- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The degradation of synthetic polymers by marine microorganisms is not as well understood as the degradation of plastics in soil and compost. Here, we use metagenomics,
metatranscriptomics and metaproteomics to study the biodegradation of an aromatic-aliphatic copolyester blend by a marine microbial enrichment culture. The culture can use the plastic film
as the sole carbon source, reaching maximum conversion to CO2 and biomass in around 15 days. The consortium degrades the polymer synergistically, with different degradation steps being
performed by different community members. We identify six putative PETase-like enzymes and four putative MHETase-like enzymes, with the potential to degrade aliphatic-aromatic polymers and
their degradation products, respectively. Our results show that, although there are multiple genes and organisms with the potential to perform each degradation step, only a few are active
during biodegradation. SIMILAR CONTENT BEING VIEWED BY OTHERS ISOLATION OF MARINE BACTERIA WITH POTENTIAL FOR POLYHYDROXYALKANOATE DEGRADATION AND OPTIMIZATION FOR ENZYME PRODUCTION Article
Open access 04 May 2025 NOVEL BACTERIAL TAXA IN A MINIMAL LIGNOCELLULOLYTIC CONSORTIUM AND THEIR POTENTIAL FOR LIGNIN AND PLASTICS TRANSFORMATION Article Open access 26 September 2022
ENRICHMENT OF LDPE-DEGRADING BACTERIAL CONSORTIA: COMMUNITY SUCCESSION AND ENHANCED DEGRADATION EFFICIENCY THROUGH VARIOUS PRETREATMENT METHODS Article Open access 20 November 2024
INTRODUCTION Three fifty million tons of plastics were produced worldwide exclusively in 20171. Over 250,000 tons of them were released to and found floating in the oceans2,3. In 2015, it
was estimated that by 2025 the plastic waste in the ocean could increase by an order of magnitude4. Commercial production of biodegradable plastics started in the early 2000s5 as an
ecologically friendlier alternative to conventional plastics6,7,8. The manufacturing of these plastics are intended for specific applications, such as items with a shorter life time and
where recycling is not feasible9,10. Most biodegradable polymers contain ester bonds that are susceptible to enzymatic hydrolysis9,11,12. Poly(butylene adipate-co-terephthalate) (PBAT) is a
commercial aromatic-aliphatic copolyester composed of adipic acid (Ad), terephthalic acid (Te), and 1, 4-butanediol (B). It has favorable mechanical properties due to its aromatic
components, in combination with biodegradability granted by the more flexible aliphatic components13,14. The depolymerization of PBAT by soil and compost microorganisms is well-studied:
known enzymes with PBAT hydrolytic activity include cutinase-like serine hydrolases15,16,17, mostly originating from terrestrial _Actinomycetes_ and fungi. So far, only one PBAT-hydrolyzing
enzyme has been characterized from the aquatic environment14. The enzymatic hydrolysis of PBAT yields a mixture of the terephthalate-butanediol monoester (BTe), and the monomers16.
PBAT-degrading enzymes have different degrees of activity toward the BTe intermediate. The PBAT-degrading enzyme Ppest from _Pseudomonas alcaligenes_ cannot degrade BTe, which almost
completely inhibits the enzyme’s activity14. Similarly, the cutinase from _Humicula insolens_, HiC, has a much lower activity on BTe than the Thc_Cut1 from _Thermobifida cellulosilytica_,
which can degrade it efficiently to monomers16. Therefore, it was proposed that an esterase with a high specificity to the BTe could aid enzymatic PBAT hydrolysis by removing this inhibitory
degradation product14. This proposed degradation pathway is similar to the PET-degradation pathway by _Ideonella sakaiensis_18. The degradation is initiated by PETase, an _α_/_β_ hydrolase
that degrades the polymer into the monoester mono-2-hydroxyethyl terephthalate (MHET). This monoester is hydrolyzed by another _α_/_β_ hydrolase, MHETase, to form Te and ethylene glycol18.
_I. sakaiensis_ also carries a Te degradation (TPD) cluster, which converts Te into protocatechuate (PCA) in a two-step reaction18,19. After conversion into pyruvate and oxaloacetate by the
PCA degradation (PCD) cluster, the aromatic monomer is completely metabolized19. Due to the structural similarity of PBAT and PET, some PBAT-degrading enzymes can also degrade PET, albeit
more slowly17,20. It is so far unknown whether a two-step degradation pathway for PBAT with an intermediary monoester-cleaving enzyme exists. Regardless of whether their enzymes can
hydrolyze the polymer to its monomers or not, most PBAT-degrading microorganisms discovered to date cannot use the monomers as C source, and therefore are not able to degrade the polymer
into biomass and CO2. It has been stated that in case of a mature compost environment, other members of the microbial community can metabolize the released monomers21. It is possible that
such complex synthetic polymer blends would be mineralized by consortia rather than single microorganisms in the environment. Mechanisms of cooperative degradation however have not yet been
investigated. Due to the structural similarity of PBAT degradation intermediates to those of PET, we hypothesized that the complete PBAT degradation by a microbial consortium may follow a
similar pathway to PET degradation by _I. sakaiensis_. In this study, we investigate the mineralization of a commercial PBAT-based blend film (PF) by a marine enrichment culture. PF is
composed of PBAT blended with a copolyester of Te, B, and sebacic acid (Se) (instead of Ad), and also contains a small amount of polylactic acid. In this framework, we elucidate the
degradation pathway of this biodegradable plastic with an integrated metaomic approach. The abundance and distribution of individual microorganisms in the consortium, their potential roles
in the PF degradation process, and the relation to catabolic genes from other environments are investigated. Our study describes a marine microbial consortium that synergistically degrades a
complex biodegradable copolymer and proposes a mechanism for the biodegradation. RESULTS We enriched a marine microbial community in an artificial marine medium supplemented with PF as sole
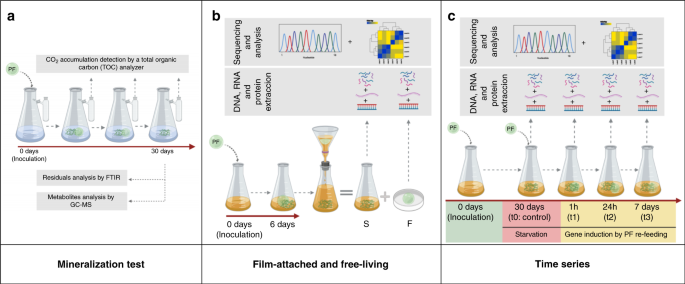
carbon source (named I1 culture). To elucidate which microorganisms and genes play a role in PF biodegradation, three independent experiments were carried out (Fig. 1): the first
experimental setup (a) aimed to detect biodegradation products and CO2 production due to microbial activity. In the second experiment (b) we aimed to analyze differences between the
film-attached (F) and free-living bacteria (S) through metaomics. Finally a time series experiment (c) was performed to identify putative genes and proteins needed for the biodegradation of
PF through metaomics. MINERALIZATION OF THE PF AND MONOMERS The mineralization tests were carried out for 30 days to ensure complete biodegradation (Fig. 1a). However, disintegration of PF
was already visually observed after six days. Within a month, three independent experiments showed that the communities converted around 60% (Fig. 2a (a1, a2, a3)) of the initial carbon
content supplied in the films into CO2. The highest degradation rate was registered between days 6 and 10 (11–16% mineralized/day), reaching a plateau after 15 days. Although most of the
film seemed to be disintegrated, some solid residues remained in these cultures. Release of PF oligomers and monomers, and the composition of solid residues remaining after complete
mineralization were further analyzed. Metabolites were extracted from the cells and cell-free culture supernatants after eight and twenty days of incubation with PF. After eight days of
incubation, the cell biomass contained low amounts of the monomers Ad, Se, and Te, as well as the monoester BTe. After twenty days, the intracellular amounts of Ad and Te were 95% reduced
and Se and BTe were depleted below detection limit (Table S1). Monomers and oligomers were undetectable in the extracellular environment (data not shown). In parallel, the solid residues
remaining after PF degradation were collected, analyzed by FTIR and contrasted to plastic samples incubated in the absence of the I1 culture (Fig. S1). It was observed that the main ester
peak of PF at 1710–1750 cm−1 disappeared completely in most of the biological replicates. Peaks corresponding to the solid CaCO3 mineral filler were detected in all samples, as well as those
that potentially belong to biofilm components (detailed peak analysis is given in Fig. S1). The plastic samples that were incubated in the culture medium for 30 days without the addition of
the I1 culture did not show any change in the FTIR spectra (Fig. S1a). Based on the FTIR spectra, we conclude that the remaining solid residues are CaCO3 mineral filler with attached
biofilm, and almost all of the polyester was degraded. Before PF disintegration, the biofilm-forming ability of the community was analyzed. Scanning electron microscopy (SEM) imaging
revealed that the PF surface was colonized by the I1 community already after three days (Fig. 2b). At this time, we observed pits distributed uniformly across the PF surface resulting from
microbial activity. After six days, larger holes and craters were formed. Microbial biofilm surrounded by exopolysaccharides resided in these holes. The main microbial morphotype were rod
shaped cells of about 2 μm. At this time, the film was very fragile and began to disintegrate. In addition to the mineralization of the film itself, the mineralization of each monomer was
measured separately. The maximum conversion to CO2 was 50%, 59%, 29%, and 40% for B, Te, Se, and Ad acid, respectively (Fig. S2). The CO2 production reached a plateau after 10 days.
TAXONOMIC AND FUNCTIONAL PROFILING OF THE I1 COMMUNITY In the following section, the results of the bioinformatic analyses of the experiments as illustrated in Fig. 1b and c are described.
For each experiment, DNA, RNA, and proteins were extracted from the same sample. Metagenomes were assembled, binned and taxonomically classified. The reads were mapped to the bins for
abundance estimation. Metagenome binning yielded 32 bins of >95% completeness and <10% contamination (Table S2). Taxonomic profiling revealed that the I1 culture is a diverse
community composed mainly of Alphaproteobacteria, Gammaproteobacteria and Flavobacteria, as well as Actinobacteria albeit in lower numbers. The abundance profiles remained stable throughout
the time series experiment (Fig. 3a). The six most abundant bins in this experiment were three of the family Rhodobacteraceae, i.e., bin 17 and 20 (_Pseudooceanicola_ spp.), bin 10
(unclassified Rhodobacteraceae bacterium), one _Marinobacter_ (bin 32), one _Aequorivita_ (bin 1) and one of the family Micavibrionaceae (bin 3). These bins comprised around 80% of the
binned population (Fig. S3). The microbial community composition of the biofilm on the plastic surface was contrasting to the free-living community composition (experimental setup
illustrated in Fig. 1b). Relative abundances of Alphaproteobacteria (bins 2, 13, 14, 17, 18, 23, 26, 27) strains and a few Bacteroidia (bin 1, 30), Actinobacteria (bin 6) and
Gammaproteobacteria (bin 12) strains increased in the free-living fraction compared to film-attached communities (Fig. 3a, Fig. S4). In contrast, the Gammaproteobacteria _Marinobacter_ sp.
(bin 32) and Marinicellaceae (bin 28), the Bacteroidium _Geldibacter_ sp. (bin 16), the Acidomicrobium _Ilumatobacter_ (bin 4), Phycisphaerae (bin 5), and an Alphaproteobacterium belonging
to Micavibrionaceae (bin 3) were enriched in the film-attached fraction. These observations were supported by correlating their abundance distribution. The abundances of the film-associated
taxa (bins 3, 5, 16, 28, 32) are significantly positively-correlated to each other. Similarly, the abundances of free-living taxa (1, 2, 9, 10, 12, 13, 14, 15, 17, 21, 23, 26, 30, 33) are
positively correlated with each other. Abundances of film-associated bins are negatively-correlated to the abundances of most free-living taxa (Fig. 3b). Overall, the four most abundant bins
made up 75–80% of the binned population in both film-attached and free-living fractions (Fig. S3). This scenario changed when the monomers were used as sole carbon sources instead of PF.
When B was given as sole carbon source, _Marinobacter_ sp. (bin 7) was the most abundant bacterium, comprising 80% of the binned community. Similarly, _Saccharospirillum_ sp. (bin 12)
dominated the cultures with Te, comprising 80% of the binned community (Fig. S5). Both bacteria were present in low amounts (≤2%) when the culture was growing with PF as carbon source. The
addition of Ad and Se triggered the growth of multiple bacteria, where bins 1, 13 and 32 were the most abundant, comprising 65% of the community in both cases. Interestingly, bins 10 and 17,
which were among the most abundant bacteria throughout the time series and the free-living community, were barely traceable when single monomers were given as carbon source (Fig. S3).
GENOMIC CONTEXT OF THE PUTATIVE PF DEPOLYMERASES The functional analysis of the metagenomes revealed a diverse gene repertoire which is potentially related to polymer degradation. Six
orthologues of the three known PETases (named as PETase-like enzymes (Ples)); _I. sakaiensis_ PETase (A0A0K8P6T7, GAP38373)18 (IsPETase), leaf compost cutinase (AEV21261.1)22 and
_Thermobifida fusca_ cutinase (ADV92528.1)23 were recovered from the two _Marinobacter_ bins 21 (Ple200 and Ple201) and 32 (Ple611, Ple453, Ple628 and Ple629) (Table 1). The Ples show a
45–50% amino acid (aa) identity to the known PETases except Ple453 (29–32%) (Table 1). All Ples carry the common lipase/esterase consensus motif G-X1-S-X2-G, the IsPETase catalytic triad
His237, Ser160, and Asp20624 (Fig. S6a) and were classified as _α_/_β_ hydrolases25. All proteins carry signal peptides for periplasmic localization via the Sec pathway. Similar to the
tandem cutinases _est1_ and _est119_ of _Thermobifida alba_ AHK11926, the genes encoding for Ple200 and Ple201, as well as Ple628 and Ple629 have a tandem structure. In both cases, the genes
were separated by 359 nucleotides (nt) without interjacent ORFs. The intergenic spaces of each tandem pair had different sequences. These both contained putative transcription termination
sites, indicating that the genes may be independently transcribed. Ple200 and Ple201 from bin 21 are very closely related to Ple628 and Ple629 from bin 32, with an aa identity of 90% between
Ple200 and Ple628 or Ple201 and Ple629, respectively. The aa identity between each tandem pair is 74–75%. The 71 kbp contig with the _ple_ genes in bin 21 has a similar sequence (85% nt
identity) and high synteny to the corresponding region in bin 32. Most Ples clustered phylogenetically together with related genes from two other _Marinobacter_ species, Ple453 being more
distantly related to these, as well as to the known PETases (Fig. S7a). GENOMIC CONTEXT OF THE PUTATIVE DOWNSTREAM DEGRADATION GENES In addition to the Ples, four genes encoding MHETase-like
enzymes (Mles) were recovered from the metagenome (Table 1). These have a 29–47% aa identity to the _I. sakaiensis_ MHETase (A0A0K8P8E7) and carry the MHETase catalytic triad27 (Fig. S6b).
Like Ples, Mles carry a signal Sec peptide. Three of them could be allocated to a certain host: Mle800, Mle267, and Mle288 (Table 1). Mle267 and Mle800 have a 60% aa identity to each other.
They are however distantly related to both Mle046 and Mle288 (Fig. S7b). The fourth Mle, Mle046, has the highest identity to the IsMHETase (Table 1). The _mle046_ gene is 100% identical to
the homolog present on the _Celeribacter manganoxidans_ plasmid pDY25-B (CP021406.1). In the I1 metagenome, _mle046_ is located on an unbinned 107 kbp contig (contig 279). This contig also
carries a conjugation-related operon, and thus possibly belongs to a conjugative plasmid. This operon is closely related (>90%) to those from several conjugative plasmids from marine
Alphaproteobacteria, including pDY25-B (92% nt identity). On pDY25-B, the _mle046_ homolog and eight other ORFs are flanked on either side by a Tn7 transposase, forming a putative composite
transposon. The _mle046_ is located at the end of contig 279, and is also flanked by a Tn7-type transposase on the right side. The two left-flanking ORFs, as well as the left-flanking
transposase which are present on the pDY25-B transposon are missing from this contig. The rest or this transposon is almost identical (99% nt identity) to the pDY25-B transposon (Fig. S8).
The genomic context of this unbinned Mle therefore suggests that it is located on an Alphaproteobacterial conjugative plasmid, and has been acquired via a transposable element. There was no
evidence of acquisition via horizontal gene transfer for the other Mles. Two different TPD clusters were found in the I1 metagenome (Table 1). These were located on bin 12 (named sTPD), as
well as the unbinned 21 kbp-contig 1092 (named pTPD). Both clusters are orthologous to those from _Comamonas_ sp. E619 and _I. sakaiensis_18 (Table 1). The pTPD cluster lacks the
terephthalate permease gene _tphC_28 which is present in _Comamonas_ and _Ideonella_ (Fig. S9). No homologs of the _tphC_ were present in the I1 metagenome. The pTPD cluster has a 100% nt
identity to the TPD cluster on pDY25-B. The contig 1092 carries a gene encoding for the plasmid partitioning protein ParA with a 85% nt identity to the _parA_ on the _Phaeobacter piscinae_
plasmid pP18i (CP010724.1). The genomic context of the unbinned pTPD cluster indicates that, like _mle046_, the pTPD cluster may be part of an Alphaproteobacterial plasmid. The sTPD cluster
has a different structure to those of _Comamonas_ and _Ideonella_, as it only contained the _tphA2A3B_ genes, missing the reductase component _tphA1_ (Table 1). Adjacent to the sTPD genes,
homologs of _pht2345_ genes for phthalate degradation29 are present (Fig. S9). The abundance of bin 12 when Te is added as sole C source indicates that this organism can use Te as C source,
despite the missing _tphA1_. Phylogenetically, TphA2 subunits from I1 form three distinct clades: pTphA2 (Tpad1092), sTphA2 (Tpad12), and _Ideonella_/_Comamonas_ TphA2 and relatives
clustering separately from each other (Fig. S7c). The conversion of PCA to pyruvate and oxaloacetate for complete terephthalate degradation can be accomplished via PCA 4,5-19,30,31, PCA
2,3-32 or PCA 3,4-33 cleavage. In the I1 metagenome, diverse protocatechuate 4,5- and 2,3-dioxygenase subunits were found. These, with the exception of those from _Saccharospirillum_ all
belonged to Alphaproteobacteria (Table S4). GENE EXPRESSION AND PROTEIN BIOSYNTHESIS DURING THE TIME SERIES The metatranscriptome and -proteome of the I1 community was characterized
following the addition of fresh PF substrate after a starvation period (Fig. 1c). At each sampling point, about 70% of all genes were detected (cutoff ≥ 10 reads in at least two of the
biological replicates) in the metatranscriptome, and around 6% were detected in the metaproteome (exact numbers given in Fig. S10). Only a small portion of the entire metatranscriptome was
significantly up/downregulated during the time series (Wald test, <0.05 _p_-value, >2-fold change) compared to t0, corresponding to 0.4/0.9% of the transcripts for the first time
point, 4.7/5% for the second, and 5.4/5.4% for the third. The bins which were highly abundant in the metagenome (bins 10, 17, 20, and 32) contributed with the highest amount of upregulated
genes at this time point. Bin 17 and 20 showed continued transcript upregulation after one week, while bin 10 and 32 had a reduced number of upregulated transcripts at the last time point.
For each time point, the downregulated transcripts were mainly contributed by low abundance members of the I1 community (Fig. 4a). In total 8126 protein groups were identified from the
metaproteome, from which the highest amount of proteins (67%) were detected only after 7 days of incubation in the presence of new polymer films. The remaining time points showed a core of
protein groups (≥3049, 38%) present at every time point. The highest number of upregulated protein groups (921 proteins) was consistently present after 7 days (Fig. S10). We found that the
most active bacteria in terms of protein biosynthesis were Alphaproteobacteria and Gammaproteobacteria throughout the entire experiment, especially of the genus _Marinobacter_. Between these
two bacterial groups, the number of upregulated protein groups at every time point was greater for Gammaproteobacteria than for Alphaproteobacteria (Fig. 4a). The addition of PF after a
starvation period triggered the upregulation of several genes related to central metabolism, supporting that this substrate is used as carbon source. In addition, biofilm formation-related
pathways (ko02025) were upregulated on the transcript level at the first and last time points, but not on protein level. Bacterial secretion systems (ko03070) showed increased transcription
and translation (Fig. S11), which may be due to an increase in the production of secreted proteins such as Ples and Mles. The _ple628_, _ple629_, and _ple453_, are the only _ple_ genes that
whose expression significantly increased in the metatranscriptome. The increase happened exclusively at the second time point. Consequently, these were also the only Ples detected in the
metaproteome. Ple628 and Ple453 were highly abundant and upregulated throughout the time series (Fig. 5). The remaining Ples were absent from the proteome and possibly did not play a
significant role in the depolymerization of PF. Due to competition in biofilms it is possible that redundant and cost-intensive metabolic functions are halted to favor selection for
syntrophy. Similar to the Ples, only one Mle (_mle046_), was upregulated in the metatranscriptome at the second and third time point. Interestingly, Mle046 was the most abundant protein
group of the I1 metaproteome throughout the time series. It was also the only Mle protein that could be detected (Fig. 5). Both TPD clusters on contig 1092 (_ptpd_) and bin 12 (_stpd_) were
transcribed during PF degradation. The _stpd_ genes were significantly upregulated at the third time point, while the _pht_ genes of bin 12 were upregulated at the first time point. Only
sTphA2 and sTphA3 proteins were present, whereby the latter protein was downregulated at the last time point (Fig. 5). The pht proteins were not detected. The _ptpd_ gene expression did not
significantly change during the last two time points. The pTPD proteins, like Mle046, were highly abundant in the metaproteome at all time points without significant protein fold changes.
Only six PCD subunits were detected in the proteome. These belonged to bins 10, 17, and 20, as well as two unbinned subunits whose nearest relatives originate from Alphaproteobacteria (Fig.
S12). Generally, the transcription of the subunits was upregulated throughout the time series while the protein biosynthesis was downregulated. No PCD proteins from bin 12 were detected.
GENE EXPRESSION AND PROTEIN BIOSYNTHESIS: FILM VS. FREE-LIVING BACTERIA The taxonomic composition, gene expression, and protein biosynthesis profiles of film-attached and free-living
community were significantly different. About 10% of all transcripts and 5.4% of the proteins were upregulated in the film-attached community, while 12% of the transcripts and 5% of the
proteins were upregulated in the free-living community. In total, about 25%, 28%, 16% of the genes/transcripts/proteins respectively were unique to the free-living community, and 11%, 13%,
16% unique to the film-attached community (Fig. S10). Most transcripts of the film-attached community were contributed by bins 32, 28, 21, and 5, representing both high and low-abundance
members (Fig. 4b). Yet, the majority of the proteins in the film-attached community were however produced by bin 32, and to a lesser extent by bin 21, the most abundant members. For the
free-living community, transcript contribution from the high-abundance members bin 10, 17, and 21 were lower than their contribution to protein biosynthesis. The lower-abundant bins 12 and
13 were active in this fraction, contributing to both gene expression and protein biosynthesis. We have observed that pathways related to biofilm formation, bacterial secretion, quorum
sensing and flagellar assembly were enriched in the biofilm metatranscriptome, but not in the metaproteome. Rather, the metaproteome showed an enrichment of fatty acid metabolism proteins,
which may be related to the metabolism of dicarboxylic acids via _β_-oxidation34,35,36 (Fig. S11). The expression of all _ple_ genes was restricted to the film-attached community. The
_ple453_ and _ple628_ genes were upregulated in the film-attached community compared to the free-living fraction, and consequently more proteins were synthesized in the biofilm (Fig. 5).
Similar to the time series experiment, neither Ple200 nor Ple201 could be detected in any of the fractions. Ple629 was detected in the film-attached community, in contrast to the time series
experiment where this protein could not be detected. The abundance of bin 32 in the biofilm, its metabolic potential, and gene expression profile suggests that this species could be the PF
depolymerizing species in the consortium. The _mle046_ gene was highly expressed in both film-attached and free-living communities without a significant difference in expression levels,
however, more Mle046 was detected in the free-living community (4-fold). Other Mle gene expression levels were low and proteins were not detected. The _ptpd_ transcripts and proteins were
present in both film-attached and free-living communities. Their expression and biosynthesis levels were either similar in both fractions or upregulated in the free-living community. On the
other hand, the _stpd_ and _pht_ transcripts were upregulated in the free-living community. Consistent with the time series experiment, only sTph and not pht proteins were detected. These
were upregulated or only present in the free-living community (Fig. 5). Only two PCA dioxygenase subunits were detected in the proteomes during this experiment. These were not significantly
up or downregulated in the film or free-living community (Fig. S12). Although the transcripts of PCA dioxygenase subunits from bins 10 and 12 were upregulated in the film community; these
were not detected in the proteome. DISCUSSION In this study, we used a multi-omics approach to elucidate the biodegradation potential of a commercial aliphatic-aromatic polyester blend by a
marine microbial consortium. Our data shows that both the polymers and its monomers can be mineralized by the consortium, and points towards a synergistic biodegradation process within the
I1 community. Labor division within microbial consortia to completely degrade complex anthropogenic chemicals has been demonstrated before37,38,39. In the I1 culture, different members of
the community perform depolymerization, breakdown of intermediates, and the aromatic monomers as proposed in Fig. 6. The bacterial community on PF is comparable to what has been found on
biofilms colonizing other plastics particles and is mostly comprised of _Gammaproteobacteria_40,41. Some members of this class are early colonizers of marine substrates40,42 and have been
found colonizing plastics43,44,45,46,47. The data suggests that, among the Gammaproteobacteria, the genus _Marinobacter_ plays a crucial role in PF degradation. The hydrocarbonoclastic
capability of some _Marinobacter_ species48,49,50, as well as their abundance on the film and their corresponding activity point towards this potential. The genomes of both _Marinobacter_
bins (bin 21 and 32) encoded for Ples. Bin 32 possibly performs the initial depolymerization of PF (Fig. 6), allowing access to the rather hydrophobic substrate40. Bin 32, which lacks Te
degradation genes, was abundant when the culture received the dicarboxylic acid monomers as sole carbon source. Moreover, pathways related to the metabolism of dicarboxylic acids were
enriched in the biofilm metaproteome where this bacterium is abundant. Thus, it is likely that after depolymerization, this microorganism grows on the available dicarboxylic acid monomers. A
cultivation-dependent approach may not accurately represent how the degradation process would occur in the environment, however our results suggest that the marine aliphatic-aromatic
polyester degradation genes are highly similar to those identified in terrestrial microorganisms. Within the free-living fraction, Alphaproteobacteria comprised the highest abundance, mainly
by members of the Rhodobacteraceae family. Rhodobacteraceae thrive in marine environments51,52 and several of them can alternate between free-living and attached lifestyles53,54,55. This
lifestyle is especially apparent for bins 10 and 20, which are equally abundant in biofilm and supernatant. Alphaproteobacteria in the I1 community are therefore likely to feed on the
soluble oligomers and monomers after depolymerization. The highly-expressed _mle_ and _tpd_ genes belong to Alphaproteobacterial hosts, as well as the majority of the _pcd_ genes. We
therefore assume that Alphaproteobacteria play a key role in the mineralization of aromatic oligmers and monomers. We have observed that bins 12 and 7, which outgrow the other community
members when Te and B are given as sole carbon source, have a low abundance (<2%) when the community is growing on PF. After PF cleavage by the Ples, B and Te could be available as the
monoester BTe at first rather than as free monomers, similar to the release of MHET by IsPETase18. The intracellular presence of BTe during the PF degradation further indicates that this
monoester is taken up and used as a C source rather than being cleaved into the monomers extracellularly first (see proposed alternative mechanism in Fig. S13). Bins 7 and 12 are thus
outcompeted by _mle046_-encoding organisms when the culture is growing on PF. Since both _mle046_ and _ptpd_ genes are located on plasmids, it is hard to state exactly which community
members use BTe as growth substrate. Bins 10 and 17 are very abundant when the community is growing on the PF, but become nearly undetectable when monomers are supplied as C source. Based on
this observation, we postulate that either one or both of these bacteria are the hosts of the _mle046_ and _ptpd_-carrying plasmids. These bacteria possibly take up and use BTe as a C
source, but are outgrown by bin 12 and bin 7 when monomers Te and B are freely available. When the community grows on PF as the C source, the bin 12 and 7 possibly exist as secondary
degraders. Taxa with lower abundance also showed an increase in activity throughout the degradation process. Downstream degradation products and leaking metabolites could be metabolized by
lower abundant taxa such as bin 9 (Sandaracinaceae), which contributed most of the upregulated proteins at the last sampling point. Members of this family consume low molecular weight
compounds in marine ecosystems (i.e., acetate, ethanol)56. Based on the gene expression and protein biosynthesis patterns of the depolymerases and downstream degradation genes, we conclude
that PF depolymerization is performed by the film-attached community. Aromatic oligomers and monomers are degraded by both the film and free-living community, thus sharing the task of
complete PF mineralization. METHODS INOCULUM SOURCE Marine seawater and eulittoral sediment samples were collected from three different sources: Helgoland, Germany (54∘ 11’ 17.0" N 7∘
52’ 50.1" E), near Athens, Greece (37∘ 53’ 33" N, 23∘ 24’ 30" E) and Elba, Italy (42∘ 44’ 00.8" N 10∘ 09’ 14.4" E) (November 2016). Samples were stored in the dark
at 4 ∘C until further use. REAGENTS PF (ecovio® FT 2341) was supplied by BASF (Ludwigshafen, Germany). The monomers were purchased from Sigma Aldrich (Missouri, USA) with the highest purity
grade available. CULTURE CONDITIONS All cultivations were performed in a mineral media as described before for _Pirellula_ (600a. M13a DSMZ medium)57 with some modifications: Artificial sea
water (ASW 1X), Hutner’s salts and metals solutions were prepared according to DSMZ 590 medium. The carbon and nitrogen sources described for these media (600a. M13a and 590 DSMZ medium)
were omitted and instead NH4Cl (1 mM) and K2HPO4 (0.1 mM) were used. The pH of cultures supplied with either Se or Ad were maintained with HEPES buffer instead of bicarbonate. The enrichment
cultures were set up with 50 mL ASW medium supplemented with 10 mg of PF and 0.01% tryptone. To prepare the inoculum, 1 g of each sediment and 10 mL of each seawater sample were pooled,
vortexed for 2 min and allowed to settle. One mililiter of the supernatant was used to inoculate each culture. After film disintegration was observed (ca. two months), 1 mL of the liquid
culture was transferred into fresh ASW medium with 10 mg of PF, but without the addition of tryptone. After four transfers, an enrichment culture capable of disintegrating the plastic film
in a time frame of six days was obtained and named I1. The culture was routinely maintained in ASW medium with PF as carbon source. Cultivations were performed at pH 7.0 and incubated in a
MaxQTM 4000 orbital shaker (Fisher Scientific, Schwerte, Germany) at 22 ∘C, 120 rpm. To measure copolymer and monomer mineralization, the I1 community was inoculated in 25 mL of mineral
media containing 50 mg of carbon per liter of either PF, Te, B, Ad, or Se. Each flask had a volume of 250 mL and contained an external CO2 trap. Copolymer or monomer mineralization were
measured from three biological replicates. In addition, three negative controls consisting of cultures without a carbon source were also included in the experiment. All cultivations were
performed at pH 7.0 and incubated in a MaxQTM 4000 orbital shaker (Fisher Scientific, Schwerte, Germany) at 22 ∘C, 120 rpm. Cultures were harvested for metagenomics in stationary phase after
the carbon source was depleted. Cultures were centrifuged in a HeraeusTM MultifugeTM X3 (Fischer Scientific, Schwerte, Germany) at 4 ∘C, 8000 rpm for 10 min. Differences between
film-attached communities and free-living bacteria were analyzed in 200 ml of mineral media supplied with 5 × 5 cm PF. The experiment consisted of three biological replicates that were
incubated in a MaxQTM 4000 orbital shaker (Fisher Scientific, Schwerte, Germany) at 22 ∘C, 120 rpm. Before disintegration of the PF, the two fractions of the bacterial communities were
separated by filtration. The filtrate was consequently centrifuged in a HeraeusTM MultifugeTM X3 (Fischer Scientific,Schwerte, Germany) at 4 ∘C, 8000 rpm for 10 min. The films and recovered
pellets were flash-frozen in liquid nitrogen and stored at −80 ∘C until DNA, RNA and protein extraction. Gene expression and protein biosynthesis were analyzed in response to the presence of
PF in a time series experiment. For this, we used 67.2 ± 0.1 mg of PF in 200 mL of mineral media. The incubation was carried out in three 2 L baffled flasks in a MaxQTM 4000 orbital shaker
(Fisher Scientific, X, Germany) at 22 ∘C, 70 rpm (instead of 120 rpm). Cultures were harvested during starvation (control: t0), immediately after 1 h of PF addition (t1), after 24 h (t2),
and after 7 days (t3). The last sample was taken right before the disintegration of the film. At each time point, 30 mL of a free-living and attached biofilm mixture was extracted and
centrifuged in a HeraeusTM MultifugeTM X3 (Fischer Scientific, Schwerte, Germany) at 4 ∘C, 8000 rpm for 10 min. The bacterial pellets were flash frozen in liquid nitrogen and stored at −80
∘C until DNA, RNA, and protein extraction. For all experiments, cultures previously grown with PF as the pre-inoculum were used. BACTERIAL GROWTH AND CO2 PRODUCTION The extent of
mineralization was calculated based on CO2 formation. The CO2 formed in the mineralization tests were measured as inorganic carbon using a TOC-L analyzer and data collection was retrieved by
using the TOC-Control L v. 1.06 software (Shimadzu, Kyoto, Japan). Before the analysis, the released CO2 was entrapped in 4 mL of 0.1 mM NaOH to form NaHCO3 and diluted 10× with Milli-Q
water. The carbon content was determined using a calibration curve of NaHCO3. DETECTION OF PF RESIDUES AND BYPRODUCTS (FTIR; GC-MS) Residues originated from PF mineralization tests were
analyzed by FTIR using a PerkinElmer Spectrum 100 FT-IR Spectrometer (PerkinElmer, Massachusetts, USA). The scans were performed from 4000 to 650 cm−1 with a resolution of 2,00 cm−1. The
resulting spectra were visualized and analyzed with Spectrum Quant software v. 10.4 (PerkinElmer, Massachusetts, USA). Figures were further edited with the software Spectragryph v. 1.2.14
(https://www.effemm2.de/spectragryph/). The monoester BTe was used as a GC-MS standard in addition to the monomers. This compound was synthesized following the procedure described by Perz et
al.58. The identity was confirmed with 1H NMR obtained on a Bruker Avance III platform. The purity was estimated to be 93%. 1H NMR (500 MHz, DMSO-d6) _δ_ 13.34 (s, 6H), 8.06 (s, 28H), 4.45
(s, 6H), 4.38 (s, 2H), 4.31 (t, _J_ = 6.6 Hz, 13H), 3.89 (q, _J_ = 1.0 Hz, 1H), 3.45 (d, _J_ = 12.9 Hz, 13H), 3.31 (s, 3H), 1.93–1.87 (m, 1H), 1.82–1.67 (m, 13H), 1.61–1.50 (m, 13H).
Metabolites were extracted from the bacterial pellets as described before59 with minor modifications. Briefly, cell pellets were resuspended in 500 μL methanol (containing 5 mg/mL
13C5-ribitol as internal standard) and cells were lysed in an ultrasonic bath at 70 ∘C for 15 min. The same volume of water was added, the sample was mixed and centrifuged for 5 min at
12,000×_g_ to remove residual plastic film/particulate material. The supernatant was vigourously mixed with 500 μL of chloroform, centrifuged for 5 min at 17,000 × _g_ and 800 μL of the
polar phase were dried in vacuum. GC-MS analysis was performed on an Agilent GC-MSD system (7890B coupled to a 5977 GC) equipped with a high-efficiency source (HES) and a PAL RTC system
using a two-step derivatization with a methoxyamine hydrochloride solution (20 mg mL−1 in pyridine) and N-methyl-N-(trimethylsilyl)-trifluoracetamide. The autosampler was operated by the
Maestro 1.5.4.2/3.5 (Gerstel) software. Targeted GC-MS analysis was performed on an Agilent GC-MSD system (7890B coupled to a 5977 GC) (Agilent Technologies, California, USA) equipped with a
high-efficiency source (HES) and a PAL RTC system as previously described59 using authentic standards of adipic acid, sebacic acid, terephthalic acid and BTe. Data was analyzed with the
MassHunter GC/MS Acquisition B.07.06.2704 (Agilent Technologies, California, USA). FIELD EMISSION SCANNING ELECTRON MICROSCOPY (FESEM) PF-attached bacteria were fixed with 5% formaldehyde
and 2% glutaraldehyde in HEPES buffer (0.1 M HEPES, 0.01 M CaCl2, 0.01 M MgCl2, 0.09 M sucrose, pH 6.9) on ice, then washed twice with TE buffer (20 mM TRIS, 2 mM EDTA, pH 6.9). The treated
samples were then dehydrated with graded series of ethanol (10, 30, 50, 70, 90, 100%) on ice for 10 min for each step and allowed to reach room temperature before another change in 100%
ethanol. Consecutively, samples were then subjected to critical-point drying with liquid CO2 (CPD 030, Bal-Tec AG, Balzers, Liechtenstein). Dried samples were covered with an approximately 8
nm thick gold-palladium film by sputter coating (SCD 500 Bal-Tec, Balzers, Liechtenstein) before examination in a field emission scanning electron microscope Zeiss Merlin (Carl Zeiss AG,
Oberkochen, Germany) using the Everhart-Thornley SE-detector and the Inlens SE-detector in a 75:25 ratio with an acceleration voltage of 5 kV. Images were stored digitally with SEMSmart
software v. 5.05. DNA, RNA, AND PROTEIN EXTRACTION, PURIFICATION AND QUANTIFICATION DNA, RNA and proteins were extracted from most of the experiments as shown in the experimental setup (Fig.
1). Only DNA was extracted from the monomer mineralization tests. Total DNA, RNA and proteins were extracted from all tests simultaneously by using the Quick-DNA/RNA Miniprep Plus Kit (Zymo
Research, California, USA) according to the manufacturer’s instructions. To asses the integrity of isolated DNA and RNA, 5 μL of each sample was loaded in an agarose gel and visualized by
using Intas Gel v. 0.2.14 software. To remove possible RNA contamination, the DNA samples were additionally treated with RNase A (AppliChem, Darmstadt, Germany) and purified by using DNA
Clean and Concentrator kit (Zymo Research, California, USA) following manufacturer’s instructions. Quality of DNA and RNA was additionally analyzed via Nanodrop by using the Nanodrop 2000 v.
1.5 software (Nanodrop 2000, Thermo Fischer Scientific, Waltham, USA). The fractions of proteins isolated with Quick-DNA/RNA Miniprep Plus Kit (Zymo Research, California, USA) were further
precipitated with acetone according to the manufacturer’s instructions. Purified total DNA, RNA, and proteins were quantified to determine concentration by using Qubit RNA BR, dsDNA BR, and
Protein Assay kits and measured on a Qubit 3.0 Fluorometer (Invitrogen, California, USA). DNA AND RNA SEQUENCING The DNA sequencing library was generated from 200 ng DNA using NEBNext Ultra
II FS DNA Library Prep Kit for Illumina (New England BioLabs, Massachusetts, USA) according to manufacturer’s protocols including PCR amplification with four cycles. The libraries were
sequenced on Illumina NovaSeq 6000 using the NovaSeq 6000 S1 PE Reagent Kit (300 cycles) (Illumina, California, USA) with an average of 1E7 reads per DNA sample. Data was analyzed and
converted to FASTQ files using the NovaSeq Control Software v1.6, RTA Version 3.4.4 and bcl2fastq v2.20.0.422. Quality and integrity of total RNA was controlled on Agilent Technologies 2100
Bioanalyzer (Agilent Technologies, Waldbronn, Germany). The RNA sequencing library was generated from 250 ng total RNA using Ribo-off rRNA Depletion Kit (Bacteria) (Vazyme BioTech, Nanjing,
China) for rRNA depletion followed by NEBNextⓇ UltraTM II Directional RNA Library Prep Kit (New England BioLabs, Massachusetts, USA) according to the manufacturer’s protocols. The libraries
were sequenced on Illumina NovaSeq 6000 using NovaSeq 6000 S1 PE Reagent Kit (100 cycles) (Illumina, California, USA) with an average of 2E7 reads per RNA sample. METAPROTEOMICS BY NANO
LC-MS/MS The protein precipitates were dissolved in SDS-PAGE sample loading buffer, loaded on an SDS-gel and run for 10 min. The gel pieces were cut, washed and incubated with 25 mM
1,4-dithiothreitol (in 20 mM ammonium bicarbonate) for 1 h and 100 mM iodoacetamide (in 20 mM ammonium bicarbonate) for 30 min. The pieces were further destained, dehydrated and
proteolytically cleaved overnight at 37 ∘C with trypsin (Promega, Walldorf, Germany). The digested peptides were extracted and desalted using ZipTip-C18 tips (Merck Millipore, Darmstadt,
Germany). Afterwards, the peptide lysates were re-suspended in 0.1% formic acid and injected to a nanoliquid chromatography mass spectrometry (nanoLC-MS/MS). Mass spectrometric analysis of
peptides was performed on a Q Exactive HF mass spectrometer (Fisher Scientific, Massachusetts, USA) coupled with a TriVersa NanoMate (Advion, Ltd., Harlow, UK) source in LC chip coupling
mode. LC gradient, ionization mode and mass spectrometry mode have been used as described before60. Data resulting from LC-MS/MS experiments were analyzed using the Proteome Discoverer v.
1.4 (Fischer Scientific, Massachusetts, USA) using SEQUEST HT. As a database, the protein-coding sequences of the metagenome were used. Search settings were set to trypsin (Full), max.
missed cleavage: 2, precursor mass tolerance: 10 ppm, fragment mass tolerance: 0.02 Da. The false discovery rates (FDR) were determined with the node Percolator61 embedded in Proteome
Discoverer and the FDR threshold was set at a peptide level of <1%. The same threshold was set for the protein FDR. DATA ANALYSIS The metagenomes were processed with MetaWRAP v. 1.262.
In brief, the reads were trimmed with Trim Galore! v. 0.6.463 and overrepresented sequences comprising technical artifacts were filtered out. All reads were co-assembled with MetaSPAdes v.
3.13.064 which yielded in 75,703 contigs (174.8 Mbp with a N50 of 118,039). Gene calling and annotation was performed on the metagenome with Prokka v. 1.1465. Binning of the assembled
sequences was carried out with CONCOCT v. 1.0.066, MaxBin v. 2.2.567 and Metabat v. 2.12.168, which were subsequently refined with MetaWRAP. Completeness and contamination of the refined
bins was assessed with checkm v. 1.0.1269, the taxonomy of the bins was assigned with GTDBtk v. 0.3.270. The trimmed reads were mapped on the assembled metagenome with the Subread aligner71
from the RSubread package v. 1.34.772. Abundance estimation was performed with samtools v. 1.773. The correlation of the relative abundance of the bins was visualized with the ggcorrplot
package v. 0.1.3.99974 using Pearson correlation with a p-value significance cutoff of 0.05 and hierarchical clustering. Metatranscriptomics reads were trimmed with Trim Galore! v. 0.6.463
and rRNA reads were removed with SortMeRNA v. 2.1b75. Subsequently, the remaining reads were mapped on the assembled metagenome the Subread aligner71 from the RSubread package v. 1.34.772
(Table S5). Differential gene expression analysis was conducted with DeSEQ2 v. 1.24.076. In brief, genes were normalized with the median of ratios method77. The differential gene expression
analysis was performed by fitting the negative binomial model for each gene and subsequent hypothesis testing with the Wald test. The shrunken log2 foldchanges (LFC) were calculated using
the adaptive t prior shrinkage estimator with apeglm v. 1.6.078. The _p_-values were adjusted for multiple testing using the Benjamini and Hochberg method79. A gene is considered to be
significantly differentially expressed with ≤two-fold change and an adjusted _p_ value < 0.05 with a false discovery rate (FDR) < 0.05. For metaproteomic quantification the redundant
proteins were grouped in protein groups by applying the strict parsimony principle. Only the protein groups that explain at least one unique identified peptide were reported. Only the
peptides that were not shared between different proteins or protein groups were used for the protein quantification through the Top3 approach implemented in the Proteome Discoverer v. 1.4
(Fischer Scientific, Massachusetts, USA). The protein data were further log2 transformed and normalization of the peptides were manually performed by dividing the transformed values by the
median of the sample and multiplying by the mean of entire data set80,81 with Microsoft Excel 2010 v. 14.0.7252.5000. For both comparative metatranscriptomic and proteomic analyses, fold
changes were calculated relative to t0 and free-living fraction for the time series and film-attached communities analysis respectively. The replicate consistency was determined by analyzing
the distribution of the normalized counts (for MT) and normalized area (for MP), respectively (Fig. S14a). Unsupervised clustering of the metatranscriptomes and metatranscriproteomes was
done and visualized with ComplexHeatmap v. 2.1.082 (Fig. S14b). The input data was the normalized count numbers of each biological replicate. Circos plots were created with the online tool
Circos Table Viewer83. KEGG over-representation tests were carried out and visualized using ClusterProfiler v. 3.14.384. All R packages were used with R v. 3.6.085. All tools were run with
default parameters. Protein alignments were performed with T-Coffee online platform86 (http://tcoffee.crg.cat/apps/tcoffee/do:regular, last visited 30.09.2020) and similarities were shaded
with BOXSHADE v. 3.2 online source (https://embnet.vital-it.ch/software/BOX_form.html). Phylogenetic trees were constructed for Ples, Mles and TPADO based on their aminoacidic sequences and
compared to other homologous proteins. For this, the sequences obtained in this study together with homologous sequences were aligned with ClustalW v. 2.187 by using the Geneious_Ⓡ_ Prime
v.2019.1.1 software (https://www.geneious.com). BLOSUM was selected as the scoring matrix and gaps were set at 10 (gap open cost) and 0.1 (gap extend cost) per element. The trees were
generated with GeneiousⓇ Prime v. 2019.1.1 (https://www.geneious.com) by using Jukes-Cantor distance model and built with the Neighbor–Joining method88. No outgroups were included in any of
the phylogenetic trees. The number of bootstrap replicates were 1000. Newick phylogenetic trees were further midpoint rooted and edited with iTOL v. 5.589. REPORTING SUMMARY Further
information on research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The metagenomic and metatranscriptomic raw data have been
deposited at EBI Metagenomics/MGnify: PRJEB37199, the metaproteomics data at the ProteomeXchange Consortium via PRIDE with the identifier PXD018391. KEGG
(https://www.genome.jp/kegg/pathway.html) and Uniprot (https://www.uniprot.org/) databases were used for protein annotation. All other data are available from the corresponding author upon
request. Source data are provided with this paper. REFERENCES * Plastics Europe. Plastics-the facts 2018
https://www.plasticseurope.org/application/files/6315/4510/9658/Plastics_the_facts_2018_AF_web.pdf (2018). * Eriksen, M. et al. Plastic pollution in the world’s oceans: More than 5 trillion
plastic pieces weighing over 250,000 tons afloat at sea. _PLoS ONE_ 9, 1–15 (2014). Google Scholar * Van Sebille, E. et al. A global inventory of small floating plastic debris. _Environ.
Res. Lett_. 12, 124006 (2015). * Jambeck, J. R. et al. Plastic waste inputs from land into the ocean. _Science_ 347, 768–771 (2015). Article ADS CAS PubMed Google Scholar * Platt, D. K.
_Biodegradable Polymers: Market Report_. (Smithers rapra limited, Shrewsbury, UK, 2006). Google Scholar * Chen, G.-Q. A microbial polyhydroxyalkanoates (pha) based bio- and materials
industry. _Chem. Soc. Rev._ 38, 2434–2446 (2009). Article ADS CAS PubMed Google Scholar * Siegenthaler, K. O., Künkel, A., Skupin, G. & Yamamoto, M. Ecoflex and ecovioⓇ:
biodegradable, performance-enabling plastics. In _Synthetic Biodegradable Polymers._ (eds Rieger, B. et al.) 91–136 (Springer Berlin Heidelberg, Berlin, Heidelberg, 2012). * Byrom, D.
Polymer synthesis by microorganisms: technology and economics. _Trends Biotechnol._ 5, 246–250 (1987). Article CAS Google Scholar * Gross, R. A. & Kalra, B. Biodegradable polymers for
the environment. _Science_ 297, 803–807 (2002). Article ADS CAS PubMed Google Scholar * Brodhagen, M., Peyron, M., Miles, C. & Inglis, D. A. Biodegradable plastic agricultural
mulches and key features of microbial degradation. _Appl. Microbiol. Biotechnol._ 99, 1039–1056 (2015). Article CAS PubMed Google Scholar * Mueller, R.-J. Biological degradation of
synthetic polyesters-enzymes as potential catalysts for polyester recycling. _Process Biochem._ 41, 2124–2128 (2006). Article ADS CAS Google Scholar * Zumstein, M. T., Kohler, H. E.,
McNeill, K. & Sander, M. High-throughput analysis of enzymatic hydrolysis of biodegradable polyesters by monitoring cohydrolysis of a polyester-embedded fluorogenic probe. _Environ. Sci.
Technol._ 51, 4358–4367 (2017). Article ADS CAS PubMed Google Scholar * Witt, U., Müller, R.-J. & Deckwer, W.-D. New biodegradable polyester-copolymers from commodity chemicals
with favorable use properties. _J. Environ. Polym. Degrad._ 3, 215–223 (1995). Article CAS Google Scholar * Wallace, P. W. et al. PpEst is a novel PBAT degrading polyesterase identified
by proteomic screening of Pseudomonas pseudoalcaligenes. _Appl. Microbiol. Biotechnol._ 101, 2291–2303 (2017). Article CAS PubMed Google Scholar * Thumarat, U., Nakamura, R., Kawabata,
T., Suzuki, H. & Kawai, F. Biochemical and genetic analysis of a cutinase-type polyesterase from a thermophilic Thermobifida alba AHK119. _Appl. Microbiol. Biotechnol._ 95, 419–430
(2012). Article CAS PubMed Google Scholar * Perz, V. et al. Substrate specificities of cutinases on aliphatic-aromatic polyesters and on their model substrates. _N. Biotechnol._ 33,
295–304 (2016). Article CAS PubMed Google Scholar * Kleeberg, I., Welzel, K., Vandenheuvel, J., Muller, R. J. & Deckwer, W. D. Characterization of a new extracellular hydrolase from
Thermobifida fusca degrading aliphatic-aromatic copolyesters. _Biomacromolecules_ 6, 262–270 (2005). Article CAS PubMed Google Scholar * Yoshida, S. et al. A bacterium that degrades and
assimilates poly(ethylene terephthalate). _Science_ 351, 1196–1199 (2016). Article ADS CAS PubMed Google Scholar * Sasoh, M. et al. Characterization of the terephthalate degradation
genes of Comamonas sp. strain E6. _Appl. Environ. Microbiol._ 72, 1825–1832 (2006). Article CAS PubMed PubMed Central Google Scholar * Gouda, M. K., Kleeberg, I., van den Heuvel, J.,
Müller, R. J. & Deckwer, W. D. Production of a polyester degrading extracellular hydrolase from Thermomonospora fusca. _Biotechnol. Prog._ 18, 927–934 (2002). Article CAS PubMed
Google Scholar * Müller, R.-J., Kleeberg, I. & Deckwer, W.-D. Biodegradation of polyesters containing aromatic constituents. _J. Biotechnol._ 86, 87–95 (2001). Article PubMed Google
Scholar * Sulaiman, S. et al. Isolation of a novel cutinase homolog with polyethylene terephthalate-degrading activity from leaf-branch compost by using a metagenomic approach. _Appl.
Environ. Microbiol._ 78, 1556–1562 (2012). Article CAS PubMed PubMed Central Google Scholar * Herrero Acero, E. et al. Enzymatic surface hydrolysis of PET: Effect of structural
diversity on kinetic properties of cutinases from Thermobifida. _Macromolecules_ 44, 4632–4640 (2011). Article ADS CAS Google Scholar * Austin, H. P. et al. Characterization and
engineering of a plastic-degrading aromatic polyesterase. _Proc. Natl Acad. Sci. USA_ 115, E4350–E4357 (2018). Article CAS PubMed PubMed Central Google Scholar * Ollis, D. L. et al. The
alpha/beta hydrolase fold. _Protein Eng._ 5, 197–211 (1992). Article CAS PubMed Google Scholar * Thumarat, U. et al. Comparison of genetic structures and biochemical properties of
tandem cutinase-type polyesterases from Thermobifida alba AHK119. _J. Biosci. Bioeng._ 120, 491–497 (2015). Article CAS PubMed Google Scholar * Palm, G. J. et al. Structure of the
plastic-degrading Ideonella sakaiensis MHETase bound to a substrate. _Nat. Commun._ 10, 1717 (2019). Article ADS CAS PubMed PubMed Central Google Scholar * Hosaka, M. et al. Novel
tripartite aromatic acid transporter essential for terephthalate uptake in Comamonas sp. strain E6. _Appl. Environ. Microbiol._ 79, 6148–6155 (2013). Article CAS PubMed PubMed Central
Google Scholar * Nomura, Y., Nakagawa, M., Ogawa, N., Harashima, S. & Oshima, Y. Genes in pht plasmid encoding the initial degradation pathway of phthalate in Pseudomonas putida. _J.
Ferment. Bioeng._ 74, 333–344 (1992). Article CAS Google Scholar * Kersten, P. J., Dagley, S., Whittaker, J. W., Arciero, D. M. & Lipscomb, J. D. 2-pyrone-4,6-dicarboxylic acid, a
catabolite of gallic acids in Pseudomonas species. _J. Bacteriol._ 152, 1154–1162 (1982). CAS PubMed PubMed Central Google Scholar * Kamimura, N. et al. Characterization of the
protocatechuate 4,5-cleavage pathway operon in Comamonas sp. strain E6 and discovery of a novel pathway gene. _Appl. Environ. Microbiol._ 76, 8093–8101 (2010). Article CAS PubMed PubMed
Central Google Scholar * Crawford, R. L., Bromley, J. W. & Perkins-Olson, P. E. Catabolism of protocatechuate by Bacillus macerans. _Appl. Environ. Microbiol._ 37, 614–618 (1979).
Article CAS PubMed PubMed Central Google Scholar * Harwood, C. S. & Parales, R. E. The beta-ketoadipate pathway and the biology of self-identity. _Annu. Rev. Microbiol._ 50, 553–590
(1996). Article CAS PubMed Google Scholar * Chapman, P. J. & Duggleby, R. G. Dicarboxylic acid catabolism by bacteria. _Biochem. J._ 103, 7–9c (1967). Article Google Scholar *
Parke, D., Garcia, M. A. & Ornston, L. N. Cloning and genetic characterization of dca genes required for beta-oxidation of straight-chain dicarboxylic acids in Acinetobacter sp. strain
ADP1. _Appl. Environ. Microbiol._ 67, 4817–4827 (2001). Article CAS PubMed PubMed Central Google Scholar * Kallscheuer, N., Polen, T., Bott, M. & Marienhagen, J. Reversal of
_β_-oxidative pathways for the microbial production of chemicals and polymer building blocks. _Metab. Eng._ 42, 33–42 (2017). Article CAS PubMed Google Scholar * Yu, K. et al. An
integrated meta-omics approach reveals substrates involved in synergistic interactions in a bisphenol A (BPA)-degrading microbial community. _Microbiome_ 7, 16 (2019). * de Souza, M. L. et
al. Molecular basis of a bacterial consortium: interspecies catabolism of atrazine. _Appl. Environ. Microbiol._ 64, 178–184 (1998). Article PubMed PubMed Central Google Scholar * Pelz,
O. et al. Towards elucidation of microbial community metabolic pathways: unravelling the network of carbon sharing in a pollutant-degrading bacterial consortium by immunocapture and isotopic
ratio mass spectrometry. _Environ. Microbiol._ 1, 167–174 (1999). Article CAS PubMed Google Scholar * Dussud, C. et al. Colonization of non-biodegradable and biodegradable plastics by
marine microorganisms. _Front. Microbiol._ 9, 1571 (2018). Article PubMed PubMed Central Google Scholar * Kirstein, I. V., Wichels, A., Gullans, E., Krohne, G. & Gerdts, G. The
plastisphere-uncovering tightly attached plastic ‘specific’ microorganisms. _PLoS ONE_ 14, 1–17 (2019). Article CAS Google Scholar * Lee, J.-W., Nam, J.-H., Kim, Y.-H., Lee, K.-H. &
Lee, D.-H. Bacterial communities in the initial stage of marine biofilm formation on artificial surfaces. _J. Microbiol._ 46, 174–182 (2008). Article CAS PubMed Google Scholar * Zettler,
E. R., Mincer, T. J. & Amaral-Zettler, L. A. Life in the ‘plastisphere’: Microbial communities on plastic marine debris. _Environ. Sci. Technol._ 47, 7137–7146 (2013). Article ADS CAS
PubMed Google Scholar * Sekiguchi, T. et al. Biodegradation of aliphatic polyesters soaked in deep seawaters and isolation of poly (caprolactone)-degrading bacteria. _Polym. Degrad.
Stab._ 96, 1397–1403 (2011). Article CAS Google Scholar * Sekiguchi, T. et al. Isolation and characterization of biodegradable plastic degrading bacteria from deep-sea environments.
_JAMSTEC Rep. Res. Dev__._ 11, 33–41 (2011). Article Google Scholar * Oberbeckmann, S., Osborn, A. M. & Duhaime, M. B. Microbes on a bottle: Substrate, season and geography influence
community composition of microbes colonizing marine plastic debris. _PLoS ONE_ 11, 1–24 (2016). Article CAS Google Scholar * Oberbeckmann, S., Loeder, M. G., Gerdts, G. & Osborn, A.
M. Spatial and seasonal variation in diversity and structure of microbial biofilms on marine plastics in northern european waters. _FEMS Microbiol. Ecol._ 90, 478–492 (2014). Article CAS
PubMed Google Scholar * Gauthier, M. J. et al. Marinobacter hydrocarbonoclasticus gen. nov., sp. nov., a new, extremely halotolerant, hydrocarbon-degrading marine bacterium. _Int. J. Syst.
Bacteriol._ 42, 568–576 (1992). Article CAS PubMed Google Scholar * Kleindienst, S. et al. Chemical dispersants can suppress the activity of natural oil-degrading microorganisms. _Proc.
Natl Acad. Sci. USA_ 112, 14900–14905 (2015). Article ADS CAS PubMed PubMed Central Google Scholar * Gao, W. et al. Marinobacter nanhaiticus sp. nov., polycyclic aromatic
hydrocarbon-degrading bacterium isolated from the sediment of the South China Sea. _Antonie Van. Leeuwenhoek_ 103, 485–491 (2013). Article CAS PubMed Google Scholar * Garrity, G. M.,
Bell, J. A. & Lilburn, T. Rhodobacteraceae family. nov. 1–2 (American Cancer Society, 2015). * Pujalte, M. J., Lucena, T., Ruvira, M. A., Arahal, D. R. & Macián, M. C. The family
Rhodobacteraceae. In (eds Rosenberg, E., DeLong, E. F., Lory, S., Stackebrandt, E. & Thompson, F.) _The Prokaryotes: Alphaproteobacteria and Betaproteobacteria._ 439–512 (Springer Berlin
Heidelberg, Berlin, Heidelberg, 2014). * Michael, V. et al. Biofilm plasmids with a rhamnose operon are widely distributed determinants of the ‘swim-or-stick’ lifestyle in roseobacters.
_ISME J._ 10, 2498–2513 (2016). Article CAS PubMed PubMed Central Google Scholar * Belas, R., Horikawa, E., Aizawa, S.-I. & Suvanasuthi, R. Genetic determinants of Silicibacter sp.
TM1040 motility. _J. Bacteriol._ 191, 4502–4512 (2009). Article CAS PubMed PubMed Central Google Scholar * D’Alvise, P. W., Magdenoska, O., Melchiorsen, J., Nielsen, K. F. & Gram,
L. Biofilm formation and antibiotic production in Ruegeria mobilis are influenced by intracellular concentrations of cyclic dimeric guanosinmonophosphate. _Environ. Microbiol._ 16, 1252–1266
(2014). Article CAS PubMed Google Scholar * Probandt, D. et al. Permeability shapes bacterial communities in sublittoral surface sediments. _Environ. Microbiol._ 19, 1584–1599 (2017).
Article CAS PubMed Google Scholar * Gade, D., Stührmann, T., Reinhardt, R. & Rabus, R. Growth phase dependent regulation of protein composition in Rhodopirellula baltica. _Environ.
Microbiol._ 7, 1074–1084 (2005). Article CAS PubMed Google Scholar * Perz, V. et al. Substrate specificities of cutinases on aliphatic-aromatic polyesters and on their model substrates.
_N. Biotechnol._ 33, 295–304 (2016). Article CAS PubMed Google Scholar * Will, S. E. et al. Day and Night: metabolic profiles and evolutionary relationships of six axenic non-marine
cyanobacteria. _Genome Biol. Evol._ 11, 270–294 (2018). Article CAS PubMed Central Google Scholar * Haange, S. et al. Disease development is accompanied by changes in bacterial protein
abundance and functions in a refined model of dextran sulfate sodium (dss)-induced colitis. _J. Proteome Res._ 18, 1774–1786 (2019). Article CAS PubMed Google Scholar * Käll, L.,
Canterbury, J., Weston, J., Noble, W. & Maccoss, M. Semi-supervised learning for peptide identification from shotgun proteomics datasets. _Nat. Methods_ 4, 923–5 (2007). Article CAS
PubMed Google Scholar * Uritskiy, G. V., DiRuggiero, J. & Taylor, J. MetaWRAP-a flexible pipeline for genome-resolved metagenomic data analysis. _Microbiome_ 6, 158 (2018). Article
PubMed PubMed Central Google Scholar * Krueger, F. Trim galore. https://github.com/FelixKrueger/TrimGalore (2019). * Nurk, S., Meleshko, D., Korobeynikov, A. & Pevzner, P. A.
metaSPAdes: a new versatile metagenomic assembler. _Genome Res._ 27, 824–834 (2017). Article CAS PubMed PubMed Central Google Scholar * Seemann, T. Prokka: rapid prokaryotic genome
annotation. _Bioinformatics_ 30, 2068–2069 (2014). Article CAS PubMed Google Scholar * Alneberg, J. et al. Binning metagenomic contigs by coverage and composition. _Nat. Methods_ 11,
1144–1146 (2014). Article CAS PubMed Google Scholar * Wu, Y. W., Simmons, B. A. & Singer, S. W. MaxBin 2.0: an automated binning algorithm to recover genomes from multiple
metagenomic datasets. _Bioinformatics_ 32, 605–607 (2016). Article CAS PubMed Google Scholar * Kang, D. D. et al. MetaBAT 2: an adaptive binning algorithm for robust and efficient genome
reconstruction from metagenome assemblies. _PeerJ_ 7, e7359 (2019). Article PubMed PubMed Central Google Scholar * Parks, D. H., Imelfort, M., Skennerton, C. T., Hugenholtz, P. &
Tyson, G. W. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. _Genome Res._ 25, 1043–1055 (2015). Article CAS PubMed PubMed
Central Google Scholar * Chaumeil, P. A., Mussig, A. J., Hugenholtz, P. & Parks, D. H. GTDB-Tk: a toolkit to classify genomes with the Genome Taxonomy Database. _Bioinformatics_
(2019). * Liao, Y., Smyth, G. K. & Shi, W. The Subread aligner: fast, accurate and scalable read mapping by seed-and-vote. _Nucleic Acids Res._ 41, e108 (2013). Article CAS PubMed
PubMed Central Google Scholar * Liao, Y., Smyth, G. K. & Shi, W. The R package Rsubread is easier, faster, cheaper and better for alignment and quantification of RNA sequencing reads.
_Nucleic Acids Res._ 47, e47 (2019). Article CAS PubMed PubMed Central Google Scholar * Li, H. et al. The Sequence Alignment/Map format and SAMtools. _Bioinformatics_ 25, 2078–2079
(2009). Article CAS PubMed PubMed Central Google Scholar * Kassambara, A. ggcorrplot: Visualization of a correlation matrix using ggplot2. (2019). * Kopylova, E., Noé, L. & Touzet,
H. SortMeRNA: fast and accurate filtering of ribosomal RNAs in metatranscriptomic data. _Bioinformatics_ 28, 3211–3217 (2012). Article CAS PubMed Google Scholar * Love, M. I., Huber, W.
& Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. _Genome Biol._ 15, 550 (2014). Article CAS PubMed PubMed Central Google Scholar *
Anders, S. & Huber, W. Differential expression analysis for sequence count data. _Genome Biol._ 11, R106 (2010). Article CAS PubMed PubMed Central Google Scholar * Zhu, A., Ibrahim,
J. G. & Love, M. I. Heavy-tailed prior distributions for sequence count data: removing the noise and preserving large differences. _Bioinformatics_ 35, 2084–2092 (2019). Article CAS
PubMed Google Scholar * Benjamini, Y. & Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. _J. R. Stat. Soc. B_ 57, 289–300
(1995). MathSciNet MATH Google Scholar * Välikangas, T., Suomi, T. & Elo, L. L. A systematic evaluation of normalization methods in quantitative label-free proteomics. _Brief.
Bioinform._ 19, 1–11 (2016). PubMed Central Google Scholar * Chawade, A., Alexandersson, E. & Levander, F. Normalyzer: a tool for rapid evaluation of normalization methods for omics
data sets. _J. Proteome Res._ 13, 3114–3120 (2014). Article CAS PubMed PubMed Central Google Scholar * Gu, Z., Eils, R. & Schlesner, M. Complex heatmaps reveal patterns and
correlations in multidimensional genomic data. _Bioinformatics_ 32, 2847–2849 (2016). Article CAS PubMed Google Scholar * Krzywinski, M. I. et al. Circos: an information aesthetic for
comparative genomics. _Genome Res._ 9, 1639–1645 (2009). Article CAS Google Scholar * Yu, G., Wang, L.-G., Han, Y. & He, Q.-Y. clusterprofiler: an R package for comparing biological
themes among gene clusters. _OMICS_ 16, 284–287 (2012). Article CAS PubMed PubMed Central Google Scholar * R Core Team. _R: A Language and Environment for Statistical Computing. R
Foundation for Statistical Computing._ Vienna, Austria (2019). * Notredame, C., Higgins, D. G. & Heringa, J. T-coffee: a novel method for fast and accurate multiple sequence alignment.
_J. Mol. Biol._ 302, 205–217 (2000). Article CAS PubMed Google Scholar * Larkin, M. et al. Clustal W and Clustal X version 2.0. _Bioinformatics_ 23, 2947–2948 (2007). Article CAS
PubMed Google Scholar * Saitou, N. & Nei, M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. _Mol. Biol. Evol._ 4, 406–425 (1987). CAS PubMed Google
Scholar * Letunic, I. & Bork, P. Interactive Tree Of Life (iTOL): an online tool for phylogenetic tree display and annotation. _Bioinformatics_ 23, 127–128 (2006). Article CAS PubMed
Google Scholar Download references ACKNOWLEDGEMENTS The authors thank Anja Heuer (Microbial Biotechnology group, DSMZ) and Gesa Martens (Bacterial Metabolomics group, DSMZ) for technical
assistance, Kathleen Eismann (Molecular System Biology department, UFZ), and Silke Reinecke (Natural Product Chemistry group, HZI), for providing us with technical expertise and support.
Manfred Rohde (Central Facility for Microscopy, HZI) is acknowledged for the generation the SEM images, and Mark Brönstrup (Chemical Biology department, HZI) is acknowledged for providing
access to the FTIR spectrometer. In addition, the authors acknowledge the use of de.NBI cloud and the support by the High Performance and Cloud Computing Group at the Zentrum für
Datenverarbeitung of the University of Tübingen and the Federal Ministry of Education and Research (BMBF) through grant no 031 A535A. The authors thank BASF SE for financial support. FUNDING
Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Junior Research Group Microbial Biotechnology, Leibniz Institute DSMZ-German
Collection of Microorganisms and Cell Cultures, Inhoffenstraße 7B, 38124, Braunschweig, Germany Ingrid E. Meyer-Cifuentes & Başak Öztürk * Department of Biological Oceanography, Leibniz
Institute of Baltic Sea Research, Seestraße 15, D-18119, Rostock, Germany Johannes Werner * Department of Molecular Systems Biology, Helmholtz-Centre for Environmental Research-UFZ,
Permoserstraße 15, 04318, Leipzig, Germany Nico Jehmlich * Junior Research Group Bacterial Metabolomics, Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures,
Inhoffenstraße 7B, 38124, Braunschweig, Germany Sabine E. Will & Meina Neumann-Schaal Authors * Ingrid E. Meyer-Cifuentes View author publications You can also search for this author
inPubMed Google Scholar * Johannes Werner View author publications You can also search for this author inPubMed Google Scholar * Nico Jehmlich View author publications You can also search
for this author inPubMed Google Scholar * Sabine E. Will View author publications You can also search for this author inPubMed Google Scholar * Meina Neumann-Schaal View author publications
You can also search for this author inPubMed Google Scholar * Başak Öztürk View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS I.E.M.C.
designed and performed experiments, analyzed the data, and wrote the paper; J.W. carried out the bioinformatic analysis of the metagenomes and metatranscriptomes; N.J. performed the
metaproteomic analysis by nano LC-MS; M.N. and S.W. performed the metabolite analysis by GC-MS; B.Ö designed the experiments, analyzed the data, wrote the paper, and supervised the project.
All authors have contributed to the editing of the paper and agree on the final version. CORRESPONDING AUTHOR Correspondence to Başak Öztürk. ETHICS DECLARATIONS COMPETING INTERESTS The
authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Georg Guebitz and the other, anonymous, reviewers for their contribution
to the peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Meyer-Cifuentes, I.E., Werner, J., Jehmlich, N. _et
al._ Synergistic biodegradation of aromatic-aliphatic copolyester plastic by a marine microbial consortium. _Nat Commun_ 11, 5790 (2020). https://doi.org/10.1038/s41467-020-19583-2 Download
citation * Received: 12 August 2020 * Accepted: 13 October 2020 * Published: 13 November 2020 * DOI: https://doi.org/10.1038/s41467-020-19583-2 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative