- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Synthetic biology is a powerful tool to create therapeutics which can be rationally designed to enable unique and combinatorial functionalities. Here we utilize non-pathogenic _E
coli_ Nissle as a versatile platform for the development of a living biotherapeutic for the treatment of cancer. The engineered bacterial strain, referred to as SYNB1891, targets
STING-activation to phagocytic antigen-presenting cells (APCs) in the tumor and activates complementary innate immune pathways. SYNB1891 treatment results in efficacious antitumor immunity
with the formation of immunological memory in murine tumor models and robust activation of human APCs. SYNB1891 is designed to meet manufacturability and regulatory requirements with built
in biocontainment features which do not compromise its efficacy. This work provides a roadmap for the development of future therapeutics and demonstrates the transformative potential of
synthetic biology for the treatment of human disease when drug development criteria are incorporated into the design process for a living medicine. SIMILAR CONTENT BEING VIEWED BY OTHERS
ENGINEERED _E. COLI_ NISSLE 1917 FOR DELIVERY OF BIOACTIVE IL-2 FOR CANCER IMMUNOTHERAPY Article Open access 02 August 2023 PROBIOTIC NEOANTIGEN DELIVERY VECTORS FOR PRECISION CANCER
IMMUNOTHERAPY Article Open access 16 October 2024 REPROGRAMMING THE TUMOR IMMUNE MICROENVIRONMENT USING ENGINEERED DUAL-DRUG LOADED _SALMONELLA_ Article Open access 06 August 2024
INTRODUCTION As the immuno-oncology field matures and as more therapies are evaluated in the clinic, our appreciation for the complexity of tumor-immune cell interactions deepens. While
immunotherapies have become the standard of care for numerous cancers, significant unmet medical need persists primarily for the 55–87% of patients failing to respond to checkpoint
inhibitors1. Combinatorial therapies seek to expand response rates2, with an emerging interest in innate immune agonists, such as toll-like receptor (TLR)3,4 and STimulator of INterferon
Genes (STING) agonists5,6,7, to drive intratumoral antigen-presenting cell (APC) activation and tumor-antigen presentation to effector T cells. Small molecule STING (smSTING) agonists are of
particular interest because of their role in the production of type I interferons (IFNs) and the subsequent generation of antitumor immunity5,6,7. Recent findings are beginning to elucidate
a potential shortcoming of smSTING agonists in that they may lead to activation of STING in “off-target” cell types, such as effector T cells, resulting in apoptosis8 and ultimately
impeding the formation of immunological memory9. Different means of targeting STING agonists to intratumoral APCs could reduce these non-targeted, systemic effects and improve the overall
efficacy of such approaches. Certain bacteria are an ideal vector for targeting STING agonism to APCs as they are actively phagocytosed and have the added benefit of triggering complementary
immune pathways through the stimulation of pattern-recognition receptors (PRRs), such as the TLRs. Use of bacterial-based therapies in cancer dates back to 1891 with the identification and
testing of Coley’s toxin10. More recently, numerous studies have demonstrated the intrinsic capacity of various bacteria to selectively colonize tumors, primarily localizing to the hypoxic
tumor core and sometimes leading to tumor regressions11,12,13,14,15,16,17. These therapies primarily focused on the use of attenuated pathogenic bacteria. Non-pathogenic bacteria with long
historical use as probiotics and established safety profiles in humans, such as _Escherichia coli_ Nissle 1917 (_Ec_N) or Symbioflor-2, represent more favorable bacterial chassis for the
development of a living cancer therapeutics17,18. In particular, _Ec_N has a variety of advantageous features, including increased serum sensitivity19, susceptibility to a broad range of
antibiotics18, its defined genomic landscape and most importantly its engineerability18,20. In this study, we evaluate the utility of _Ec_N as a platform for localized modulation of the
tumor microenvironment (TME), demonstrating tumor-restricted colonization, intratumoral metabolic activity, localized immune activation, and impact on tumor growth. Then using synthetic
biology techniques, we engineered a strain of _Ec_N, referred to as SYNB1891, to target STING activation to phagocytic APCs in the tumor and to trigger complementary innate immune pathways
via the bacterial chassis. Synthetic biology represents a promising means to develop therapies with complex, rationally designed functionalities21,22. While these techniques have been
utilized extensively to demonstrate proof of concept for many approaches, most fail to implement design elements which would support efficient translation into clinical evaluation. With the
criteria of a clinical product at the forefront of our design process, we demonstrate the step-by-step incorporation of design elements to create a living therapeutic which produces the
STING-agonist cyclic di-AMP (CDA), contains dual safety and biocontainment features and is amenable to large-scale manufacturing. Here we evaluate the mechanism of action and pharmacological
properties of the final clinical candidate in relevant murine and human model systems. Importantly, SYNB1891 maintains full functionality after the incorporation of all these critical
design elements which makes it safe, scalable for manufacturing and suitable for testing in humans from a regulatory perspective. RESULTS _E. COLI_ NISSLE AS AN ONCOLOGY THERAPEUTIC VECTOR
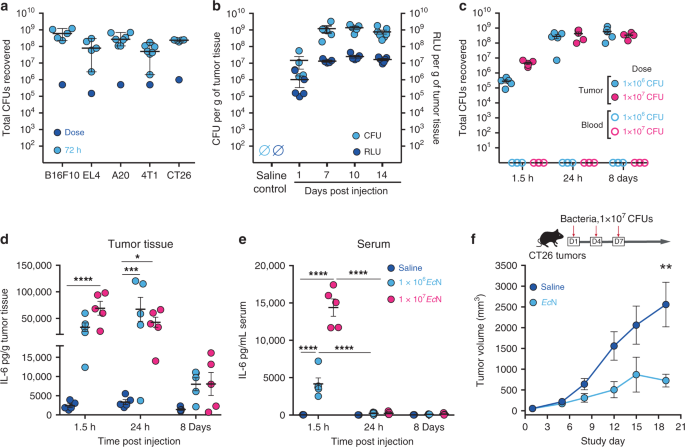
In agreement with previous studies23,24,25,26,27, upon intratumoral (i.t.) delivery _Ec_N expands and colonizes a wide variety of murine tumor types, including B16.F10, EL4, A20, 4T1, and
CT26 syngeneic tumors in wildtype immunocompetent mice (Fig. 1a). In tumors _Ec_N exhibited a rapid expansion of 100–1000 fold, reaching steady state between 24 and 72 h, and remained
localized to the tumor as bacteria were not detected in blood (Fig. 1a–c). To evaluate _Ec_N persistence and metabolic activity, we used an engineered strain containing the _LuxABCDE_
bioluminescent reporter cassette (_Ec_N-Lux)28. Following i.t. injection, _Ec_N-Lux expanded in tumors, persisted and exhibited consistent metabolic activity (measured as bioluminescence)
for up to 14 days (Fig. 1b). Bioluminescence also enabled qualitative monitoring of bacterial localization within the tumor and surrounding tissue on a macroscopic scale. Following i.t.
injection bioluminescence is detected within and throughout the tumor mass; however, it is not detected in the subcutaneous space surrounding the tumor up to 72 h post-injection
(Supplementary Fig. 1a). Intratumoral treatment resulted in dose-dependent increases in IL-6 and TNFα in both tumor and serum at early time points (Fig. 1d, e and Supplementary Fig. 1b, c),
yet, the magnitude and duration of the response were substantially higher within tumors. Finally, i.t. administration of _Ec_N resulted in a significant delay in tumor growth compared to
saline injection controls but did not result in complete tumor regressions (Fig. 1f). Collectively, these data demonstrate the utility of _Ec_N as a delivery vector for the treatment of
cancer which exhibits localization, persistence, metabolic activity within tumor tissue and a moderate level of anti-tumor activity. Such attributes would be important for the sustained
delivery of relevant immunological therapeutic payloads. ENGINEERING A STING AGONIST-PRODUCING LIVE THERAPEUTIC STING bridges innate and adaptive immunity through activation of APCs,
production of type I IFNs, neoantigen cross-presentation to cytotoxic T cells and subsequently the initiation of tumor-specific T cell responses5,6,7. Cyclic di-nucleotides (CDNs) bind to
and activate STING, triggering type I IFNs29,30. Physiologically, CDNs can originate from host cyclic GMP-AMP synthase (cGAS) following detection of cytosolic double-stranded DNA29 or when
produced by invading, intracellular bacteria. While the CDN product of cGAS, 2′3′-cGAMP, is made by eukaryotic cells, prokaryotes produce and utilize other CDNs, such as CDA, cyclic-di-GMP
and 3′3′-cGAMP, for critical signaling processes31. We developed a STING-agonist-producing _Ec_N therapeutic strain by systematically prototyping and incorporating the various components
necessary for a therapy intended for large-scale manufacturing and translation to the clinic. These steps included selection of a CDN-producing enzyme, selection of an inducible promoter,
incorporation of auxotrophies for safety and biocontainment, and removal of any antibiotic resistances used during strain engineering and development. A panel of three CDA-producing enzymes
(encoded by _dncV_ from _Vibrio cholerae_, _cdaS_ from _Bacillus subtilis_ and _dacA_ from _Listeria monocytogenes_) were expressed in _Ec_N under a tetracycline-inducible promoter (Ptet)
and the resulting levels of CDA production were evaluated in vitro. Diadenylate cyclase (DacA) was selected for further evaluation as it produced the highest levels of CDA following gene
induction in _Ec_N (Fig. 2a). Co-culture of _dacA-_expressing _Ec_N (SYN-Ptet-_dacA_) with the RAW 264.7 immortalized macrophage cell line resulted in a dose-dependent secretion of IFNβ1,
whereas non-induced SYN-Ptet-_dacA_ did not (Fig. 2b). To evaluate activity in vivo, SYN-Ptet-_dacA_ or non-engineered _Ec_N were i.t. administered followed by intraperitoneal (i.p.)
