- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Flavin-mediated photocatalytic oxidations are established in synthetic chemistry. In contrast, their use in reductive chemistry is rare. Deazaflavins with a much lower reduction
potential are even better suited for reductive chemistry rendering also deazaflavin semiquinones as strong reductants. However, no direct evidence exists for the involvement of these radical
species in reductive processes. Here, we synthesise deazaflavins with different substituents at C5 and demonstrate their photocatalytic activity in the dehalogenation of _p_-halogenanisoles
with best performance under basic conditions. Mechanistic investigations reveal a consecutive photo-induced electron transfer via the semiquinone form of the deazaflavin as part of a
triplet-correlated radical pair after electron transfer from a sacrificial electron donor to the triplet state. A second electron transfer from the excited semiquinone to _p_-halogenanisoles
triggers the final product formation. This study provides first evidence that the reductive power of excited deazaflavin semiquinones can be used in photocatalytic reductive chemistry.
SIMILAR CONTENT BEING VIEWED BY OTHERS DIOXYGEN COMPATIBLE ELECTRON DONOR-ACCEPTOR CATALYTIC SYSTEM AND ITS ENABLED AEROBIC OXYGENATION Article Open access 29 February 2024 FORMATION AND
DEGRADATION OF STRONGLY REDUCING CYANOARENE-BASED RADICAL ANIONS TOWARDS EFFICIENT RADICAL ANION-MEDIATED PHOTOREDOX CATALYSIS Article Open access 06 January 2023 PHOTOCATALYTIC CO2
REDUCTION WITH AMINOANTHRAQUINONE ORGANIC DYES Article Open access 25 February 2023 INTRODUCTION Flavins, in the form of flavin mononucleotide (FMN) or flavin adenine dinucleotide (FAD), act
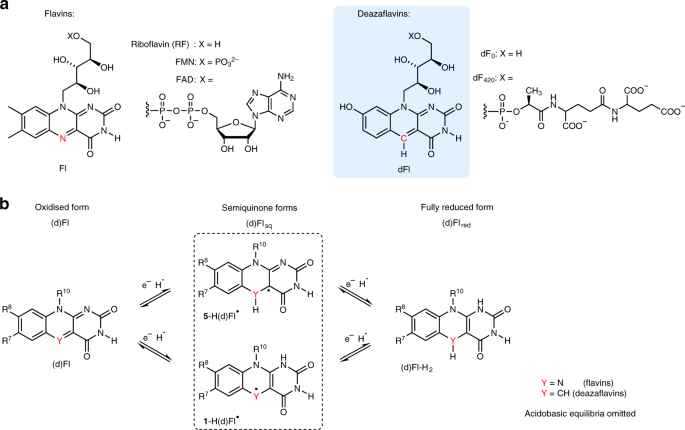
as cofactors in various types of enzymes1,2. Some organisms also use deazaflavins (dFls; dF0 or dF420, see Fig. 1a) where the _N_-5 atom of the isoalloxazine ring is replaced by a carbon
atom3,4. Despite their structural similarity, their roles in biological systems differ substantially3,5. Flavins (Fls) form semiquinones (Flsqs) (Fig. 1b), which are transiently stable in
solution6 and more stable in protein environments7,8. Flsqs are typically involved in one-electron redox processes1,9. Fully reduced flavin (Flred) participates in two-electron reactions or,
similarly as Flsq, interacts readily with molecular oxygen (O2), forming reactive oxygen species (ROS) or flavin hydroperoxide (FlOOH)10,11,12,13. FlOOH is the key agent in oxygenations
mediated by flavin-dependent monooxygenases14,15. In contrast to Fls, dFls serve exclusively as two-electron carriers, thus behaving rather like nicotinamide adenine dinucleotide (phosphate)
(NAD(P)H)3,5. Fully reduced deazaflavins (dFlreds) are considerably stable against oxidation by oxygen, thus avoiding formation of ROS10. This is one reason why dFls have been tested as
native coenzyme (FAD or FMN) substitutes16,17,18. Both Fls and dFls absorb visible light enabling their involvement in light-dependent processes19,20,21,22. Fl cofactors in their fully
reduced form participate in photo-induced electron transfer (PET) reactions in DNA photolyases repairing pyrimidine dimers formed upon exposure to ultraviolet (UV) light23,24. Fls in their
oxidised form are responsible for bacterial bioluminescence25 or perform various functions in photoreceptors7,26,27,28. dFl dF0 in its oxidised form serves as a light-harvesting antenna in
photolyases29. Inspired by nature, artificial Fl derivatives as well as flavoenzymes have been applied in photoredox catalysis and photobiocatalysis, mainly in oxidative transformations or
in energy transfer processes6,20,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44. Photoenzymatic radical polymerisation employing highly reducing excited FADH*− known from photolyases is rare
and a very recent example of a photoreductive process with Fl45. In the dFl series, dFlred has been found to be generated from dFl by PET from ethylenediaminetetraacetic acid used as
sacrificial reductant46. This procedure has been utilised in the NAD(P)H-independent regeneration of FADH2 from FAD16,47. dFl belong to the redox cofactors with the lowest redox potential;
the first reduction potential of dFl [_E_(dFl/dFlsq)] is even more negative than that of Fls by 0.5 V3. Therefore, dFls are better suited for reductive chemistry than Fls. The very negative
first reduction potential renders the dFl semiquinone (dFlsq) as a strong reducing agent. Despite this fact, there is no precedence of reductive processes involving this radical species.
This might result from the very low stability of the dFlsq free in the solution3,5,48. Here we demonstrate that the reducing power of excited dFl radicals can be used in the presence of two
substrates: (i) a sacrificial electron donor, e.g. diisopropyl(ethyl)amine (DIPEA), allowing the photochemical formation of the radical dFlsq and (ii) a substrate for reduction,
_p_-halogenanisole, undergoing dehalogenation via the excited radical dFlsq*. RESULTS DESIGN AND SYNTHESIS A series of 5-dFls 1–5 was synthesised with different substituents in position 5
(H-, Me-, _i_Pr-, CF3-, and Ph-) via two different routes depending on the substituent (Fig. 2 and Supplementary Notes 1 and 2). Derivatives 1–4 were obtained following route A starting from
3,4-dimethoxyaniline (6). Reductive amination yielded compound 7, which was subsequently coupled with 6-chloro-3-methylpyrimidine-2,4(1_H_,3_H_)-dione to give access to the key intermediate
8. Depending on the reaction conditions for the following step, the corresponding products 1–4 were obtained. As this synthetic method did not allow the synthesis of the phenyl derivative
5, route B was developed. In this three-component microwave-assisted procedure, adapted from Tu et al.49, compound 5 was obtained from a reaction of benzaldehyde with
_N_-butyl-3,4-dimethoxyaniline (7) and _N_-methylbarbituric acid (9). Following this procedure, the phenyl derivative could be isolated in both the oxidised (5ox) and fully reduced form
(5red), which reflects the low sensitivity of the dFlred against O23,50. This observation is further supported by incubation of 5red in darkness for 19 h in non-degassed acetonitrile (ACN)
where no considerable formation of 5ox is observed via absorption spectroscopy (Supplementary Note 3). However, light excitation of 5red allows to overcome the activation barrier for
re-oxidation by O2 resulting in almost stoichiometric conversion to 5ox (Supplementary Note 3). PHOTOPHYSICAL AND ELECTROCHEMICAL CHARACTERISATION Compounds 1–3 have similar UV/visible (Vis)
absorption and emission spectra in ACN giving similar electronic transition energies of these dFls with the usual blue-shift of around 40 nm compared to isoalloxazines (Fig. 2 and
Supplementary Fig. 1)51. In contrast, derivative 4 with the strong electron withdrawing group -CF3 shows a red-shifted electronic transition by 26 nm in ACN compared to isoalloxazines. The
compounds 1–4 are all highly emitting with fluorescence quantum yields ranging from 67% to 85% in ACN. Their emission lifetimes in ACN range between 6.8 and 11.1 ns (Supplementary Note 2).
In agreement with literature reports4, cyclo-voltammetry (CV) shows more negative redox potential of the first reduction step of the dFls compared to tetraacetyl riboflavin (TARF)
(_E_(TARF/TARFsq) = −0.79 V, ref. 39) by 0.13–0.52 V (Supplementary Note 4). The phenyl derivative 5 shows deviating characteristics compared to the other dFls. While the electronic
transition energies are also blue-shifted compared to isoalloxazines and the redox potential of the first reduction step is also more negative compared to TARF, its emission properties are
significantly different. The fluorescence quantum yield reaches only 16% and its excited singlet state lifetime is considerably reduced being only 1.7 ns (Supplementary Note 2). Furthermore,
compound 5 is the only derivative that forms a thermally stable fully reduced form 5red, which is only re-oxidised by light as discussed above (Supplementary Note 3). PHOTODEHALOGENATION
Owing to the strong reduction potential of dFl derivatives compared to their Fl analogues, we investigated their use in the reductive photoredox catalysis for the dehalogenation of aryl
halides, which is considered as a benchmark reaction because of their very negative redox potentials. For instance, with a one-electron reduction potential of −2.75 V52, _p_-bromoanisole
(_p_-BA) is beyond the limits of most organic photoredox catalysts53,54, or it requires combination of electrocatalysis and photocatalysis for dehalogenation55,56. We achieved an almost
quantitative yield of _p_-BA dehalogenation using the phenyl derivative 5 (6 mM, 8 mol% relative to substrate), when irradiating at 385 nm in the presence of a sacrificial reducing agent,
here we used DIPEA, and caesium carbonate (Cs2CO3) (Table 1). The anisole yield is independent on the oxidation state of the photocatalyst, i.e. 5ox or 5red, since the dFlred 5red is
re-oxidised by O2 and light as described above (Supplementary Note 3). Noteworthy, 5ox also showed photo-dehalogenation of _p_-chloroanisole, whose redox potential is even more negative
(_E_red = −2.88 V, ref. 52). We next investigated the influence of the solvent and the excitation wavelength on the total product yield (Supplementary Note 5). Interestingly, when
dimethylformamide was used as solvent under otherwise identical conditions the product yield decreased by almost 50%. With respect to the excitation wavelength, the best yield, ca. 80%, was
obtained for wavelengths ≤400 nm. When using light of 455 nm, only 50% yield could be obtained. As a control, in the absence of photocatalyst, DIPEA, or irradiation, no reaction occurs.
Recording the product build-up in case of 5ox showed that 60% conversion was observed already after 6 h. Furthermore, we investigated the effect of basicity on the dehalogenation efficiency
of _p_-BA (Table 2). Without any base, only yields between 10% and 30% are observed for all photocatalysts. In the presence of Cs2CO3, under otherwise identical conditions, the yield was
increased to >60% for the dFls 3 and 5, while the reactivity of dFls 1 and 4 remained unchanged or increased to only 38% for the methyl derivative 2. For the dFls 1 and 5, we then tested
bases with increasing basicity, i.e. NaHCO3, Cs2CO3, and KOtBu. While the activity of 5 was significantly enhanced by Cs2CO3 or KOtBu, the reactivity of 1 only increased in case of KOtBu.
These findings already indicate an important acidobasic equilibrium of a potential intermediate species of the photocatalyst that affects the overall performance. Thus a deprotonated form of
one key intermediate seems to be beneficial (A more detailed discussion can be found in Supplementary Note 6). EXCITED SINGLET STATE REACTION RESULTS IN A LOSS CHANNEL The reactivity of the
excited states of the compounds 1 and 5ox with the sacrificial substrate DIPEA was investigated by time-resolved absorption and emission spectroscopy in the absence of any additional base,
since the main focus was on the understanding of the underlying photocatalytic reaction mechanism (Further studies on the role of specific deprotonation states is currently ongoing in our
laboratories and will be presented elsewhere.). In both cases, the excited state quenching was observed without the formation of any additional transient species (Fig. 3 and Supplementary
Note 7). This indicates the formation of a singlet born radical pair that recombines faster than it is formed owing to spin-allowed radical pair recombination (Fig. 3e and Supplementary Note
7). Such a fast recombination reaction was already observed in the Fl photo-oxidation of aromatic alcohols and represents a pure loss channel for photocatalytic applications6. In case of
the reaction between DIPEA and the excited singlet of the dFl, the bi-molecular reactions rates for 1 and 5ox under low substrate concentrations (<200 mM DIPEA) are (1.05 ± 0.01) × 1010
M−1 s−1 and (9.0 ± 0.1) × 109 M−1 s−1, respectively (see Stern–Volmer analysis in Supplementary Note 7). These values are smaller by 56% and 47%, respectively, compared to the theoretical
diffusion limited bi-molecular reaction limit (see Supplementary Note 7 for a detailed discussion), which might indicate a geometrical preference for reactive encounters allowing for
necessary interactions, and thus enabling a high probability for the reaction to proceed. In the presence of only the actual substrate _p_-BA (with a concentration of 100 mM, which is
sufficiently high for diffusion controlled encounters with the excited singlet state of the photocatalyst), the transient absorption (TA) data of 1 and 5ox resemble those of the
photocatalysts alone demonstrating no reaction between _p_-BA and the corresponding excited states (Fig. 3c and Supplementary Note 7). In addition, in case of the stable fully reduced form,
5red, also no reaction between the excited singlet state and the substrate _p_-BA is observed (Supplementary Note 7). TRIPLET STATE REACTION FORMS TRANSIENTLY STABLE SEMIQUINONE TA
spectroscopy in the ns to μs range was used in order to study the triplet state formation of 5ox (Fig. 4a, e as well as further details in Supplementary Notes 2 and 8). In comparison to
isoalloxazines, its yield is low being only ca. 11% (Supplementary Table 1). In the presence of the substrate _p_-BA (50 mM), no reaction is observed with either the excited singlet or
triplet state of 5ox (Fig. 4b, e). In contrast, in the presence of the sacrificial electron donor DIPEA (50 mM) electron transfer (eT) from DIPEA to the triplet state occurs forming the
semiquinone 5sq (Fig. 4c, f), whose spectrum agrees well with the electrochemically generated spectrum (Fig. 5g and Supplementary Note 9). The yield for eT can be determined from the
corresponding decay rates to \(\Phi _{{\mathrm{eT}}} = 1 - k_0k_{\mathrm{q}}^{ - 1} = 87\%\), where _k_0 is the total decay rate constant of the triplet in the absence and _k_q in the
presence of DIPEA. Considering the concentration of DIPEA (50 mM), the corresponding bi-molecular reaction rate with the triplet state is given to be (8.7 ± 0.1) × 108 M−1 s−1 (_k_eT =
_Φ_eT_k_q ([DIPEA])−1). However, in the available spectral window of our experiment, we do not observe spectral signatures of the corresponding DIPEA radical cation. The assignment as the
semiquinone radical is further proven by recording its electron paramagnetic resonance spectrum under photo-stationary conditions (Supplementary Note 10). In the presence of both _p_-BA and
DIPEA, again, only the triplet reaction with DIPEA is observed, but no indication for a reaction between _p_-BA and 5sq is found (Fig. 4d, f). In summary, while the excited singlet state
reaction with DIPEA results in a singlet born spin correlated radical pair, 1[5sq, DIPEA•+], which recombines faster than it is formed owing to spin allowance, the triplet reaction with
DIPEA involves a triplet born radical pair, 3[5sq, DIPEA•+], which is spin forbidden for recombination, thus allowing its accumulation (Fig. 4g). REACTIVITY OF THE EXCITED DFLSQ In the next
step, we investigated the reactivity of 5sq in its excited state 5sq* towards the dehalogenation of _p_-BA. For this purpose, we generated the semiquinone form 5sq electrochemically without
the need of the light-induced reaction with DIPEA by applying constantly −1.2 V to the sample in ACN and followed its reactivity by UV/Vis absorption over time under the following four
conditions: (1) alone in the dark, (2) in the dark and the presence of _p_-BA (250 mM), (3) alone in the light (_λ_exc = 385 nm), and (4) in the light (_λ_exc = 385 nm) and in the presence
of _p_-BA (250 mM). Alone in the dark, initially the concentration of 5ox depletes completely and 5sq is formed simultaneously (Fig. 5a). On longer delays, the concentration of 5sq reduces
by ca. 18% with the simultaneous formation of 5red indicating partial disproportionation of two 5sq molecules into 5ox and 5red19. The former does not recover considerably because the
electrode-driven one electron reduction immediately forms 5sq. Using the species spectra obtained from the spectro-electrochemical experiment (Fig. 5g and Supplementary Note 9), the sequence
of spectra can be perfectly decomposed in order to obtain the corresponding concentration–time profiles of the contributing species (Fig. 5c). Repeating this experiment in the dark but now
in the presence of _p_-BA, exactly the same behaviour is observed within the experimental error (Fig. 5b, c) demonstrating no reaction between _p_-BA and either of the dFl redox species in
their ground states. This additionally confirms the results of the TA spectroscopy. However, in the experiments with simultaneous illumination of the sample, significantly different kinetics
are observed compared to the situation in the dark. In the absence of _p_-BA but presence of light, again, a rapid reduction of 5ox is observed (Fig. 5d). However, under these conditions
5sq does not accumulate to the same extent (here only to 75% in the transient maximum) as in the case without illumination. Instead, a significantly enhanced formation of 5red up to 70% in
the transient maximum is observed, indicating a light-induced second eT from the electrode to the excited 5sq*. Again, these data can be decomposed well with the known species spectra of the
three redox states of 5 (Fig. 5f). In the final experiment with light and _p_-BA, again, different kinetics are observed compared to the three experiments described before. In this case,
5ox is only converted to ca. 75%. This is accompanied by a significantly reduced accumulation of 5sq and 5red (Fig. 5e), thus indicating a reaction between _p_-BA and 5sq in its excited
state reforming 5ox. Using only the known species-associated spectra (SAS) of the dFl intermediates, this time, the decomposition of the data shows significant deviations. Closer inspection
of the arising absorption band at 300 nm reveals an asymmetric shape compared to the expected absorption band of 5red indicating some underlying spectral contributions of a different species
than those already known from the dFls. After subtraction of a fitted linear combination of the known spectral features of dFl, an absorption spectrum with a single absorption band peaking
at 330 nm remains (magenta spectrum in Fig. 5g). Considering a potential radical anion of _p_-BA as an intermediate arising after eT from excited 5sq* to _p_-BA, we calculated the absorption
spectrum of _p_-BA•‒ quantum chemically using a high level of theory, i.e. state-averaged XMCQDPT-CASSCF(12,12), considering also the solvent environment by the polarisable continuum model
(PCM) for ACN (see Supplementary Note 11). As seen in Fig. 5g and Supplementary Fig. 12a, the calculated spectrum matches the measured spectrum accurately, so that we assign this spectrum to
_p_-BA•‒. Considering the _p_-BA•‒ spectrum together with the already determined species spectra for all dFl intermediates now results in a good fit with deviation within the experimental
error. Since the fit does not depend on the scaling of the _p-_BA•‒ spectrum, we scaled the spectrum arbitrarily so that the resulting concentration–time profile stays below all Fl
contributions. Furthermore, the quantum chemical calculation revealed a significantly longer bond length between the bromine and the adjacent carbon atom in _p_-BA•‒ (2.86 Å) than in _p_-BA
(1.92 Å) as well as a significantly reduced dissociation energy being close to thermal energy fluctuations (Supplementary Fig. 12b), which explains well the observed final dissociation of
the bromine during the dehalogenation, which is triggered by a single electron reduction step as summarised in Fig. 5h (Further details are given in Supplementary Note 11.). Analogous
experiments were conducted with the non-substituted dFl 1. However, in contrast to the experiments with 5, the semi-reduced form could not be observed on the time scale of the measurements.
The failure of detecting the semi-reduced form 1sq indicates that its lifetime is significantly shorter compared to that of 5sq. This agrees with the observation of a significantly lower
yield in the dehalogenation using 1 compared to 5, since less 1sq may be accumulated. PHOTOCATALYTIC REACTION MECHANISM BASED ON CONSECUTIVE PET (CONPET) In summary, our results can be
compiled to the photocatalytic reaction mechanism depicted in Fig. 6. With respect to the redox potential neither 5red (_E_(5sq/5red) = −1.6 V) nor 5sq (_E_(5ox/5sq) = −1.41 V) can reduce
_p_-BA (_E_red = −2.75 V) in their ground states. The participation of the excited fully reduced form 5red* is not observed most likely due to very efficient intrinsic deactivation of the
excitation energy as seen in fast deactivation kinetics on a ps time scale (Supplementary Note 7 and Supplementary Fig. 8). In the presence of a sacrificial electron donor DIPEA, we observe
different reactivities for either the excited singlet or triplet state of 5ox. In the excited singlet state reaction, a singlet born spin correlated radical pair, 1[5sq, DIPEA•+], is formed,
which recombines faster than it is formed. Accordingly, this reaction represents a pure loss channel. In contrast, eT to the triplet state results in a triplet born radical pair, 3[5sq,
DIPEA•+], that is spin forbidden for recombination, thus allowing the accumulation of the considerably stable 5sq. As a consequence, 5sq can be excited by a second photon (_E_*(5ox/5sq) =
−3.3 V), which enables a conPET57 from 5sq* to the substrate, _p_-BA, regenerating 5ox in its ground state and closing the photocatalytic cycle. The dissociation energy for the bond between
the bromine and the adjacent carbon in _p_-BA•‒ is significantly reduced leading to thermally driven dissociation into Br– and the aryl radical. Finally, anisole is formed via hydrogen
abstraction from either a DIPEA radical cation or a solvent molecule57. Owing to the lack of the observation of any protonated 5sq form (see Supplementary Note 6), the radical anion of 5sq
is the active species. DISCUSSION We have described the detailed photocatalytic mechanism of a reductive dehalogenation of _p_-halogenanisole by dFls. The key point is the conPET via the
anionic semiquinone form of the dFl. In doing so, we have revealed that dFls are valuable photocatalysts for reductive chemistry. In summary, after photoexcitation of dFl followed by
intersystem crossing a sacrificial electron donor, at a moderate concentration, will enable a first eT to the triplet state resulting in a considerably stable anionic semiquinone. Additional
excitation of the anionic semiquinone provides sufficient energy for a second eT process from the semiquinone to compounds with highly negative redox potentials, such as
_p_-halogenanisoles. Finally, downstream reactions resulting in product formation occur spontaneously. The action spectrum of the conPET reaction comprises the absorption of two different
coloured photons by the two key photocatalytic forms of dFl, where one is formed from the other having a distinct lifetime in the ns regime. Therefore, a concrete timed illumination of the
photocatalytic system with short and intense pulses at the appropriate excitation wavelengths, which are temporally optimised to the lifetime of the key intermediates, should allow further
improvement on the efficiency. This aspect is of general importance for all conPET-type reactions and should be addressed in future work. To our knowledge, this study shows for the first
time that the reductive power of excited dFlsq can be used in photocatalytic reductive chemistry. Moreover, because of its very negative potential, this species seems to belong to the most
powerful reductants53,55,56,58,59,60,61. Therefore, this study expands the photocatalytic toolbox for new chemical transformations and should serve as a guide for engineering photocatalysts
of the next generation. METHODS MATERIALS Starting materials and reagents were purchased from commercial suppliers (Sigma Aldrich, Alfa Aesar, Acros, Fluka, VWR, or Fluorochem) and were used
without further purification. Solvents were used as p.a. grade or dried and distilled according to literature known procedures. NUCLEAR MAGNETIC RESONANCE (NMR) SPECTROSCOPY NMR spectra
were recorded at room temperature using a Bruker Avance 300 (300 MHz for 1H, 75 MHz for 13C), a Bruker Avance 400 (400 MHz for 1H, 101 MHz for 13C), an Agilent 400-MR DDR2 (400 MHz for 1H
and 101 MHz for 13C), or a Varian Mercury Plus 300 (300 MHz for 1H, 75 MHz for 13C) with internal solvent signal as reference. All chemical shifts are reported in δ-scale as parts per
million (multiplicity, coupling constant _J_, number of protons) relative to the solvent residual peaks as the internal standard (IS). NMR spectra are available in Supplementary Figs. 13–22.
MASS SPECTROMETRY The mass spectrometric measurements were performed at the Central Analytical Laboratory of the University of Regensburg or at the Central Analytical Laboratory of UCT,
Prague on a Finnigan MAT 95, ThermoQuest Finnigan TSQ 7000, Finnigan MATSSQ 710A, Agilent Q-TOF 6540 UHD, or a LTQ Orbitrap Velos (Thermo Fisher Scientific). GAS CHROMATOGRAPHY (GC) GC
measurements were performed on a GC 7890 from Agilent Technologies. Data acquisition and evaluation was done with Agilent ChemStation Rev.C.01.04. A capillary column HP-5MS/30 m × 0.25
mm/0.25 μM film and helium as carrier gas (flow rate of 1 mL/min) were used. The injector temperature (split injection: 40:1 split) was 280 °C, detection temperature 300 °C (FID). The GC
oven temperature programme was adjusted as follows: the initial temperature of 40 °C was kept for 3 min and was increased at a rate of 15 °C/min until the injection temperature of 280 °C was
reached. After 5 min, the temperature was further increased at a rate of 25 °C/min until the final temperature of 300 °C was reached and kept for 5 min. Using 4-methylanisole as an IS, we
obtained the GC calibration given in Supplementary Fig. 23 for the quantitative analysis. CYCLO-VOLTAMMETRY CV measurements were performed with the three-electrode potentiostat galvanostat
(PGSTAT302N, Metrohm Autolab). The control of the measurement instrument, the acquisition, and processing of the CV data were performed with the software Metrohm Autolab NOVA 1.10.4.
Electrochemical studies were carried out under argon atmosphere in ACN containing 0.1 M tetra-_N_-butylammonium hexafluorophosphate using ferrocene/ferrocenium (Fc/Fc+) as an internal
reference. A glassy carbon electrode (working electrode), platinum wire counter electrode, and Ag quasi-reference electrode were employed. SPECTRO-ELECTROCHEMISTRY Measurements were
performed in an Ottle Cell (Optically transparent thin-layer electro-chemical cell), pathlength = 0.02 cm, working electrode: Pt minigrid, counter electrode: Pt minigrid, pseudo reference
electrode: Ag wire. Samples were prepared by degassing a solution of 5ox in ACN (3 mM) via bubbling of Argon for several minutes. The substrate, _p_-BA, was added via a Hamilton syringe
resulting in a solution of 250 mM. A constant electric field of −1.2 V was applied to the cell, and UV/Vis absorption spectra were recorded every 5 s (using an Agilent 8453 spectrometer).
Irradiation was done via a handheld LED (_λ_max = 385 nm) for a time of ca. 2 s in between each spectrum. Spectro-electrochemical data are given in Fig. 5 and Supplementary Note 9. GENERAL
IRRADIATION CONDITIONS Samples were irradiated at 254 nm (UV handlamp from Herolab GmbH Laborgeräte, 254 nm, 8 W), at 365 nm (UV handlamp from Herolab GmbH Laborgeräte, 365 nm, 8 W), at 370
nm (Opulent Starboard, Luminus SST-10-UV-A130, _λ_max = 370 nm, _I_typ = 500 mA, _I_max = 1.0 A, _Φ_typ = 875 mW), at 385 nm (Opulent Starboard, Luminus SST-10-UV-A130, _λ_max = 385 nm,
_I_typ = 500 mA, _I_max = 1.5 A, _Φ_typ = 1015 mW), or at 455 nm (CREE XLamp Me-C LED, _λ_max = 455 nm, _I_max = 700 mA). The corresponding emission spectra of each LED are given in
Supplementary Fig. 24 or in the corresponding figures containing sequences of UV/Vis absorption spectra. STATIONARY UV/VIS ABSORPTION AND EMISSION SPECTROSCOPY UV/Vis absorption (Cary 60 or
Cary 100 UV-Vis spectrophotometer; Agilent) and UV/Vis emission spectra (Horiba FluoroMax-4 spectrofluorometer) were recorded at room temperature. Fluorescence quantum yields were determined
with a Hamamatsu C9920-02 system equipped with a Spectralon integrating sphere. The quantum yield accuracy is <10% according to the manufacturer. STEPWISE ILLUMINATION AND RECORDING OF
ABSORPTION SPECTRA Stationary UV/Vis absorption spectra were recorded using an Agilent Cary 50 UV/Vis spectrophotometer in a sample cell of 10 × 10 mm that can be flanged to the freeze pump
and thaw apparatus and closed after degassing. A sample of 5RED was illuminated using an UV handlamp (UV handlamp from Herolab GmbH Laborgeräte, 254 nm, 365 nm, 8 W) providing either 254 or
365 nm. Continuous light was delivered onto the sample that was reproducibly positioned in ca. 2 cm distance without any further optics allowing for direct comparison of data sets recorded
under different sample preparation conditions. After each illumination period, an absorption spectrum was recorded directly afterwards. The sample volume of 3 mL was continuously stirred
during the illumination periods providing a homogeneously illuminated sample. TIME-RESOLVED UV/VIS EMISSION SPECTROSCOPY A self-constructed time correlated single photon counting set-up62
was used to record emission decay data at single detection wavelength. A quartz cuvette with four optical windows of the dimension 2 mm × 10 mm was used. The sample was excited along the 2
mm pathlength, and the emission was recorded orthogonally to this. The optical density of the sample was set to ca. 0.1 at the excitation wavelength over 2 mm pathlength. NS TO MS
TIME-RESOLVED UV/VIS ABSORPTION SPECTROSCOPY The streak camera set-up described first in refs. 62,63 was adapted as follows. The third harmonic of a Nd:YAG laser (10 Hz, Surelite II,
Continuum) pumping an Optical Parametric Oscillator (OPO, Continuum) tuned to 450 nm (10 mJ, ca. 10 ns) was used for sample excitation. As a probe light, a pulsed 150 W Xe-flash lamp
(Applied Photophysics) was used that was focussed three times via toric mirror optics: (i) before probe shutter, (ii) into sample, and (iii) into spectrograph. The entire white light probe
pulse was analysed by a combination of a spectrograph (200is, Bruker) and a streak camera (C7700, Hamamatsu Photonics). The use of mechanical shutters enabled the recording of a sequence of
three individual data sets: (i) an image (_D_FL) with both flash lamp and laser, (ii) an image (_D_0) without any incoming light, and (iii) an image (_D_F) only with the flash lamp. One
hundred such sequences were recorded, and corresponding data sets were averaged. Then the TA was calculated as: $$\Delta {\mathrm{OD = log}}\left( {\frac{{D_{\mathrm{F}} -
D_0}}{{D_{{\mathrm{FL}}} - D_0}}} \right).$$ A 10-mL sample was stepwise cycled by a peristaltic pump (ecoline, ISMATEC) through a flow cell with a pathlength of 2 mm for pump and 10 mm for
probe beams (dimensions: 2 mm × 10 mm × 30 mm, Starna) ensuring a total replacement of the sample prior to each individual measurement. No photocatalyst degradation was observed under the
used conditions. SUB-PS AND SUB-NS PUMP/SUPERCONTINUUM PROBE SPECTROSCOPY Ultrafast broadband TA experiments were carried out using UV/Vis pump–supercontinuum probe spectroscopy at 1 kHz
repetition frequency. In short, a Ti-sapphire amplifier system (Coherent Libra) was used to generate 800 nm with 1.2 mJ pulses at 1 kHz. The output was split into three parts of which only
two were used: (1) Ca. 50% of the 800 nm pulses were used to pump a collinear Optical Parametric Amplifier (OPA, TOPAS-800-fs, Light Conversion) tuned to pump pulses centred at ca. 450 nm
(ca. 100 fs, ca. 400 nJ at the sample position) for sample excitation. (2) Ca. 10% were used to pump a non-colinear OPA (in-house build) tuned to pulses centred at ca. 530 nm (ca. 100 fs,
ca. 5 μJ at the CaF2 position) for generation of supercontinuum white light probe pulses by focussing into a moving CaF2 disc of 1-mm thickness giving a probe spectrum ranging from 310 to
700 nm. Pump pulses were delayed via a motorised delay line equipped with an open corner cube mirror up to 2 ns. Two complementary high-speed spectrographs (Entwicklungsbüro EB Stresing) for
signal and reference recording were used. The pump and probe pulses were focussed colinearly into the sample to spot sizes of ca. 80 and 60 μm full width at half maximum, respectively. For
longer delays reaching out from ns to μs time ranges, a similar spectrometer was used in which the pump laser was electronically delayed relative to the probe laser. A detailed description
can be found in ref. 64. The relative polarisations between the pump and probe were set by a half-wave plate in the pump beam path to magic angle (54.71°) for observations of pure population
changes or to either parallel (0°) or orthogonal (90°) for observation of the anisotropy. The averaged pre-_t_0 laser scatter signal was subtracted from the data and the ca. 1 ps chirp of
the white light was corrected for prior to data analysis using the coherent artefact as an indicator for time zero at each wavelength. Throughout the probe range, the spectral resolution was
better than 4 nm and the temporal resolution was better than 150 fs. Ten individual scans with averaging 100 spectra per time point were typically recorded. The time axis—within total 500
points—was linear between −1 and 2 ps and logarithmic from 2 ps to the maximum time delay ensuring that the dynamics on every time scale will have equal weighting in the fitting analysis. In
the sub-ps TA set-up, 10 mL of the sample were cycled through an in-house build cell with a pathlength of 100 μm for pump and probe beams. In sub-ns TA set-up, 10 mL of the sample were
cycled through a flow cell (Starna) with a pathlength of 2 mm for pump and probe beams. In all cases, all scans resulted in reproducible data sets. In addition, the integrity of the sample
was checked by recording stationary absorption spectra before and after each measurement. No photocatalyst degradation was observed under the used conditions. The shown data correspond to
one representative measurement. No smoothing or filtering procedures were applied to the data. TA DATA ANALYSIS AND MODELLING Singular value decomposition (SVD)-based rank analysis and
global fitting were performed using an in-house written programme described previously62,63. In brief, the linear least squares problem $$\chi^{2}=\parallel{\mathbf{\Delta}} {\mathbf{A}} -
{\mathbf{FB}}\parallel^{2}={\mathrm{Min}}$$ is solved, where ∆A is the time-resolved absorption data matrix, F is the matrix containing the analytical functions accounting for the temporal
changes in the data, i.e. exponential decays (convoluted with the instrument response, typically a Gaussian function), and B is the matrix with the to be determined spectra. Further
optimisation of _χ_2 is achieved by optimising the rate constants in F by a nonlinear least squares algorithm. As a result of such fits, the so-called decay-associated difference spectra (in
matrix B) and their associated optimised rate constants are obtained. These are the unique result of the global fit, and this treatment does not require any model for the kinetics involved
in the transient processes. The number of exponentials in the global fit is determined by the SVD-based rank analysis, which is described elsewhere65. The model that relates the actual
species kinetics to the elementary function is applied afterwards resulting in SAS. The shape of the SAS in terms of identity with well-known spectra or following physical laws decides about
the appropriateness of the model. This step does not change the _χ_2 value found in the global fit, and therefore, this procedure has the advantage that all interpretation is performed with
the same quality of fit. As an alternative analysis, known species spectra, taken either from literature or recorded in this work, were taken in order to decompose the recorded
time-resolved data matrix using the transpose of the data matrix and using the basis spectra instead of analytical functions. The resulting concentration–time profiles inform about the
appropriateness of the basis spectra and the physical reasonability, i.e. total sum of species being constant to 1. QUANTUM CHEMICAL CALCULATIONS Quantum chemical calculations on all
molecular moieties were performed using either the Firefly QC package66, which is partially based on the GAMESS (US)67 source code, or the Orca package68,69. All ground state structures were
optimised on the level of restricted open shell density functional theory (ROHF-DFT) using the B3LYP functional and the aug-cc-pDVZ basis set. Complete active space self-consistency field
(CASSCF) theory was used in order to calculate the static correlation energy. The highest 12 contributing _π_ and _n_ electrons were included into the CAS, distributed over 12 molecular
orbitals (MO), and energy averaging over 10 states with equal weights, i.e. CASSCF(12,12)10, was performed. In order to calculate the dynamic correlation energy, extended multi-configuration
quasi-degenerate perturbation theory (XMCQDPT) was used on top of the CASSCF optimised MOs70. In all cases, an intruder state avoidance denominator shift of 0.02 was used. The absorption
spectrum for 5sq was calculated via unrestricted open shell time-dependent DFT (TD-DFT) with the aug-cc-pDVZ basis set. Solvent effects were taken into account with the conductor-like PCM
for the TD-DFT calculation and with the PCM for the XMCQDPT-CASSCF calculations. Relaxed potential energy surfaces along the C-Br bond in _p_-BA and _p_-BA•− were performed on the DFT
(B3LYP) level of theory with the aug-cc-pVDZ basis set. DATA AVAILABILITY The authors declare that the data supporting the findings of this study are available within the paper and
Supplementary Information, as well as from the authors upon reasonable request. REFERENCES * Walsh, C. T. & Wencewicz, T. A. Flavoenzymes: versatile catalysts in biosynthetic pathways.
_Nat. Prod. Rep._ 30, 175–200 (2013). Article CAS PubMed Google Scholar * Joosten, V. & Van Berkel, W. J. H. Flavoenzymes. _Curr. Opin. Chem. Biol._ 11, 195–202 (2007). Article CAS
PubMed Google Scholar * Greening, C. et al. Physiology, biochemistry, and applications of F420 and FO-dependent redox reactions. _Microbiol. Mol. Biol. Rev._ 80, 451–493 (2016). Article
CAS PubMed PubMed Central Google Scholar * Walsh, C. Naturally occurring 5-deazaflavin coenzymes: biological redox roles. _Acc. Chem. Res._ 19, 216–221 (1986). Article CAS Google
Scholar * Hemmerich, P., Massey, V. & Fenner, H. Flavin and 5-deazaflavin: a chemical evaluation of ‘modified’ flavoproteins with respect to the mechanisms of redox biocatalysis. _FEBS
Lett._ 84, 5–21 (1977). Article CAS PubMed Google Scholar * Megerle, U. et al. Unraveling the flavin-catalyzed photooxidation of benzylic alcohol with transient absorption spectroscopy
from sub-pico- to microseconds. _Phys. Chem. Chem. Phys._ 13, 8869–8880 (2011). Article CAS PubMed Google Scholar * Kutta, R. J., Archipowa, N. & Scrutton, N. S. The sacrificial
inactivation of the blue-light photosensor cryptochrome from _Drosophila melanogaster_. _Phys. Chem. Chem. Phys._ 20, 28767–28776 (2018). Article CAS PubMed PubMed Central Google Scholar
* Kutta, R. J., Archipowa, N., Johannissen, L. O., Jones, A. R. & Scrutton, N. S. Vertebrate cryptochromes are vestigial flavoproteins. _Sci. Rep._ 7, 44906 (2017). Article ADS CAS
PubMed PubMed Central Google Scholar * Massey, V. The chemical and biological versatility of riboflavin. _Biochem. Soc. Trans._ 28, 283–296 (2000). Article CAS PubMed Google Scholar *
Holtmann, D. & Hollmann, F. The oxygen dilemma: a severe challenge for the application of monooxygenases? _ChemBioChem_ 17, 1391–1398 (2016). Article CAS PubMed PubMed Central
Google Scholar * Insińska-Rak, M. & Sikorski, M. Riboflavin interactions with oxygen—a survey from the photochemical perspective. _Chem. Eur. J._ 20, 15280–15291 (2014). Article PubMed
CAS Google Scholar * Gelalcha, F. G. Heterocyclic hydroperoxides in selective oxidations. _Chem. Rev._ 107, 3338–3361 (2007). Article CAS PubMed Google Scholar * Gadda, G. Oxygen
activation in flavoprotein oxidases: the importance of being positive. _Biochemistry_ 51, 2662–2669 (2012). Article CAS PubMed Google Scholar * Huijbers, M. M. E., Montersino, S.,
Westphal, A. H., Tischler, D. & van Berkel, W. J. H. Flavin dependent monooxygenases. _Arch. Biochem. Biophys._ 544, 2–17 (2014). Article CAS PubMed Google Scholar * Torres Pazmiño,
D. E., Winkler, M., Glieder, A. & Fraaije, M. W. Monooxygenases as biocatalysts: classification, mechanistic aspects and biotechnological applications. _J. Biotechnol._ 146, 9–24 (2010).
Article PubMed CAS Google Scholar * Zilly, F. E., Taglieber, A., Schulz, F., Hollmann, F. & Reetz, M. T. Deazaflavins as mediators in light-driven cytochrome P450 catalyzed
hydroxylations. _Chem. Commun._ 46, 7152–7154 (2009). Article CAS Google Scholar * Su, Q., Boucher, P. A. & Rokita, S. E. Conversion of a dehalogenase into a nitroreductase by
swapping its flavin cofactor with a 5-deazaflavin analogue. _Angew. Chem. Int. Ed._ 56, 10862–10866 (2017). Article CAS Google Scholar * Taglieber, A., Schulz, F., Hollmann, F., Rusek, M.
& Reetz, M. T. Light-driven biocatalytic oxidation and reduction reactions: scope and limitations. _ChemBioChem_ 9, 565–572 (2008). Article CAS PubMed Google Scholar * Duchstein,
H.-J., Fenner, H., Hemmerich, P. & Knappe, W.-R. (Photo)chemistry of 5-deazaflavin. _Eur. J. Biochem._ 95, 167–181 (1979). Article CAS PubMed Google Scholar * Schmermund, L. et al.
Photo-biocatalysis: biotransformations in the presence of light. _ACS Catal._ 9, 4115–4144 (2019). Article CAS Google Scholar * Iwata, T. et al. Hydrogen bonding environments in the
photocycle process around the flavin chromophore of the AppA-BLUF domain. _J. Am. Chem. Soc._ 140, 11982–11991 (2018). Article CAS PubMed Google Scholar * Silva, E. & Edwards, A. M.
(eds) _Flavins: Photochemistry and Photobiology_ (RSC Publishing, 2006). * Sancar, A. Mechanisms of DNA repair by photolyase and excision nuclease (Nobel Lecture). _Angew. Chem. Int. Ed._
55, 8502–8527 (2016). Article CAS Google Scholar * Sancar, A. Structure and function of DNA photolyase and cryptochrome blue-light photoreceptors. _Chem. Rev._ 103, 2203–2238 (2003).
Article CAS PubMed Google Scholar * Lee, J., Müller, F. & Visser, A. J. W. G. The sensitized bioluminescence mechanism of bacterial luciferase. _Photochem. Photobiol._ 95, 679–704
(2019). Article CAS PubMed Google Scholar * Conrad, K. S., Manahan, C. C. & Crane, B. R. Photochemistry of flavoprotein light sensors. _Nat. Chem. Biol._ 10, 801 (2014). Article CAS
PubMed PubMed Central Google Scholar * Zollitsch, T. M. et al. Magnetically sensitive radical photochemistry of non-natural flavoproteins. _J. Am. Chem. Soc._ 140, 8705–8713 (2018).
Article CAS PubMed Google Scholar * Cashmore, A. R., Jarillo, J. A., Wu, Y.-J. & Liu, D. Cryptochromes: blue light receptors for plants and animals. _Science_ 284, 760–765 (1999).
Article ADS CAS PubMed Google Scholar * Kiontke, S., Gnau, P., Haselsberger, R., Batschauer, A. & Essen, L.-O. Structural and evolutionary aspects of antenna chromophore usage by
class II photolyases. _J. Biol. Chem._ 289, 19659–19669 (2014). Article CAS PubMed PubMed Central Google Scholar * Lee, S. H., Choi, D. S., Kuk, S. K. & Park, C. B.
Photobiocatalysis: activating redox enzymes by direct or indirect transfer of photoinduced electrons. _Angew. Chem. Int. Ed._ 57, 7958–7985 (2018). Article CAS Google Scholar * König, B.,
Kümmel, S., Svobodová, E. & Cibulka, R. Flavin photocatalysis. _Phys. Sci. Rev._ 3, 1–17 (2018). Article Google Scholar * Metternich, J. B., Mudd, R. J. & Gilmour, R. _Science of
Synthesis: Photocatalysis in Organic Synthesis_, Vol. 1 (Thieme, 2018). * Zelenka, J., Cibulka, R. & Roithová, J. Flavinium catalysed photooxidation: detection and characterization of
elusive peroxyflavinium intermediates. _Angew. Chem. Int. Ed._ 58, 15412–15420 (2019). Article CAS Google Scholar * Ramirez, N. P., König, B. & Gonzalez-Gomez, J. C. Decarboxylative
cyanation of aliphatic carboxylic acids via visible-light flavin photocatalysis. _Org. Lett._ 21, 1368–1373 (2019). Article CAS PubMed Google Scholar * März, M. et al.
Azodicarboxylate-free esterification with triphenylphosphine mediated by flavin and visible light: method development and stereoselectivity control. _Org. Biomol. Chem._ 16, 6809–6817
(2018). Article PubMed Google Scholar * Mühldorf, B. & Wolf, R. C-H photooxygenation of alkyl benzenes catalyzed by riboflavin tetraacetate and a non-heme iron catalyst. _Angew. Chem.
Int. Ed._ 55, 427–430 (2016). Article CAS Google Scholar * Hering, T., Mühldorf, B., Wolf, R. & König, B. Halogenase-inspired oxidative chlorination using flavin photocatalysis.
_Angew. Chem. Int. Ed._ 55, 5342–5345 (2016). Article CAS Google Scholar * Hartman, T. & Cibulka, R. Photocatalytic systems with flavinium salts: from photolyase models to synthetic
tool for cyclobutane ring opening. _Org. Lett._ 18, 3710–3713 (2016). Article CAS PubMed Google Scholar * Mühldorf, B. & Wolf, R. Photocatalytic benzylic C-H bond oxidation with a
flavin scandium complex. _Chem. Commun._ 51, 8425–8428 (2015). Article Google Scholar * Mojr, V. et al. Tailoring flavins for visible light photocatalysis: organocatalytic [2+2]
cycloadditions mediated by a flavin derivative and visible light. _Chem. Commun._ 51, 12036–12039 (2015). Article CAS Google Scholar * Feldmeier, C., Bartling, H., Magerl, K. &
Gschwind, R. M. LED-illuminated NMR studies of flavin-catalyzed photooxidations reveal solvent control of the electron-transfer mechanism. _Angew. Chem. Int. Ed._ 54, 1347–1351 (2015).
Article CAS Google Scholar * Metternich, J. B. & Gilmour, R. A bio-inspired, catalytic E → Z isomerization of activated olefins. _J. Am. Chem. Soc._ 137, 11254–11257 (2015). Article
CAS PubMed Google Scholar * Zelenka, J. et al. Combining flavin photocatalysis and organocatalysis: metal-free aerobic oxidation of unactivated benzylic substrates. _Org. Lett._ 21,
114–119 (2019). Article CAS PubMed Google Scholar * Zhang, W., Carpenter, K. L. & Lin, S. Electrochemistry broadens the scope of flavin photocatalysis: photoelectrocatalytic
oxidation of unactivated alcohols. _Angew. Chem. Int. Ed._ 59, 409–417 (2020). Article CAS Google Scholar * Zhou, F., Li, R., Wang, X., Du, S. & An, Z. Non-natural photoenzymatic
controlled radical polymerization inspired by DNA photolyase. _Angew. Chem. Int. Ed._ 58, 9479–9484 (2019). Article CAS Google Scholar * Link, P. A. J., van der Plas, H. C. & Müller,
F. Photoreduction of 5-deazaflavins [1,3,9-triaza-anthracene-2(3H),4(10H)-diones]. _J. Chem. Soc. Chem. Commun._ 17, 1385–1387 (1986). Article Google Scholar * van Schie, M. M. C. H. et
al. Deazaflavins as photocatalysts for the direct reductive regeneration of flavoenzymes. _Mol. Catal._ 452, 277–283 (2018). Article CAS Google Scholar * Goldberg, M., Pecht, I., Kramer,
H. E. A., Traber, R. & Hemmerich, P. Structure and properties of 5-deazaflavin radicals as compared to natural flavosemiquinones. _Biochim. Biophys. Acta_ 673, 570–593 (1981). Article
CAS PubMed Google Scholar * Shi, F. et al. A facile and efficient synthesis of novel pyrimido[5,4-b][4,7]phenanthroline-9,11(7H,8H,10H,12H)-dione derivativesviamicrowave-assisted
multicomponent reactions. _J. Heterocycl. Chem._ 46, 563–566 (2009). Article CAS Google Scholar * Khalafi-Nezhad, A., Sarikhani, S., Shahidzadeh, E. S. & Panahi, F. l-Proline-promoted
three-component reaction of anilines, aldehydes and barbituric acids/malononitrile: regioselective synthesis of 5-arylpyrimido[4,5-b]quinoline-diones and
2-amino-4-arylquinoline-3-carbonitriles in water. _Green Chem._ 14, 2876–2884 (2012). Article CAS Google Scholar * Edmondson, D. E., Barman, B. & Tollin, G. Importance of the N-5
position in flavine coenzymes. Properties of free and protein-bound 5-deaza analogs. _Biochemistry_ 11, 1133–1138 (1972). Article CAS PubMed Google Scholar * Neumeier, M. et al.
Dichromatic photocatalytic substitutions of aryl halides with a small organic dye. _Chem. Eur. J._ 24, 105–108 (2018). Article CAS PubMed Google Scholar * Bardagi, J. I., Ghosh, I.,
Schmalzbauer, M., Ghosh, T. & König, B. Anthraquinones as photoredox catalysts for the reductive activation of aryl halides. _Eur. J. Org. Chem._ 1, 34–40 (2018). Article CAS Google
Scholar * Ghosh, I. & König, B. Chromoselctive photocatalysis: controlled bond activation through light-color regulation of redox potentials. _Angew. Chem. Int. Ed._ 55, 7676–7679
(2016). Article CAS Google Scholar * Kim, H., Kim, H., Lambert, T. H. & Lin, S. Reductive electrophotocatalysis: merging electricity and light to achieve extreme reduction potentials.
_J. Am. Chem. Soc._ 142, 2087–2092 (2020). Article CAS PubMed PubMed Central Google Scholar * Cowper, N. G. W., Chernowsky, C. P., Williams, O. P. & Wickens, Z. K. Potent
reductants via electron-primed photoredox catalysis: unlocking aryl chlorides for radical coupling. _J. Am. Chem. Soc._ 142, 2093–2099 (2020). Article CAS PubMed Google Scholar * Ghosh,
I., Ghosh, T., Bardagi, J. I. & König, B. Reduction of aryl halides by consecutive visible light-induced electron transfer processes. _Science_ 346, 725–728 (2014). Article ADS CAS
PubMed Google Scholar * Meyer, A. U., Slanina, T., Heckel, A. & König, B. Lanthanide ions coupled with photoinduced electron transfer generate strong reduction potentials from visible
light. _Chem. Eur. J._ 23, 7900–7904 (2017). Article CAS PubMed Google Scholar * Kerzig, C., Guo, X. & Wenger, O. S. Unexpected hydrated electron source for preparative visible-light
driven photoredox catalysis. _J. Am. Chem. Soc._ 141, 2122–2127 (2019). Article CAS PubMed Google Scholar * MacKenzie, I. A. et al. Discovery and characterization of an acridine radical
photoreductant. _Nature_ 580, 76–80 (2020). Article CAS PubMed ADS Google Scholar * Cibulka, R. Strong chemical reducing agents produced by light. _Nature_ 580, 31–32 (2020). Article
CAS PubMed ADS Google Scholar * Kutta, R. J. _Blitzlichtphotolyse - Untersuchung zu LOV-Domänen und Photochromen Systemen_. Dissertation, Naturwissenschaftliche Fakultät IV -Chemie und
Pharmazie- der Universität Regensburg (2012). * Kutta, R. J., Langenbacher, T., Kensy, U. & Dick, B. Setup and performance of a streak camera apparatus for transient absorption
measurements in the ns to ms range. _Appl. Phys. B_ 111, 203–216 (2013). Article ADS CAS Google Scholar * Baudisch, B. _Time Resolved Broadband Spectroscopy from UV to NIR_.
Dissertation, Ludwig-Maximilians-Universität München (2018). * Lanzl, K., Sanden-Flohe, M. V., Kutta, R. J. & Dick, B. Photoreaction of mutated LOV photoreceptor domains from
_Chlamydomonas reinhardtii_ with aliphatic mercaptans: implications for the mechanism of wild type LOV. _Phys. Chem. Chem. Phys._ 12, 6594–6604 (2010). Article CAS PubMed Google Scholar
* Granovsky, A. A. Firefly version 8. http://classic.chem.msu.su/gran/firefly/index.html (1997). * Schmidt, M. W. et al. General atomic and molecular electronic structure system. _J. Comput.
Chem._ 14, 1347–1363 (1993). Article CAS Google Scholar * Neese, F. The ORCA program system. _WIREs Comput. Mol. Sci._ 2, 73–78 (2012). Article CAS Google Scholar * Neese, F. Software
update: the ORCA program system, version 4.0. _WIREs Comput. Mol. Sci._ 8, e1327 (2018). Article Google Scholar * Granovsky, A. A. Extended multi-configuration quasi-degenerate
perturbation theory: the new approach to multi-state multi-reference perturbation theory. _J. Chem. Phys._ 134, 214113 (2011). Article ADS PubMed CAS Google Scholar Download references
ACKNOWLEDGEMENTS This project was supported by the Czech Science Foundation (project No 19-09064S), the Deutsche Forschungsgemeinschaft (KO 1537/18-1) and the European Research Council (ERC)
under the European Union’s Horizon 2020 research and innovation programme (grant agreement 741623). R.J.K. thanks the Deutsche Forschungsgemeinschaft for a Research Fellow stipend (KU
3190/1-1, KU 3190/2-1) supporting this project. The authors are grateful to Professor Eberhard Riedle (BioMolekulare Optik, Ludwig-Maximillians-Universität München) for use of the ns to μs
transient absorption apparatus. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Institute of Organic Chemistry, University of Regensburg, 93040, Regensburg, Germany Andreas Graml &
Burkhard König * Department of Organic Chemistry, University of Chemistry and Technology, Prague, 16628, Prague, Czech Republic Tomáš Neveselý & Radek Cibulka * Institute of Physical and
Theoretical Chemistry, University of Regensburg, 93040, Regensburg, Germany Roger Jan Kutta Authors * Andreas Graml View author publications You can also search for this author inPubMed
Google Scholar * Tomáš Neveselý View author publications You can also search for this author inPubMed Google Scholar * Roger Jan Kutta View author publications You can also search for this
author inPubMed Google Scholar * Radek Cibulka View author publications You can also search for this author inPubMed Google Scholar * Burkhard König View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS R.J.K., R.C., and A.G. wrote the paper with input from all of the authors. A.G. and T.N. performed the entire synthesis of all
used compounds and conducted all photocatalytic dehalogenation reactions. A.G. did the electrochemical and EPR characterisation. R.J.K. together with A.G. and T.N. designed the
spectro-electrochemistry experiment, which A.G. performed. R.J.K. together with A.G. and T.N. collected the stationary spectroscopic data. R.J.K. performed all time-resolved spectroscopic
experiments and conducted the computational studies. Experimental data were analysed by R.J.K. together with A.G., computational data were analysed by R.J.K., and results were discussed by
all authors. The project was conceived by R.C., R.J.K., and B.K. CORRESPONDING AUTHORS Correspondence to Roger Jan Kutta, Radek Cibulka or Burkhard König. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks the anonymous reviewers for their contribution to the peer
review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Graml, A., Neveselý, T., Jan Kutta, R. _et al._ Deazaflavin reductive
photocatalysis involves excited semiquinone radicals. _Nat Commun_ 11, 3174 (2020). https://doi.org/10.1038/s41467-020-16909-y Download citation * Received: 16 March 2020 * Accepted: 02 June
2020 * Published: 23 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16909-y SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative