- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Since the discovery of the Verwey transition in magnetite, transition metal compounds with pyrochlore structures have been intensively studied as a platform for realizing remarkable
electronic phase transitions. We report on a phase transition that preserves the cubic symmetry of the β-pyrochlore oxide CsW2O6, where each of W 5_d_ electrons are confined in
regular-triangle W3 trimers. This trimer formation represents the self-organization of 5_d_ electrons, which can be resolved into a charge order satisfying the Anderson condition in a
nontrivial way, orbital order caused by the distortion of WO6 octahedra, and the formation of a spin-singlet pair in a regular-triangle trimer. An electronic instability due to the unusual
three-dimensional nesting of Fermi surfaces and the strong correlations of the 5_d_ electrons characteristic of the pyrochlore oxides are both likely to play important roles in this
charge-orbital-spin coupled phenomenon. SIMILAR CONTENT BEING VIEWED BY OTHERS IDEAL SPIN-ORBIT-FREE DIRAC SEMIMETAL AND DIVERSE TOPOLOGICAL TRANSITIONS IN Y8COIN3 FAMILY Article Open access
15 November 2024 COBALT-BASED MAGNETIC WEYL SEMIMETALS WITH HIGH-THERMODYNAMIC STABILITIES Article Open access 04 January 2021 STABILIZATION OF THREE-DIMENSIONAL CHARGE ORDER THROUGH
INTERPLANAR ORBITAL HYBRIDIZATION IN PR_X_Y1−_X_BA2CU3O6+_Δ_ Article Open access 19 October 2022 INTRODUCTION Understanding the phase transitions of crystalline solids is a central issue in
materials science. Electronic phase transitions in transition metal compounds with pyrochlore structures, made of three-dimensional networks of corner-sharing tetrahedra, have posed
challenging questions in materials science since their discovery. The classical example is magnetite Fe3O4, which was reported to show a metal−insulator transition accompanied by a charge
order of Fe at 119 K, called the Verwey transition1. Although many studies of this transition have been made, full understanding of its ground state has not yet been reached, and relevant
studies based on new perspectives are continuing2. Recently, metal−insulator transitions accompanied by all-in-all-out-type magnetic order in 5_d_ oxides, such as Cd2Os2O7 and Nd2Ir2O7, have
attracted considerable attention3,4,5, in terms of a ferroic order of extended magnetic octapoles and the formation of Weyl fermions in solids3,6,7,8,9. As described above, rich physics
appears in pyrochlore systems, which might be caused by the high crystal symmetry and a large number of atoms in a unit cell, resulting in the self-organization of _d_ electrons in various
forms. In this study, we report self-organization of 5_d_ electrons at the electronic phase transition of β-pyrochlore oxide CsW2O6, discovered by using high-quality single crystals. CsW2O6
was first synthesized by Cava et al., which was reported to have a cubic lattice with _Fd_\(\bar 3\)_m_ space group at room temperature10. In this structure, W atoms form a pyrochlore
structure and have 5.5+ valence with a 5_d_0.5 electron configuration. Electrical resistivity measurement of polycrystalline samples suggested that a metal−insulator transition occurs at 210
K11. The crystal structure of the insulating phase was reported to have orthorhombic _Pnma_ space group11; however, this space group was suggested to be incorrect by a theoretical study12.
Electronic structure calculations on the _Fd_\(\bar 3\)_m_ phase pointed out that there is a strong nesting of the Fermi surfaces, which induces a symmetry lowering to the _P_4132 space
group. Recent photoemission experiments of thin films suggested that the valence of W in the insulating phase disproportionates into 5+ and 6+13. RESULTS A PHASE TRANSITION AT 215 K We
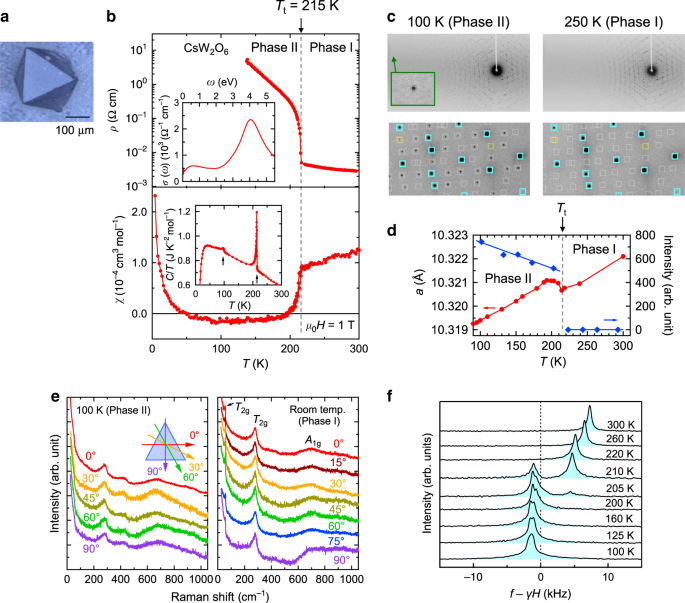
prepared single crystals of CsW2O6 (Fig. 1a) and W-deficient CsW1.835O6 in a quartz tube (see “Method” section). As shown in Fig. 1b, the electrical resistivity, _ρ_, of a single crystal of
CsW2O6 strongly increases below _T_t = 215 K with decreasing temperature, as in the cases of a polycrystalline sample and a thin film11,13. This increase is accompanied by a small but
obvious temperature hysteresis, indicating that a first-order phase transition occurs at _T_t. Here, the phases above and below _T_t are named Phase I and II, respectively. The magnetic
susceptibility, _χ_, shown in Fig. 1b strongly decreases below _T_t, which is identical to the polycrystalline case11. However, the line widths of the 133Cs-NMR spectra in Phase II do not
show any significant broadening compared to those in Phase I, as shown in Fig. 1f, indicating that the decrease of _χ_ in Phase II is not caused by antiferromagnetic order. Figure 1c shows
single-crystal X-ray diffraction (XRD) patterns of CsW2O6 measured at 250 (Phase I) and 100 K (Phase II). Each of the diffraction spots at 250 K were indexed on the basis of a cubic cell of
_a_ = 10.321023(7) Å with _Fd_\(\bar 3\)_m_ space group, consistent with previous reports10,11. In the diffraction pattern at 100 K, more diffraction spots appear. All these spots were
indexed on the basis of cubic _P_213 space group with a lattice constant of _a_ = 10.319398(6) Å, which is almost identical to _a_ of Phase I. This change of diffraction spots occurs at
_T_t, as seen in the temperature dependence of the intensity shown in Fig. 1d. Moreover, in Phase II, diffraction spots do not split into multiple spots nor do they change their shapes, even
in the high-angle region, as shown in Fig. 1c. Laue class and crystal system determined by the observed reflections clearly indicated that a structural change that preserves the cubic
symmetry occurs at _T_t and the Phase II has the Laue class of _m_\(\bar 3\) (see Supplementary Note 1 and Supplementary Fig. 3). As seen in the polarization dependence of the Raman spectra
of (111) surface measured at 100 K (Phase II) and room temperature (Phase I) shown in Fig. 1e, the spectra of Phase II are independent of the polarization angle same as in Phase I,
indicating the presence of three-fold rotational symmetry perpendicular to (111), consistent with the inferred cubic symmetry. These results mean that the _Pnma_ structural model proposed
based on the powder diffraction data is incorrect11. In addition, the proposed _Pnma_ structure has a pseudo-tetragonal distortion of approximately 0.03%, which was not observed in the
present study, as shown in Supplementary Figs. 2 and 4. This result is also supported by the 133Cs-NMR data on the single crystals of CsW2O6 discussed below. In a polycrystalline sample of
CsW2O6, W-deficient CsW1.835O6 always exists as an impurity phase. We believe that the fact that the single crystals of CsW2O6 and W-deficient CsW1.835O6 were separately prepared and the
diffraction and physical property measurements were performed by using the high-quality single crystals played a crucial role for elucidating the nature of Phase II. The crystallographic
parameters of CsW2O6 at 250 and 100 K determined by the structural analyses are shown in Supplementary Tables 2 and 4, respectively. Physical properties and crystallographic parameters of
CsW1.835O6 are also shown in Supplementary Fig. 6 and Supplementary Table 6. A point to be noted in the Raman spectra shown in Fig. 1e is a peak appeared at ~55 cm−1, reflecting the rattling
of Cs+ ions14 (see Supplementary Note 6). CRYSTAL STRUCTURE OF PHASE II Here we discuss the solved crystal structure of Phase II. In Phase I with the _Fd_\(\bar 3\)_m_ space group, each of
Cs, W, and O atoms occupies one site, where the Cs and W atoms form diamond and pyrochlore structures, respectively (Fig. 2a). In Phase II with the _P_213 space group, the Cs atoms occupy
two different sites and form a zinc-blende structure, as shown in Fig. 2b. This was further confirmed by the two peaks in 133Cs-NMR spectra correspond to the two Cs sites, which appear as a
small peak split in the 200, 160, and 125 K data shown in Fig. 1f. On the other hand, W atoms occupy two sites with a 1:3 ratio in Phase II, as shown in Fig. 2b, c, which is incompatible
with the W5+–W6+ charge order with a 1:1 ratio of W5+ and W6+ atoms. According to the bond valence sum calculation for the W–O distances determined from single-crystal XRD analyses15, the
valences of the W(1) and W(2) atoms are estimated to be 6.07(3) and 5.79(3) at 100 K (Phase II), respectively. Considering that the reliable bond valence sum parameters of W6+ are available
but those of W5+ are not, it is natural for the W(1) atoms to be W6+ without 5_d_ electrons. In this case, the valence of the W(2) atoms becomes 5.33+ with 5_d_2/3 electron configurations.
The above discussion indicates that a charge order with a noninteger valence occurs at _T_t. In fact, single crystals of W-deficient CsW1.835O6, where all W atoms have 6+ valence without
5_d_ electrons, do not show the transition at _T_t (see Supplementary Fig. 6). In Phase II, the W(2) atoms form a three-dimensional network of small and large regular triangles, which are
alternately connected by sharing their corners, as shown in Fig. 2b. Although the difference of sizes between the large and small triangles are about 2%, arrangements of the occupied 5_d_
orbitals are completely different between them, resulting in a W3 trimer on a small triangle, as discussed later. If there was no alternation of the W3 triangles, the W sublattice would
possess a hyperkagome structure16, as shown in Fig. 2c. The presence of the alternation indicates that ‘breathing hyperkagome’ structure is formed during Phase II17. DISCUSSION The charge
order in Phase II of CsW2O6 is interesting in that the “Anderson condition” is maintained in an unusual way. Anderson pointed out that magnetite has an infinite number of charge ordering
patterns, where all the tetrahedra in a pyrochlore structure have the same total charge, i.e., so-called Anderson condition, and this macroscopic degeneracy strongly suppresses the
transition temperature of the Verwey transition18. This situation can be interpreted as geometrical frustration of electronic charges. However, not only magnetite, but also other
mixed-valent pyrochlore systems, such as CuIr2S4 and AlV2O4, were reported to show a charge order that violated the Anderson condition19,20,21,22,23. In them, the energy gained by _σ_
bonding between _d_ orbitals of adjacent atoms was expected to be large enough to compensate for the loss of Coulomb energy due to violation of the Anderson condition, because spinel-type
compounds comprised the edge-shared octahedra24,25. In contrast, the charge order of CsW2O6 satisfies the Anderson condition, where each tetrahedron consists of three W5.33+ and one W6+
atoms. However, this charge order is different to that proposed by Anderson and Verwey, which has integer valences with a 1:1 ratio18,26. Hyperkagome-type orders often appear in pyrochlore
systems with a 1:3 ratio of two kinds of atoms, such as the uuud spin structure of the half magnetization plateau of Cr spinel oxides and the atomic order in B-site ordered spinel oxides
A2BB_'_3O827,28,29. As far as we are aware, CsW2O6 is the only example to show a hyperkagome-type order where the formation of this order is nontrivial. It is an alternative way to
relieve the geometrical frustration based on the traditional problem in condensed matter physics. Why does such unusual charge order occur in CsW2O6? A key to understand this question is
hidden in Fermi-surface instability of the electronic band structure of Phase I. The left panel of Fig. 3 shows the band structure of Phase I and the right panel shows four overlapping band
structures, which are depicted after the parallel shifts of electronic bands corresponding to a change of the primitive cell from face-centered cubic to primitive cubic. As seen in the right
panel of Fig. 3, band crossing occurs close to all points where electronic bands touch the Fermi energy _E_F, suggesting that the Fermi surfaces are well nested by the parallel shift of the
electronic bands, corresponding to the loss of centering operations. This situation can be called “three-dimensional nesting”, which means that a large electronic energy is gained by the
structural change associated with the above symmetry change. The electronic instability and nesting of the Fermi surfaces in Phase I was also pointed out in the previous study12. It is quite
rare for cubic compounds to have such well-nested Fermi surfaces, except for the filled-skutterudite PrRu4P12. PrRu4P12 shows a metal−insulator transition accompanied by a structural change
from body-centered cubic to primitive cubic30,31 and has a Fermi-surface instability corresponding to this structural change32. The above discussion indicates that three-dimensional nesting
is likely an essential ingredient for the 215 K transition. However, if it is the only driving force, a structural change from _Fd_\(\bar 3\)_m_ to _P_4132 or _P_4332, which are maximal
non-isomorphic subgroups with primitive cubic lattices, must occur, as also discussed in the previous theoretical study12. In this case, the W(2) atoms should form a uniform hyperkagome
structure. This study also suggested that the band gap does not open at the Fermi energy in the _P_4132 and _P_4332 cases12, which is inconsistent with the observed insulating nature of the
Phase II. In reality, the space group of Phase II is _P_213, which is a subgroup of _P_4132 and _P_4332, and W(2) atoms form a breathing hyperkagome structure, where the size of a small
triangle is 2% smaller than that of a large triangle. The _P_213 space group is also different from orthorhombic _P_212121 proposed in ref. 12. In addition, whether the band structure
calculated with the structural parameters of Phase II is gapped or not might be important for understanding of the underlying physics of the phase transition. At present, however, the
calculated results with an overlapped gap have not yet completely converged, due to the large number of atoms in a primitive unit cell. Phenomenologically, orientation of the occupied 5_d_
orbitals is important for the symmetry lowering from _P_4132/_P_4332 (uniform hyperkagome) to _P_213 (breathing hyperkagome). For a W(2)O6 octahedron of Phase II shown in Fig. 2e, the two
apical W(2)-O bonds (gray) are 3–8% shorter than the other four equatorial bonds (blue), indicating that the octahedron is uniaxially compressed. This compression is comparable to the
typical Jahn–Teller distortion in _t_2g electron systems, and the 5_d_ orbitals lying in this equatorial plane should be occupied by electrons. Schematic pictures of the occupied 5_d_
orbitals in small and large triangles are shown in the right and left panels of Fig. 2f, respectively. There is considerable overlap between the occupied 5_d_ orbitals in the small triangle
via an O 2_p_ orbital. In contrast, there is little overlap in a large triangle, indicating that two electrons in three W(2) atoms are confined in a W3 trimer in the small triangle. This
regular-triangle trimer formation might be understood as a three-centered-two-electron (3c2e) bond formation, where two electrons are accommodated in a molecular orbital made of three W 5_d_
orbitals (and the O 2_p_ orbitals hybridize with them). In this case, it is natural to have a nonmagnetic ground state. This regular-triangle trimer is essentially different to those of
famous LiVO2 and LiVS2, where two electrons are shared by two V atoms along each side of a triangle33,34,35. Na3Ir3O8 was pointed out to have regular-triangle Ir3 molecules formed on a
hyperkagome structure36. However, the Ir3 molecules are connected each other, which is essentially different from the fact that the W3 trimers in CsW2O6 are isolated, as seen in Fig. 2b.
Stabilization of the electronic energy by the formation of multiple-centered bonds, where a few electrons are shared by many atoms, often occurs in electron-deficient molecules or cluster
compounds37. To our knowledge, CsW2O6 is the only example where this type of bond formation appears as a phase transition. The formation of regular-triangle trimers itself is also
surprising, because the 3c2e bond usually has a bent shape. According to previous reports, only the H3+ ion has a regular-triangle shape in triatomic molecules formed by the 3c2e bond.
Moreover, H3+ is an interstellar material and it is not stable on Earth, having been observed in astronomical spectra38. This regular-triangle shape of the trimer might be related to its
internal structure, a part of which appears in the atomic displacement parameters (ADPs). As shown in Fig. 2d, the O atoms bridging W(2) atoms in a W3 trimer (O(1) site) in Phase II have
large ADPs perpendicular to the W-W bond. The ADPs of the other O atoms are typical values, suggesting that the ADPs of the O(1) site do not increase by the structural instability of the
β-pyrochlore structure, but rather by the electronic instability of the trimer. The large ADPs perpendicular to the W-W bond indicate that there is a strong fluctuation that changes the
W(2)–O(1)–W(2) angle. In pyrochlore oxides, the change of this angle has a large effect on the orbital overlap39. Therefore, this fluctuation can be interpreted as a strong fluctuation to a
state in which one of the W-W bonds becomes stronger, or in the extreme, to a state in which a W2 dimer is formed. Since ADPs of the W(2) site have typical values, it is unlikely that the
dimers statically and randomly form on the trimers. Instead, the dimer might dynamically fluctuate or resonate. For a complete understanding of the internal structure of the trimers, it
would be desirable to directly observe their dynamical properties in a future study. For the formation of this trimer, electron correlation of the 5_d_ electrons in CsW2O6 might be another
essential factor. The optical conductivity spectra of CsW2O6 measured at room temperature deduced from the reflectivity using the Kramers–Kronig transformation40, shown in the inset of Fig.
1b, exhibit a broad peak at around 0.6 eV. Extrapolation of the spectra to zero frequency coincides with _ρ_ = 3 mΩ cm at room temperature (the main panel of Fig. 1b). This result indicates
that there is no, or negligibly small, Drude contribution in the spectra, and the conducting carriers are trapped by something with an energy scale of 0.6 eV, resulting in the loss of
coherency, which is supported by _dρ_/_dT_ < 0 in Phase I. Absence of a peak in the far-infrared region indicates that this localization is not due to disorder, but might be reminiscent
of the spectra of lightly-carrier-doped Mott insulators41,42,43. As seen in the band structure shown in Fig. 3a, there are flat parts near _E_F and the energy bands have a narrow width of
0.7 eV, suggesting the presence of a strong electron correlation for a 5_d_ electron system. In 5_d_ or 4_d_ pyrochlore oxides, the 5_d_/4_d_ electrons often have moderately-strong electron
correlation because of the small orbital overlap due to the bent metal–oxygen–metal bonds. In fact, the optical conductivities of Nd2Ir2O7 and Sm2Mo2O7 indicate the presence of incoherent
_d_ electrons9,44, similar to the case of CsW2O6. As a result, 5_d_ pyrochlore oxides often show an electronic phase transition with the order of electronic degrees of freedom. Nd2Ir2O7 with
_J_eff = 1/2 and Cd2Os2O7 with _S_ = 3/2, without charge and orbital degrees of freedom, showed a magnetic order accompanied by a metal−insulator transition3,4,5. Instead, for CsW2O6, the
trimers are formed with the help of the electron correlation as in the case of LiVO245. In the trimer of CsW2O6, the two 5_d_ electrons form a spin-singlet pair, resulting in the nonmagnetic
and insulating ground state. This is an alternative type of self-organization of _d_ electrons realized in a strongly correlated 5_d_ oxide. Finally, we will discuss another structural
transition at 90 K. Phase II looks like a ground state, where most of the degrees of freedom have been lost, but surprisingly another phase transition occurs at 90 K. By indexing the
diffraction spots in the single-crystal XRD data of Phase II, the crystal structure below 90 K, named Phase III, was found to have monoclinic _P_21 space group with a four-times-larger (2 ×
1 × 2) unit cell than that of Phase II, as shown in Supplementary Fig. 1. The procedure performed for the determination of the size of unit cell and space group of Phase III is described in
Supplementary Notes 2 and 3. As seen in the inset of Fig. 1b, the heat capacity divided by temperature, _C_/_T_, shows a small but obvious peak, which corresponds to the entropy change of
~0.4 J K−1 mol−1, at ~90 K, indicating the presence of a bulk phase transition. The _P_21 space group is different from _Pnma_ and _P_212121 space groups proposed in the previous
studies11,12. The atomic positions in Phase III have not yet been determined, because of tiny monoclinic distortion and domain formation, but it is clear that the structural change at 90 K
is small, as seen in Supplementary Fig. 2. In addition, _χ_ does not exhibit an anomaly at 90 K. These results suggest that the 90 K transition is not caused by the spin, charge, and/or
orbital order different to the 215 K transition. What mechanism gives rise to the 90 K transition? The diffuse scattering that appears in the single-crystal XRD patterns might provide a hint
to answering this question. In the single-crystal XRD patterns of Phases I, II, and III shown in Fig. 4, there are diffuse scatterings at the same positions, which follow the extinction
rule of _h_ + _l_ = 4_n_ (for a cubic unit cell) and connect the superlattice spots that emerged in Phase III. This suggests that the structural change from Phase II to III and the diffuse
scatterings have the same origin. The same diffuse scattering pattern also appeared in CsW1.835O6 (Supplementary Fig. 7) and CsTi0.5W1.5O646, which are isostructural to CsW2O6, but only have
W6+ atoms without 5_d_ electrons, suggesting that they are independent of the 215 K transition and might be caused by the structural instability of the β-pyrochlore structure itself. This
discussion also implies that the 215 K transition is irrelevant to this instability and is purely electronic driven. In conclusion, we found that regular-triangle W3 trimers are formed at
the 215 K transition in β-pyrochlore oxide CsW2O6, as determined using structural- and electronic-property measurements of high-quality single crystals. This transition represents the
self-organization of 5_d_ electrons, where geometrical frustration is relieved in a nontrivial way that satisfies the traditional Anderson condition and results in the quite rare cubic−cubic
structural transition. This type of electronic transition is not only unusual, but is only partly understood by the first principles calculations, suggesting that it might be a spin-,
charge-, and orbital-coupled phase transition occurring beyond the existing electronic phase transitions of pyrochlore systems. The above finding shows that the exploration of geometrically
frustrated 5_d_ compounds will lead to the discovery of further interesting electronic phenomena, such as odd-parity multipoles and spin–charge–orbital entangled quantum liquids. METHODS
SAMPLE PREPARATION Single crystals of CsW2O6 were prepared by crystal growth in an evacuated quartz tube under a temperature gradient. A mixture of a 3:1:3 molar ratio of Cs2WO4 (Alfa Aeser,
99.9%), WO3 (Kojundo Chemical Laboratory, 99.99%), and WO2 (Kojundo Chemical Laboratory, 99.99%), with a combined mass of 0.1 g, was sealed in an evacuated quartz tube with 0.1 g of CsCl
(Wako Pure Chemical Corporation, 99.9%). The hot and cold sides of the tube were heated to, and then kept at 973 K and 873 K, respectively, for 96 h, and then the furnace was cooled to room
temperature. The mixture was put on the hot side. The obtained single crystals had an octahedral shape with {111} faces with edges of at most 1 mm. Powder samples of CsW2O6 were prepared by
the solid-state reaction method described in the previous studies10,11. The obtained powder was sintered at 773 K for 10 min using a spark plasma sintering furnace (SPS Syntex). Single
crystals of W-deficient CsW2−_x_O6 were prepared using the flux method. A mixture of a 3:1:3 molar ratio of Cs2WO4 (Alfa Aeser, 99.9%), WO3 (Kojundo Chemical Laboratory, 99.99%), and WO2
(Kojundo Chemical Laboratory, 99.99%), with the combined mass of 0.1 g, and 0.2 g of CsCl (Wako Pure Chemical Corporation, 99.9%) were put in an alumina crucible, which was sealed in an
evacuated quartz tube. The tube was heated to, and then kept at 923 K for 48 h, and then slowly cooled to 873 K at a rate of −0.5 K/h. The obtained single crystals have similar octahedral
shape and mostly have a larger size than those of CsW2O6. The value of the W deficiency, _x_, was estimated to be 0.165 via a structural analysis using the single-crystal XRD data, meaning
that the chemical composition of the single crystal is CsW1.835O6, where the W atoms have no 5_d_ electrons. MEASUREMENTS AND FIRST PRINCIPLES CALCULATIONS The electrical resistivity and
magnetization measurements of the CsW2O6 and CsW1.835O6 single crystals were performed using a Physical Property Measurement System (PPMS, Quantum Design) and Magnetic Property Measurement
System (Quantum Design), respectively. The normal incident reflectivity of (111) surface of a CsW2O6 single crystal was taken at room temperature using a Fourier-type interferometer
(0.005−1.6 eV, DA-8, ABB Bomem; 0.06−1.0 eV, FT-IR6100, Jasco) and a grating spectrometer (0.46−5.8 eV, MSV-5200, Jasco) installed with a microscope40,47. As a reference mirror, we used
either evaporated Au (far- to near-IR region), Ag (near-IR to visible region), or Al (near- to far-UV region) films on a glass plate. The heat capacity of the CsW2O6 sintered sample was
measured using the relaxation method with the PPMS. Single-crystal XRD experiments of the CsW2O6 and CsW1.835O6 samples were performed at BL02B1 in the SPring-8 synchrotron radiation
facility in Japan. The experimental conditions are shown in Supplementary Tables 1, 3, and 5. SORTAV and SHELXL were used for merging the reflection data and the structural
refinement48,49,50. A part of crystal structure views were drawn using VESTA51. Powder XRD experiments of CsW2O6 were performed at BL02B2 in SPring-8. Synchrotron X-rays with energies of
15.5 and 25 keV were used for the measurements below and above 150 K, respectively. Rietveld analyses of the powder XRD data were performed using GSAS. Raman scattering spectra of the CsW2O6
single crystals were measured using a diode-pumped CW solid-state laser with a wavelength of 5614 Å. 133Cs-NMR measurements of a CsW2O6 single crystal were conducted in a magnetic field of
8 T. The NMR spectra were obtained by Fourier transforming the free induction decay signal. The band structure calculations of Phase I of CsW2O6 were performed using the full potential
linear augmented plane wave (FLAPW) method with a local density approximation. The experimentally obtained structural parameters shown in Supplementary Tables 1 and 2 were used for the
calculations. DATA AVAILABILITY The data that support the findings of this study are available on request from the authors. REFERENCES * Verwey, E. J. W. Electronic conduction of magnetite
(Fe3O4) and its transition point at low temperatures. _Nature_ 144, 327–328 (1939). Article ADS CAS Google Scholar * Senn, M. S., Wright, J. P. & Attfield, J. P. Charge order and
three-site distortions in the Verwey structure of magnetite. _Nature_ 481, 173–176 (2012). Article ADS CAS Google Scholar * Yamaura, J. et al. Tetrahedral magnetic order and the
metal-insulator transition in the pyrochlore lattice of Cd2Os2O7. _Phys. Rev. Lett._ 108, 247205 (2012). Article ADS CAS PubMed Google Scholar * Tomiyasu, K. et al. Emergence of
magnetic long-range order in frustrated pyrochlore Nd2Ir2O7 with metal–insulator transition. _J. Phys. Soc. Jpn._ 81, 034709 (2012). Article ADS CAS Google Scholar * Sagayama, H. et al.
Determination of long-range all-in-all-out ordering of Ir4+ moments in a pyrochlore iridate Eu2Ir2O7 by resonant x-ray diffraction. _Phys. Rev. B_ 87, 100403 (2013). Article ADS CAS
Google Scholar * Hiroi, Z., Yamaura, J., Hirose, T., Nagashima, I. & Okamoto, Y. Lifshitz metal–insulator transition induced by the all-in/all-out magnetic order in the pyrochlore oxide
Cd2Os2O7. _APL Mater._ 3, 041501 (2015). Article ADS CAS Google Scholar * Tian, Z. et al. Field-induced quantum metal-insulator transition in the pyrochlore iridate Nd2Ir2O7. _Nat.
Phys._ 12, 134–138 (2016). Article CAS Google Scholar * Wan, X., Turner, A. M., Vishwanath, A. & Savrasov, S. Y. Topological semimetal and Fermi-arc surface states in the electronic
structure of pyrochlore iridates. _Phys. Rev. B_ 83, 205101 (2011). Article ADS CAS Google Scholar * Ueda, K. et al. Variation of charge dynamics in the course of metal-insulator
transition for pyrochlore-type Nd2Ir2O7. _Phys. Rev. Lett._ 109, 136402 (2012). Article ADS CAS PubMed Google Scholar * Cava, R. J. et al. Cs8.5W15O48 and CSW2O6—members of a new
homologous series of cesium tungsten oxides. _J. Solid State Chem._ 103, 359–365 (1993). Article ADS CAS Google Scholar * Hirai, D. et al. Spontaneous formation of zigzag chains at the
metal-insulator transition in the β-pyrochlore CsW2O6. _Phys. Rev. Lett._ 110, 166402 (2013). Article ADS PubMed CAS Google Scholar * Streltsov, S. V., Mazin, I. I., Heid, R. &
Bohnen, K. P. Spin-orbit driven Peierls transition and possible exotic superconductivity in CsW2O6. _Phys. Rev. B_ 94, 241101 (2016). Article ADS Google Scholar * Soma, T., Yoshimatsu,
K., Horiba, K., Kumigashira, H. & Ohtomo, A. Electronic properties across metal-insulator transition in β-pyrochlore-type CsW2O6 epitaxial films. _Phys. Rev. Mater._ 2, 115003 (2018).
Article CAS Google Scholar * Hasegawa, T., Takasu, Y., Ogita, N. & Udagawa, M. Raman scattering in KOs2O6. _Phys. Rev. B_ 77, 064303 (2008). Article ADS CAS Google Scholar *
Brown, I. D. & Altermatt, D. Bond-valence parameters obtained from a systematic analysis of the inorganic crystal structure database. _Acta Crystallogr._ B41, 244–247 (1985). Article
CAS Google Scholar * Okamoto, Y., Nohara, M., Aruga-Katori, H. & Takagi, H. Spin-liquid state in the _S_ = 1/2 hyperkagome antiferromagnet Na4Ir3O8. _Phys. Rev. Lett._ 99, 137207
(2007). Article ADS PubMed CAS Google Scholar * Okamoto, Y., Nilsen, G. J., Attfield, J. P. & Hiroi, Z. Breathing pyrochlore lattice realized in _A_-site ordered spinel oxides
LiGaCr4O8 and LiInCr4O8. _Phys. Rev. Lett._ 110, 097203 (2013). Article ADS PubMed CAS Google Scholar * Anderson, P. W. Ordering and antiferromagnetism in ferrites. _Phys. Rev._ 102,
1008–1013 (1956). Article ADS CAS Google Scholar * Wright, J. P., Attfield, J. P. & Radaelli, P. G. Long range charge ordering in magnetite below the Verwey transition. _Phys. Rev.
Lett._ 87, 266401 (2001). Article ADS CAS PubMed Google Scholar * Radaelli, P. G. et al. Formation of isomorphic Ir3+ and Ir4+ octamers and spin dimerization in the spinel CuIr2S4.
_Nature_ 416, 155–158 (2002). Article ADS CAS PubMed Google Scholar * Horibe, Y. et al. Spontaneous formation of vanadium “molecules” in a geometrically frustrated crystal: AlV2O4.
_Phys. Rev. Lett._ 96, 086406 (2006). Article ADS CAS PubMed Google Scholar * Browne, A. J., Kimber, S. A. J. & Attfield, J. P. Persistent three- and four-atom orbital molecules in
the spinel AlV2O4. _Phys. Rev. Mater._ 1, 052003 (2017). Article Google Scholar * Okamoto, Y. et al. Band Jahn–Teller instability and formation of valence bond solid in a mixed-valent
spinel oxide LiRh2O4. _Phys. Rev. Lett._ 101, 086404 (2008). Article ADS PubMed CAS Google Scholar * Hiroi, Z. Structural instability of the rutile compounds and its relevance to the
metal–insulator transition of VO2. _Prog. Solid State Chem._ 43, 47–69 (2015). Article CAS Google Scholar * Attfield, J. P. Orbital molecules in electronic materials. _APL Mater._ 3,
041510 (2015). Article ADS CAS Google Scholar * Verwey, E. J. W., Haaymann, P. W. & Romeijn, F. C. Physical properties and cation arrangement of oxides with spinel structures I.
cation arrangement in spinels. _J. Chem. Phys._ 15, 174–180 (1947). Article ADS CAS Google Scholar * Matsuda, M. et al. Spin–lattice instability to a fractional magnetization state in
the spinel HgCr2O4. _Nat. Phys._ 3, 397–400 (2007). Article CAS Google Scholar * Kawai, H., Tabuchi, M., Nagata, M., Tukamoto, H. & West, A. R. Crystal chemistry and physical
properties of complex lithium spinels Li2MM′3O8 (M=Mg, Co, Ni, Zn; M′=Ti, Ge). _J. Mater. Chem._ 8, 1273–1280 (1998). Article CAS Google Scholar * Strobel, P., Palos, A. I., Anne, M.
& Cras, F. L. Structural, magnetic and lithium insertion properties of spinel-type Li2Mn3MO8 oxides (M = Mg, Co, Ni, Cu). _J. Mater. Chem._ 10, 429–436 (2000). Article CAS Google
Scholar * Sekine, C., Uchiumi, T., Shirotani, I. & Yagi, T. Metal-insulator transition in PrRu4P12 with skutterudite structure. _Phys. Rev. Lett._ 79, 3218–3221 (1997). Article ADS
CAS Google Scholar * Lee, C.-H. et al. Structural phase transition accompanied by metal–insulator transition in PrRu4P12. _J. Phys.: Condens. Matter_ 13, L45–L48 (2001). ADS CAS Google
Scholar * Harima, H., Takegahara, K., Curnoe, S. H. & Ueda, K. Theory of metal-insulator transition in praseodymium skutterudite compounds. _J. Phys. Soc. Jpn._ 71, 70–73 (2002).
Article Google Scholar * Pen, H. F., van den Brink, J., Khomskii, D. I. & Sawatzky, G. A. Orbital ordering in a two-dimensional triangular lattice. _Phys. Rev. Lett._ 78, 1323–1326
(1997). Article ADS CAS Google Scholar * Katayama, N., Uchida, M., Hashizume, D., Niitaka, S., Matsuno, J., Matsumura, D., Nishihata, Y., Mizuki, J., Takeshita, N., Gauzzi, A., Nohara,
M. & Takagi, H. Anomalous metallic state in the vicinity of metal to valence-bond solid insulator transition in LiVS2. _Phys. Rev. Lett._ 103, 146405 (2009). Article ADS CAS PubMed
Google Scholar * Kojima, K., Katayama, N., Tamura, S., Shiomi, M. & Sawa, H. Vanadium trimers randomly aligned along the _c_-axis direction in layered LiVO2. _Phys. Rev. B_ 100, 235120
(2019). Article ADS CAS Google Scholar * Takayama, T. et al. Spin-orbit coupling induced semi-metallic state in the 1/3 hole-doped hyper-kagome Na3Ir3O8. _Sci. Rep._ 4, 6818 (2014).
Article CAS PubMed PubMed Central Google Scholar * Müller, U. _Inorganic Structural Chemistry_ (pp. 128–149. John Wiley & Sons, Chichester, UK, 2007). Google Scholar * Oka, T.
Observation of the infrared spectrum of H3 +. _Phys. Rev. Lett._ 45, 531–534 (1980). Article ADS CAS Google Scholar * Subramanian, M. A., Aravamudan, G. & Subba Rao, G. V. Oxide
pyrochlores. _Prog. Solid State Chem._ 15, 55–143 (1983). Article CAS Google Scholar * Jönsson, P. E. et al. Correlation-driven heavy-fermion formation in LiV2O4. _Phys. Rev. Lett._ 99,
167402 (2007). Article ADS PubMed CAS Google Scholar * Uchida, S. et al. Optical spectra of La2−_x_Sr_x_CuO4: effect of carrier doping on the electronic structure of the CuO2 plane.
_Phys. Rev. B_ 43, 7942–7954 (1991). Article ADS CAS Google Scholar * Imada, M., Fujimori, A. & Tokura, Y. Metal-insulator transition. _Rev. Mod. Phys._ 70, 1039–1263 (1998). Article
ADS CAS Google Scholar * Basov, D. N., Averitt, R. D., van der Marel, D., Dressel, M. & Haule, K. Electrodynamics of correlated electron materials. _Rev. Mod. Phys._ 83, 471–541
(2011). Article ADS CAS Google Scholar * Kézsmárki, I. et al. Charge dynamics near the electron-correlation induced metal-insulator transition in pyrochlore-type molybdates. _Phys. Rev.
Lett._ 93, 266401 (2004). Article ADS PubMed CAS Google Scholar * Yoshitake, J. & Motome, Y. Trimer formation and metal–insulator transition in orbital degenerate systems on a
triangular lattice. _J. Phys. Soc. Jpn._ 80, 073711 (2011). Article ADS CAS Google Scholar * Thorogood, G. J., Saines, P. J., Kennedy, B. J., Withers, R. L. & Elcombe, M. M. Diffuse
scattering in the cesium pyrochlore CsTi0.5W1.5O6. _Mater. Res. Bull._ 43, 787–795 (2008). Article CAS Google Scholar * Takenaka, K. et al. Anisotropic optical spectra of
BaCo1−_x_Ni_x_S2: effect of Ni substitution on the electronic structure of the Co1−_x_Ni_x_S plane. _Phys. Rev. B_ 63, 115113 (2001). Article ADS CAS Google Scholar * Blessing, R. H.
Data reduction and error analysis for accurate single crystal diffraction intensities. _Crystallogr. Rev._ 1, 3–58 (1987). Article Google Scholar * Blessing, R. H. DREADD—data reduction
and error analysis for single-crystal diffractometer data. _J. Appl. Crystallogr_. 22, 396–397 (1989). Article Google Scholar * Sheldrick, G. M. A short history of _SHELX_. _Acta
Crystallogr._ A64, 112–122 (2008). Article ADS MATH CAS Google Scholar * Momma, K. & Izumi, F. _VESTA 3_ for three-dimensional visualization of crystal, volumetric and morphology
data. _J. Appl. Crystallogr._ 44, 1272 (2011). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS The authors are grateful to Y. Yamakawa, A. Yamakage, A. Koda, R. Kadono, J.
Matsuno, and D. Hirai for helpful discussions and T. Fujii for his help with experiments. Y.O. is also grateful for collaboration with Y. Nagao, J. Yamaura, M. Ichihara, Z. Hiroi, and M.
Yoshida in the early stage of this work using polycrystalline samples. This work was partly carried out under the Visiting Researcher Program of the Institute for Solid State Physics, the
University of Tokyo and supported by JSPS KAKENHI (Grant Number: 18H04314, 19H05823, 16H03848, 15H05886, 15H05882, 20K03829, 17H02918, 18H04310). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS
* Department of Applied Physics, Nagoya University, Furo-cho, Chikusa-ku, Nagoya, 464-8603, Japan Yoshihiko Okamoto, Haruki Amano, Naoyuki Katayama, Hiroshi Sawa, Kenta Niki, Rikuto Mitoka,
Yasunori Yokoyama, Yuto Nakamura, Hideo Kishida & Koshi Takenaka * Department of Physics, Kobe University, Rokkodai 1-1, Nada-ku, Kobe, 657-8501, Japan Hisatomo Harima * Graduate School
of Integrated Arts and Sciences, Hiroshima University, Kagamiyama 1-7-1, Higashi-Hiroshima, 739-8521, Japan Takumi Hasegawa & Norio Ogita * Institute for Solid State Physics, University
of Tokyo, Kashiwanoha 5-1-5, Kashiwa, 277-8581, Japan Yu Tanaka & Masashi Takigawa * National Institute for Materials Science (NIMS), Sakura 3-13, Tsukuba, 305-0003, Japan Kanji Takehana
& Yasutaka Imanaka Authors * Yoshihiko Okamoto View author publications You can also search for this author inPubMed Google Scholar * Haruki Amano View author publications You can also
search for this author inPubMed Google Scholar * Naoyuki Katayama View author publications You can also search for this author inPubMed Google Scholar * Hiroshi Sawa View author publications
You can also search for this author inPubMed Google Scholar * Kenta Niki View author publications You can also search for this author inPubMed Google Scholar * Rikuto Mitoka View author
publications You can also search for this author inPubMed Google Scholar * Hisatomo Harima View author publications You can also search for this author inPubMed Google Scholar * Takumi
Hasegawa View author publications You can also search for this author inPubMed Google Scholar * Norio Ogita View author publications You can also search for this author inPubMed Google
Scholar * Yu Tanaka View author publications You can also search for this author inPubMed Google Scholar * Masashi Takigawa View author publications You can also search for this author
inPubMed Google Scholar * Yasunori Yokoyama View author publications You can also search for this author inPubMed Google Scholar * Kanji Takehana View author publications You can also search
for this author inPubMed Google Scholar * Yasutaka Imanaka View author publications You can also search for this author inPubMed Google Scholar * Yuto Nakamura View author publications You
can also search for this author inPubMed Google Scholar * Hideo Kishida View author publications You can also search for this author inPubMed Google Scholar * Koshi Takenaka View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS The samples were prepared by K.N., R.M., and Y.O. Single crystal XRD experiments were performed by
H.A., H.S., and N.K. Electrical resistivity was measured by K.N., Y.O., and K. Takenaka. Reflectivity measurements were done by Y.Y., K. Takenaka, K. Takehana, Y.I., K.N., Y.N., and H.K.
Magnetic susceptibility and heat capacity were measured by Y.O., K.N., and R.M. Raman scattering experiments were performed by T.H., R.M., and N.O. NMR experiments were performed by Y.T. and
M.T. First principles calculations were performed by H.H. The manuscript was drafted by Y.O. and revised by all the authors. CORRESPONDING AUTHOR Correspondence to Yoshihiko Okamoto. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Alexander Yaresko and the other,
anonymous, reviewer(s) for their contribution to the peer review of this work. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Okamoto, Y., Amano, H., Katayama, N. _et al._ Regular-triangle trimer and charge
order preserving the Anderson condition in the pyrochlore structure of CsW2O6. _Nat Commun_ 11, 3144 (2020). https://doi.org/10.1038/s41467-020-16873-7 Download citation * Received: 03
December 2019 * Accepted: 29 May 2020 * Published: 19 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16873-7 SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative



![[withdrawn] category 1 licence application (part 2)](https://www.gov.uk/assets/static/govuk-opengraph-image-03837e1cec82f217cf32514635a13c879b8c400ae3b1c207c5744411658c7635.png)