injection of anhydrous tetracycline (aTc) into B16.F10 melanoma tumor-bearing mice. B16.F10 was selected as a relevant tumor models as this represents a poorly inflamed tumor which is
typically refractory to immunotherapy in mice32,33. Similar to results obtained in vitro, production of CDA by engineered _Ec_N resulted in a gain of IFNβ1 induction, compared to
non-engineered _Ec_N, in vivo (Fig. 2c). Additionally, SYN-Ptet-_dacA_ treatment significantly decreased tumor growth by eight days post-treatment initiation (Supplementary Fig. 2a–c). While
treatment with both engineered and non-engineered _Ec_N led to early increases in innate associated cytokines (like TNFα, GM-CSF, IL-6, IL-1β and CCL2) 24 h post dose initiation
(Supplementary Fig. 2d), only SYN-Ptet-_dacA_ treatment resulted in a shift to expression of T-cell associated cytokines (like IL-2, Granzyme B and IFNγ) at 8 days post dose initiation
(Supplementary Fig. 2e). Collectively, these data suggest the engineered expression of CDA in _Ec_N enables the additional activation of type I IFNs and possibly the initiation of
efficacious, T-cell antitumor immunity in vivo. Tightly controlled induction of the _dacA_ gene circuit is critical from a manufacturing perspective as rapid depletion of adenosine
triphosphate (ATP) and/or production of the bacterial signaling molecule CDA could hinder large-scale biomass production and bacterial fitness, and as such the use of an inducible promoter
becomes crucial. Since the utilization of tetracyclines as an induction agent is not desirable for clinical studies, we evaluated several other promoter/inducer systems. To assess activity
in vivo we introduced inducible promoter-GFP cassettes into an _Ec_N strain which constitutively expressed mCherry (RFP) and systemically administered induction agents following bacterial
colonization of B16.F10 tumors. This approach allowed for the detection of RFP+ bacteria by flow cytometry from tumor cell suspensions and quantification of gene induction by GFP (Fig. 2d).
Utilizing this approach, we evaluated the activity of the salicylate (Psal), cumate (Pcmt) and fumarate-and-nitrate reductase (PfnrS) promoters, in combination with their associated
agonists, salicylic acid, cumate, and hypoxia, respectively. While all promoters showed functionality within an hour of administration of their respective inducing agent, the hypoxia
inducible PfnrS34,35 was selected for further development as this promoter showed the highest levels of intratumoral payload induction, with 65–95% of all bacteria induced. These data are in
agreement with the results reported for _Salmonella typhimurium_ where a set of hypoxia sensitive promoters, such as the Nitrate Reductase promoter, were shown to enable tumor-specific
induction compared to the spleen36. Furthermore, PfnrS does not require administration of an exogenous agent due to the hypoxic nature of the TME and oxygen concentration can be tightly
controlled during bacterial expansion in fermenters. The feasibility of hypoxia inducible CDA production was confirmed for _Ec_N containing the PfnrS-_dacA_ circuit under anaerobic
conditions in vitro (Fig. 2e). From a safety and regulatory perspective, biocontainment controls are critical elements of a bacterial-based live therapeutic for clinical use37,38. The
introduction of a thymidine (thy) auxotrophy by deletion of the thymidylate synthase gene (_thyA_) has been shown to be effective as a biocontainment mechanism, as free thy is not readily
available in most extracellular spaces37. We found that the TME contains a sufficient concentration of free thy for the _Ec_N _thyA_ mutant to proliferate and colonize (Supplementary Fig.
2f). Diaminopimelic acid (dap) is a component of the bacterial cell wall and is not produced by eukaryotes, and therefore we hypothesized that a dap auxotrophic strain would be unable to
survive in a mammalian host environment. Indeed, deletion of the _dapA_ gene (encoding 4-hydroxy-tetrahydropicolinate synthase) resulted in a mutant strain of _Ec_N that was unable to expand
or persist within tumors and was cleared over time (Supplementary Fig. 2f). We thus engineered a dual safety mechanism by introducing both _dapA_ and _thyA_ deletions to prevent
intratumoral and extra-tumoral bacterial proliferation, respectively, and the inability of a double mutant to proliferate in vivo was confirmed in a variety of tumor types (Fig. 2f and
Supplementary Fig. 2g). To ensure stability during manufacturing and to meet regulatory guidelines the PfnrS-_dacA_ circuit was inserted into the genome of the _dapA_, _thyA_ double mutant
and all antibiotic resistance genes were removed. The final clinical candidate strain, referred to as SYNB1891, maintained its anaerobically inducible production of CDA (Fig. 2g),
dose-dependent biological activity when co-cultured with RAW 264.7 macrophage cells in vitro (Fig. 2h), inherent sensitivity to human serum (Supplementary Fig. 2h) and sensitivity to a wide
panel of antibiotics currently utilized in the clinic (Supplementary Fig. 2i). In summary, the selection and validation of these various modular components constituted our clinical candidate
strain, SYNB1891, consisting of an engineered _Ec_N with dual _dapA_ and _thyA_ auxotrophies, the genomic integration of the _dacA_ gene under the control of the anaerobically inducible
PfnrS promoter and removal of antibiotic resistance genes (Fig. 2i). MECHANISMS OF SYNB1891-MEDIATED TYPE I INTERFERON INDUCTION To begin deconvoluting the mechanisms of action of SYNB1891,
we treated murine bone marrow-derived dendritic cells (BMDCs) from wild-type (WT), _Tmem173__gt_ (STING−/−) or _Tlr4__lps-del_ (TLR4−/−) mice and incorporated relevant pathway controls, such
as purified lipopolysaccharide (LPS) and a benchmark selective smSTING-agonist, 2′3′-c-di-AM(PS)2 (Rp, Rp), utilized at a concentration (5 μg/ml) similar to what has been previously
reported6. Type I interferon production by BMDCs in response to SYNB1891 was greatly dependent on STING signaling, as STING−/− BMDCs failed to induce high levels of IFNβ1 expression (Fig. 3a
and Supplementary Fig. 3a). _Ec_N chassis itself and LPS induced low levels of IFNβ1 expression in a TLR4-dependent but STING-independent manner (Fig. 3a and Supplementary Fig. 3a),
suggesting that TLR4 activation may play a minor role in type I IFN induction in response to SYNB1891. IFNβ1 expression in response to smSTING agonist was preserved in TLR4−/− BMDCs and lost
in STING−/− BMDCs (Fig. 3a and Supplementary Fig. 3a). Additionally, SYNB1891 and smSTING-agonist treatment resulted in similar levels of upregulation for the co-stimulatory marker _cd86_
as compared to controls (Supplementary Fig. 3b). Despite a peak ~1.5-fold increase in IFNβ1 production compared to SYNB1891 (Fig. 3a), smSTING-agonist resulted in far less or no production
of additional proinflammatory cytokines like IL-6, IL-12, TNFα and IL-1β up to 18 h post-treatment (Fig. 3b, c and Supplementary Fig. 3c–g). LPS-TLR4 signaling may contribute to the
expression of these additional cytokines, as lack of TLR4 signaling significantly blunted expression of _Il6_ and _Il12a_ mRNA in response to SYNB1891, control _Ec_N and LPS (Fig. 3b, c).
Collectively, SYNB1891 treatment resulted in the greatest production for all analyzed cytokines relative to _Ec_N control, suggesting that both the bacterial chassis and the engineered
expression of CDA play important roles in the overall immune-activating mechanism of action of SYNB1891 (Supplementary Fig. 3c–g). Next, we used the phagocytosis inhibitor cytochalasin D to
evaluate the contribution of active cellular uptake of SYNB1891 towards its overall mechanism of action in macrophages (RAW cells) and BMDCs. Cytochalasin D inhibits actin polymerization and
prevents phagocytosis, yet it has minimal effect on endocytosis or pinocytosis of soluble small molecules39,40. To quantify phagocytosed bacteria, nuclei and F-actin staining were used to
identity individual BMDCs and demarcate their cellular border, respectively. Co-culture of murine BMDCs with SYNB1891 modified to express GFP (SYNB1891-_gfp_), showed many bacterial cells
were internalized (e.g. co-localized with F-actin staining) and residing within mature phagosomes that contained lysosome-associated membrane protein (LAMP-1) (Fig. 3d, e). Pretreatment with
cytochalasin D significantly inhibited the number of bacterial cells observed within BMDCs (Fig. 3f, g) and abrogated expression of IFNβ1 following treatment with SYNB1891 in both cell
types (Fig. 3h, j and Supplementary Fig. 3h, k). In contrast, cytochalasin D did not significantly impact IFNβ1 induction following treatment with smSTING-agonist (Fig. 3h, j and
Supplementary Fig. 3h, k). Inhibition of phagocytosis did not significantly impact TNFα production in response to bacterial or LPS treatment (Supplementary Fig. 2j), yet decreased IL-6
expression by 2.5-5 fold (Fig. 3i, k). These data suggest that _Ec_N can trigger proinflammatory cytokines secretion from the surface of the phagocytic cell, although internalization is
required to optimally enhance signaling, induce IFNβ1 and activate STING. Relative expression levels of each cytokine and the impact of phagocytosis on their expression by _Ec_N, SYNB1891
and smSTING-agonist treatments are summarized in Supplementary Fig. 3l. Collectively, these data suggest that SYNB1891 is phagocytosed and active uptake is required for STING-dependent
induction of type I IFN responses, thus providing a natural mechanism for preferential activation of APCs. Moreover, the data demonstrate the pleiotropic activity provided by the _Ec_N
chassis of SYNB1891 which activates parallel innate immune signaling pathways, through PRRs like TLR4, and results in the expression of complementary proinflammatory cytokines. SYNB1891
TRIGGERS MULTIPLE INNATE IMMUNE PATHWAYS IN VIVO To evaluate its activity in vivo, we delivered a single i.t. injection of SYNB1891 at various dose levels into established B16.F10 murine
melanoma tumors and evaluated its pharmacokinetics and pharmacodynamics over time. As expected, the _dapA_ mutation incorporated into SYNB1891 resulted in its clearance from the TME over
time, with bacteria undetectable by colony forming unit (CFU) assay in blood at all time points (Fig. 4a and Supplementary Fig. 4b, c). Quantitative PCR (qPCR) using SYNB1891 specific probes
was utilized as a secondary means to detect bacteria presence in the blood independent of cell viability. While SYNB1891 DNA was detected in the majority of mice injected intravenously (as
a positive control) up to 24-h post dose, only 1 of 5 mice receiving i.t. injection at the highest dose had detectable levels of SYNB1891 in blood at 2- and 6-h (Supplementary Fig. 4d, e)
post-injection. SYNB1891 treatment delayed tumor growth and resulted in undetectable tumors in some animals (Fig. 4b). Intratumoral production of CDA was detected at 24- and 72-h post dose,
confirming the functional activity of SYNB1891 in vivo (Fig. 4c). Type I IFNs (Fig. 4d, e) as well as a variety of proinflammatory cytokines, including TNFα, IL-6, IL-1β, IFNγ and GM-CSF
(Fig. 4f–j), were induced in a dose-dependent manner, highlighting the dual activity of both CDA and the _Ec_N bacterial chassis. In agreement with previous reports utilizing
smSTING-agonists41, significant upregulation of IL-15 was also observed following treatment with SYNB1891 (Fig. 4k). Collectively, these data demonstrate the dose-dependent pharmacology of
SYNB1891, its target engagement in vivo and its utility as a potent inducer of localized inflammation and its potential anti-tumor activity. SYNB1891 GENERATES EFFICACIOUS ANTI-TUMOR
IMMUNITY Durable responses are a hallmark of successful immunotherapy. To evaluate the efficacy of SYNB1891 we treated B16.F10 tumor-bearing mice with three i.t. injections over the course
of a week and monitored them long-term. SYNB1891 treatment resulted in a dose-dependent significant delay in tumor growth compared to saline injection controls and drove durable tumor
rejections for 30–40% of animals (Fig. 5a and Supplementary Fig. 5a–c). While injection of control _Ec_N resulted in a significant delay of tumor growth, it did not result in complete tumor
rejection. Utilizing a benchmark smSTING-agonist at a previously reported efficacious dose (50 μg per animal)6, we performed a head-to-head comparison of the long-term efficacy of the
bacterial chassis alone (Control _Ec_N), STING activation alone (smSTING Agonist) or engineered STING agonist in a bacterial chassis (SYNB1891). While both STING-agonist approaches were
effective at controlling tumor growth for up to 2 weeks, SYNB1891 treatment resulted in greater long-term efficacy (40% survival) compared to treatment with smSTING agonist (10% survival)
(Fig. 5b and Supplementary Fig. 5d, e). To extend our findings to additional tumor types, we performed similar studies on A20 B cell lymphoma tumors. Treatment of A20 tumors with SYNB1891
resulted in a dose-dependent effect on tumor control with 108, 5 × 108 and 109 CFU doses resulting in 30%, 50%, and 80% of mice with complete tumor rejections, respectively (Fig. 5c and
Supplementary Fig. 6a, b). To evaluate the contribution of T cells towards SYNB1891 efficacy in the A20 model, we depleted CD4+ T cells or CD8+ T cells prior-to treatment initiation and
throughout the course of the study using depleting antibodies. While mice treated with either isotype control or a CD4+ T cell-depleting antibody exhibited a 40-50% complete response rate,
0% of mice receiving a CD8+ T cell-depleting antibody survived long-term (Fig. 5d). Finally, SYNB1891-treated animals that had remained A20 tumor-free for at least 60 days were rechallenged
with A20 cells on the contralateral flank. Unlike age-matched naïve controls which showed rapid tumor growth, all cured mice remained tumor-free after re-challenge (Fig. 5e and Supplementary
Fig. 6c). These data suggest that SYNB1891 treatment results in the activation of CD8+ T cells which are required for long-term efficacy and likely the formation of protective immunological
memory. Collectively, these data demonstrate the complementary contributions of both the _Ec_N bacterial chassis and the production of the STING agonist CDA towards the generation of an
efficacious and durable antitumor immune response in two distinct murine tumor models (B16F10 and A20) in two genetic backgrounds (C57BL/6 and BALB/c). SYNB1891 ACTIVATES MULTIPLE HUMAN
STING ALLELES To assess activity of SYNB1891 in human APCs, we first evaluated STING pathway induction utilizing modified human monocyte THP-1 cell lines containing an interferon-regulatory
factor (IRF) reporter. SYNB1891 treatment resulted in robust dose-dependent IRF induction which was lost in STING−/− cells (Fig. 6a) and reduced in cGAS−/− cells (Fig. 6b). In contrast to
murine APCs where bacterial chassis did not induce significant amount of IFN-β1, treatment of human THP-1 cells with non-engineered control _Ec_N led to moderate, dose-dependent STING
pathway induction (Fig. 6b). However, bacterial chassis alone completely lost the ability to stimulate IRF in cGAS−/− cells. (Fig. 6b). These data suggest a dual mechanism of action for
SYNB1891 in the stimulation of STING in human APCs which includes direct activation of STING via CDA produced by SYNB1891, and activation of cGAS, likely through detection of cytoplasmic
bacterial DNA (Supplementary Fig. 7a). Treatment of THP-1 cells containing an NF-κB reporter with SYNB1891 also led to a robust dose-dependent activation of the NF-κB pathway, which was
partially reduced, ~1.6-fold, in STING−/− cells (Fig. 6c). These results also suggest that SYNB1891 triggers NF-κB induction through STING-dependent and STING-independent pathways. Next, we
evaluated STING-pathway activity across a panel of THP-1 cell lines which contained three of the most prevalent human _TMEM173_ (STING) variants: R232 representing 57.9%, HAQ representing
20.4% and H232 representing 13.7% of alleles found in the human population, respectively42. SYNB1891 treatment resulted in ~9-to-28-fold IRF induction across all three alleles compared to
non-stimulated or STING−/− controls (Fig. 6d). In agreement with previous studies6, the HAQ allele showed the highest level of induction with the R232 and H232 alleles showing intermediate
and lower activity, respectively. Next, we evaluated SYN1891 activity and the contribution of phagocytosis in primary human, monocyte-derived dendritic cells (DCs). Similar to results
obtained in murine APCs, treatment of primary human DCs with SYNB1891 resulted in robust expression of _IFNB1_, the expression of various proinflammatory cytokines (such as _IL6, TNFa,
IL-1B,_ and _IL12A_) and the upregulation of various DC maturation makers (like _CD86_ and _CD40_) (Fig. 6e, f and Supplementary Fig. 7b–l). While treatment with either control _Ec_N or
SYNB1891 resulted in similar levels of expression for the various proinflammatory cytokines and DC maturation markers, SYNB1891 treatment led to significantly higher induction of _IFNB1_.
Both _IFNB1_ and _IL6_ expression were decreased following pretreatment with cytochalasin D, suggesting that optimal stimulation of the STING and NF-κB pathways requires active bacterial
phagocytosis by human APCs (Fig. 6e, f). Treatment with up to 25 μg/mL smSTING-agonist resulted in comparable levels of _CD86_ expression, however lower levels of _IFNB1_, _CD40_, _IL6_, and
_IL12A_ expression relative to SYNB1891, with no detection of _IL1B_ above background (Fig. 6e, f and Supplementary Fig. 7b–l). Together, these results confirm the translation of our
findings from mouse models to human APCs for the induction of type I IFNs and NF-κB-associated cytokines following SYNB1891 treatment, and for a range of _TMEM173_ allelic variants
representing a large majority of the human population. DISCUSSION In this study, we used synthetic biology techniques and drug development criteria to design and create an engineered
bacterial strain capable of localized, targeted STING activation and a product suitable for manufacturing and evaluation in human clinical trials. SYNB1891’s functionality was rationally
designed by incorporating the _dacA_, CDA-producing enzyme, in a tumor tropic bacterial chassis to target STING activation to APCs in the TME. In addition, as a Gram-negative bacterial
chassis _Ec_N triggers complementary proinflammatory pathways through PRR activation. Safety and biocontainment features were incorporated by controlling bacterial proliferation inside and
outside of tumors through two distinct auxotrophies, _thyA_ and _dapA_, and by removing antibiotic resistance genes to enable clearance of bacteria by available treatments. Lastly, by
utilizing the PfnrS promoter to control _dacA_ expression, STING-agonist production is repressed to generate biomass during manufacturing and later induced upon entry into the hypoxic TME.
The resulting biotherapeutic, SYNB1891, was shown to produce high levels of CDA and induce potent type I IFN production in a phagocytosis-dependent manner in both, mouse and human APCs.
Mechanistically, smSTING agonists non-specifically penetrate a variety of cell types while a bacterial vector like SYNB1891 preferentially reaches the intracellular space of APCs via active
phagocytosis. This is potentially an important feature to avoid “off-target” STING-pathway activation in non-APCs, such as T cells where STING activation could be detrimental to the
formation of antitumor immunity8,9. In murine tumor models, SYNB1891 treatment resulted in efficacious anti-tumor immunity, long-term efficacy, and the establishment of immunological memory.
Accordingly, while a recent study demonstrated that high doses of smSTING agonist can have inhibitory effects on the development of immunological memory9, all A20-tumor bearing mice treated
and cured by SYNB1891 were resistant to secondary tumor challenge, regardless of dose level. Beyond STING activation, SYNB1891 was found to trigger expression of additional proinflammatory
cytokines which may result in superior long-term efficacy. Due to _Ec_N’s intrinsic tumor tropism and serum sensitivity, intratumoral injection of this immunostimulatory vector results in a
cytokine response concentrated to the TME. This is advantageous from a safety perspective to minimize systemic exposure, but also from a biological perspective to generate defined chemokine
gradients to steer immune cell trafficking into the tumor. Finally, translation of results from mouse models to humans is often a great challenge. Immune reactivity of mouse versus human
cells to the synthetic smSTING agonist DMXAA is one relevant example, with promising preclinical results in mouse models but a lack of activity as a human STING agonist43. Moreover, the
_TMEM173_ gene (encoding hSTING) presents in the human population in several common alleles with varying binding properties to agonistic molecules42. SYNB1891 treatment induced type I IFN
production in human dendritic cells and in human monocytes containing a variety of the most common human STING (_TMEM173_) gene variants. Important differences between murine and human
systems were uncovered, including the contribution of cGAS towards STING activation following SYNB1891 treatment of human APCs. Beyond the specific description of SYNB1891 and its mechanism
of action in the context of immuno-oncology, our study demonstrates the value of synthetic biology to rationally design microorganisms to fight human disease. A variety of bacterial based
therapies have previously been investigated for the treatment of cancer, with the majority using attenuated pathogens such as _Salmonella typhimurium_, _Clostridium novyi_, and _Listeria
monocytogenes_11. _Listeria-_based approaches typically use attenuated strains as a vaccine vector, taking advantage of the intrinsic capacity of the bacterium to forcefully invade APCs and
engineering expression of shared tumor-associated antigens or unique neoepitopes personalized to each patient44,45,46,47. Similar to traditional vaccine approaches, this requires the a
priori identification of efficacious target antigens. While _Salmonella-_ and _Clostridium-_based therapeutics also require the use of highly attenuated strains, such approaches primarily
take advantage of the oncolytic potential of these pathogenic bacteria48. Clinically these oncolytic strains have either failed to exhibit robust efficacy or have been hindered by
dose-limiting toxicities11. In contrast, SYNB1891 uses the non-pathogenic strain _Ec_N, which has a potentially advantageous safety profile and significantly broader set of tools for genetic
manipulation and engineering. Using synthetic biology techniques, we rationally designed an _Ec_N strain that expresses the STING-agonist CDA and, following intratumoral injection, delivers
targeted activation to APCs in the TME in order to generate and support antitumor immune responses. Since previous bacterial based therapies with compelling preclinical evidence have failed
to demonstrate efficacy in patients, clinical testing of SYNB1891 is critical to assess the translatability of our findings to human disease. A Phase I clinical trial of SYNB1891
intratumoral injection in patients with percutaneous accessible advanced or metastatic malignancies has already been initiated (ClinicalTrials.gov Identifier: NCT04167137). While safety and
tolerability will first be assessed for the percutaneous route of administration, future investigations with radiologically guided intratumoral injection would enable treatment of visceral
lesions. Positive indications for safety and efficacy would provide support for the development of additional engineered strains with rationally designed functionalities tailored to the
needs of specific cancer and patient subtypes. Although the application of synthetic biology to human therapeutics is still in its infancy, our work highlights its potential and provides
guidance for the development of future approaches intended for clinical evaluation. METHODS DATA REPORTING No statistical methods were used to predetermine sample size. Mice utilized for in
vivo experiments were randomized based on tumor volume and investigators were not blinded to allocation during experiments or outcome assessments. MICE SPF C57BL/6, BALB/c, STING−/−
(C57BL/6J-_Tmem173__gt_/J) and TLR4−/− (B6.B10ScN-_Tlr4__lps-del_/JthJ) female mice were purchased from Jackson Laboratory. Animals were maintained under specific pathogen-free conditions in
a climate controlled holding room at an ambient temperature of 21 ± 2 °C with relative humidity ranges between 30% and 70% and automated controlled 12 h dark/light cycle. Mice were used for
the experiments at 7 to 9 weeks of age. Animal housing and all procedures related to in vivo experiments were reviewed and approved by Mispro’s Institutional Animal Care and Use Committee
(Mispro Biotech Services, 400 Technology Square, Cambridge, MA, 02139) in accordance with the Animal Welfare Act. GROWTH AND PRE-INDUCTION OF STRAINS IN SHAKE FLASKS For preparation of
cultures at small scale using flasks, cells were inoculated in 4 ml of 2-YT media containing appropriate antibiotics and/or supplements in a 14-ml culture tube at 37 °C with shaking (250
rpm) overnight. The next day, cell cultures were diluted 1:100 in 40 ml of fresh 2-YT media in 125-mL baffled flasks at 37 °C with shaking (250 rpm) for 2–3 h, with appropriate antibiotics
and/or supplements. For gene circuit induction, depending on the promoter system, cells were induced for 4–5 h with the addition of anhydrotetracycline (Sigma; aTc; 100 ng/ml final
concentration) or by placing flasks in an anaerobic chamber (Coy) supplying an atmosphere of 85% nitrogen, 10% carbon dioxide and 5% hydrogen. Following induction, cells were washed and spun
down by centrifugation at 4000_g_ for 10 min at 4 °C. Cell pellets were utilized to analyze for cyclic-di-AMP by LC-MS/MS (see, _Cyclic-di-AMP (CDA) quantification by LC-MS/MS_). If cell
stocks needed to be stored for future usage, they were harvested by centrifugation, washed with cold PBS buffer, resuspended in formulation buffer containing 15% glycerol and PBS, and stored
at −80 °C. All bacterial stocks utilized for in vitro or in vivo assays were first validated to contain >90% viability, purity as determined by growth in the presence/absence of
antibiotics or metabolites associated with strain auxotrophy and for functionality looking for expression of payload following induction. GROWTH AND INDUCTION OF STRAINS IN BIOREACTORS Cells
were inoculated in 50 mL of FM3 medium supplemented with diaminopimelic acid and thymidine in a 500-mL Ultra-Yield flask (Thomson). Cells were grown at 37 °C with shaking at 350 rpm
overnight. Next day, 110 mL of the overnight culture was used to inoculate 4 L of FM3 in an Eppendorf BioFlow 115 bioreactor (starting OD600 of ~0.5). The fermenter was controlled at 60%
dissolved oxygen with agitation, air, and oxygen supplementation, and controlled to pH 7 using ammonium hydroxide. Cells were harvested after 5 h and at OD600 ~30. Cells were harvested by
centrifugation at 4500_g_ for 30 min at 4 °C, resuspended in formulation buffer, and stored at −80 °C. OD600 was measured on a BioPhotometer Plus spectrophotometer (Eppendorf). Before use,
frozen stocks were evaluated for viability (>90%), purity and functionality as described above. BACTERIAL STRAIN CONSTRUCTION List of strains used in this publication provided in
Supplementary Table 1. _Escherichia coli_ Nissle 1917 (_Ec_N), designated as SYN001 here, was purchased from the German Collection of Microorganisms and Cell Cultures (DSMZ Braunschweig, _E.
coli_ DSM 6601). SYN001 with strep resistance was obtained by streaking ~ 1011 cells of SYN001 on LB plate containing 300 ng/mL streptomycin and taking the single colony formed to have
mutated to be strep resistant and designated as SYN094. Plasmid harboring the _luxABCDE_ operon under the control of constitutive promoter was synthesized and subcloned into pST vector by
Genewiz. Three different cyclic dinucleotide synthase genes _dncV_, _dacA_, and _cdaS_ were codon optimized, synthesized by IDT and subcloned into a Synlogic vector containing kanamycin
resistant gene, a medium copy number origin of replication p15A and under the regulatory control of the Ptet promoter. Similarly, gene encoding GFP protein under the control of Ptet, Psal or
Pcmt promoters were synthesized by IDT or Genewiz and subcloning into the same Synlogic vector with p15A origin and kanamycin resistance. The gene encoding GFP protein under the control of
PfnrS37,38 promoter was also subcloned into a Synlogic vector containing ampicillin resistant gene, a low copy number origin of replication pSC101 and constitutive promoter driven gene
encoding mCherry fluorescent protein. These plasmids were used to electroporate SYN001 or SYN094 with integrated cassette into _malEK_ locus to constitutively express mCherry protein. After
the electroporation (Eporator, Eppendorf, 1.8-kV pulse, 1-mm gap length electro-cuvettes) the transformed cells were selected as colonies on LB agar (Sigma, L2897) containing proper
antibiotics. For deletion of the _dapA_ gene, _thyA_ gene and phage fragment (Φ), a single round of PCR was performed using pKD3 or pKD4 as the template DNA37,38. The primers were designed
to generate a dsDNA fragment that contained homology adjacent to the targeted gene locus or fragment in the _Ec_N chromosome and a chloramphenicol or kanamycin resistance gene flanked by frt
sites. The resulting knockout fragment typically included ~40–60 base pairs of _Ec_N homology on its 5′ and 3′ end (DNA Sequences for Genomic Deletions). Parent strain, which contains the
plasmid pKD46, was transformed with the knockout fragment by electroporation. Colonies were selected on LB agar containing proper antibiotics (30 ng/mL chloramphenicol or 100 ng/mL
kanamycin) and proper auxotrophy supplements (diaminopimelate (100 µg/mL) and/or thymidine (3 mM) and correct recombination events were verified by PCR. A verified colony was saved for
future operations. A plasmid containing constitutively expressed GFP protein was constructed similarly as described above into Synlogic vector with pSC101 origin and ampicillin resistance
gene and transformed into the control Nissle strain with _dapA_, _thyA_ and phage knockouts. Cyclic-di-AMP producing strain was created by insertion of genes into the _Ec_N chromosome. The
intergenic locus exo/cea was identified as suitable integration site. Chromosomal insertions into the _Ec_N genome were carried out using the well-characterized lambda Red recombineering
approach. For this insertion, (1) a pKD4-based plasmid containing ~1000 bp of 5′ and 3′ _Ec_N genome homology for recombination was built, followed by (2) insertion of the Pfnrs_-dacA_
fragment into the plasmid by isothermal assembly (HiFI DNA Assembly Master Mix, NEB), (3) amplification of the insertion fragment from the plasmid by PCR (including _Ec_N homology regions
and kanamycin resistance cassette, Q5 High-Fidelity Master Mix, NEB), (4) recombineering of the insertion fragment by electroporation via pKD46 and subsequent pKD46 removal, and (5) the
removal of antibiotic resistance cassettes via pCP20 and subsequent pCP20 removal. All DNA sequences and detailed scheme for genomic insertion and deletions used in the construction of
clinical candidate strain SYNB1891 are available upon request. Complete genome sequence of E.coli Nissle 1917 is listed in Genbank/EMBL/DDBJ accession number: CP007799. Plasmid maps and DNA
sequences for CDA-producing enzymes are listed in the Supplementary Table 2 and Supplementary Fig. 8. The genome map, manipulated genome sequence of SYNB1891 and corresponding primers are
available in Supplementary Fig. 9 and Supplementary Tables 3–7. Engineered strains described in this manuscript can be made available subject to a Material Transfer Agreement (MTA), which
can be requested by contacting the corresponding authors. All requests will be reviewed by Synlogic to verify whether the request is subject to any intellectual property or confidentiality
obligations. ANTIBIOTIC SENSITIVITY TEST Sensitivity of SYNB1891 to a panel of commonly utilized antibiotics was evaluated by Boston Analytical laboratory (Salem, NH) in accordance with
Clinical and Laboratory Standard Institute (CLSI) Chapter M100 “Performance Standards for Antimicrobial Susceptibility Testing.” Three antibiotic resistance trials were performed using an
independent organism preparation and assay set up. SYNB1891 was grown on LB with 100 µg/mL DAP and 10 mM Thymidine agar and a suspension was prepared then adjusted to a known concentration
using the spectrophotometer. Lawn plates were prepared using the adjusted concentration. A total of 16 Antibiotic disks were assessed. For each disk tested, a lawn plate was prepared by
inoculating the surface of an LB/DAP/Thymidine agar plate with 200 μL of the adjusted suspension. Each disk was aseptically placed in the center of the plate. Sample plates were incubated at
30–35 °C for 18–24 h. A negative control was prepared by placing a blank disk on an inoculated plate. Following incubation, the zones of inhibition were measured using calibrated calipers.
Each unrounded result was documented in millimeters. ANEROBIC IN VITRO CIRCUIT INDUCTION The cryovials were thawed on ice and mixed well before measuring OD density. 1010 cells were taken
into 1 mL PBS in a deep well plate, mixed by pipetting (duplicate each sample). Plate was spun down at 2750_g_, 4 °C for 10 min. Media was discarded and the plate with pellets were stored at
−80 °C as time zero control samples. For induction of the circuit to produce cyclic-di-AMP, 500 µL culture from the thawed cryovial was added into 3.5 mL minimum media (1 × M9 + 50 mM MOPS
+ 2% glucose + 100 ng/mL diaminopimelic acid + 3 mM thymidine) and recovered by shaking at 37 °C, 250 rpm for 30 min. The culture was then moved into anaerobic chamber to incubate statically
for 2 h, at 37 °C. When finishing incubation, the tubes were brought out, mixed by vertexing and OD density was measured. Similar to time zero control samples, 1010 cells were taking into 1
mL PBS in a deep well plate, mixed well by pipetting (duplicate each sample), pelleted by centrifugation and stored at −80 °C. Samples were then sent for LC-MS/MS analysis of cyclic-di-AMP
(see, _Cyclic-di-AMP (CDA) quantification by LC-MS/MS_). For the further in vitro experiments, induced bacteria were washed twice in sterile PBS and bacterial numbers were counted by
Cellometer X2 (Nexcelom Bioscience). SUBCUTANEOUS TUMOR MODELS AND IN VIVO STUDIES B16.F10 melanoma (ATCC, CRL-6475), EL4 T-cell lymphoma (ATCC, TIB-39), A20 B-cell lymphoma (ATCC, TIB-208),
4T1 mammary carcinoma (ATCC, CRL-2539) and CT26 colon carcinoma (ATCC, CRL-2638) cells were cultured under standard conditions as specified by ATCC (37 °C incubator, 5% CO2, humidified) in
their recommended media formulations. Adherent cell lines were harvested following trypsinization. All cell lines were rinsed in Dulbecco’s phosphate-buffered (DPBS) saline twice to remove
excess FBS and resuspended in DPBS for implantation. The following number of cells were implanted for the indicated cell lines: B16.F10 (2 × 105), EL4 (5 × 105), A20 (1 × 106), 4T1 (1 × 105)
and CT26 (1 × 106). Mice were injected subcutaneously using a 26-gauge needle into the shaved, left or right flank and tumors were allowed to establish until they reached between 40 and 300
mm3, as indicated for each experiment. Mice were then randomized into treatment groups based on tumor volume in order to create groups with approximately equal average tumor volumes.
Intratumoral injections involved direct insertion of the needle into the largest section of the tumor and the slow, consistent release of contents. Intravenous injections were performed via
the tail vein. For the efficacy studies tumor-bearing mice were treated on Study days 1, 4, and 7. As indicated, 50 μL of either bacteria, smSTING agonist or saline (as injection control)
were injected into tumors. Tumor volume (_V_) was measured via electronic caliper where _V_ = length × width2 × 0.5. Mice having tumors exceeding 2000 mm3 were euthanized per IACUC protocol.
Unless otherwise stated, once 50% or more of mice in an experimental group were removed from study no further tumor measurements were taken and all remaining mice in said group were
euthanized, with the exception of any mice having no palpable tumor mass. All complete responder (C.R.) mice, or those having no palpable tumor by the indicated timepoint, were monitored
until at least study day 60 and at least 50 days post the final treatment dose to ensure no tumor relapse was observed. In some studies T cells were depleted by intraperitoneal injection of
200 μg of antibody resuspended in DPBS (final volume 100 μl) 1 day before bacterial treatment initiation and twice weekly throughout the study with an isotype matched antibody injected as
control using the following antibodies: rat IgG2b isotype control (clone: LTF-2, BioXcell, BP0090), anti CD4 (clone: GK1.5, BioXcell, BP0003-1), or anti CD8α (clone: 2.43, BioXcell, BP0061).
TUMOR HOMOGENATES At the indicated time points mice were euthanized and tumors were removed via dissection. Harvested tumors were placed in sterile beadbug homogenizer tubes prefilled with
2.8 mm stainless steel beads (Sigma, Z763829) containing 500 μl sterile DPBS, weighed and homogenized for 5 min utilizing a Fast Prep-24 homogenizer (MP Biomedicals). Whole tumor homogenates
were utilized for the quantification of cyclic-di-AMP and bacterial quantification (see below). Tumor supernatants were utilized for the analysis of intratumoral cytokines and were prepared
as follows. Triton-X100 (Thermo Scientific, BP151-100) was diluted in DPBS to generate a 10% Triton-X100 stock solution and added to tumor homogenates to make a final concentration of 1%
Triton-X100. Treated homogenates were pipetted vigorously and vortexed to mix and centrifuged at 10,000_g_ for 10 min. Tumor supernatants were then collected, transferred to a 96-well plate,
treated with HALT protease inhibitor (Thermo Scientific, 1861278), frozen and stored at −80 °C to allow for quantification of all samples and time points in the same run. BACTERIAL
QUANTIFICATION To perform colony unit forming (CFUs) assays, whole tumor homogenates or blood collected by cardiac puncture and treated with EDTA were transferred to 96-well plates, 10x
serial diluted into DPBS and 10 μl of diluted samples were streaked across LB-agarose plates. Depending on the antibiotic resistances or auxotrophies of the bacterial strain being
quantified, LB-agarose plates were supplemented with 3 mM thymidine (Sigma, T1895), 100 μg/mL diaminopimelic acid (Sigma, D1377), 30 μg/mL streptomycin (Sigma, S9137-100G) or 300 μg/mL
chloramphenicol (Sigma, C0378-100G). Plates were incubated at 37 °C overnight and the number of colonies formed was quantified from the lowest dilution where 30 colonies or greater were
present. CFU bacterial quantification exhibited a limit of detection of ~100 CFUs per milliliter of sample with a limit of quantification of ~3000 CFUs per milliliter. Relative
bioluminescence from 100 μL tumor homogenate in a 96-well opaque plate, compared to background from saline treated control, was measured using the Synergy Neo Microplate Reader (BioTek
Instruments). To detect bacterial luminescence for excised tumor and/or surrounding tissue, samples were placed in a G:Box (Syngene) gel doc system. Images under white light were captured to
delineate the border between the B16F10 tumors and the surround healthy subcutaneous skin tissue. Bacterial luminescence was capture in the absence of light with full aperture and a 2 min
exposure time. For the detection of SYNB1891 bacterial DNA in blood and tumor a SYNB1891 probe specific qPCR assay was developed and validated using good laboratory practices standards at
QPS, LLC, Newark DE, USA. Tumors and EDTA treated blood were snap frozen and shipped to QPS for analysis. The qPCR analysis was performed using customized SYNB1891 forward, reverse primers
and probe (Life Technologies, ThermoFisher Scientific) according to analytical procedure (method)′ number 1813-1804. SYNB1891 primer/probe specific sequences were as follows: Forward primer
“5′- AGGATACTCATGTTGGAAAAGTCCAT-3′”; Reverse primer “5′- CCCGCATCCCACGCTAAC-3′”; Probe “5′-6FAM CTTGCCCCCCTTATATMGBNFQ-3′”. The qPCR detection limit was 10 copies of SYNB1891 DNA per 1 µg of
total DNA with a quantification limit of 25 copies per sample. CYTOKINE QUANTIFICATION Tumors were harvested and tumor supernatants were isolated as described above. Blood was collected by
cardiac puncture and was transferred to a serum separator tube containing EDTA (BD, 365967). Serum was isolated by allowing the blood to clot for 30 min at room temperature followed by
centrifugation for 90 s at 15,000_g_. Serum layer was transferred into a 96-well plate, treated with HALT protease inhibitor (Thermo Scientific, 1861278) and stored at −80 °C until analysis.
IL-6, TNFα, IL-1β, MCP-1, IL-2, Granzyme B and IFN-γ from tumor supernatants and serum level of IL-6 and TNFα were quantified using a custom MILLIPLEX mouse cytokine magnetic bead panel kit
(MILLIPLEX Multiplex Assay, Millipore Sigma) following manufacturer’s instructions and analyzed on a MAGPIX analyzer (Luminex). IFNβ was quantified from culture media and tumors using the
VeriKine mouse interferon beta ELISA kit (PBL) following manufacturer’s instructions. IL-6 and TNFα were quantified from culture media using mouse Quantikine ELISA kits (R&D systems)
following manufacturer’s instructions. All ELISAs were run on a 96-well plate and analyzed via colorimetric readout using the Synergy Neo Microplate Reader (BioTek Instruments). Intratumoral
levels of IFNα, IL-6, GM-CSF and IL-15 were quantified using Biolegend’s Mouse Inflammation LEGENDplex Custom flow analyte kit (Biolegend) following manufacturer’s instructions and were
analyzed on a Cytoflex LX flow cytometer (Bechman Coutler). EVALUATION OF INDUCIBLE PROMOTERS IN VIVO B16.F10 tumors were implanted and allowed to reach a volume of 100–200 mm3. Mice were
randomized based on tumor volume into various experimental groups and intratumorally injected with either saline or 5 × 105 CFUs of bacteria constitutively expressing mCherry (RFP) and
containing a green fluorescence protein (GFP) cassette under the control of an inducible promoter. 48 h post-bacterial injection mice were administered with either PBS (as control), 10 μg
anhydrous tetracycline (aTc), 6 mg _p_-isopropylbenzoate (Sigma; cumate, cmt) or 100 mM sodium salicylate (Sigma; aspirin, sal) via intraperitoneal injection. Mice were euthanized 1 or 16 h
post-induction, tumors were harvested by dissection and placed into single cell suspension by mechanical disruption though a 40 μM cell strainer. Samples were placed in a 96-well round
bottom plate and analyzed on a Macsquant VYB flow cytometer (Miltenyi Biotec). Bacteria were differentiated from mouse cells via size and expression of RFP, and the percentage of induced
cells (GFP+) was quantified. Flow cytometer settings and gating strategy are shown in Supplementary Fig. 10. CYCLIC-DI-AMP (CDA) QUANTIFICATION BY LC-MS/MS To quantify CDA production in
vitro, bacteria were grown and induced in an anerobic chamber as described above (See, _Growth and pre-induction of strains in shake flasks_). Bacterial cells were harvested by
centrifugation, rinsed twice with DPBS, counted using a Cellometer X2 (Nexcelom), 1010 cells transferred to a 96-well plate and frozen at −80 °C. Tumors were harvested and homogenized as
described above. Tumor homogenates were transferred to a 96-well plate, frozen and stored at −80 °C. CDA was then quantified from bacterial pellets or tumor homogenates by LC-MS/MS. In
brief, tumor homogenate was extracted with 9 parts methanol, vortexed, and centrifuged at 2300_g_ for 5 min at 4 °C. Supernatants were diluted 10-fold with 0.1% formic acid prior to
analysis. CDA was separated and detected on a Vanquish UHPLC/TSQ Altis LC-MS/MS system (Thermo Fisher Scientific). Samples were injected (1 µL) and separated on an Ace Excel 2 µm 100 Å
C18-AR 100 × 2.1 mm column (Advanced Chromatography Technologies Ltd.) and analyte eluted using a linear gradient of 0 to 98% B mixed with A from 1 to 3 min (A: 0.1% formic acid; B:
acetonitrile, 0.1% FA) at 0.6 mL/min at 50 °C. CDA was detected using selected reaction monitoring (SRM) of a compound specific mass transition 659.1 > 136 m/z in positive electrospray
ionization mode. Peaks were integrated and peak areas used to calculate concentrations of the unknowns using a 1/X weighted linear 7-point standard curve from 0.032 to 250 ng/mL using
Xcalibur Quan Browser (Thermo Fisher Scientific). HUMAN SERUM (COMPLEMENT) SENSITIVITY Fresh healthy human serum samples from three donors were obtained from Research Blood Components LLC,
Watertown, MA. Aliquots of serum from each donor were heat inactivated at 56 °C for 30 min (after aliquots reached the temperature). Induced SYNB1891-cmR bacteria (see _Anerobic_ in vitro
_circuit induction_) were resuspended at a concentration 1 × 107 cells/ml and 50 µl of bacteria were added to 450 µl of human serum (untreated or heat inactivated). Next, bacteria were
incubated at 37 °C with shaking for _T_ = 0, 45, 90, and 180 min and then plated in serial dilutions for CFU counting on LB-agarose plates supplemented with thymidine, diaminopimelic acid
and chloramphenicol (see _Bacterial quantification from_ in vivo _samples_) to determine susceptibility to serum lysis. MACROPHAGE AND DENDRITIC CELL CULTURES Murine RAW264.7 macrophage cell
line (ATCC) was maintained according to ATCC’s recommendations in DMEM-GlutaMAX media containing 10% FBS and penicillin-streptomycin (100 IU/ml and 100 mg/ml). For generation of murine bone
marrow-derived dendritic cells, bone marrow cells were sterilely harvested from the tibia and femur, red blood cell lysed using sterile Red Blood Cell Lysing Buffer Hybri-Max (Sigma) and
frozen in FBS containing 10% DMSO (Sigma) at −80 °C. Bone marrow cells were thawed, rinsed to remove DMSO and resuspended at a concentration of 5 × 105 cells/mL in culture media RPMI
1640-GlutaMax containing 5% FBS, 50uM 2-mercaptoethanol, penicillin-streptomycin (100 IU/ml and 100 mg/ml) and 20 ng/ml GM-CSF (BioLegend). After day 3, all non-adherent cells were removed,
and adherent dendritic cell clusters were cultured in the fresh medium for another 6 days before use. Every other day half of the medium was replaced with fresh culture medium. MICROSCOPY
For microscopic imaging of BMDCs, cells were plated on poly-d-l-lysine covered glass coverslips in 24 well plate at concentration of 5 × 105 cells/well in RPMI 1640-GlutaMax media containing
5% FBS without antibiotics. After cells adhered, in some wells cells were pre-treated with Cytochalasin D (10 μM) for 1 h. Then live pre-induced SYNB1891_-gfp_ bacteria at MOI of 25 were
added to BMDCs. Plates were spun at 350g for 2 min to allow bacteria to come in contact with DCs and incubated at 37 °C for 1 h to allow phagocytosis. After 1 h non-internalized bacteria
were washed out by rinsing three times with ice-cold DPBS and cells were fixed for 20 min at RT in 4% PFA in PBS. Cells were rinsed 2-3 times with 1 mL PBS (1 min each) and permeabilized for
10 min with permeabilization buffer (0.2% saponin in PBS, 0.03 M sucrose, 1% BSA). Then cells were treated for 60 min with blocking buffer (2% goat serum, 1% BSA, 0.1% cold fish skin
gelatin, 0.1% saponin and 0.05% Tween-20 in PBS, pH7.2) and stained with primary antibody against GFP (rabbit anti-GFP, Abcam, Cat. # ab6556) and LAMP-1 (rat anti-mouse-LAMP-1 (1D4B),
eBioscience, Cat. # 14-1071-82) at 1:250 dilution in dilution buffer (PBS, 0.05% Tween-20, 1%BSA, 0.1% saponin) overnight at 4 °C. Next day cells were washed 3 times, 5 min each, in 1 ml of
dilution buffer and stained with secondary antibody donkey anti-rabbit IgG (Alexa488, ThermoFisher Scientific,. # A-21206) and goat anti-rat IgG (Alexa 647, ThermoFisher Scientific, #
A-21247) in dilution buffer for 60 min at RT at dilution 1:750. Next, BMDCs were washed 3 times, 5 min each, in 1 mL of dilution buffer, cell nuclei and F-actin were stained with NucBlue and
ActinRed 555 Probes (ThermoFisher Scientific) according to the manufacturer’s protocol. After staining, BMDCs were rinsed two times with DPBS, coverslips were mount with ProLong® Gold
Antifade Mountant (ThermoFisher Scientific) and dried at RT overnight. Cells were imaged using 40 × (aperture 0.75) and 60 × (aperture 1.42) objectives with EVOS™ FL Auto 2 Imaging System
(Invitrogen). Post-acquisition contrast adjustment was performed using the ImageJ software. A representative example for the quantification of bacteria co-localized within dendritic cells is
shown in Supplementary Fig. 11. The average number of bacteria per dendritic cell in a field of view (FOV) was quantified and shown as the ratio of bacteria internalized by dendritic cells
to the total number of dendritic cells per FOV. CYTOKINE RESPONSES IN MACROPHAGES AND DENDRITIC CELLS RAW264.7 macrophages or BMDCs were seeded in 24 well plate at concentration of 5 × 105
cells/well in 0.5 mL of corresponding media (see _Macrophage and dendritic cell cultures)_ without growth cytokines and antibiotics. Human monocyte-derived primary DCs (StemCell
Technologies, 70041) were defrosted and prepared for downstream application according to the manufacturer’s protocol. Human DCs were seeded in 96-well plate at concentration of 1 × 105
cells/well in 0.2 mL of RPMI 1640-GlutaMax media containing 10% FBS. Two hours later after cells adhered, cells were stimulated with the following treatments in triplicate: live control
bacteria _Ec_N or live induced SYNB1891 bacteria at MOI of 25; ultrapure LPS (Invivogen) at concentration of 100 ng/mL; stable smSTING agonist (2′3′-c-di-AM(PS)2 (Rp,Rp), Invivogen) at
concentration of 5 or 25 μg/mL. Plates were spun at 350_g_ for 2 min to allow bacteria to come in contact with DCs or macrophages and incubated at 37 oC for 1 h to allow internalization of
treatments. After 1 h non-internalized bacteria, LPS or smSTING agonists were washed out two times with warm DPBS, fresh media containing antibiotics were added and cells were incubated at
37 oC. Depending on the readout, cells or culture media were harvested for analysis at 2, 4, 6, or 18 h post-treatment. Macrophages and DCs incubated in media only were utilized as a
negative control. Cell supernatants and cells were collected and analyzed for the presence of cytokine proteins and gene expression. Quantikine ELISA kits (R&D Systems) were used to
detect mouse cytokines IL-6 and TNF-α via standard colorimetric readout or custom MILLIPLEX mouse cytokine magnetic bead panel kits (MILLIPLEX Multiplex Assay, Millipore Sigma) were used to
detect IL-6, IL-1β, and IL-12p70 and analyzed on a MAGPIX analyzer (Luminex). PBL AssayScience ELISA was used to detect mouse IFN-β1 via standard colorimetric readout. In some experiments
cells were pre-treated for 1 h with Cytochalasin D (10 μM in corresponding media) before stimulation to stop phagocytosis. Cytochalasin D was present in the media during first hour of
stimulation until wash. RNA ISOLATION, CDNA PREPARATION, AND QPCR ANALYSIS Cellular RNA was isolated with TurboCapture 96 mRNA Kit (Qiagen) according to the manufacturer’s protocol. For cDNA
preparation, cDNA was synthesized from isolated RNA using High-Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Thermo Fisher Scientific) according to manufacturer’s protocol.
For 20 μl reaction, 2X RT master mix was prepared as follows: 10X RT Buffer 2 μL, 25X dNTP mix 0.8 μL,10X Random primers 2 μL, MultiScribe RT (50 U/μl) 1 μL, RNAase inhibitor 1 μL,
Nuclease-free H2O 3.2 μL, and mRNA 10 μL. PCR plate was run 10 min at 25 °C and 120 min at 37 °C in T100 Thermal Cycler (Bio-Rad). For the quantification of mouse _Ifnb1_ (Mm00439552_s1
Taqman probe), mouse _Il6_ (Mm00446190_m1), mouse _Il12a_ (Mm00434169_m1), mouse _cd86_ (Mm00444540_m1), human _IFNB1_ (Hs01077958_s1), human _IL6_ (Hs00174131_m1), human _TNF_
(Hs00174128_m1), human _CD86_ (Hs01567026_m1), human _CD40_ (Hs01002915_g1), human _IL-12A_ (Hs01073447_m1), and human _IL-1B_ (Hs01555410_m1) mRNA expression, a 10 μL qPCR reaction was
performed using Taqman assay probes and TaqMan Fast Advanced Master Mix, containing the following components: 2X Master Mix 5 μL, Taqman gene probe (20×) 0.5 μL (FAM-labeled), Taqman mouse
or human β-actin (endogenous control)* probe (20×) 0.5 μL (VIC-labeled), cDNA 4 μL. Reaction was run in MicroAmp™ Optical 384-Well Reaction Plate 20 s at 95 °C and 40 cycles (1 s—95 °C, 20
s—60 °C) in QuantStudio 6 Real Time PCR System (Applied Biosystems). Gene expression was analyzed by using comparative CT Method (ΔΔCT Method). The Prism7.0 c software (GraphPad Software,
San Diego, CA) was used for all statistical analysis. HUMAN MONOCYTE THP-1 REPORTER CELL LINE ASSAY THP1-Dual cells (thpd-nfis) containing an IRF-luciferase reporter and NF-κB-alkaline
phosphatase (SEAP) reporter, THP1-Dual KO-cGAS (thpd-kocgas) cells with the stable knockout of the cGAS gene and THP1-Dual KO-STING (thpd-kostg) cells with the stable knockout of the STING
gene were purchased from InVivoGen and cultured following the manufacturers protocols. THP-1 cells lines containing the following TMEM173 (STING) alleles were utilized: H232 (InVivoGen,
thpd-h232), HAQ-THP1 endogenous allele (InVivoGen, thpd-nfis) and R232 (InVivoGen, thpd-r232). Cells were harvested, pelleted and resuspended in 50% fresh RPMI 1640-GlutaMax media containing
10% FBS (RPMI-10) and 50% supernatant from expansion cell culture, and 1 × 105 cells in 225 μL per well were transferred to a 96-well cell culture plate. Bacteria were pre-induced to
express CDA (as described above), rinsed three times in cold DPBS and resuspended in RPMI-10. Bacteria (at the indicated ratios) or media alone were added to the THP-1 cells to reach a total
volume of 250 μL. Cells were incubated overnight, pelleted and supernatant was transferred to a 96-well white opaque plate for luciferase or 96-well transparent plate for phasphotase
activity detection. Quanti-Luc (InVivoGen, rep-qlc1) or Quanti-Blue (InVivoGen, rep-qbs1) solutions were reconstituted and utilized following manufacturers protocol to assay luciferase or
phosphatase activity of THP-1 culture media. Luminescence or optical density signal was measured using the Synergy Neo Microplate Reader (BioTek Instruments). The Prism7.0 c software
(GraphPad Software, San Diego, CA) was used for all statistical analysis. DATA ANALYSIS The Prism7.0 c software (GraphPad Software, San Diego, CA) was used for all statistical analysis. The
details of the statistical tests carried out are indicated in the respective figure legends. Values were compared using either a Student’s _t_ test to compare two groups or one-way ANOVA
with Tukey’s multiple comparisons posttests to compare the variance in the multiple group means with one independent factor (e.g. treatment group). We employed two-way ANOVA with Tukey’s
multiple comparisons posttests when we had the effect of two factors (e.g. treatment and time (or genotype)) on a dependent variable. In in vivo experimental cases where some mice were
removed from study for a treatment group upon reaching a study endpoint, we performed comparisons between the groups using one-way ANOVA with Tukey’s multiple comparisons posttests at the
indicated timepoint. For Kaplan–Meier survival experiments, we performed a log-rank (Mantel-Cox) test. REPORTING SUMMARY Further information on research design is available in the Nature
Research Reporting Summary linked to this article. DATA AVAILABILITY Complete genome sequence of E.coli Nissle 1917 is listed in Genbank/EMBL/DDBJ accession number: CP007799. Plasmid maps
and DNA sequences for CDA-producing enzymes are listed in the Supplementary Table 2 and Supplementary Fig. 8. The genome map, manipulated genome sequence of SYNB1891 and corresponding
primers are available in Supplementary Fig. 9 and Supplementary Tables 3–7. Engineered strains described in this manuscript can be made available subject to a Material Transfer Agreement
(MTA), which can be requested by contacting the corresponding authors. All requests will be reviewed by Synlogic to verify whether the request is subject to any intellectual property or
confidentiality obligations. Additional data underlying the figures and supplementary information are available from the corresponding authors on reasonable request. REFERENCES * Darvin, P.,
Toor, S. M., Sasidharan Nair, V. & Elkord, E. Immune checkpoint inhibitors: recent progress and potential biomarkers. _Exp. Mol. Med._ 50, 1–11 (2018). Article PubMed CAS Google
Scholar * Zou, W., Wolchok, J. D. & Chen, L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. _Sci. Transl. Med._ 8, 328rv4
(2016). Article PubMed PubMed Central CAS Google Scholar * Iribarren, K. et al. Trial Watch: Immunostimulation with Toll-like receptor agonists in cancer therapy. _Oncoimmunology_ 5,
e1088631 (2016). Article PubMed CAS Google Scholar * Adams, S. Toll-like receptor agonists in cancer therapy. _Immunotherapy_ 1, 949–964 (2009). Article CAS PubMed Google Scholar *
Fu, J. et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. _Sci. Transl. Med._ 7, 283ra52 (2015). Article PubMed PubMed Central Google
Scholar * Corrales, L. et al. Direct activation of STING in the tumor microenvironment leads to potent and systemic tumor regression and immunity. _Cell Rep._ 11, 1018–1030 (2015). Article
CAS PubMed PubMed Central Google Scholar * Woo, S.-R. et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. _Immunity_ 41, 830–842
(2014). Article CAS PubMed PubMed Central Google Scholar * Larkin, B. et al. Cutting edge: activation of STING in T cells induces type I IFN responses and cell death. _J. Immunol._ 199,
397–402 (2017). Article CAS PubMed Google Scholar * Sivick, K. E. et al. Magnitude of therapeutic STING activation determines CD8+ T cell-mediated anti-tumor Immunity. _Cell Rep._ 25,
3074–3085.e5 (2018). Article CAS PubMed Google Scholar * McCarthy, E. F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. _Iowa Orthop. J._ 26, 154–158
(2006). PubMed PubMed Central Google Scholar * Felgner, S., Kocijancic, D., Frahm, M. & Weiss, S. Bacteria in cancer therapy: renaissance of an old concept. _Int J. Microbiol._ 2016,
8451728 (2016). Article PubMed PubMed Central Google Scholar * Zhou, S., Gravekamp, C., Bermudes, D. & Liu, K. Tumour-targeting bacteria engineered to fight cancer. _Nat. Rev.
Cancer_ 18, 727–743 (2018). Article CAS PubMed PubMed Central Google Scholar * Staedtke, V. et al. Clostridium novyi-NT can cause regression of orthotopically implanted glioblastomas in
rats. _Oncotarget_ 6, 5536–5546 (2015). Article PubMed PubMed Central Google Scholar * Zhao, M. et al. Tumor-targeting bacterial therapy with amino acid auxotrophs of GFP-expressing
Salmonella typhimurium. _Proc. Natl Acad. Sci. USA_ 102, 755–760 (2005). Article ADS CAS PubMed PubMed Central Google Scholar * Danino, T., Lo, J., Prindle, A., Hasty, J. & Bhatia,
S. N. In vivo gene expression dynamics of tumor-targeted bacteria. _ACS Synth. Biol._ 1, 465–470 (2012). Article CAS PubMed PubMed Central Google Scholar * Leschner, S. et al. Tumor
invasion of _Salmonella enterica_ serovar Typhimurium is accompanied by strong hemorrhage promoted by TNF-alpha. _PLoS ONE_ 4, e6692 (2009). Article ADS PubMed PubMed Central CAS Google
Scholar * Kocijancic, D. et al. Therapy of solid tumors using probiotic Symbioflor-2: restraints and potential. _Oncotarget_ 7, 22605–22622 (2016). Article PubMed PubMed Central Google
Scholar * Sonnenborn, U. & Schulze, J. The non-pathogenic _Escherichia coli_ strain Nissle 1917—features of a versatile probiotic. _Microb. Ecol. Health Dis._ 21, 122–158 (2009). CAS
Google Scholar * Grozdanov, L. et al. A single nucleotide exchange in the wzy gene is responsible for the semirough O6 lipopolysaccharide phenotype and serum sensitivity of _Escherichia
coli_ strain Nissle 1917. _J. Bacteriol._ 184, 5912–5925 (2002). Article CAS PubMed PubMed Central Google Scholar * Gurbatri, C. R. et al. Engineered probiotics for local tumor delivery
of checkpoint blockade nanobodies. _Sci. Transl. Med_. 12, eaax0876 (2020). Article CAS PubMed PubMed Central Google Scholar * Bashor, C. J. & Collins, J. J. Understanding
biological regulation through synthetic biology. _Annu. Rev. Biophys._ 47, 399–423 (2018). Article CAS PubMed Google Scholar * Kitada, T., DiAndreth, B., Teague, B. & Weiss, R.
Programming gene and engineered-cell therapies with synthetic biology. _Science_ 359, eaad1067 (2018). * Zhang, Y. et al. _Escherichia coli_ Nissle 1917 targets and restrains mouse B16
melanoma and 4T1 breast tumors through expression of azurin protein. _Appl. Environ. Microbiol._ 78, 7603–7610 (2012). Article CAS PubMed PubMed Central Google Scholar * Loessner, H. et
al. Drug-inducible remote control of gene expression by probiotic _Escherichia coli_ Nissle 1917 in intestine, tumor and gall bladder of mice. _Microbes Infect._ 11, 1097–1105 (2009).
Article CAS PubMed Google Scholar * Westphal, K., Leschner, S., Jablonska, J., Loessner, H. & Weiss, S. Containment of tumor-colonizing bacteria by host neutrophils. _Cancer Res._
68, 2952–2960 (2008). Article CAS PubMed Google Scholar * Stritzker, J. et al. Tumor-specific colonization, tissue distribution, and gene induction by probiotic _Escherichia coli_ Nissle
1917 in live mice. _Int. J. Med. Microbiol._ 297, 151–162 (2007). Article CAS PubMed Google Scholar * Morrissey, D., O’Sullivan, G. C. & Tangney, M. Tumour targeting with
systemically administered bacteria. _Curr. Gene Ther._ 10, 3–14 (2010). Article CAS PubMed Google Scholar * La Rosa, S. L., Diep, D. B., Nes, I. F. & Brede, D. A. Construction and
application of a luxABCDE reporter system for real-time monitoring of Enterococcus faecalis gene expression and growth. _Appl. Environ. Microbiol._ 78, 7003–7011 (2012). Article PubMed CAS
Google Scholar * Diner, E. J. et al. The innate immune DNA sensor cGAS produces a noncanonical cyclic dinucleotide that activates human STING. _Cell Rep._ 3, 1355–1361 (2013). Article
CAS PubMed PubMed Central Google Scholar * Jin, L. et al. MPYS is required for IFN response factor 3 activation and type I IFN production in the response of cultured phagocytes to
bacterial second messengers cyclic-di-AMP and cyclic-di-GMP. _J. Immunol._ 187, 2595–2601 (2011). Article CAS PubMed Google Scholar * Krasteva, P. V. & Sondermann, H. Versatile modes
of cellular regulation via cyclic dinucleotides. _Nat. Chem. Biol._ 13, 350–359 (2017). Article CAS PubMed PubMed Central Google Scholar * Kocak, E. et al. Combination therapy with
anti-CTL antigen-4 and anti-4-1BB antibodies enhances cancer immunity and reduces autoimmunity. _Cancer Res._ 66, 7276–7284 (2006). Article CAS PubMed Google Scholar * Sánchez-Paulete,
A. R. et al. Cancer immunotherapy with immunomodulatory Anti-CD137 and anti-PD-1 monoclonal antibodies requires BATF3-dependent dendritic cells. _Cancer Discov._ 6, 71–79 (2016). Article
PubMed CAS Google Scholar * Durand, S. & Storz, G. Reprogramming of anaerobic metabolism by the FnrS small RNA. _Mol. Microbiol._ 75, 1215–1231 (2010). Article CAS PubMed PubMed
Central Google Scholar * Boysen, A., Møller-Jensen, J., Kallipolitis, B., Valentin-Hansen, P. & Overgaard, M. Translational regulation of gene expression by an anaerobically induced
small non-coding RNA in _Escherichia coli_. _J. Biol. Chem._ 285, 10690–10702 (2010). Article CAS PubMed PubMed Central Google Scholar * Leschner, S. et al. Identification of
tumor-specific Salmonella Typhimurium promoters and their regulatory logic. _Nucleic Acids Res._ 40, 2984–2994 (2012). Article CAS PubMed Google Scholar * Kurtz, C. B. et al. An
engineered E. coli Nissle improves hyperammonemia and survival in mice and shows dose-dependent exposure in healthy humans. _Sci. Transl. Med_. 11, eaau7975 (2019). Article CAS PubMed
Google Scholar * Isabella, V. M. et al. Development of a synthetic live bacterial therapeutic for the human metabolic disease phenylketonuria. _Nat. Biotechnol._ 36, 857–864 (2018). Article
CAS PubMed Google Scholar * Ip, W. K. E. et al. Phagocytosis and phagosome acidification are required for pathogen processing and MyD88-dependent responses to _Staphylococcus aureus_.
_J. Immunol._ 184, 7071–7081 (2010). Article CAS PubMed Google Scholar * Tse, S. M. L. et al. Differential role of actin, clathrin, and dynamin in Fc gamma receptor-mediated endocytosis
and phagocytosis. _J. Biol. Chem._ 278, 3331–3338 (2003). Article CAS PubMed Google Scholar * Anthony, S. M. et al. Inflammatory signals regulate IL-15 in response to lymphodepletion.
_J. Immunol._ 196, 4544–4552 (2016). Article CAS PubMed Google Scholar * Yi, G. et al. Single nucleotide polymorphisms of human STING can affect innate immune response to cyclic
dinucleotides. _PLoS ONE_ 8, e77846 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Adli, DaeiFarshchi et al. overview on Vadimezan (DMXAA): the vascular disrupting
agent. _Chem. Biol. Drug Des._ 91, 996–1006 (2018). Article CAS Google Scholar * Flickinger, J. C., Rodeck, U. & Snook, A. E. Listeria monocytogenes as a vector for cancer
immunotherapy: current understanding and progress. _Vaccines (Basel)_ 6, E48 (2018). * Gunn, G. R. et al. Two Listeria monocytogenes vaccine vectors that express different molecular forms of
human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. _J.
Immunol._ 167, 6471–6479 (2001). Article CAS PubMed Google Scholar * Singh, R., Dominiecki, M. E., Jaffee, E. M. & Paterson, Y. Fusion to Listeriolysin O and delivery by Listeria
monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. _J. Immunol._ 175, 3663–3673 (2005). Article CAS PubMed Google Scholar *
Shahabi, V. et al. Development of a Listeria monocytogenes based vaccine against prostate cancer. _Cancer Immunol. Immunother._ 57, 1301–1313 (2008). Article CAS PubMed Google Scholar *
Wang, Y. et al. Oncolytic bacteria and their potential role in bacterium-mediated tumour therapy: a conceptual analysis. _J. Cancer_ 10, 4442–4454 (2019). Article CAS PubMed PubMed
Central Google Scholar Download references ACKNOWLEDGEMENTS Drs. James J. Collins, Roger Geiger, Filip Janku, Timothy K. Lu, Neil Segal, Dmitriy Zamarin and Weiping Zou for discussions and
comments on the manuscript. Megan McPhillips, Makswell Vittands, Nate Scaffidi and Alice Gao for technical assistance. Timothy Mayville, Scott Hamilton, Michael Jessel and Justin Wong for
bacterial fermentation process development and material generation. AUTHOR INFORMATION Author notes * These authors contributed equally: Daniel S. Leventhal, Anna Sokolovska, Ning Li.
AUTHORS AND AFFILIATIONS * Synlogic, Inc., 301 Binney street, suite 402, Cambridge, Massachusetts, 02142, United States Daniel S. Leventhal, Anna Sokolovska, Ning Li, Christopher Plescia,
Starsha A. Kolodziej, Carey W. Gallant, Rudy Christmas, Jian-Rong Gao, Michael J. James, Andres Abin-Fuentes, Munira Momin, Christopher Bergeron, Adam Fisher, Paul F. Miller, Kip A. West
& Jose M. Lora Authors * Daniel S. Leventhal View author publications You can also search for this author inPubMed Google Scholar * Anna Sokolovska View author publications You can also
search for this author inPubMed Google Scholar * Ning Li View author publications You can also search for this author inPubMed Google Scholar * Christopher Plescia View author publications
You can also search for this author inPubMed Google Scholar * Starsha A. Kolodziej View author publications You can also search for this author inPubMed Google Scholar * Carey W. Gallant
View author publications You can also search for this author inPubMed Google Scholar * Rudy Christmas View author publications You can also search for this author inPubMed Google Scholar *
Jian-Rong Gao View author publications You can also search for this author inPubMed Google Scholar * Michael J. James View author publications You can also search for this author inPubMed
Google Scholar * Andres Abin-Fuentes View author publications You can also search for this author inPubMed Google Scholar * Munira Momin View author publications You can also search for this
author inPubMed Google Scholar * Christopher Bergeron View author publications You can also search for this author inPubMed Google Scholar * Adam Fisher View author publications You can
also search for this author inPubMed Google Scholar * Paul F. Miller View author publications You can also search for this author inPubMed Google Scholar * Kip A. West View author
publications You can also search for this author inPubMed Google Scholar * Jose M. Lora View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS
J.M.L. conceived and supervised the project. D.S.L., A.S., and J.M.L. planned experiments, analyzed data and wrote the paper with assistance from K.A.W.; N.L., P.F.M., A.F., and J.G.
designed and performed bacterial engineering and in vitro bacterial analyses. A.S., C.P., and C.G. designed and performed in vitro immunological assays and analyzed the data. S.K., R.C., and
D.L. performed in vivo assays. K.A.W. supervised in vivo assays and analyzed data. M.J. performed mass spectrometry analyses. C.B., M.M., and A.A-F. devised fermentation conditions and
provided essential materials. CORRESPONDING AUTHORS Correspondence to Daniel S. Leventhal, Anna Sokolovska or Jose M. Lora. ETHICS DECLARATIONS COMPETING INTERESTS All authors are or were
employees of Synlogic, Inc. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_ thanks Siegfried Weiss and the other, anonymous, reviewer(s) for their contribution to the
peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Leventhal, D.S., Sokolovska, A., Li, N. _et al._ Immunotherapy with
engineered bacteria by targeting the STING pathway for anti-tumor immunity. _Nat Commun_ 11, 2739 (2020). https://doi.org/10.1038/s41467-020-16602-0 Download citation * Received: 29 October
2019 * Accepted: 11 May 2020 * Published: 01 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16602-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative