducting layered nickelate films, with different cation and dopant chemistry,
structures, growth methods, reduction conditions, and crystalline quality. Not only did we find no significant concentrations of hydrogen in superconducting films, but we were also unable
to use excessive reduction temperature or time to force significant amounts of hydrogen into these structures. These results are consistent with first-principles calculations which show that
hydrogen incorporation is energetically unfavorable in both infinite-layer and quintuple-layer nickelates. At most, we observed increased concentrations by a factor of two from the trace
amounts already present within the substrates. Furthermore, hydrogen, as hydride or hydroxide ions (H− and OH−), was more likely to be found in SrTiO3 caps or in the substrates than in the
nickelate films themselves. This propensity for hydrogen to appear in higher concentrations in SrTiO3 capping layers and SrTiO3/nickelate interfaces is interesting in the context of recent
work showing the important role such capping layers can play in facilitating the reduction process21. It should be noted that our measurements generally reveal as-grown samples with hydrogen
levels at or below the SIMS detection limit prior to reduction, although it is, of course, not possible to completely eliminate hydrogen from any material system. CaH2 does appear to
introduce hydrogen into the system, as evidenced by changes in both film and substrate levels in as-grown La0.78Ca0.22NiO3 and reduced La0.78Ca0.22NiO2 films, for example. However,
topotactic reduction appears unable to introduce hydrogen into these nickelates at the levels near ANiO2H0.25 (A = La,Sr,Nd,Ca) previously cited as critical doping for superconductivity27.
At such high levels of incorporated hydrogen, Ding et al. observed H− intensities approximately 40× to 60× the substrate concentration, and approaching a factor of 200× to 600× near the film
surface. We find no evidence of such large relative H− intensities in the films studied in this work. Therefore, although superconductivity is highly sensitive to reduction optimization,
this is likely due to the crystalline quality and oxygen stoichiometry, and not hydrogen stoichiometry. Of course, this study does not demonstrate that superconductivity requires the
complete absence of incorporated hydrogen. This work instead indicates that many films appear resistant to hydrogen infiltration and that superconductivity may be readily realized at very
low hydrogen levels for which no theoretical evidence supports hydrogen-mediated superconductivity. _Note added:_ Recently, independent SIMS experiments performed by Zeng et al.40 and
Gonzalez et al.41 also concluded that extensive hydrogen incorporation is unnecessary for superconductivity in the infinite-layer nickelates, in agreement with this work. METHODS SAMPLE
SYNTHESIS: THIN FILM DEPOSITION AND REDUCTION LA1−_X_(CA,SR)_X_NIO2 FILMS Thin films, ≈ 6 nm thick, of the infinite-layer nickelates La0.78Ca0.22NiO2 and La0.8Sr0.2NiO2 were grown on SrTiO3
(001) substrates using pulsed laser deposition (PLD) and CaH2 topotactic reduction33,34. SrTiO3 (001) substrates were etched with hydrofluoric acid and annealed in air at 900 °C for 90 min
before deposition. This is to maximize the TiO2 termination which serves to minimize disordered Ruddlesden–Popper type growth. We first grow the perovskite phase using PLD with the following
optimal set of parameters: _T_growth = 575 °C, \({{{{\rm{P}}}}}_{{O}_{2}}\) = 150 mTorr (1 Torr = 133.322 Pa), _J_ = 2.5 J/cm2. Afterwards, the film was post-annealed at growth temperature
under the same oxygen partial pressure for 10 min followed by cooling at 8 °C/minute. The topotactic phase transition to the infinite-layer phase was mediated by the substrate strain and
performed in the same PLD vacuum chamber with a base pressure of less than 1 × 10−6 Torr. The reduction environment was achieved by heating approximately 0.1 g of CaH2 powder to obtain a (H2
and other species) pressure in the range of approximately 0.1–0.3 Torr. Samples are annealed at 340 °C for 1 h. After reduction, samples were capped with amorphous SrTiO3 at room
temperature using PLD to protect the surface from reoxidation. Mild oxidation damage to the top nickelate surface can be expected in this process. ND6NI5O12 AND NDNIO2 FILMS We use
ozone-assisted molecular beam epitaxy (MBE) to synthesize the precursor Nd6Ni5O16/NdGaO3 (110) and NdNiO3/SrTiO3 (001) films in Figs. 3 and 4, respectively. To calibrate the nickel and
neodymium elemental fluxes, we synthesize NiO on MgO (001) and Nd2O3 on yttria-stabilized zirconia (YSZ (111)), then measure the film thickness via X-ray reflectivity. Next, we synthesize
NdNiO3/LaAlO3 (001) and use the _c_-axis lattice constant and film thickness to refine the Nd/Ni ratio and monolayer dose, respectively. Using the optimized neodymium and nickel shutter
times from the synthesis of NdNiO3/LaAlO3, we synthesize the Ruddlesden–Popper nickelates _via_ monolayer shuttering. Both NdNiO3 and Ruddlesden–Popper nickelates are synthesized at a
substrate temperature of 500–600 °C with approximately 1.0 × 10−6 Torr distilled ozone (Heeg Vacuum Engineering). The MBE synthesis conditions and calibration scheme are described in Refs.
10,22; similar techniques were also used in Refs. 42,43. The perovskite and Ruddlesden-Popper films are reduced to the square-planar phase via CaH2 topotactic reduction. The following
methods are similar to those used elsewhere10,20,44. First, the as-grown films are cut into identical pieces, and the pieces to be reduced are tightly wrapped in aluminum foil (All-Foils) to
avoid direct contact between the film and CaH2. Each film is then placed in a borosilicate tube (Chemglass Life Sciences) with approximately 0.1 g of CaH2 pieces (>92%, Alfa Aesar). The
borosilicate tube is pumped down to <0.5 mTorr, sealed, and then heated for several hours at 290 °C in a convection oven (Heratherm, Thermo Fisher Scientific) with a 10 °C min−1 heating
rate. ND0.8SR0.2NIO3 FILMS Polycrystalline targets of NdNiO3 and Nd0.8Sr0.2NiO3 were prepared by the liquid-mix technique45,46. A 10 nm thick Nd0.8Sr0.2NiO3 films were grown on (001) SrTiO3
substrates using a Neocera PLD system equipped with an in-situ RHEED (Staib Instruments, Germany). The depositions were conducted using a KrF excimer laser operating at 2 Hz with a fluence
of 1.5 J cm−2. During the deposition, a dynamic oxygen pressure of 150 mTorr was maintained, and the substrate temperature was 735 °C. The optional 10 nm thick SrTiO3 capping layer was grown
at the same condition as the film. After the deposition, all samples were in-situ annealed at the deposition temperature in an oxygen atmosphere of 500 Torr for 30 min and subsequently
cooled to room temperature at a rate of 15 °C min−1. The as-grown films were sealed in evacuated (approximately 1 mTorr) quartz ampoules with 0.1 g CaH2 powder (90%–95%, Thermo Scientific
Chemicals). The ampoules were then baked in a muffle furnace at 600 °C for up to 10 h. The temperature ramp rate was fixed at 10 °C min−1. Once the ampoules were opened, the reduced films
were immediately rinsed in _n_-butanol and isopropanol in an ultrasonic bath for 3 min. X-RAY DIFFRACTION X-ray diffraction (XRD) measurements were performed at room temperature before and
after reduction by each group on commercially available X-ray diffractometers using Cu K_α_1 (_λ_ = 1.5406 Å) radiation. X-RAY REFLECTOMETRY X-ray reflectometry (XRR) measurements were
performed at ambient conditions in a horizontal configuration using a Rigaku SmartLab diffractometer. The incident beam was collimated using the parallel beam slit and an incident slit of 30
μm height to improve _Q_-resolution. The Cu K_α_1 wavelength (_λ_ = 1.5406 Å) was isolated by using a Ge-(220) × 2 monochromator. The scattered beam was further collimated by a 0.2 mm
receiving slit. The data were reduced using the _reductus_ web-service47 and fit to a slab model using Refl1D48. TIME-OF-FLIGHT SECONDARY ION MASS SPECTROSCOPY Time-of-flight SIMS was
performed using an IONTOF IV (Münster, Germany) equipped with a 20 keV \({{{{\rm{Ar}}}}}_{2600\pm 1000}^{+}\) cluster source for sputtering, a 30 keV \({{{{\rm{Bi}}}}}_{3}^{+}\) liquid metal
ion source for analysis, and a time-of-flight mass analyzer. Depth profiling was performed in non-interlaced mode with 1 scan of analysis with a lateral resolution of (128 × 128) pixels, 10
scans of sputtering, and at least 0.5 s of charge compensation per cycle, where both the analysis and sputter rasters were kept inside a (500 × 500) μm area. The corresponding ion doses
were 1.9 × 109 ions/cm2 (0.12 pA) for Bi3+, and between 2.1 × 1014 ions/cm2 to 2.6 × 1014 ions/cm2 (5.1–6.4 nA) per cycle for the cluster source due to day-to-day fluctuations in the beam
current. On especially insulating samples or substrates, a small drop of silver paint was used to electrically contact the sample surface to the sample holder for further charge
compensation. For reliable detection of H− ions, contributions from residual gases were minimized by keeping the chamber pressure below 5 × 10−7 Pa. Both negative and positive ions were
analyzed at separate spots, and the signal rastered over multiple spots was averaged after normalizing for the highest intensity ion unique to the substrate (\({{{{\rm{TiO}}}}}_{2}^{-}\) or
\({{{{\rm{GaO}}}}}_{2}^{-}\)). Spectra were analyzed using SurfaceLab to define a region of interest, perform mass calibrations, identify peaks with the appropriate compounds, and extract
the total integrated peak intensity as a function of sputter time. As many molecular compounds can have similar mass, peak assignments were made carefully, considering factors such as mass
offset, isotopic distribution, and similarity in profile shape to other known oxide and hydroxide species. ELECTRON MICROSCOPY All cross-sectional STEM specimens were prepared by the
standard focused ion beam (FIB) lift-out procedure and imaged in high-angle annular dark-field (HAADF)-STEM configuration. The instrument, processing, and experimental details for specific
samples are as follows: * Nd6Ni5O12. Preparation: Thermo Fisher Scientific Helios G4 UX and FEI Helios 660 FIBs. Imaging: probe-corrected Thermo Fisher Scientific Spectra 300 X-CFEG
operating at 300 kV, 19 mrad convergence semi-angle, 33 mrad inner collection angles. * NdNiO2. Preparation: FEI Helios 660 FIBs with final polishing at 5 kV accelerating voltage and 41 pA
probe current. Imaging: Thermo Fisher 615 Scientific Titan Themis Z G3 operating at 200 kV, 18.9 mrad convergence semi-angle, and 68 (280) mrad inner (outer) collection angles. *
SrTiO3-capped (Nd,Sr)NiO3−_x_ and (Nd,Sr)NiO2. Preparation: Thermo Fisher Scientific Helios G4 UXe PFIB Dual Beam, with final polishing at 5 kV accelerating voltage. Imaging: Thermo Fisher
Scientific Spectra 200 operating at 200 kV, 25 mrad convergence semi-angle, 54 (200) mrad inner (outer) collection angles, and probe current of approximately 20 pA. Energy dispersive X-ray
spectroscopy (EDS) chemical mapping was performed on the same instrument with Bruker Dual-X X-ray detectors an electron beam current of approximately 100 pA. COMPUTATIONAL METHODS
Density-functional theory (DFT)-based calculations were performed to theoretically explore the energetics of topotactic hydrogen in the infinite-layer nickelate _R_NiO2 (_R_ = La, Nd, both
doped and undoped) as well as in the quintuple-layer nickelate Nd6Ni5O12. For _R_NiO2H_δ_, _R_0.75(Sr,Ca)0.25NiO2H_δ_, and Nd6Ni5O12H_δ_ (_δ_ = 0, 1) structural relaxations were performed
using the VASP code49,50,51 with the the Perdew–Burke–Ernzerhof version of the generalized gradient approximation (GGA-PBE)52. For the infinite-layer materials (_R_NiO2 and
_R_0.75(Sr,Ca)0.25NiO2) up to a 2 × 2 × 2 supercell was used to accommodate the appropriate H content and/or (Sr,Ca)-doping level. We place the topotatic-H at the positions of the (removed)
apical oxygens as this was shown to be the most energetically favorable position for H-incorporation from previous works27,28,29,30. GGA-PBE was chosen as it provides lattice constants in
close agreement with experimental data, as shown in Supplementary Note H. A _Γ_-centered 13 × 13 × 15 (9 × 9 × 11) _k_-mesh was used for the 1 × 1 × 1 unit cells (2 × 2 × 2 supercells) with
a 0.05 eV Gaussian smearing. For Nd6Ni5O12, a _Γ_-centered 9 × 9 × 9 _k_-mesh with a 0.05 eV Gaussian smearing was used. The size of the plane-wave basis sets was set with a kinetic energy
cut-off of 520 eV. For _R_ = Nd, we have used a pseudopotential where the Nd(4_f_) electrons are frozen in the core. To compute the chemical potential of hydrogen (_μ_[H]), we optimized an
H2 dimer in 153 Å3 box with energy cutoff set to 325 eV. DATA AVAILABILITY The data supporting this study have been deposited in Figshare. CODE AVAILABILITY Analysis was performed by
open-source Python packages, including NumPy, matplotlib, and SciPy. SIMS data reduction was performed using the commercial software SurfaceLab 7. All density-functional theory calculations
were performed with the Vienna Ab-Initio Simulation Package (VASP). REFERENCES * Anisimov, V. I., Bukhvalov, D. & Rice, T. M. Electronic structure of possible nickelate analogs to the
cuprates. _Phys. Rev. B_ 59, 7901–7906 (1999). Article ADS CAS Google Scholar * Chaloupka, J. & Khaliullin, G. Orbital order and possible superconductivity in LaNiO3/LaMO3
superlattices. _Phys. Rev. Lett._ 100, 016404 (2008). Article ADS PubMed Google Scholar * Hansmann, P. et al. Turning a nickelate fermi surface into a cupratelike one through
heterostructuring. _Phys. Rev. Lett._ 103, 016401 (2009). Article ADS CAS PubMed Google Scholar * Hansmann, P., Toschi, A., Yang, X., Andersen, O. K. & Held, K. Electronic structure
of nickelates: From two-dimensional heterostructures to three-dimensional bulk materials. _Phys. Rev. B_ 82, 235123 (2010). Article ADS Google Scholar * Han, M. J., Wang, X., Marianetti,
C. A. & Millis, A. J. Dynamical mean-field theory of nickelate superlattices. _Phys. Rev. Lett._ 107, 206804 (2011). Article ADS CAS PubMed Google Scholar * Li, D. et al.
Superconductivity in an infinite-layer nickelate. _Nature_ 572, 624–627 (2019). Article ADS CAS PubMed Google Scholar * Osada, M. et al. A superconducting praseodymium nickelate with
infinite layer structure. _Nano Lett._ 20, 5735–5740 (2020). Article ADS CAS PubMed Google Scholar * Osada, M., Wang, B. Y., Lee, K., Li, D. & Hwang, H. Y. Phase diagram of infinite
layer praseodymium nickelate Pr1−_x_Sr_x_NiO2 thin films. _Phys. Rev. Mater._ 4, 121801 (2020). Article CAS Google Scholar * Wei, W., Vu, D., Zhang, Z., Walker, F. J. & Ahn, C. H.
Superconducting Nd1−_x_Eu_x_NiO2 thin films using in situ synthesis. _Sci. Adv._ 9, eadh3327 (2023). Article CAS PubMed PubMed Central Google Scholar * Pan, G. A. et al.
Superconductivity in a quintuple-layer square-planar nickelate. _Nat. Mater._ 21, 160–164 (2022). Article ADS CAS PubMed Google Scholar * Zeng, S. et al. Superconductivity in
infinite-layer nickelate La1−_x_Ca_x_NiO2 thin films. _Sci. Adv._ 8, eabl9927 (2022). Article CAS PubMed PubMed Central Google Scholar * Osada, M. et al. Nickelate superconductivity
without rare-earth magnetism: (La,Sr)NiO2. _Adv. Mater._ 33, 2104083 (2021). Article CAS Google Scholar * Fowlie, J. et al. Intrinsic magnetism in superconducting infinite-layer
nickelates. _Nat. Phys._ 18, 1043–1047 (2022). Article CAS Google Scholar * Lee, K. et al. Linear-in-temperature resistivity for optimally superconducting (Nd,Sr)NiO2. _Nature_ 619,
288–292 (2023). Article ADS CAS PubMed Google Scholar * Lane, C. et al. Competing incommensurate spin fluctuations and magnetic excitations in infinite-layer nickelate superconductors.
_Commun. Phys._ https://doi.org/10.1038/s42005-023-01213-0 (2023). * Lu, H. et al. Magnetic excitations in infinite-layer nickelates. _Science_ 373, 213–216 (2021). Article ADS CAS PubMed
Google Scholar * Zeng, S. et al. Phase diagram and superconducting dome of infinite-layer Nd1−_x_Sr_x_NiO2 thin films. _Phys. Rev. Lett._ 125, 147003 (2020). Article ADS CAS PubMed
Google Scholar * Zhou, X. et al. Experimental progress on the emergent infinite-layer Ni-based superconductors. _Mater. Today_ 55, 170–185 (2022). Article CAS Google Scholar * Ferenc
Segedin, D. et al. Limits to the strain engineering of layered square-planar nickelate thin films. _Nat. Commun._ 14, 1468 (2023). Article ADS CAS PubMed PubMed Central Google Scholar
* Lee, K. et al. Aspects of the synthesis of thin film superconducting infinite-layer nickelates. _APL Mater._ https://doi.org/10.1063/5.0005103 (2020). * Parzyck, C. T. et al. Synthesis of
thin film infinite-layer nickelates by atomic hydrogen reduction: clarifying the role of the capping layer. _APL Mater._ 12, 031132 (2024). Article ADS CAS Google Scholar * Pan, G. A. et
al. Synthesis and electronic properties of Nd_n_+1Ni_n_O3_n_+1 Ruddlesden-Popper nickelate thin films. _Phys. Rev. Mater._ 6, 055003 (2022). Article CAS Google Scholar * Momma, K. &
Izumi, F. VESTA: a three-dimensional visualization system for electronic and structural analysis. _J. Appl. Crystallogr._ 41, 653–658 (2008). Article ADS CAS Google Scholar * Parzyck, C.
T. et al. Absence of 3_a_0 charge density wave order in the infinite-layer nickelate NdNiO2. _Nat. Mater._ https://doi.org/10.1038/s41563-024-01797-0 (2024). * Wei, W. et al. Solid state
reduction of nickelate thin films. _Phys. Rev. Mater._ 7, 013802 (2023). Article CAS Google Scholar * Hayward, M. A., Green, M. A., Rosseinsky, M. J. & Sloan, J. Sodium hydride as a
powerful reducing agent for topotactic oxide deintercalation: synthesis and characterization of the nickel(i) oxide LaNiO2. _J. Am. Chem. Soc._ 121, 8843–8854 (1999). Article CAS Google
Scholar * Ding, X. et al. Critical role of hydrogen for superconductivity in nickelates. _Nature_ 615, 50–55 (2023). Article ADS CAS PubMed Google Scholar * Si, L. et al. Topotactic
hydrogen in nickelate superconductors and akin infinite-layer oxides ABO2. _Phys. Rev. Lett._ 124, 166402 (2020). Article ADS CAS PubMed Google Scholar * Si, L., Worm, P. & Held, K.
Fingerprints of topotactic hydrogen in nickelate superconductors. _Crystals_ https://www.mdpi.com/2073-4352/12/5/656 (2022). * Si, L., Worm, P., Chen, D. & Held, K. Topotactic hydrogen
forms chains in _A__B_O2 nickelate superconductors. _Phys. Rev. B_ 107, 49–51 (2023). Article Google Scholar * Qin, C., Jiang, M. & Si, L. Effects of different concentrations of
topotactic hydrogen impurities on the electronic structure of nickelate superconductors. _Phys. Rev. B_ 108, 155147 (2023). Article ADS CAS Google Scholar * Di Cataldo, S., Worm, P., Si,
L. & Held, K. Absence of electron-phonon-mediated superconductivity in hydrogen-intercalated nickelates. _Phys. Rev. B_ 108, 174512 (2023). Article ADS Google Scholar * Chow, L. E.
et al. Pairing symmetry in infinite-layer nickelate superconductor. Preprint at _arXiv_ https://doi.org/10.48550/arXiv.2201.10038 (2023). * Chow, L. E. et al. Pauli-limit violation in
lanthanide infinite-layer nickelate superconductors. Preprint at _arXiv_ https://doi.org/10.48550/arXiv.2204.12606 (2022). * Sun, W. et al. Evidence for anisotropic superconductivity beyond
pauli limit in infinite-layer lanthanum nickelates. _Adv. Mater._ 35, 2303400 (2023). Article CAS Google Scholar * Sheffels, S. et al. Insight on hydrogen injection and GdOx/Co interface
chemistry from in operando neutron reflectometry and secondary ion mass spectrometry. _Appl. Phys. Lett._ https://doi.org/10.1063/5.0128835 (2023). * Lu, Q. et al. Engineering magnetic
anisotropy and emergent multidirectional soft ferromagnetism in ultrathin freestanding LaMnO3 films. _ACS Nano_ 16, 7580–7588 (2022). Article CAS PubMed Google Scholar * Orvis, T. et al.
Electron doping BaZrO3 via topochemical reduction. _ACS Appl. Mater. interfaces_ 11, 21720–21726 (2019). Article CAS PubMed Google Scholar * Eltes, F. et al. Low-loss BaTiO3-Si
waveguides for nonlinear integrated photonics. _ACS Photonics_ 3, 1698–1703 (2016). Article CAS Google Scholar * Zeng, S. et al. Origin of a Topotactic Reduction Effect for
Superconductivity in Infinite-Layer Nickelates. _Phys. Rev. Lett._ 133, 066503 (2024). * Gonzalez, M. et al. Absence of hydrogen insertion into highly crystalline superconducting infinite
layer nickelates. _Phys. Rev. Materials_ 8, 084804 (2024). * Li, Z. et al. Epitaxial growth and electronic structure of Ruddlesden-Popper nickelates (La_n_+1Ni_n_O3_n_+1, _n_ = 1-5). _APL
Mater._ 8, 091112 (2020). Article ADS CAS Google Scholar * Sun, W. et al. Electronic and transport properties in Ruddlesden-Popper neodymium nickelates Nd_n_+1Ni_n_O3_n_+1 (_n_=1-5).
_Phys. Rev. B_ 104, 184518 (2021). Article ADS CAS Google Scholar * Li, Y. et al. Impact of cation stoichiometry on the crystalline structure and superconductivity in nickelates. _Front.
Phys._ 9, 719534 (2021). Article Google Scholar * Patel, R. K. et al. Hole doping in a negative charge transfer insulator. _Commun. Phys._ 5, 216 (2022). Article CAS Google Scholar *
Patel, R. K. et al. Epitaxial stabilization of ultra thin films of high entropy perovskite. _Appl. Phys. Lett._ 116, 071601 (2020). Article ADS CAS Google Scholar * Maranville, B.,
Ratcliff II, W. & Kienzle, P. _r_eductus: a stateless Python data reduction service with a browser front end. _J. Appl. Crystallogr._ 51, 1500–1506 (2018). Article ADS CAS Google
Scholar * Kirby, B. J. et al. Phase-sensitive specular neutron reflectometry for imaging the nanometer scale composition depth profile of thin-film materials. _Curr. Opin. Colloid Interface
Sci._ 17, 44–53 (2012). Article CAS Google Scholar * Kresse, G. & Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set.
_Phys. Rev. B_ 54, 11169–11186 (1996). Article ADS CAS Google Scholar * Kresse, G. & Hafner, J. Ab initio molecular dynamics for liquid metals. _Phys. Rev. B_ 47, 558–561 (1993).
Article ADS CAS Google Scholar * Kresse, G. & Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. _Phys. Rev. B_ 59, 1758–1775 (1999). Article ADS
CAS Google Scholar * Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. _Phys. Rev. Lett._ 77, 3865–3868 (1996). Article ADS CAS PubMed Google
Scholar Download references ACKNOWLEDGEMENTS D.F.S., G.A.P., and J.A.M. acknowledge support from the US Department of Energy, Office of Basic Energy Sciences, Division of Materials
Sciences and Engineering, under Award No. DE-SC0021925. L.E.C. and A.A. acknowledge support from the Ministry of Education (MOE), Singapore, under its Tier-2 Academic Research Fund (AcRF),
Grants No. MOET2EP50121-0018 and MOE-T2EP50123-0013, and the SUSTech-NUS Joint Research Program. J.R. and M.S. acknowledge support from an ARO MURI program with award no. W911NF-21-1-0327,
and the National Science Foundation of the United States under grant number DMR-2122071. R.K.P. and S. Middey acknowledge MHRD, Government of India for financial support under the STARS
research funding scheme (grant number: STARS/APR2019/PS/156/FS). J.A.M., A.S.B., and H.L. acknowledge support from NSF grant no. DMR-2323971. I.E. and Y.Z. were supported by the Rowland
Institute at Harvard. K.J. and Y.T.S. acknowledge support from USC Viterbi startup funding and the USC Research and Innovation Instrumentation Award. B.H.G. and L.F.K acknowledge support
from the Platform for the Accelerated Realization, Analysis, and Discovery of Interface Materials (PARADIM) and the Packard Foundation. Materials growth and electron microscopy were
supported in part by PARADIM under NSF Cooperative Agreement no. DMR-2039380. Focused ion beam sample preparation was performed in part at the Harvard University Center for Nanoscale Systems
(CNS); a member of the National Nanotechnology Coordinated Infrastructure Network (NNCI), which is supported by the National Science Foundation under NSF award no. ECCS-2025158.
Transmission electron microscopy was carried out in part through the use of MIT.nano’s facilities. Electron microscopy data were acquired in part at the Core Center of Excellence in Nano
Imaging at USC. Electron microscopy was performed in part at the Cornell Center for Materials Research (CCMR) Shared Facilities, which are supported by the NSF MRSEC Program (No.
DMR-1719875). P.P.B. and P.Q. received funding from the NRC RAP. G.A.P. acknowledges additional support from the Paul and Daisy Soros Fellowship for New Americans. D.F.S. and G.A.P
acknowledge support from the NSF Graduate Research Fellowship Grant DGE-1745303. We thank Kyuho Lee for insightful discussions. We also thank Kerry Sieben for X-ray assistance. We thank
Hanjong Paik for supporting the growth of the _n_ = 5 superconducting sample. Research was performed in part at the NIST Center for Nanoscale Science and Technology. Certain commercial
equipment, instruments, software, or materials are identified in this paper in order to specify the experimental procedure adequately. Such identifications are not intended to imply
recommendation or endorsement by NIST, nor it is intended to imply that the materials or equipment identified are necessarily the best available for the purpose. AUTHOR INFORMATION Author
notes * These authors contributed equally: Purnima P. Balakrishnan, Dan Ferenc Segedin, Lin Er Chow AUTHORS AND AFFILIATIONS * NIST Center for Neutron Research, National Institute of
Standards and Technology, Gaithersburg, MD, 20899, USA Purnima P. Balakrishnan, P. Quarterman & Alexander J. Grutter * Department of Physics, Harvard University, Cambridge, MA, 02138,
USA Dan Ferenc Segedin, Grace A. Pan, Qi Song & Julia A. Mundy * Department of Physics, Faculty of Science, National University of Singapore, Singapore, 117551, Singapore Lin Er Chow
& A. Ariando * Material Measurement Laboratory, National Institute of Standards and Technology, Gaithersburg, MD, 20899, USA Shin Muramoto * Mork Family Department of Chemical
Engineering and Materials Science, University of Southern California, Los Angeles, CA, 90089, USA Mythili Surendran, Koushik Jagadish, Yu-Tsun Shao & Jayakanth Ravichandran * Core Center
for Excellence in Nano Imaging, University of Southern California, Los Angeles, CA, 90089, USA Mythili Surendran & Jayakanth Ravichandran * Department of Physics, Indian Institute of
Science, Bengaluru, 560012, India Ranjan K. Patel & Srimanta Middey * Department of Physics, Arizona State University, Tempe, AZ, 85287, USA Harrison LaBollita & Antia S. Botana *
The Rowland Institute at Harvard, Harvard University, Cambridge, MA, 02138, USA Yang Zhang & Ismail El Baggari * Core Center for Excellence in Nano Imaging, University of Southern
California, 925 Bloom Walk, Los Angeles, CA, 90089, USA Yu-Tsun Shao * School of Applied and Engineering Physics, Cornell University, Ithaca, NY, 14853, USA Berit H. Goodge & Lena F.
Kourkoutis * Kavli Institute at Cornell for Nanoscale Science, Ithaca, NY, 14853, USA Berit H. Goodge & Lena F. Kourkoutis * Max Planck Institute for Chemical Physics of Solids, 01187,
Dresden, Germany Berit H. Goodge * Ming Hsieh Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, 90089, USA Jayakanth Ravichandran Authors
* Purnima P. Balakrishnan View author publications You can also search for this author inPubMed Google Scholar * Dan Ferenc Segedin View author publications You can also search for this
author inPubMed Google Scholar * Lin Er Chow View author publications You can also search for this author inPubMed Google Scholar * P. Quarterman View author publications You can also search
for this author inPubMed Google Scholar * Shin Muramoto View author publications You can also search for this author inPubMed Google Scholar * Mythili Surendran View author publications You
can also search for this author inPubMed Google Scholar * Ranjan K. Patel View author publications You can also search for this author inPubMed Google Scholar * Harrison LaBollita View
author publications You can also search for this author inPubMed Google Scholar * Grace A. Pan View author publications You can also search for this author inPubMed Google Scholar * Qi Song
View author publications You can also search for this author inPubMed Google Scholar * Yang Zhang View author publications You can also search for this author inPubMed Google Scholar *
Ismail El Baggari View author publications You can also search for this author inPubMed Google Scholar * Koushik Jagadish View author publications You can also search for this author
inPubMed Google Scholar * Yu-Tsun Shao View author publications You can also search for this author inPubMed Google Scholar * Berit H. Goodge View author publications You can also search for
this author inPubMed Google Scholar * Lena F. Kourkoutis View author publications You can also search for this author inPubMed Google Scholar * Srimanta Middey View author publications You
can also search for this author inPubMed Google Scholar * Antia S. Botana View author publications You can also search for this author inPubMed Google Scholar * Jayakanth Ravichandran View
author publications You can also search for this author inPubMed Google Scholar * A. Ariando View author publications You can also search for this author inPubMed Google Scholar * Julia A.
Mundy View author publications You can also search for this author inPubMed Google Scholar * Alexander J. Grutter View author publications You can also search for this author inPubMed Google
Scholar CONTRIBUTIONS P.P.B., S. Muramoto, and A.J.G. performed and analyzed ToF-SIMS measurements. P.P.B., A.J.G., and P.Q. performed XRR and analyzed and interpreted all reflectometry
data. L.E.C. deposited and reduced La0.8Sr0.2NiO3 and La0.78Ca0.22NiO3 films, and performed electronic characterization. NdNiO3 and Nd6Ni5O16 films were fabricated by G.A.P., D.F.S., and
Q.S., while G.A.P. and D.F.S. performed reduction and electronic characterization. Nd0.8Sr0.2NiO3 films were deposited and initially characterized by R.K.P. and S. Middey, and reduced by
M.S. TEM measurements were performed and analyzed by Y.Z., I.E., K.J., Y.T.S., B.H.G., and L.F.K. H.L. and A.S.B. performed the theoretical calculations. The study was designed by A.J.G.,
P.P.B., P.Q., D.F.S., J.A.M., A.A., and J.R. The paper was written by P.P.B. and A.J.G. with input from all authors. CORRESPONDING AUTHORS Correspondence to Purnima P. Balakrishnan,
Jayakanth Ravichandran, A. Ariando, Julia A. Mundy or Alexander J. Grutter. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW
INFORMATION _Nature Communications_ thanks Steve Harvey and the anonymous, reviewer(s) for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION PEER REVIEW FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Balakrishnan, P.P., Ferenc Segedin, D., Chow, L.E. _et al._ Extensive hydrogen incorporation is not necessary for superconductivity in topotactically reduced
nickelates. _Nat Commun_ 15, 7387 (2024). https://doi.org/10.1038/s41467-024-51479-3 Download citation * Received: 26 March 2024 * Accepted: 07 August 2024 * Published: 27 August 2024 * DOI:
https://doi.org/10.1038/s41467-024-51479-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative





![[withdrawn] mgn 215 new requirements for emergency escape breathing devices](https://www.gov.uk/assets/static/govuk-opengraph-image-03837e1cec82f217cf32514635a13c879b8c400ae3b1c207c5744411658c7635.png)
