- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Hyaluronan is widely used in cosmetics and pharmaceutics. Development of robust and safe cell factories and cultivation approaches to efficiently produce hyaluronan is of many
interests. Here, we describe the metabolic engineering of _Corynebacterium glutamicum_ and application of a fermentation strategy to manufacture hyaluronan with different molecular weights.
_C. glutamicum_ is engineered by combinatorial overexpression of type I hyaluronan synthase, enzymes of intermediate metabolic pathways and attenuation of extracellular polysaccharide
biosynthesis. The engineered strain produces 34.2 g L−1 hyaluronan in fed-batch cultures. We find secreted hyaluronan encapsulates _C. glutamicum_, changes its cell morphology and inhibits
metabolism. Disruption of the encapsulation with leech hyaluronidase restores metabolism and leads to hyper hyaluronan productions of 74.1 g L−1. Meanwhile, the molecular weight of
hyaluronan is also highly tunable. These results demonstrate combinatorial optimization of cell factories and the extracellular environment is efficacious and likely applicable for the
production of other biopolymers. SIMILAR CONTENT BEING VIEWED BY OTHERS REGULATING CELLULAR METABOLISM AND MORPHOLOGY TO ACHIEVE HIGH-YIELD SYNTHESIS OF HYALURONAN WITH CONTROLLABLE
MOLECULAR WEIGHTS Article Open access 28 February 2025 PRODUCTION AND PURIFICATION OF HIGHER MOLECULAR WEIGHT CHONDROITIN BY METABOLICALLY ENGINEERED _ESCHERICHIA COLI_ K4 STRAINS Article
Open access 06 August 2020 STRUCTURAL INSIGHTS INTO TRANSLOCATION AND TAILORED SYNTHESIS OF HYALURONAN Article Open access 25 September 2024 INTRODUCTION Hyaluronan (hyaluronic acid or HA)
is a negatively charged, non-sulfated glycosaminoglycan comprising repeating uridine diphosphate glucuronate (UDP-GlcA) and uridine diphosphate N-acetylglucosamine (UDP-GlcNAc) disaccharide
units. It is a natural substance in vertebrates and mainly found in the eyes, joints and skin, where it absorbs large amounts of water for joint lubrication1, cell coating or repair of
damaged skin tissue2. In addition to vertebrates, some pathogenic microorganisms, such as group A _Streptococcus_3 and _Pasteurella multocida_ type A strains4, produce HA as the major
component of their capsules for protection against exterior damage. Due to its biocompatibility, hygroscopicity and non-immunogenicity, HA and its derivatives are important to the cosmetics,
pharmaceutical, biomedical, and food industries5,6,7,8. Currently, commercial production of HA is mainly dependent on fermentation of group A _Streptococcus_9; however, high risk of
pathogenicity to livestock and contamination by exotoxins in the HA product hinder its broader application. The development of metabolic engineering allows the engineering of non-pathogenic
_Escherichia coli_ strains for heterologous HA production10,11. To further eliminate potential safety concerns, many generally recognized as safe (GRAS) strains, such as _Bacillus
subtilis_12,13,14,15,16, _Lactococcus lactis_17,18,19,20, _Corynebacterium glutamicum_21,22, and _Pichia pastoris_23, have been engineered as alternative producers of HA. Additionally,
cell-free systems have been exploited to produce HA at specific molecular weight (MW)24,25. Various strategies have been adopted to construct these HA producers, with selection of HA
synthases and host strains representing the most critical step, as variations in HA synthase sequence, structural conformation26, or host cell metabolic capacities make differences in HA
yield or MW. HA synthase activities have been improved by protein engineering24,27 or modification of the microenvironment of the enzymatic reaction, including the membrane lipid
composition28. Moreover, metabolic engineering strategies such as overexpressing enzymes of intermediate metabolic pathways (e.g., UDP-glucose 6-dehydrogenase or glucosamine-1-phosphate
N-acetyltransferase) or blocking the synthesis of unwanted metabolites (e.g., l-lactate) were adopted to drive the generation of intermediate metabolites required for HA synthesis29,30. In
view of the GRAS status and metabolic capacity, _C. glutamicum_ as the Gram-positive model organism has been engineered as an HA producer. In 2014, Hoffmann et al.21 first constructed the HA
biosynthetic pathway by expressing _Streptococcus equi_ subsp. _zooepidemicus_ HA synthase (seHasA) in _C. glutamicum_. The engineered strain produced 1.2 g L−1 of HA. In 2016, Cheng et
al.31 co-expressed the codon-optimized _Streptococcus dysgalactiae_ subsp. _equisimilis ssehasA_ gene along with endogenous _ugdA_ in _C. glutamicum_ to produce 8.3 g L−1 HA in fed-batch
cultivation. Additionally, deletion of _ldh_, encoding lactate dehydrogenase, increased HA production to 21.6 g L−1 in fed-batch culture29. A follow-up study enhanced the HA yield to 28.7 g
L−1 by attenuating the glycolysis pathway, pentose phosphate pathway and the dehydrogenation of pyruvate22. In the present study, we engineer _C. glutamicum_ for high-yield HA production by
selecting the most productive HA synthase, overexpressing enzymes of the intermediate pathways to convert glucose into the HA building blocks UDP-GlcA and UDP-GlcNAc and decreasing
endogenous extracellular polysaccharide biosynthesis. The engineered strain produces 34.2 g L−1 HA in fed-batch cultures. Our analysis of cell morphology reveals that the secreted HA forms
an HA capsule-like layer, which is subsequently found to restrict nutrient uptake and inhibit HA synthesis. To relieve this inhibition effect, we supplement leech hyaluronidase (LHYal,
hydrolase)32 to the fed-batch culture to disrupt cell encapsulation and decrease broth viscosity. This strategy significantly promotes glucose uptake and HA production. RESULTS CONSTRUCTION
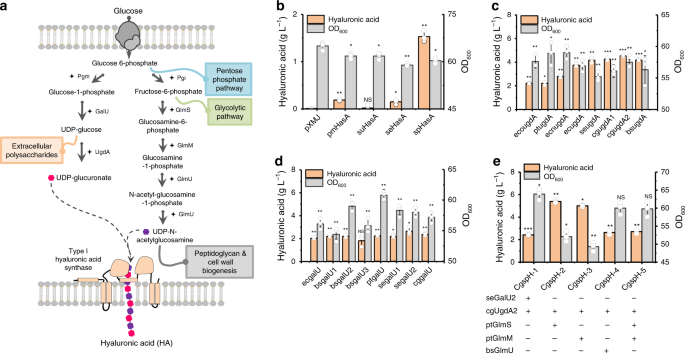
OF HA-PRODUCING _C. GLUTAMICUM_ STRAINS HA synthase polymerizes HA chains using two building blocks: UDP-GlcA and UDP-GlcNAc (Fig. 1a). Two types of bacterial HA synthases have been
identified: type I synthase is a bifunctional enzyme that polymerizes the HA chain, as well as transfers HA across the cell membrane33,34,35 (Supplementary Fig. 1); and type II HA synthase,
found only in _P. multocida_36, is a cytosolic glycosyltransferase with high affinity to the cytoplasmic membrane (Supplementary Fig. 1). Here, HA synthases from _P. multocida_ (pmHasA), _S.
pyogenes_ (spHasA), _S. uberis_ (suHasA), and_ S. equi_ subsp. _zooepidemicus_ HasA (seHasA) were selected and overexpressed individually in the host strain _C. glutamicum_ ATCC 13032.
Comparatively, spHasA generated the highest HA titer (1.5 g L−1) in shake-flask cultivation, which was 10-fold and 7-fold higher than that of seHasA and pmHasA, respectively (Fig. 1b).
Almost no HA was synthesized by suHasA (Fig. 1b). Protein alignment showed seHasA21 and sseHasA22,29,31 share the same protein sequence. In comparison, there is 72% sequence identity between
seHasA and spHasA and 71% sequence identity between suHasA and spHasA (Supplementary Fig. 2a). Western Blot demonstrated suHasA expressed very poorly in _C. glutamicum_ while the expression
levels of spHasA and seHasA are similar (Supplementary Fig. 2b). To further explore the reason of the distinct HA synthesis capabilities between seHasA and spHasA, we replaced the most
dissimilar region of seHasA from spHasA, the first transmembrane helix Leu7-Val25 of seHasA with the corresponding transmembrane helix Thr7-Met25 of spHasA35 to generate seHasAThr7-Met25.
The mutant seHasAThr7-Met25 was expressed to the same level of the wild-type seHasA in _C. glutamicum_ (Supplementary Fig. 2b). However, seHasAThr7-Met25 produced much higher amount of HA
(0.87 g L−1, Supplementary Fig. 2c), suggesting the first tranmembrane helix of type I HA synthase should play critical roles in regulating HA synthesis. After determining spHasA as the best
performing HA synthase, all genes encoding enzymes related to intermediate metabolite-synthesis pathways, including _galU_, _ugdA_, _glmS_, _glmM_, and _glmU_, from five species (_C.
glutamicum_ ATCC13032, cg; _B. subtilis_ 168, bs; _S. equi_ subsp. _zooepidemicus _WSH-24, se; _Pseudomonas putida_ KT2440, pt; and _E. coli_ MG1655, ec) were selected and individually
overexpressed to examine their contributions to HA formation. In particular, _ugdA_ genes (also known as _kfiD_) found in the heparosan-biosynthesis genome island of _E. coli_ O10:K5:H4 ATCC
23506 (eco) and _E. coli_ Nissle 1917 (ecn) (Supplementary Fig. 3) were investigated. We found that UDP-glucose 6-dehydrogenase (UgdA) from different species exhibited different abilities
to promote HA biosynthesis (4.5 g L−1, Fig. 1c). Endogenous UgdA encoded by _cgugdA2_ in _C. glutamicum_ showed the best performance in enhancing HA biosynthesis as compared with GalU from
_S. equi_ subsp. _zooepidemicus_, GlmS, GlmM from _P. putida_ and GlmU from _B. subtilis_, which showed similar capabilities at stimulating HA biosynthesis (around 2 g L−1) (Fig. 1d and
Supplementary Fig. 4a–c). Therefore, we overexpressed _cgugdA2_ along with_ S. equi_ subsp. _zooepidemicus_ _ galU2_ (_segalU2_), _P. putida glmS_ (_ptglmS_) and _glmM_ (_ptglmM_) and _B.
subtilis glmU_ (_bsglmU_), respectively, finding that co-overexpression of _cgugdA2_ with _ptglmS_ or _ptglmM_ improved HA production to 5.4 g L−1 and 5.0 g L−1, respectively, in shake-flask
cultivations (Fig. 1e). By contrast, other combinations failed to further improve the HA synthesis (Fig. 1e). ENHANCEMENT OF HA PRODUCTION BY ATTENUATING THE BIOSYNTHESIS OF EXTRACELLULAR
POLYSACCHARIDES We then investigated a pathway competing with HA synthesis, specifically the biosynthesis of extracellular polysaccharides, which is involved in consumption of key
intermediate metabolites such as UDP-glucose37, the precursor of UDP-GlcA (Fig. 1a). The corynebacterial cell wall contains a peptidoglycan-attached arabinogalactan structure, which is
enveloped by mycolic acid and an outer layer of extracellular polysaccharides, proteins and lipids38 (Fig. 2a). The main components of corynebacterial extracellular polysaccharides (also
considered as capsular polysaccharides or cell-surface polysaccharides) are arabinomannan, mannan and glucan38,39. However, the genes required for biosynthesis of _C. glutamicum_
cell-surface polysaccharides remain largely unknown. Taniguchi et al.40 reported that overexpression of _C. glutamicum_ sigma factor SigD induced secretion of carbohydrate compounds and
raised the expression levels of three glycosyltransferases genes (_cg0420_, _cg0532_, and _cg1181_). Following genome analysis, we found that _cg0420_ locates in close proximity to the
_wzx_, _wzy_, and _wzz_ genes (Fig. 2b). Because the Wzx/Wzy-dependent assembly pathway synthesizes the majority of bacterial cell-surface polysaccharides (Fig. 2a)41, we speculated that
_cg0420_ may participate in biosynthesis of _C. glutamicum_ extracellular polysaccharides. Prior to _cg0420_ deletion, we found three other putative glycosyltransferase genes within the same
genomic region (_cg0424_, _cg0419_, and _cg0438_) (Fig. 2b and Supplementary Fig. 5). After many rounds of trials, we did not obtain single deletions for _cg0420_, _cg0419_, or _cg0438_.
However, we were able to delete _cg0424_ to generate strain Delcg0424. Basing on Delcg0424, we further deleted _cg0420_ and constructed strain Delcg0420,0424. It was found that neither
single _cg0424_ deletion nor double deletion of _cg0420_ and _cg0424_ impaired cell growth (Fig. 2c). Sugar constituent analysis of the Delcg0424 extracellular polysaccharides showed that
the concentration of mannose decreased by 32% while the concentration of arabinose was not affected (Fig. 2d). The concentrations of mannose and arabinose released from strain Delcg0420,0424
extracellular polysaccharides hydrolysis decreased by 24% and 47% respectively, comparing to that of the strain Delcg0424 (Fig. 2d). Thus, Cg0424 and Cg0420 probably have the activities of
mannosyltransferase and arabinosyltransferase to catalyze the formation of mannan and arabinomannan, respectively. Moreover, the synthesis of _C. gultamicum_ cell-surface polysaccharides
probably involves more glycosyltransferases, as the double deletion of _cg0420_ and _cg0424_ does not abolish the synthesis of mannan or arabinomannan. Deletion of these putative
glycosyltransferase genes positively influenced HA synthesis, as HA yield increased by 14.9% with double deletion of _cg0424_ and _cg0420_ to 6.4 g L−1 in shake-flask cultures (Table 1). The
optimal engineered _C. glutamicum_ strain CgspH-7 underwent fed-batch cultivation. After consuming 166 g L−1 glucose, the HA titer reached 34.2 g L−1 (Fig. 2e) and the broth turned viscous
(Supplementary Table 1). HA ACCUMULATION ENCAPSULATES _C. GLUTAMICUM_ CELLS HA is produced as the main component of some bacterial capsules and is attached to the cell surface to provide
protection and virulence. To determine how HA is released into culture broth and its distribution on the _C. glutamicum_ surface, we visualized the HA-producing strains CgspH-2 and CgspH-7
by phase-contrast microscopy and found that both strains exhibited cell aggregation along with HA accumulation (Fig. 3a). Moreover, the engineered _C. glutamicum_ strains showed a chain-like
morphology (Fig. 3a and Supplementary Fig. 6) resembling that of the native HA-producing _Streptococcus_ species. To visualize HA distribution, we performed negative staining with nigrosin,
a classical capsule-staining dye42. Secreted HA formed a capsule-like layer, with HA-producing cells encapsulated (Fig. 3a), whereas no similar structure was observed in the control strain
_C. glutamicum_ pXMJ-pEC carrying blank vectors. As a consequence, we proposed a model of the encapsulation process (Fig. 3b). HA molecules were not secreted directly into the medium but
rather knitted into a slime layer encapsulating the dividing cells. Additionally, the HA molecules are gradually released from the outer layer and dissolved in the culture broth. In the
presence of adequate glucose, the size of the cell capsule grow continuously (Fig. 3a between time points 16 and 28 hour), whereas decreased glucose availability at the late growth phase
made HA secretion slow down and HA encapsulation decay (Fig. 3a, time point 32 hour). THE HA CAPSULE LAYER RESTRICTS NUTRIENT UPTAKE The HA capsule layer of _Streptococcus_ plays important
roles in resisting phagocytosis and protecting the cells from desiccation and drying3. However, the influence of the HA capsule layer on cell metabolism remains unclear. Therefore, we used
recombinant LHYal with high substrate specificity to disrupt the HA capsule layer of the native HA producer _S. equi_ subsp. _zooepidemicus_ WSH-24 to study the impact of HA capsule on cell
metabolism9. Supplementation with LHYal (6000 U mL−1) disrupted the HA capsule of _S. equi_ subsp. _zooepidemicus_ (Fig. 4a). Additionally, the disruption of capsule sped up glucose
consumption, stimulated cell growth and significantly increased HA yield (Fig. 4b). This result demonstrates that the presence of an HA capsule inhibited nutrient uptake and cell growth. The
influence could be relieved by disruption of cell encapsulation by LHYal treatment (Fig. 4c). Moreover, we supplied different concentrations of high-MW HA (1.3 MDa) to cultures of _C.
glutamicum_ ATCC 13032, a non-HA producer, to examine the impact of HA that released into culture broth on cell growth and metabolism. In order to represent better the natural phenomenon,
the HA supplemented here is produced by the natural HA producer _S. equi_ subsp. _zooepidemicus_. We found significant decreases in cell growth and glucose consumption in the presence of 5 g
L−1 HA and 10 g L−1 HA (Fig. 4d). By contrast, supplementing equal concentrations of HA pretreated with 10000 U mL−1 of LHYal for 8 hours did not slow down cell growth or glucose
consumption (Fig. 4d). These results suggest that increased concentration of high-MW HA released into cultures also restricts nutrient uptake. ELIMINATING THE CAPSULE-LIKE LAYER PROMOTES HA
PRODUCTION We then determined whether elimination of the capsule-like layer of CgspH-7 would also be beneficial to cell growth and HA production. We supplemented 6000 U mL−1 LHYal to CgspH-7
shake-flask cultures upon initiation of HA accumulation. As expected, cell encapsulation did not show up when LHYal was supplemented and the chain-structured cell aggregates partially
recovered to the natural rode shape of _C. glutamicum_ (Fig. 5a). As a result, the glucose consumption and cell growth of CgspH-7 were enhanced and HA synthesis was accordingly promoted. No
such effects were observed on the control strain pXMJ-pEC (Fig. 5b). Furthermore, addition of LHYal to fed-batch cultures of _C. glutamicum_ CgspH-7 not only improved HA production, but also
resulted in stronger metabolic activities and prolonged exponential phases (Fig. 6a–c). Supplementation of LHYal to a final concentration of 1500, 3000, or 6000 U mL−1 increased the total
consumed glucose from 166 g L−1 (Fig. 2e) to 316 g L−1 (Fig. 6a), 424 g L−1 (Fig. 6b), and 440 g L−1 (Fig. 6c), respectively and increases in the OD600 from 172 (Fig. 2e) to 215, 253, and
231 (Fig. 6a–c), respectively. Additionally, this improved HA production to 46.2 g L−1 (Fig. 6a), 57.5 g L−1 (Fig. 6b), and 74.1 g L−1 (Fig. 6c), whereas the weight average MW of HA
decreased to 155 kDa, 91 kDa and 53 kDa, respectively (Table 2). These results demonstrated that elimination of the capsule-like layer recovered cell metabolism rate and improved HA
production. DISCUSSION Construction of biosynthesis pathways of HA and other glycosaminoglycans in genetically engineered microbes represents a green and safe method for production of these
invaluable compounds. To construct an efficient HA-producing cell factory, selection of a proper HA synthase is critical, given that HA productivity and MW are largely determined by synthase
activity19. Most HA synthases are found in vertebrates, whereas bacterial HA synthases are mainly found in the group A _Streptococcus_3. Streptococcal HasA has four transmembrane helices
and two membrane-associated helices33,34,35; therefore, it is conceivable that the membrane microenvironment influences HasA activity. In the present study, we found that spHasA displayed
stronger HA-synthesis activities in _C. glutamicum_ and resulted in higher HA accumulation relative to HasA from the other species (Fig. 1b). The differences in the sequences of these
enzymes were primarily found in the transmembrane region (Supplementary Fig. 2a), suggesting that spHasA might be better suited to the _C. glutamicum_ cytoplasmic membrane. Recently,
Westbrook et al.27 successfully enhanced the activity of _Streptococcus equisimilis_ HasA (sseHasA) in _B. subtilis_ by regulating membrane cardiolipin content and distribution.
Additionally, previous studies engineered _E. coli_ species as alternative HA producers10,11,43. However, _E. coli_ exhibit a low degree of activity by heterologously expressed HA synthases,
possibly because of the inherent incompatibility between HA synthase and the Gram-negative _E. coli_ cell structure: _E. coli_ cells have two membranes, whereas the type I HA synthase from
Gram-positive _Streptococcus_ secretes HA chains only through the inner membrane. Protein engineering of HasA44, especially changes in the transmembrane region, might represent a possible
direction for further improvements in HA production (Supplementary Fig. 2b, c). In addition to overexpressing UgdA and GlmS for precursor generation (Fig. 1a), deletion of the competing
pathway to increase the supply of UDP-glucuronate and UDP-GlcNAc is a commonly used strategy22,29. In _C. glutamicum_, there is less availability of UDP-GlcA relative to UDP-GlcNAc, given
that overexpression of UgdA improved HA yield by 2-fold (Fig. 1c). Additionally, UDP-GlcNAc is naturally produced for peptidoglycan biosynthesis (Fig. 1a), which is critical for cell
survival. UDP-glucose, the precursor of UDP-GlcA, is used to construct the outmost layer of the _C. glutamicum_ cell wall38, which should be less important to the cell than peptidoglycans
for maintaining the cell-envelope structure. A previous report showed that _cg0420_ expression enhanced the biosynthesis of extracellular polysaccharides40. In the present study, we selected
four putative genes encoding glycosyltransferases (_cg0420_, _c__g0424_, _cg0419_, and _cg0438_) and located in close proximity to _galU_ and _ugdA_ for deletion, finding that loss of
_cg0424_ and _cg0420_ reduced cell-surface polysaccharides such as mannan and arabinomannan (Fig. 2d) and enhanced HA production (Table 1) without impairing cell growth (Fig. 2c). These
results demonstrated that the enzymes encoded by _cg0420_40 and _cg0424_ are involved in extracellular polysaccharide synthesis and that the outmost layer of the _C. glutamicum_ cell wall38
comprising extracellular polysaccharides is likely not essential to cell growth. The feedback effects of HA accumulation on cell morphology and metabolism have previously been ignored. Here,
we found that during the later period of fermentation, cells of the engineered _C. glutamicum_ strain were encapsulated following HA accumulation. The formation of a capsule-like layer
(Fig. 3a) inhibited cell metabolism and growth (Fig. 4b). The morphology of the _C. glutamicum_ CgspH-7 cells resembled the chain shape of native HA-producing _Streptococcus_ species (Fig.
4a). Moreover, our results suggest that the capsule of pathogens not only protects cells against various stressors or phages or acts as a virulence factor, but also could adversely affect
cell metabolism (Fig. 4b). This could explain why capsule formation occurs under so complex regulatory processes45. In conclusion, optimization of the HA-biosynthesis pathway, inactivation
of extracellular polysaccharide biosynthesis, destruction of cell encapsulation and resolving the mass-transfer bottleneck by supplementation with LHYal resulted in an efficient _C.
glutamicum_ cell factory and a fermentation approach. This engineered strain allowed generation of an average of 74.1 g L−1 HA with an MW of 53 kDa in 5-L fed-batch cultures (Fig. 6c and
Table 2). The results and strategies reported here are likely applicable to the production of other biopolymers. METHODS PLASMIDS AND STRAINS Plasmids and strains used in this study are
listed in Supplementary Data 1 and Supplementary Data 2. We used _E. coli_ JM109 for plasmid amplification and recombinant plasmid construction. _C. glutamicum_ ATCC 13032 was used as the
parental strain for breeding all engineered _C. glutamicum_ strains. HA synthase genes (_hasA_) were amplified from the genome of _S. equi_ subsp. _zooepidemicus_ (se) or synthesized by
GENEWIZ (Suzhou, China) according to the published genome sequences of _Streptococcus pyogenes_ (sp), _Streptococcus uberis_ (su) and _Pasteurella multocida_ (pm). The _hasA_ genes were
prefixed with “se”, “sp”, “su”, and “pm” in order to indicate species origins. After amplification with the designated primers sehasA-F and sehasA-R, sphasA-F and sphasA-R, suhasA-F and
suhasA-R and pmhasA-F and pmhasA-R (Supplementary Data 3), _hasA_ genes were ligated into HindIII/BamHI linearized pXMJ19 using the T5 exonuclease DNA assembly (TDEA) method46 to generate
the plasmids pXMJ19-sehasA, pXMJ19-sphasA, pXMJ19-suhasA, and pXMJ19-pmhasA. With the former three plasmids as templates, the linear form of plasmids pXMJ19-sphasA-6His,
pXMJ19-sehasA-6His, pXMJ19-suhasA-6His, pXMJ19-sehasAThr7-Met25 were generated via PCR using designated primers listed in Supplementary Data 3. The PCR products carrying homogenous 5′ and 3′
terminals (included in the designed primers) were transformed into _E. coli_ JM109 after purification and cyclized by the endogenous DNA recombinases. The plasmid
pXMJ19-sehasAThr7-Met25-6His was constructed by PCR in the same way using pXMJ19-sehasAThr7-Met25 as template. Genes encoding enzymes capable of synthesizing HA building blocks, including
_galU_ (encoding glucose-1-phosphate uridylyltransferase), _ugdA_ (encoding UDP-glucose 6-dehydrogenase), _glmM_ (encoding phosphoglucosamine mutase), _glmS_ (encoding
l-glutamine:d-fructose-6-phosphate aminotransferase), _glmU_ (encoding bifunctional N-acetylglucosamine-1-phosphate uridyltransferase and glucosamine-1-phosphate acetyltransferase), were
amplified from different species, including _E. coli_ MG1655 (ec), _P. putida_ KT2440 (pt), _S. equi_ subsp. _zooepidemicus_ (se) and _C. glutamicum_ ATCC13032 (cg), using the designated
primers listed in Supplementary Data 3. Additionally, we cloned the _ugdA_ genes located in the heparosan synthesis gene cluster of _E. coli_ O10:K5:H4 and _E. coli_ Nissle 1917. The primers
and the amplified genes were prefixed with “ec”, “pt”, “se”, “cg”, “eco”, and “ecn” in order to indicate their species origins. These genes were assembled individually or in specified
combinations into the pEC-XK99E plasmid using the TEDA method46. Homologous regions with a length of ~750 bp were amplified upstream and downstream of _cg0420_ and _cg0424_ using primers
0420-up-F/0420-up-R, 0420-down-F/0420-down-R, and 0424-up-F/0424-up-R, 0424-down-F/0424-down-R, respectively. The homologous regions were combined with the EcoRI/BamHI linearized pK18mobSacB
plasmid with to create pK18mobSacB-0420 and pK18mobSacB-0424, which were subsequently used for gene deletions of _cg0420_ and _cg0424_. All plasmids were transferred to _C. glutamicum_ ATCC
13032 by electroporation (200 Ω, 12.5 kV cm−1, pulse duration 4 ms) and a subsequent heat shock (46 °C for 6 minutes in BHIS medium)47. Recombinant strains were selected with kanamycin
resistance. In the same way, integrations of the plasmids pK18mobSacB-0420 and pK18mobSacB-0424 into the chromosome were selected with kanamycin resistance. Segregation of the plasmids from
chromosome was counter-selected with sucrose-induced lethality47. Loss of the gene _cg0420_ or _cg0424_ was confirmed with colony PCR and DNA sequencing. MEDIUM AND CULTIVATION _E. coli_ was
cultivated in Luria-Bertani medium (tryptone 10 g L−1, NaCl 10 g L−1, yeast extract 5 g L−1, pH 7.0) at 37 °C, and _C. glutamicum_ was cultivated at 30 °C in BHIS medium [37 g L−1 Brain
Heart Infusion (Difco), 91 g L−1 sorbitol]. Competent _C. glutamicum_ cells for electroporation were prepared by cultivating _C. glutamicum_ strains in BHIS medium to OD600 of 1.5 and
washing cells three times with 10% (v/v) glycerol47. Chloramphenicol (15 mg L−1), kanamycin (25 mg L−1) or sucrose [15% (m/v)] was supplemented, as necessary. To produce HA in shake-flasks,
engineered _C. glutamicum_ strains were cultivated at 28 °C with shaking at 200 rpm in a modified glucose-corn steep powder medium29. The medium contained 40 g L−1 glucose, 20 g L−1 corn
steep powder, 20 g L−1 (NH4)2SO4, 1 g L−1 KH2PO4, 1 g L−1 K2HPO4, 0.25 g L−1 MgSO4, and 42 g L−1 MOPS [3-(N-morpholino) propanesulfonic acid]. The initial optical density at 600 nm (OD600)
of the shake-flask cultivation was 0.2, and isopropyl‐β‐d‐thiogalactoside (IPTG) was added to induce target gene expression at time point 2.5 hour. LHYal was supplemented at time point 20
hour to a final concentration of 6000 U mL−1, as necessary. Fed-batch cultivations of _C. glutamicum_ were performed in a 5-L fermenter with 2.5 L of the glucose-corn steep powder medium.
Seed cultures were prepared in glucose-corn steep powder medium containing 40 g L−1 glucose at 30 °C and shaken at 220 rpm for 8–10 hours. During fed-batch cultivations, glucose [70% (m/v)]
was fed according to real-time glucose consumption in order to dynamically maintain its concentration between 10 and 15 g L−1. Ammonia solution [14% (v/v)] was automatically fed in order to
maintain the culture pH at between 6.5 and 7.0. Air flow was maintained at ~5 vvm. LHYal was supplemented to final concentrations of 1500, 3000, or 6000 U mL−1 at time point 20 hour. _S.
equi_ subsp. _zooepidemicus _WSH-24 seed culture was grown at 37 °C and shaking at 220 rpm for 14–16 hours in medium containing 20 g L−1 glucose, 20 g L−1 yeast extract, 2 g L−1 MgSO4·7H2O,
0.1 g L−1 MnSO4·4H2O, 1.5 g L−1 Na2HPO4, 0.64 g L−1 NaH2PO4, 2.0 g L−1 KH2PO4, 0.5 g L−1 NaHCO3, 20 g L−1 CaCO3, 200 μg L−1 CaCl2, 46 μg L−1 ZnCl2, 19 μg L−1 CuSO4·5H2O, pH 7.2. Seed
cultures were inoculated into a 3-L fermenter containing 1.5 L of fermentation medium with a composition of 70 g L−1 glucose, 20 g L−1 yeast extract, 6.2 g L−1 Na2HPO4, 1.3 g L−1 K2SO4, 2 g
L−1 MgSO4·7H2O, 200 μg L−1 CaCl2, 46 μg L−1 ZnCl2, 19 μg L−1 CuSO4·5H2O, pH 7.2. For fed-batch cultivation at 37 °C, NaOH (5 M) was automatically fed to maintain pH between 6.9 and 7.1, and
air flow was maintained at ~3 vvm. LHYal (final concentration 6000 U mL−1) was supplemented at the time of seed inoculation. MEASUREMENT OF CELL GROWTH AND METABOLITE CONCENTRATION Cell
growth was monitored by changes in the OD600. Real-time glucose concentration was measured using an M-100 biosensor analyzer (Shenzhen Siemantec Technology, Shenzhen, China). To measure
total HA, culture broths were autoclaved at 121 °C for 30 minutes. Cell debris was removed by centrifugation at 16,099 × _g_ for 15 minutes. Supernatants were pooled and mixed with four
volumes of ice-cold ethanol and HA was precipitated at −30 °C overnight. Insoluble fractions were subsequently collected by centrifugation at 16,099 × _g_ for 10 minutes. Residual ethanol
was evaporated at room temperature and the semi-dry insoluble fraction was dissolved in deionized water, whereas the water-insoluble fraction was removed by centrifugation. To remove as much
impurity, these steps were repeated three times more. After appropriate dilution (5–1000 fold, depending on HA concentration), HA content was determined via carbazole assay using glucuronic
acid as an external standard48. Supernatants from _C. glutamicum_ culture harboring the blank vector pXMJ19 or pXMJ19/pEC-XK99E were used as the negative control. To measure secreted HA,
cells were removed from the broth by centrifugation at 16,099 × _g_ for 15 minutes and HA was precipitated from the supernatant for measurement, as described above. ANALYSIS OF _C.
GLUTAMICUM_ EXTRACELLULAR POLYSACCHARIDES _C. glutamicum_ ATCC 13032 and strains lacking _cg0424_ or _cg0420_ and _cg0424_ were cultivated in CGXII minimal medium47 at 28 °C for 48 h. Cells
were shaken with glass beads at 200 rpm and room temperature for 1 h to release surface exposed materials including extracellular polysaccharides38. Cells were removed by centrifugation at
7155 × _g_ for 20 minutes. Supernatants were pooled and hydrolyzed with trifluoroacetic acid at 110 °C for 2 hours. Hydrolysates containing reducing sugars were dried in vacuum chamber at 65
°C for 2 hours. Reducing sugars generated from hydrolysis of extracellular polysaccharides reacted with 1-phenyl-3-methyl-5-pyrazolone (PMP) at 70 °C for 60 minutes in the NaOH-methanol
solution to form the sugar-PMP49. The sugar constituent of _C. glutamicum_ extracellular polysaccharides was analyzed using Agilent 1200 HPLC system equipped with a SHISEIDO CAPCELL PAK C18
column (inner diameter 4.6 mm, length 250 mm, particle size 5 μm). Chemicals were separated in the column using a mobile phase of 0.1 M KH2PO4 (pH 6.8) [82% (v/v)] and acetonitrile [18%
(v/v)] with a flow rate of 1 mL min−1. Fractions were detected with absorbance at wavelength of 245 nm. PREPARATION OF RECOMBINANT LEECH HYALURONIDASE LHYal samples were prepared and
purified from recombinant _Pichia pastoris_ through Ni-sepharose affinity resin (HisTrap FF column, GE Healthcare)32. Purified LHYal was stored at −30 °C until use. Determination of the
LHYal glycoside hydrolase activity was performed routinely by the 3,5-dinitrosalicylic acid colorimetric quantification of enzymatically released reducing sugars32. WESTERN BLOT OF TYPE I
HYALURONAN SYNTHASE _C. glutamicum_ ATCC13032 expressing C terminally 6× histidine tagged spHasA, seHasA, suHasA, seHasAThr7-Met25 were digested with 20 mg mL−1 lysozyme at room temperature
for 2 hours and lysed with sonication in buffer containing 50 mM Tris-HCl, 5 mM ethylenediaminetetraacetic acid and 1 mM phenylmethanesulfonyl fluoride. Cell debris was removed by
centrifugation at 16,099 × _g_ for 15 minutes. After BCA protein concentration assay, equal amount (20 μg) of total protein samples were applied to SDS-PAGE. Afterwards, Western Blot was
performed with 1:5000 diluted YTHXBio ZA004 His-tag mouse monoclonal antibody and 1:10000 diluted horseradish peroxidase labeled YTHXBio ZM03 goat anti-mouse IgG(H + L)-HRP) (YTHX
Biotechnology, Beijing, China). ANALYSIS OF CELL MORPHOLOGY BY MICROSCOPY For phase-contrast microscopy, cells were loaded on the thin layer of an agarose pad and visualized using an Eclipse
Ni-E microscope (Nikon, Tokyo, Japan) equipped with a module for phase-contrast microscopy. To visualize cell encapsulation, cells were negatively stained with 100 mg mL−1 water-soluble
nigrosin (Sangon Biotech, Shanghai, China), air dried and visualized with Eclipse Ni-E bright field microscope. Micrographs were processed with ImageJ50. DETERMINATION OF HA EFFECTS ON CELL
METABOLISM AND GROWTH _C. glutamicum_ ATCC 13032 was grown in glucose-corn steep powder medium for 10 hours, followed by centrifugation at 1789 × _g_ for 10 minutes and resuspension with
fresh glucose-corn steep powder medium containing 20 g L−1 glucose and designated concentrations (0, 3, 5, or 10 g L−1) of HA [obtained from Bloomage Biotechnology CO, LTD (Jinan, China)
with purity higher than 98%] or HA pretreated with 10,000 U mL−1 LHYal at room temperature for 8 hours. The initial OD600 was set to 10 and cell growth and glucose consumption were measured
every 2 hours. MEASUREMENT HA WEIGHT AVERAGE MW Supernatant containing HA was isolated, as described above, using ice-cold ethanol precipitation. The crude HA samples were subjected to
further purification with anion exchange chromatography via the ÄKTA avant 25 preparative chromatography system equipped with a HiPrep Q HP 16/10 (GE Healthcare, USA) column. Column was
equilibrated with 50 mM Tris-HCl (pH 8.0); fractions were gradient eluted with 0–200 mM NaCl and monitored with absorbance at wavelength of 210 nm. Fractions were collected, freeze-dried,
dissolved with appropriate amount of water and filtrated through a 0.22 μm membrane. The weight average MW of HA was measured using high-performance size-exclusion chromatography (HPSEC)
with multi-angle laser light scattering (MALLS) analysis. Briefly, 100 μL of HA sample was injected into an HPSEC-MALLS system (equipped with Waters 515 HPLC pump, Shodex OHpak SB-806HQ and
Shodex OHpak SB-804HQ column series, DAWN HELEOS II MALLS instrument and Optilab dRI detector), fractions were separated in the column series with the mobile phase of 0.02% (m/v) NaN3 at 25
°C at a flow rate of 1.0 mL min−1. The average value of two measurements was used to calculate the final weight average MW of HA. REPORTING SUMMARY Further information on research design is
available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The authors declare that all data supporting the findings of this study are available within the
paper and its supplementary information files. The datasets generated and analyzed during the current study are also available from the corresponding author upon request. The source data
underlying Figures 1b–e, 2c–e, 3a, 4a, b, d, 5a, b, 6a–c, and Tables 1 and 2 as well as Supplementary Figures 2b, c, 4a–c, 6 and Supplementary Table 1 are provided as a Source Data file.
Source data are provided with this paper. REFERENCES * Stecco, C. et al. Hyaluronan within fascia in the etiology of myofascial pain. _Surg. Radio. Anat._ 33, 891–896 (2011). Article Google
Scholar * Averbeck, M. et al. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. _J. Invest. Dermatol._ 127,
687–697 (2007). Article CAS PubMed Google Scholar * Wessels, M. R., Moses, A. E., Goldberg, J. B. & DiCesare, T. J. Hyaluronic acid capsule is a virulence factor for mucoid group A
streptococci. _Proc. Natl Acad. Sci. USA_ 88, 8317–8321 (1991). Article ADS CAS PubMed PubMed Central Google Scholar * Pandit, K. K. & Smith, J. E. Capsular hyaluronic acid in
_Pasteurella multocida_ type A and its counterpart in type D. _Res. Vet. Sci._ 54, 20–24 (1993). Article CAS PubMed Google Scholar * Xu, X., Jha, A. K., Harrington, D. A., Farach-Carson,
M. C. & Jia, X. Hyaluronic acid-based hydrogels: from a natural polysaccharide to complex networks. _Soft Matter_ 8, 3280–3294 (2012). Article ADS CAS PubMed PubMed Central Google
Scholar * Walimbe, T., Panitch, A. & Sivasankar, P. M. A review of hyaluronic acid and hyaluronic acid-based hydrogels for vocal fold tissue engineering. _J. Voice_ 31, 416–423 (2017).
Article PubMed PubMed Central Google Scholar * Widjaja, L. K. et al. Hyaluronic acid-based nanocomposite hydrogels for ocular drug delivery applications. _J. Biomed. Mater. Res. A_ 102,
3056–3065 (2014). Article PubMed CAS Google Scholar * Kang, Z. et al. Bio-based strategies for producing glycosaminoglycans and their oligosaccharides. _Trends Biotechnol._ 36, 806–818
(2018). Article CAS PubMed Google Scholar * Liu, L., Liu, Y., Li, J., Du, G. & Chen, J. Microbial production of hyaluronic acid: current state, challenges, and perspectives. _Microb.
Cell Fact._ 10, 99 (2011). Article CAS PubMed PubMed Central Google Scholar * Mao, Z., Shin, H. D. & Chen, R. A recombinant _E. coli_ bioprocess for hyaluronan synthesis. _Appl.
Microbiol. Biotechnol._ 84, 63–69 (2009). Article CAS PubMed Google Scholar * Yu, H. & Stephanopoulos, G. Metabolic engineering of _Escherichia coli_ for biosynthesis of hyaluronic
acid. _Metab. Eng._ 10, 24–32 (2008). Article CAS PubMed Google Scholar * Westbrook, A. W., Ren, X., Moo-Young, M. & Chou, C. P. Application of hydrocarbon and perfluorocarbon oxygen
vectors to enhance heterologous production of hyaluronic acid in engineered _Bacillus subtilis_. _Biotechnol. Bioeng._ 115, 1239–1252 (2018). Article CAS PubMed Google Scholar * Chien,
L. J. & Lee, C. K. Enhanced hyaluronic acid production in _Bacillus subtilis_ by coexpressing bacterial hemoglobin. _Biotechnol. Prog._ 23, 1017–1022 (2007). CAS PubMed Google Scholar
* Jia, Y. et al. Metabolic engineering of _Bacillus subtilis_ for the efficient biosynthesis of uniform hyaluronic acid with controlled molecular weights. _Bioresour. Technol._ 132,
427–431 (2013). Article CAS PubMed Google Scholar * Jin, P., Kang, Z., Yuan, P., Du, G. & Chen, J. Production of specific-molecular-weight hyaluronan by metabolically engineered
_Bacillus subtilis_ 168. _Metab. Eng._ 35, 21–30 (2016). Article CAS PubMed Google Scholar * Widner, B. et al. Hyaluronic acid production in _Bacillus subtilis_. _Appl. Environ.
Microbiol._ 71, 3747–3752 (2005). Article CAS PubMed PubMed Central Google Scholar * Sunguroglu, C., Sezgin, D. E., Aytar Celik, P. & Cabuk, A. Higher titer hyaluronic acid
production in recombinant _Lactococcus lactis_. _Prep. Biochem. Biotechnol._ 48, 734–742 (2018). Article CAS PubMed Google Scholar * Sheng, J., Ling, P. & Wang, F. Constructing a
recombinant hyaluronic acid biosynthesis operon and producing food-grade hyaluronic acid in _Lactococcus lactis_. _J. Ind. Microbiol. Biotechnol._ 42, 197–206 (2015). Article CAS PubMed
Google Scholar * Jeeva, P., Shanmuga Doss, S., Sundaram, V. & Jayaraman, G. Production of controlled molecular weight hyaluronic acid by glucostat strategy using recombinant
_Lactococcus lactis_ cultures. _Appl. Microbiol. Biotechnol._ 103, 4363–4375 (2019). Article CAS PubMed Google Scholar * Hmar, R. V., Prasad, S. B., Jayaraman, G. & Ramachandran, K.
B. Chromosomal integration of hyaluronic acid synthesis (has) genes enhances the molecular weight of hyaluronan produced in _Lactococcus lactis_. _Biotechnol. J._ 9, 1554–1564 (2014).
Article CAS PubMed Google Scholar * Hoffmann, J. & Altenbuchner, J. Hyaluronic acid production with _Corynebacterium glutamicum_: effect of media composition on yield and molecular
weight. _J. Appl. Microbiol._ 117, 663–678 (2014). Article CAS PubMed Google Scholar * Cheng, F., Yu, H. & Stephanopoulos, G. Engineering _Corynebacterium glutamicum_ for high-titer
biosynthesis of hyaluronic acid. _Metab. Eng._ 55, 276–289 (2019). Article CAS PubMed Google Scholar * Jeong, E., Shim, W. Y. & Kim, J. H. Metabolic engineering of _Pichia pastoris_
for production of hyaluronic acid with high molecular weight. _J. Biotechnol._ 185, 28–36 (2014). Article CAS PubMed Google Scholar * Mandawe, J. et al. Directed evolution of hyaluronic
acid synthase from _Pasteurella multocida_ towards high-molecular-weight hyaluronic acid. _Chembiochem_ 19, 1414–1423 (2018). Article CAS PubMed Google Scholar * Jing, W. &
DeAngelis, P. L. Synchronized chemoenzymatic synthesis of monodisperse hyaluronan polymers. _J. Biol. Chem._ 279, 42345–42349 (2004). Article CAS PubMed Google Scholar * Schulte, S. et
al. Exploiting the diversity of streptococcal hyaluronan synthases for the production of molecular weight-tailored hyaluronan. _Appl. Microbiol. Biotechnol._ 103, 7567–7581 (2019). Article
CAS PubMed Google Scholar * Baggenstoss, B. A. et al. Hyaluronan synthase control of synthesis rate and hyaluronan product size are independent functions differentially affected by
mutations in a conserved tandem B-X7-B motif. _Glycobiology_ 27, 154–164 (2017). Article CAS PubMed PubMed Central Google Scholar * Westbrook, A. W., Ren, X., Moo-Young, M. & Chou,
C. P. Engineering of cell membrane to enhance heterologous production of hyaluronic acid in _Bacillus subtilis_. _Biotechnol. Bioeng._ 115, 216–231 (2018). Article CAS PubMed Google
Scholar * Cheng, F., Luozhong, S., Guo, Z., Yu, H. & Stephanopoulos, G. Enhanced biosynthesis of hyaluronic acid using engineered _Corynebacterium glutamicum_ via metabolic pathway
regulation. _Biotechnol. J._ 12, 1700191 (2017). Article CAS Google Scholar * Kaur, M. & Jayaraman, G. Hyaluronan production and molecular weight is enhanced in pathway-engineered
strains of lactate dehydrogenase-deficient _Lactococcus lactis_. _Metab. Eng. Commun._ 3, 15–23 (2016). Article PubMed PubMed Central Google Scholar * Cheng, F., Gong, Q., Yu, H. &
Stephanopoulos, G. High-titer biosynthesis of hyaluronic acid by recombinant _Corynebacterium glutamicum_. _Biotechnol. J._ 11, 574–584 (2016). Article CAS PubMed Google Scholar * Jin,
P., Kang, Z., Zhang, N., Du, G. & Chen, J. High-yield novel leech hyaluronidase to expedite the preparation of specific hyaluronan oligomers. _Sci. Rep._
https://doi.org/10.1038/srep04471 (2014). * Weigel, P. H. Hyaluronan synthase: the mechanism of initiation at the reducing end and a pendulum model for polysaccharide translocation to the
cell exterior. _Int. J. Cell Biol._ 2015, 367579 (2015). * DeAngelis, P. L., Papaconstantinou, J. & Weigel, P. H. Isolation of a _Streptococcus pyogenes_ gene locus that directs
hyaluronan biosynthesis in acapsular mutants and in heterologous bacteria. _J. Biol. Chem._ 268, 14568–14571 (1993). CAS PubMed Google Scholar * Heldermon, C., DeAngelis, P. L. &
Weigel, P. H. Topological organization of the hyaluronan synthase from _Streptococcus pyogenes_. _J. Biol. Chem._ 276, 2037–2046 (2001). Article CAS PubMed Google Scholar * DeAngelis, P.
L., Jing, W., Drake, R. R. & Achyuthan, A. M. Identification and molecular cloning of a unique hyaluronan synthase from _Pasteurella multocida_. _J. Biol. Chem._ 273, 8454–8458 (1998).
Article CAS PubMed Google Scholar * Donot, F., Fontana, A., Baccou, J. C. & Schorr-Galindo, S. Microbial exopolysaccharides: main examples of synthesis, excretion, genetics and
extraction. _Carbohydr. Polym._ 87, 951–962 (2012). Article CAS Google Scholar * Puech, V. et al. Structure of the cell envelope of corynebacteria: importance of the non-covalently bound
lipids in the formation of the cell wall permeability barrier and fracture plane. _Microbiology_ 147, 1365–1382 (2001). Article CAS PubMed Google Scholar * Jackson, M. The mycobacterial
cell envelope-lipids. _Cold Spring Harb. Perspect. Med._ https://doi.org/10.1101/cshperspect.a021105 (2014). * Taniguchi, H. et al. Physiological roles of sigma factor SigD in
_Corynebacterium glutamicum_. _BMC Microbiol._ 17, 158 (2017). Article PubMed PubMed Central CAS Google Scholar * Islam, S. T. & Lam, J. S. Synthesis of bacterial polysaccharides
via the Wzx/Wzy-dependent pathway. _Can. J. Microbiol._ 60, 697–716 (2014). Article CAS PubMed Google Scholar * Taubitz, I. S. & Brandis, H. A comparison between methods of
identification and serotyping of encapsulated strains of _Haemophilus influenzae_. _Zentralblatt fur Bakteriologie, Mikrobiologie, und Hyg._ 270, 83–97 (1988). CAS Google Scholar * Yu, H.,
Tyo, K., Alper, H., Klein-Marcuschamer, D. & Stephanopoulos, G. A high-throughput screen for hyaluronic acid accumulation in recombinant _Escherichia coli_ transformed by libraries of
engineered sigma factors. _Biotechnol. Bioeng._ 101, 788–796 (2008). Article CAS PubMed Google Scholar * Zhang, L. et al. Rapid evolution of hyaluronan synthase to improve hyaluronan
production and molecular mass in _Bacillus subtilis_. _Biotechnol. Lett._ 38, 2103–2108 (2016). Article CAS PubMed Google Scholar * Tzeng, Y. L., Thomas, J. & Stephens, D. S.
Regulation of capsule in _Neisseria meningitidis_. _Crit. Rev. Microbiol_ 42, 759–772 (2016). CAS PubMed Google Scholar * Xia, Y. et al. T5 exonuclease-dependent assembly offers a
low-cost method for efficient cloning and site-directed mutagenesis. _Nucleic Acids Res._ https://doi.org/10.1093/nar/gky1169 (2019). * Eggeling, L. & Bott, M. _Handbook of
Corynebacterium glutamicum_. (Taylor & Francis, 2005). * Bitter, T. & Muir, H. M. A modified uronic acid carbazole reaction. _Anal. Biochem._ 4, 330–334 (1962). Article CAS PubMed
Google Scholar * Wang, W. et al. Optimization of reactions between reducing sugars and 1-phenyl-3-methyl-5-pyrazolone (PMP) by response surface methodology. _Food Chem._ 254, 158–164
(2018). Article CAS PubMed Google Scholar * Schindelin, J. et al. Fiji: an open-source platform for biological-image analysis. _Nat. Methods_ 9, 676–682 (2012). Article CAS PubMed
Google Scholar Download references ACKNOWLEDGEMENTS This work was financially supported by the National Key R&D program of China (2018YFA0901401), the National Natural Science
Foundation of China (31970085), a grant from the Key Technologies R&D Program of Jiangsu Province (BE2019630), the Fundamental Research Funds for the Central Universities (JUSRP51707A),
the Program for Changjiang Scholars and Innovative Research Team in University (IRT_15R26), and the 111 Project. Y.W. was sponsored by the China Postdoctoral Science Foundation
(2019M651702). AUTHOR INFORMATION Author notes * These authors contributed equally: Yang Wang, Litao Hu. AUTHORS AND AFFILIATIONS * The Key Laboratory of Carbohydrate Chemistry and
Biotechnology, Ministry of Education, Jiangnan University, 214122, Wuxi, China Yang Wang, Litao Hu, Jian Chen, Guocheng Du & Zhen Kang * The Key Laboratory of Industrial Biotechnology,
Ministry of Education, School of Biotechnology, Jiangnan University, 214122, Wuxi, China Yang Wang, Litao Hu, Hao Huang, Hao Wang, Jian Chen, Guocheng Du & Zhen Kang * Bloomage
Biotechnology CO, LTD, 250000, Jinan, China Tianmeng Zhang Authors * Yang Wang View author publications You can also search for this author inPubMed Google Scholar * Litao Hu View author
publications You can also search for this author inPubMed Google Scholar * Hao Huang View author publications You can also search for this author inPubMed Google Scholar * Hao Wang View
author publications You can also search for this author inPubMed Google Scholar * Tianmeng Zhang View author publications You can also search for this author inPubMed Google Scholar * Jian
Chen View author publications You can also search for this author inPubMed Google Scholar * Guocheng Du View author publications You can also search for this author inPubMed Google Scholar *
Zhen Kang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.W. and L.H. performed the experiments. Z.K. and Y.W. designed the experiments.
H.H. purified the leech hyaluronidase. H.W. contributed to the shake-flask cultivations. T.Z. contributed to the fed-batch cultivation. Z.K., Y.W., J.C. and G.D. drafted the manuscript.
CORRESPONDING AUTHOR Correspondence to Zhen Kang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature
Communications_ thanks Lothar Eggeling, and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION REPORTING SUMMARY DESCRIPTION OF ADDITIONAL
SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SUPPLEMENTARY DATA 3 SUPPLEMENTARY DATA 4 SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, Y., Hu, L., Huang, H. _et al._ Eliminating the capsule-like
layer to promote glucose uptake for hyaluronan production by engineered _Corynebacterium glutamicum_. _Nat Commun_ 11, 3120 (2020). https://doi.org/10.1038/s41467-020-16962-7 Download
citation * Received: 19 November 2019 * Accepted: 01 June 2020 * Published: 19 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16962-7 SHARE THIS ARTICLE Anyone you share the following
link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature
SharedIt content-sharing initiative










