- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Catalyst systems populated by high-density single atoms are crucial for improving catalytic activity and selectivity, which can potentially maximize the industrial prospects of
heterogeneous single-atom catalysts (SACs). However, achieving high-loading SACs with metal contents above 10 wt% remains challenging. Here we describe a general negative pressure annealing
strategy to fabricate ultrahigh-loading SACs with metal contents up to 27.3–44.8 wt% for 13 different metals on a typical carbon nitride matrix. Furthermore, our approach enables the
synthesis of high-entropy single-atom catalysts (HESACs) that exhibit the coexistence of multiple metal single atoms with high metal contents. In-situ aberration-corrected HAADF-STEM
(AC-STEM) combined with ex-situ X-ray absorption fine structure (XAFS) demonstrate that the negative pressure annealing treatment accelerates the removal of anionic ligand in metal
precursors and boosts the bonding of metal species with N defective sites, enabling the formation of dense N-coordinated metal sites. Increasing metal loading on a platinum (Pt) SAC to 41.8
wt% significantly enhances the activity of propane oxidation towards liquid products, including acetone, methanol, and acetic acid et al. This work presents a straightforward and universal
approach for achieving many low-cost and high-density SACs for efficient catalytic transformations. SIMILAR CONTENT BEING VIEWED BY OTHERS SCALABLE TWO-STEP ANNEALING METHOD FOR PREPARING
ULTRA-HIGH-DENSITY SINGLE-ATOM CATALYST LIBRARIES Article 25 November 2021 SYNTHESIS OF ULTRAHIGH-METAL-DENSITY SINGLE-ATOM CATALYSTS VIA METAL SULFIDE-MEDIATED ATOMIC TRAPPING Article 15
July 2024 A GENERAL STRATEGY FOR PREPARING PYRROLIC-N4 TYPE SINGLE-ATOM CATALYSTS _VIA_ PRE-LOCATED ISOLATED ATOMS Article Open access 23 November 2021 INTRODUCTION The development of
advanced catalysts must meet the requirements of future sustainable chemistry, but their commercial potential is contingent on high reaction efficiency and maximal atom economy1. Single atom
catalysts (SACs), integrating atomically dispersed metal centers with tunable coordination structures over appropriate supports, exhibit remarkable activity and unique selectivity in
electrocatalysis, photocatalysis, and thermal-catalysis2,3,4,5,6,7. Moreover, the maximal atom utilization efficiency of this class of catalysts greatly improves the atom economy, especially
for noble-metal-based catalysts. Therefore, it is beneficial for sustainable chemistry8,9,10,11,12. Given these merits of SACs, tremendous efforts have been devoted to developing a variety
of synthesis methods for many technical applications13,14,15,16. Nevertheless, considering their high surface energy, the SACs are generally constructed with low metal loadings to circumvent
the aggregation of metal atoms into metal clusters or nanoparticles17. This results in a low metal areal density. Taking the Pt SACs as a typical example, they display impressive activity
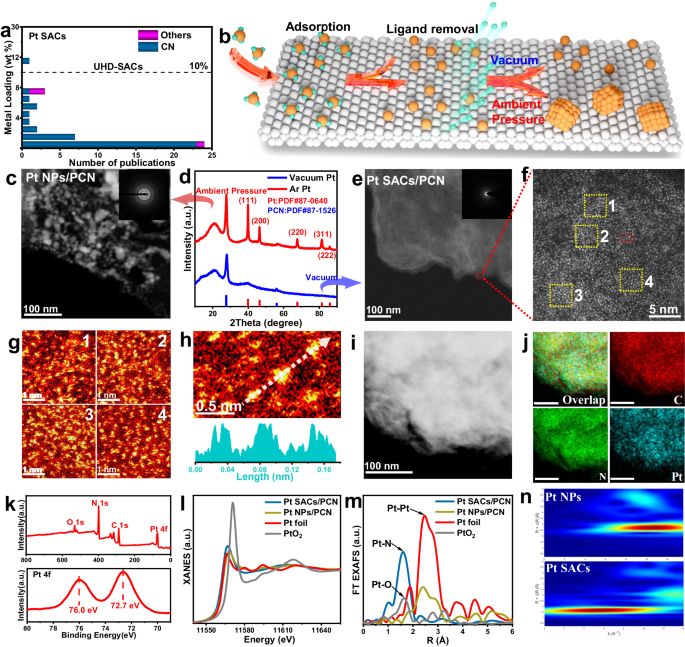
and selectivity in the thermal-driven activation of light alkane18,19,20. A literature analysis shows that the metal contents of most Pt SACs are 2 wt% or below (Fig. 1a and Supplementary
Table 1)21,22,23,24, and the Pt areal density of most catalysts are hard to surpass 1.5 atoms/nm2 (Supplementary Table 1). In this case, the SACs with insufficient areal density of active
sites not only limit their overall catalytic performance but also decrease the productivity per unit volume or mass of catalysts. Therefore, the development of a universal synthesis strategy
for accessing SACs with high metal loading and sufficient areal density is significant in this field, but challenging25,26,27,28. Recently, several strategies have been reported to
construct ultra-high-loading (UHL, higher than 10 wt%) SACs on different supports29. Wang et al adopted crosslinking carbon quantum dots as supports to provide abundant anchoring sites to
favor the formation of high densities of single metal atoms30. Lu reported a two-step annealing strategy to obtain high-loading SACs on distinct carbon and metal oxide supports31. This
strategy effectively controls the bonding of metal precursors with the carrier and prevents thermal-induced aggregation of metal into nanoparticles. Zou developed a laser planting method to
simultaneously create defects and anchor metal atoms, eventually achieving high-loading single metal atoms on carbon, TiO2, and Cu NPs32. In contrast with these pioneering works, a facile
and routine available synthetic strategy without the using of expensive equipments is highly attractive for the practical preparation and application of high-loading SACs. Herein, we report
a general negative pressure annealing approach to construct UHL-SACs libraries, consisting of 13 different transition metal SACs supported on polymeric carbon nitride (PCN) with ultra-high
metal loading. Based on the XAFS and in-situ aberration-corrected HAADF-STEM (AC HAADF-STEM), the negative pressure and thermal treatment enable fast dispersion of metal atoms over the
support rather than aggregation, which are furtherly trapped by nitrogen sites (Fig. 1b). As a result, 13 different SACs are successfully prepared on PCN with ultra-high metal loadings of
27.3–44.8 wt%. Moreover, SACs and high-entropy single atoms (HESAs) composed of multiple metal sites with high metal loadings can be also readily obtained on N-doped carbon (NC). These
evidences strongly validate the universality and scalability of the negative pressure annealing strategy for constructing a wide range of low-cost and high-areal-density SACs. RESULTS AND
DISCUSSION SYNTHESIS AND STRUCTURE INVESTIGATION OF PT UHL-SACS Given the wide applications of Pt-based catalysts33, UHL-SACs of Pt are initially investigated. The PCN is used as the
substrate firstly, which exhibits characteristic diffraction peaks at 2θ of 27.6 and 60.0° (Supplementary Fig. 1). The C 1 _s_ and N 1 _s_ XPS spectra provide additional confirmation of the
formation of C-N bonding (Supplementary Fig. 2) with a N/C atomic ratio of 1.05, establishing ample coordinate nodes for UHL-SAC fabrication. The platinum-based UHL-SACs, denoted as Pt
SACs/PCN, were synthesized by impregnating chloroplatinic acid onto PCN and subsequent annealing in a vacuum. To elucidate the crucial role of the negative pressure environment, a reference
sample was prepared by annealing in Ar flow at 101 KPa (Pt NPs/PCN). The annealing pressure shows negligible impact on the apparent morphology and chemical constitution (Supplementary Figs.
3–5) of Pt-based catalysts compared with the PCN substrate. Moreover, the color of these two samples turns black from yellow following Pt deposition (Supplementary Fig. 6). Based on ICP
analysis, the Pt contents are measured as 41.8 wt% and 40.9 wt% for Pt SACs/PCN and Pt NPs/PCN, respectively (Supplementary Table 2). The TEM image of the Pt NPs/PCN (Fig. 1c) illustrates an
accumulation of the metal particles with sizes around 10–50 nm. XRD pattern of Pt NPs/PCN (Fig. 1d) showcases the distinctive diffraction peaks characteristic of crystalline Pt, revealing
the formation of Pt particles during annealing under ambient pressure. On the contrary, an absence of discernible diffraction peaks related to crystalline Pt was observed for Pt SACs/PCN.
Additionally, the TEM image of Pt SACs/PCN (Fig. 1e and Supplementary Fig. 7) reveals no observable Pt particles, underscoring the effective prevention of metal aggregation under vacuum
annealing conditions. To delve into the local structure of Pt sites in Pt SACs/PCN, an AC HAADF-STEM measurement was employed. Figure 1f, g reveal dense bright spots assigned to isolated Pt
atoms are uniformly distributed over PCN, affirming the atomic dispersion of Pt sites on PCN randomly (Fig. 1h). Moreover, the average areal density of isolated Pt is estimated to 6.5
atoms/nm2 based on the measured BET surface area of the PCN (Supplementary Figs. 8, 9), similar with the pixel statistics of Fig. 1g (5.6 atoms/nm2). These results identify the Pt SACs/PCN
as one of the catalysts with the highest density of isolated Pt sites. Furthermore, EDS element mapping (Fig. 1i–j) affirm the uniform distribution of Pt, reinforcing the accuracy of these
statistical findings. Figure 1k presents the XPS results of Pt SACs/PCN, which reaffirms the ultra-high Pt loading (survey) with a positive oxidation state evidenced by the Pt 4_f_7/2
binding energy of 72.7 eV. The XANES spectrum of Pt SACs/PCN, positioning the white line intensity between Pt foil and PtO2, further verifies the partially positive oxidation state of Pt
(Fig. 1l). The FT EXAFS spectra (Fig. 1m) are exploited to elucidate the coordination environment of Pt sites. The Pt SACs/PCN exhibits a dominant peak assigned to Pt-N coordination at 1.6
Å, with the absence of Pt-Pt coordination at 2.5 Å. This result aligns seamlessly with wavelet transformation results (Fig. 1n), identifying the N-coordinated single-atom Pt sites in Pt
SACs/PCN. These findings serve as conclusive evidence for successfully fabricating ultra-high loading single-atom catalysts via the negative pressure annealing approach. To shed light on the
formation of Pt SACs/PCN, the structure evolution of Pt species during the annealing process was investigated by temperature-dependent in-situ AC HAADF-STEM, ex-situ XAFS, and XPS, in
vacuum and Ar condition respectively. The temperature-dependent in-situ AC HAADF-STEM images in vacuum conditions are shown in Fig. 2a and Supplementary Fig. 10. Dense bright spots are
observed at 20 °C, revealing the uniform distribution of Pt precursor on the substrate. Moreover, no clusters and particles are generated along the temperature increasing from 20 to 400 °C,
even after the sample is kept at 400 °C for 359 s. On the contrary, when annealing in the Ar flow, the atomic Pt can only be stabled below 300 °C (Fig. 2b). Observable Pt particles are
generated when the temperature reaches to 300 °C. These particles grow bigger when the temperature is further increased to 400 °C. The corresponding EDS element measurements at different
temperatures show a faster increase of Pt/Cl atomic ratio in vacuum than in Ar (Supplementary Table 3), demonstrating the accelerated Cl removal from Pt precursor in negative pressure
conditions. The coordination changes are investigated by XAFS. Figure 2c shows the Pt _L_-edge FT EXAFS spectra in vacuum conditions at different temperatures. Pt-Cl coordination is detected
at 200 °C, which transfers to Pt-N coordination at 300 °C. Moreover, no Pt-Pt coordination is detected even at 400 °C, excluding the formation of Pt-Pt bonding. For samples annealed under
Ar environment (Fig. 2d), the dominant Pt-Cl coordination at 200 °C is significantly decreased at 300 °C, companying with the formation of Pt-Pt coordination, which further takes the
predominance at 400 °C. The FT EXAFS results are in good agreement with the in-situ AC HAADF-STEM and EDS results, demonstrating the different transformation pathways of Pt precursor in
vacuum and Ar environment. The oxidation states of Pt under different annealing pressures show down-hill tendencies along the increasing temperature (Fig. 2e, f), which may due to the loss
of Pt-Cl bonding and the formation of Pt-N or Pt-Pt coordination. However, for the sample annealed in Ar, the Pt oxidation state is lower than that annealed in vacuum (Fig. 2g). This can be
attributed to the weaker electronegativity of Pt than N. These results illustrate the evolution of Pt UHL-SACs, that is Pt-Cl coordination rapidly dissociates at relatively low temperatures
to generate active Pt species, and the vacuum condition greatly suppresses the metal aggregation via promoting the Pt-N coordination at relatively high temperatures, thus enables the
formation of high-density Pt SACs. UNIVERSALLY PREPARING METAL UHL-SACS To reveal the generality of the negative pressure annealing approach, this synthetic process is extended to 12 other
single-atom metal sites on PCN (M SACs/PCN, M = V, Cr, Mn, Fe, Co, Ni, Cu, Zn, Nb, Mo, Ir and Au, Fig. 3a). The metal loadings of all these catalysts are measured between 27.3 wt% to 44.8
wt%, and the metal areal density are confirmed at a high level (Fig. 3b, Supplementary Table 2, and Supplementary Fig. 9). The white-line intensities from the XANES spectra indicate the
positive oxidation state of the metal sites in M SACs/PCN (Supplementary Fig. 11). The AC HAADF-STEM (Fig. 3c) and FT-EXAFS spectra (Fig. 3d) identify the N-coordinated single-atom metal
sites, and the absence of the metal-metal coordination excludes the formation of the metal clusters. The characterizations of XRD, TEM, EDS mapping, and XPS are shown in Supplementary Figs.
12–24, which further indicate the uniform distribution of the positive-charged isolated metal sites on the PCN substrate, and no aggregation of the metal is detected. The adaptability of the
negative pressure annealing approach on different substrates is also investigated. The NC obtained via the pyrolysis of guanine is used instead of PCN to prepare UHL-SACs (M SACs/NC, M=Pt,
Fe, Co, Ni, and Cu). The XRD of the NC substrate reveals the structure of graphitic carbon (Supplementary Fig. 25). The XPS results confirm the doping of N on carbon (Supplementary Fig. 26).
The characterizations, including AC HAADF-STEM, FT-EXAFS, EDS element mapping, XANES, XRD, and XPS (Fig. 4a–e and Supplementary Figs. 27–34), identify the N-coordinated single-atom metal
sites with positive oxidation states in these M SACs/NC. The mental contents are measured as high as 34.1 wt% (Pt), 21.5 wt% (Fe), 19.6 wt% (Co), 17.7 wt% (Ni), and 29.8 wt% (Cu)
(Supplementary Table 2), demonstrating the obtain of UHL-SACs with high metal areal density on NC substrate (Supplementary Figs. 35, 36). Moreover, high-entropy single atoms (HESACs)
containing Pt, Fe, Co, Ni, and Cu are also prepared on the NC. As shown in Fig. 4f, g and Supplementary Fig. 37, all five metals distribute uniformly on the NC as N-coordinated isolate metal
sites. The metal contents are 15.6, 3.1, 4.1, 2.3, and 7.3 wt% for Pt, Fe, Co, Ni, and Cu, respectively, resulting in an overall metal content of 32.4 wt% (Fig. 4h, Supplementary Table 2).
The positive oxidation states of the metal sites are confirmed (Supplementary Figs. 38, 39). Furthermore, no condensed matter of any metal is detected (Fig. 4i and Supplementary Figs. 40,
41). Although a limited number of metals were tested on NC, we speculate that the formation of SACs on NC follows the same evolution pathway as that on PCN, demonstrating the versatility of
this synthetic method on different N-containing carbon substrates for preparing SACs with multiple metals. Together, this work provides a universal synthetic strategy to fabricate various
UHL-SACs and even HESACs. CATALYTIC EVALUATION OF PT SACS/PCN The partial oxidation of propane to valuable liquid oxygenates represents a novel strategy to utilize this class of light
alkane34. Among the several current strategies, such as electrocatalysis35, photocatalysis36,37, thermal-derived homogeneous38, and thermal-derived heterogeneous catalysis, the exploitation
of heterogeneous catalyst shows the greatest application potential39,40,41. However, the consumption of costly oxidants poses an obstacle to it. To address this issue, a catalytic process
that can transfer propane to oxygenates with low-cost oxidants is urgently needed. Inspired by the molecular oxygen activation capacity of isolated Pt sites2, Pt SACs/PCN is evaluated in the
oxidation of propane with oxygen in this work. The reaction is performed at 175 °C in a 240 mL autoclave, with propane (5 bar) as reactant, oxygen (6 bar) as oxidant, and acetonitrile (70
ml) as solvent (Fig. 5a). The decreased pressure and the gas chromatography (GC) results indicate the consumption of propane, and the gas production is identified as CO (Supplementary Fig.
42). Interestingly, liquid productions are detected in acetonitrile, which is dominated by oxygenates (acetone, acetic, and methanol, et al_._), revealing the capacity of Pt SACs/PCN in
transforming propane into valuable liquid productions (Fig. 5b, c and Supplementary Fig. 43). These liquid productions are further quantitatively analyzed via the external standard method
(Supplementary Figs. 44, 45). As shown in Fig. 5d, the liquid product is confirmed as 37.1 mmol/gcat at 3 h, which increases with the reaction time, and reaches 71.9 and 107.6 mmol/gcat at 6
and 9 h, surpassing the low-loading Pt SACs/PCN, Pt nanoparticles (Pt NPs/PCN) and commercial Pt/C catalyst (Fig. 5e). To reveal the intrinsic activity of Pt SACs/PCN, the turnover
frequency (TOF) and mass-specific activity are confirmed as 1.6 × 10−3 molpro·molPt−1 · s−1 and 12.0 mmol/gcat/h (Fig. 5f, g and Supplementary Table 4), well-placed among select prior
reports of propane activation performance with oxygen. Interestingly, among the catalysts working with oxygen, only Pt SACs/PCN selects the pathway toward oxygenates (Fig. 5g). This may be
the first observation of heterogeneously catalytic oxidation of propane to oxygenates with oxygen, which provides a strategy to utilize propane for harvesting valuable liquid productions.
Moreover, the catalytic performance of Pt SACs/PCN shows insignificant decay after be reused five times (Fig. 5h), and the used catalyst maintains the dense isolated Pt sites (Supplementary
Fig. 46), confirming its stability. To clarify the effect of the substrates, Pt SACs on NC were also evaluated in the propane oxidation (Fig. 5e). Following a similar trend with Pt SACs/PCN,
Pt SACs/NC with higher Pt loading shows better activity than those with lower Pt loading and Pt particles, and the productions are dominated by oxygenates. These catalyst evaluations
demonstrate the potential application of high-loading Pt SACs in activating light alkanes. In conclusion, we report a general negative pressure annealing strategy to fabricate
ultrahigh-loading single-atom catalysts across a broad range of transition metals. Besides monometallic SACs, high-entropy single-atom catalysts that contain multiple metal single atoms with
high metal contents can also be obtained, proving the general applicability of the pressure annealing method. In-situ microscopic studies combined with ex-situ XAFS reveal the pivotal role
of the vacuum annealing condition in suppressing the aggregation of metal species, enabling the formation of dense N-coordinated Pt sites. Furthermore, UHL Pt SACs/PCN exhibits superior
catalytic performance in the oxidation of propane towards valuable liquid production. These findings provide valuable guidance for preparing a wide range of high-density SACs and show great
potential use in efficient catalytic transformations. METHODS Materials. Guanine, melamine, vanadyl sulfate, chromic nitrate, manganous nitrate, ferric nitrate, cobaltous nitrate, nickel
nitrate, cupric nitrate, zinc nitrate, niobium oxalate were purchased from Sinopharm Chemical Reagent Co. Molybdenum pentachloride, chloro-iridic acid, chloroplatinic acid, tetrachloro-auric
acid were purchased from Aladdin. All solutions were prepared using deionized water. All the chemicals were used without further purification. Synthesis of PCN. The mixture of melamine and
dicyandiamide (molar ratio = 7:3) was pyrolyzed in a tube furnace at 600 °C for 1.5 h (Ar flow, 100 sccm). The obtained powder (3 g) was treated in 65 wt% HNO3 (50 ml) at 80 °C for 6 h,
followed by an ultrasonic treatment for 1 h. The suspension was centrifuged and washed with deionized water to gain yellow powder, which was labeled as PCN. Synthesis of NC. Guanine was
pyrolyzed in a tube furnace at 600 °C for 1.5 h (Ar flow, 100 sccm). The obtained black powder was labeled as N-doped carbon (NC). Synthesis of Pt SACs/PCN, Pt NPs/PCN, and Pt SACs/NC. Pt
SACs/PCN were prepared via the impregnation-vacuum pyrolysis method. 20 ml chloroplatinic acid solution (0.075 mol/L) was added in 1 g PCN. The obtained suspension was ultrasonic treated for
15 min, aged at room temperature for 2 h, and dried at 80 °C for 8 h. The obtained powder was placed in a tube furnace. The tube furnace was firstly purged by 100 sccm Ar flow for 15 min.
Then, the outlet was connected to an operating mechanical pump (limiting pressure 6 × 10−2 Pa), and the inlet was closed. After 1 h of pyrolysis under vacuum conditions at 400 °C, the black
powder Pt SACs/PCN was obtained. Pt/NPs/PCN was prepared via the same procedure, except for the pyrolysis process, which was carried out in Ar flow (atmospheric pressure). Pt SACs/NC was
prepared by the impregnation-vacuum pyrolysis method, using 0.035 mol/L chloroplatinic acid solution and NC substrate. Synthesis of M SACs/PCN and M SACs/NC. M SACs/PCN (M = V, Cr, Mn, Fe,
Co, Ni, Cu, Zn, Nb, Mo, Ir and Au) was synthesized via the same method with Pt SACs/PCN, using vanadyl sulfate, chromic nitrate, manganous nitrate, ferric nitrate, cobaltous nitrate, nickel
nitrate, cupric nitrate, zinc nitrate, niobium oxalate, molybdenum pentachloride, chloro-iridic acid, and tetrachloro-auric acid as the metal precursor, respectively. The concentration of
metal precursor solution was 0.17 mol/L for V, Cr, Mn, 0.2 mol/L for Fe, Co, Ni, Cu, and Zn, 0.12 mol/L for Nb and Mo, 0.075 mol/L for Ir, and 0.045 mol/L for Au. The vacuum pyrolysis
temperature for V, Cr, Mn, Fe, Co, Ni, Cu, and Zn SACs/PCN was 500 °C. M SACs/NC (M = Fe, Co, Ni, Cu) were prepared according to the method of M SACs/PCN. The concentration of the metal
precursor solution was 0.07–0.1 mol/L. Synthesis of HESACs. HESACs were prepared according to the method of M SACs/NC. The metal precursor solution was a mixture of ferric nitrate, cobaltous
nitrate, nickel nitrate, cupric nitrate, and chloroplatinic acid. Their concentration in the solution was 0.015, 0.015, 0.01, 0.03, and 0.02 mol/L, respectively. Characterization. The
transmission electron microscopy (TEM) images and the corresponding element mapping were recorded on a JEOL-2100F FETEM. The electron acceleration energy was 200 kV. The morphologies of the
samples were measured on Thermo-Fisher Apreo S scanning electron microscope. HAADF-STEM images were recorded on the JEOL JEMARM200F TEM/STEM system. The in-situ heating aberration-corrected
high-angle annular dark field scanning transmission electron microscope (Cs-HAADF-STEM) experiments were conducted on a double-spherical aberration corrected FEI Titan Themis Z scanning
transmission electron microscope with an accelerating voltage of 300 kV combined with the in-situ heating holder provided by FEI company. The prepared precursor was dispersed onto the
in-situ heating chip purchased from FEI company. Before STEM imaging, beam shower was carried out for 15 min. A probe size of 9 was used to image. The heating rate was set as 10 oC/min. To
exclude the influence of e-beam irradiation, the images of control areas were collected only at each temperature point. The crystallographic phase of the as-synthesized materials was
characterized by X-ray powder diffractometer (XRD, Bruker D8 Advance, λ = 1.5418 Å). Raman spectroscopy (WITec alpha300R, 532 nm laser) was used to study the structure and disorder of the
catalysts. X-ray photoelectron spectroscopy (XPS) measurements were performed on a PHI 5000 VersaProbe III X-ray photoelectron spectrometer using an Al Kα X-ray source. The power is 12.5 W,
the bandpass energy of the full spectrum test is 280 eV, and the bandpass energy of the fine spectrum test is 112 eV. The spectra are calibrated by C1s (284.8 eV), and the spectral analysis
is performed by Avantage software. Inductively coupled plasma atomic emission spectroscopy (ICP-AES) was employed with PerKinElmer Optima 2100DV to detect the loading content of metal on
catalysts. X-ray Absorption Fine Structure Spectroscopy (XAFS) were collected at beamline 1W1B of Beijing Synchrotron radiation Facility and beamline BL14W1 of Shanghai Synchrotron Radiation
Facility. The acquired XAFS data were analyzed by Athena and Artemis software modules in IFEFFIT software package. Catalytic performance test. The catalyzed oxidation of propane with
molecular oxygen was carried out in a 240 mL autoclave reactor. In a typical run, 70 ml acetonitrile solvent and 100 mg catalyst were added in the reactor. And, 5 bar propane and 6 bar
oxygen was then added in the reactor According to the ideal gas equation, the added propane was about 35.4 mmol. The reaction was carried out at 175 °C. After the reaction, the reactor was
cooled to room temperature. The liquid phase was flited and analyzed by a gas chromatograph-mass spectrometer (Agilent 8890-5977B). The qualitative analysis was performed on a HP-5 MS
capillary column (inner diameter 0.25 μm, length 30 m), and the quantitative analysis was performed with FID detector (as shown in Supplementary Figs. 43, 44), using HP-5 capillary column
(0.25 μm, 30 m) and calculated by the external standard method (Supplementary Fig. 45). The gas phase was collected and analyzed by a Panna A60 gas chromatography. The yield of liquid
oxy-compounds after 9 h reaction over Pt SACs/PCN is calculated by the moles of carbon in liquid oxy-compounds (23.19 mmol carbon) and the carbon inlet (35.4 mmol propane, 106.2 mmol
carbon). DATA AVAILABILITY The data that support the findings of this study are available within the article and its Supplementary Information files. All other relevant data supporting the
findings of this study are available from the corresponding authors upon request. REFERENCES * Marion, P. et al. Sustainable chemistry: how to produce better and more from less? _Green.
Chem._ 19, 4973–4989 (2017). Article ADS CAS Google Scholar * Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. _Nat. Chem._ 3, 634–641 (2011). Article CAS PubMed
Google Scholar * Fang, X. et al. Single Pt atoms confined into a metal–organic framework for efficient photocatalysis. _Adv. Mater._ 30, 1705112 (2018). Article Google Scholar * Moliner,
M. et al. Reversible transformation of Pt nanoparticles into single atoms inside high-silica chabazite zeolite. _J. Am. Chem. Soc._ 138, 15743–15750 (2016). Article CAS PubMed Google
Scholar * Huang, Z. et al. A highly efficient pH-Universal HOR catalyst with engineered electronic structures of single Pt sites by isolated Co atoms. _Adv. Funct. Mater._ 33, 2306333
(2023). Article CAS Google Scholar * Yang, Q., Jiang, Y., Zhuo, H., Mitchell, E. M. & Yu, Q. Recent progress of metal single-atom catalysts for energy applications. _Nano Energy_ 11,
108404 (2023). Article Google Scholar * Rong, P. et al. Photocatalytic degradation of methylene Blue (MB) with Cu1-ZnO single atom catalysts on graphene-coated flexible substrates. _J.
Mater. Chem. A_ 10, 6231–6241 (2022). Article CAS Google Scholar * Shan, J. et al. Metal-metal interactions in correlated single-atom catalysts. _Sci. Adv._ 8, eabo0762 (2022). Article
CAS PubMed PubMed Central Google Scholar * Singh, B. et al. Single-atom catalysts: a sustainable pathway for the advanced catalytic applications. _Small_ 17, 2006473 (2021). Article CAS
Google Scholar * Zhang, Z. et al. Single-atom catalyst for high-performance methanol oxidation. _Nat. Commun._ 12, 5235 (2021). Article ADS CAS PubMed PubMed Central Google Scholar
* Hai, X. et al. Geminal-atom catalysis for cross-coupling. _Nature_ 622, 754–760 (2023). Article ADS CAS PubMed Google Scholar * Yu, Q. Theoretical studies of non-noble metal
single-atom catalyst Ni1/MoS2: electronic structure and electrocatalytic CO2 reduction. _Sci. China Matter_ 66, 1079–1088 (2023). Article CAS Google Scholar * Lang, R. et al.
Non-defect-stabilized thermally supplementary table single-atom catalyst. _Nat. Commun._ 10, 234 (2019). Article ADS PubMed PubMed Central Google Scholar * Zhang, Y. et al. Single-atom
Cu anchored catalysts for photocatalytic renewable H2 production with a quantum efficiency of 56%. _Nat. Commun._ 13, 58 (2022). * Li, B. et al. Heck migratory insertion catalyzed by a
single Pt atom site. _J. Am. Chem. Soc._ 145, 24126–24135 (2023). Article CAS PubMed Google Scholar * Li, H. et al. Synergetic interaction between neighbouring platinum monomers in CO2
hydrogenation. _Nat. Nanotech._ 13, 411–417 (2018). Article ADS CAS Google Scholar * Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. _Acc. Chem.
Res._ 46, 1740–1748 (2013). Article CAS PubMed Google Scholar * Serna, P. et al. Single-site vs. cluster catalysis in high temperature oxidations. _Angew. Chem. Int. Ed._ 60, 15954–15962
(2021). Article CAS Google Scholar * Marcinkowski, M. D. et al. Pt/Cu single-atom alloys as coke-resistant catalysts for efficient C-H activation. _Nat. Chem._ 10, 325–332 (2018).
Article CAS PubMed Google Scholar * Nakaya, Y., Hirayama, J., Yamazoe, S., Shimizu, K. & Furukawa, S. Single-atom Pt in intermetallics as an ultrastable and selective catalyst for
propane dehydrogenation. _Nat. Commun._ 11, 2838 (2020). Article ADS CAS PubMed PubMed Central Google Scholar * Tsounis, C. et al. Pt single atom electrocatalysts at graphene edges for
efficient alkaline hydrogen evolution. _Adv. Funct. Mater._ 32, 2203067 (2022). Article CAS Google Scholar * Tan, W. et al. Fine-tuned local coordination environment of Pt single atoms
on ceria controls catalytic reactivity. _Nat. Commun._ 13, 7070 (2022). Article ADS CAS PubMed PubMed Central Google Scholar * Kuang, P. et al. Pt single atoms supported on N-Doped
mesoporous hollow carbon spheres with enhanced electrocatalytic H2-evolution activity. _Adv. Mater._ 33, 2008599 (2021). Article CAS Google Scholar * Han, B. et al. Strong metal–support
interactions between Pt single atoms and TiO2. _Angew. Chem. Int. Ed._ 59, 11824–11829 (2020). Article CAS Google Scholar * Xiong, Y. et al. Gram-scale synthesis of high-loading
single-atomic-site Fe catalysts for effective epoxidation of styrene. _Adv. Mater._ 32, 2000896 (2020). Article CAS Google Scholar * Jiang, K. et al. Rapid melt-quenching enables general
synthesis of high-loading single-atom catalysts with bicontinuous nanoporous structure. _Adv. Mater._ 35, 2207850 (2023). Article CAS Google Scholar * Han, L. et al. A single-atom library
for guided monometallic and concentration-complex multimetallic designs. _Nat. Mater._ 21, 681–688 (2022). Article ADS CAS PubMed Google Scholar * Yang, H. et al. A Universal ligand
mediated method for large scale synthesis of transition metal single atom catalysts. _Nat. Commun._ 10, 4585 (2019). Article ADS PubMed PubMed Central Google Scholar * Wu, J., Xiong,
L., Zhao, B., Liu, M. & Huang, L. Densely populated single atom catalysts. _Small Methods_ 4, 1900540 (2020). Article CAS Google Scholar * Xia, C. et al. General synthesis of
single-atom catalysts with high metal loading using graphene quantum dots. _Nat. Chem._ 13, 887–894 (2021). Article CAS PubMed Google Scholar * Hai, X. et al. Scalable two-step annealing
method for preparing ultra-high-density single-atom catalyst libraries. _Nat. Nanotech._ 17, 174–181 (2022). Article ADS CAS Google Scholar * Wang, B. et al. Room-temperature laser
planting of high-loading single-atom catalysts for high-efficiency electrocatalytic hydrogen evolution. _J. Am. Chem. Soc._ 145, 13788–13795 (2023). Article CAS PubMed Google Scholar *
Yu, W., Porosoff, M. D. & Chen, J. G. Review of Pt-based bimetallic catalysis: from model surfaces to supported catalysts. _Chem. Rev._ 112, 5780–5817 (2012). Article CAS PubMed
Google Scholar * Zhang, H. et al. Activation of light alkanes at room temperature and ambient pressure. _Nat. Catal._ 6, 666–675 (2023). Article CAS Google Scholar * Zhang, H., Li, C.,
Lu, Q., Cheng, M.-J. & Goddard, W. A. III Selective activation of propane using intermediates generated during water oxidation. _J. Am. Chem. Soc._ 143, 3967–3974 (2021). Article CAS
PubMed Google Scholar * Ichikuni, N., Nakao, Y., Ishizuki, K., Hara, T. & Shimazu, S. Effect of local structure of Mo oxide on selective photo-oxidation of propane to acetone. _Catal.
Lett._ 143, 154–158 (2013). Article CAS Google Scholar * Sun, H., Blatter, F. & Frei, H. Oxidation of propane to acetone and of ethane to acetaldehyde by O2 in zeolites with complete
selectivity. _Catal. Lett._ 44, 247–253 (1997). Article CAS Google Scholar * Hashiguchi, B. G. et al. Main-group compounds selectively oxidize mixtures of methane, ethane, and propane to
alcohol esters. _Science_ 343, 1232–1237 (2014). Article ADS CAS PubMed Google Scholar * Liu, C.-C. et al. Catalytic oxidation of light alkanes mediated at room temperature by a
tricopper cluster complex immobilized in mesoporous silica nanoparticles. _ACS Sustain. Chem. Eng._ 6, 5431–5440 (2018). Article CAS Google Scholar * Raja, R., Jacob, C. R. &
Ratnasamy, P. Direct oxidation of propane to isopropanol. _Catal. Today_ 49, 171–175 (1999). Article CAS Google Scholar * Sakakura, S. et al. Oxygenase mimicking immobilised iron complex
catalysts for alkane hydroxylation with H2O2. _Catal. Sci. Technol._ 13, 4839–4846 (2023). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors thank the
photoemission endstation beamline 1W1B station in the Beijing Synchrotron Radiation Facility (BSRF) and beamline BL13SSW, BL14W1 in Shanghai Synchrotron Radiation Facility (SSRF) for help
with the XAFS characterizations. This work is financially supported by the National Natural Science Foundation of China (22275147 to Y.Q., 52301289 to K.L., 21902150 to Y.Q., 52122212,
12274391 and 22321001 to Y. L.), Natural Science Basic Research Program of Shaanxi (2022JM-018 to Y.W. and 2022JQ-082 to K.L.), the Youth Innovation Promotion Association of CAS (2020458 to
Y. L.), the Key Research Program of Frontier Sciences, CAS (ZDBS-LY-SLH003 to Y. L.). AUTHOR INFORMATION Author notes * These authors contributed equally: Yi Wang, Chongao Li, Xiao Han.
AUTHORS AND AFFILIATIONS * International Collaborative Center on Photoelectric Technology and Nano Functional Materials, Institute of Photonics and Photon-Technology, Northwest University,
Xi’an, Shaanxi, 710069, China Yi Wang, Chongao Li, Jintao Bai, Kunyue Leng & Yunteng Qu * Department of Chemistry, Department of Applied Chemistry, Hefei National Research Center for
Physical Sciences at the Microscale, University of Science and Technology of China, Hefei, Anhui, 230026, China Xiao Han & Yue Lin * Interdisciplinary Research Center of Biology &
Catalysis, School of Life Sciences, Northwestern Polytechnical University, Xi’an, 710000, China Xuejing Wang * Institute of High Energy Physics, Beijing, 100039, China Lirong Zheng *
Shanghai Advanced Research Institute, Chinese Academy of Science, Shanghai, 201210, China Chunxia Hong * National Key Laboratory of Continental Shale Oil, College of Chemistry and Chemical
Engineering, Northeast Petroleum University, Daqing, 163318, China Zhijun Li * Université Paris-Saclay, CentraleSupélec, ENS Paris-Saclay, CNRS, LMPS-Laboratoire de Mécanique Paris-Saclay,
8-10 rue Joliot-Curie, Gif-sur-Yvette, 91190, France Jinbo Bai Authors * Yi Wang View author publications You can also search for this author inPubMed Google Scholar * Chongao Li View author
publications You can also search for this author inPubMed Google Scholar * Xiao Han View author publications You can also search for this author inPubMed Google Scholar * Jintao Bai View
author publications You can also search for this author inPubMed Google Scholar * Xuejing Wang View author publications You can also search for this author inPubMed Google Scholar * Lirong
Zheng View author publications You can also search for this author inPubMed Google Scholar * Chunxia Hong View author publications You can also search for this author inPubMed Google Scholar
* Zhijun Li View author publications You can also search for this author inPubMed Google Scholar * Jinbo Bai View author publications You can also search for this author inPubMed Google
Scholar * Kunyue Leng View author publications You can also search for this author inPubMed Google Scholar * Yue Lin View author publications You can also search for this author inPubMed
Google Scholar * Yunteng Qu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.W., C.L., and X.H. contribute to this work equally. Y.W.
designed the basic principle and arranged the experiment. C.L. experimented. Y.Q. and K.L. worked together on the characterizations and article writing. Jinbo. B. funded this work. L.Z. and
C.H. performed the XAFS measurement. X.H. and Y.L. performed the in-situ aberration-corrected HAADF-STEM. Jintao. B., Z.L., and X.W. revised the writing work. CORRESPONDING AUTHORS
Correspondence to Kunyue Leng, Yue Lin or Yunteng Qu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. PEER REVIEW PEER REVIEW INFORMATION _Nature
Communications_ thanks Qi Yu, and the other, anonymous, reviewer for their contribution to the peer review of this work. A peer review file is available. ADDITIONAL INFORMATION PUBLISHER’S
NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW
FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Wang, Y., Li, C., Han, X. _et al._ General negative pressure annealing approach for creating ultra-high-loading single atom catalyst libraries. _Nat Commun_ 15, 5675 (2024).
https://doi.org/10.1038/s41467-024-50061-1 Download citation * Received: 25 February 2024 * Accepted: 27 June 2024 * Published: 06 July 2024 * DOI: https://doi.org/10.1038/s41467-024-50061-1
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy
to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




