- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Increased grain yield will be critical to meet the growing demand for food, and could be achieved by delaying crop senescence. Here, via quantitative trait locus (QTL) mapping, we
uncover the genetic basis underlying distinct life cycles and senescence patterns of two rice subspecies, _indica_ and _japonica_. Promoter variations in the _Stay-Green_ (_OsSGR_) gene
encoding the chlorophyll-degrading Mg++-dechelatase were found to trigger higher and earlier induction of _OsSGR_ in _indica_, which accelerated senescence of _indica_ rice cultivars. The
_indica_-type promoter is present in a progenitor subspecies _O. nivara_ and thus was acquired early during the evolution of rapid cycling trait in rice subspecies. _Japonica OsSGR_ alleles
introgressed into _indica_-type cultivars in Korean rice fields lead to delayed senescence, with increased grain yield and enhanced photosynthetic competence. Taken together, these data
establish that naturally occurring _OsSGR_ promoter and related lifespan variations can be exploited in breeding programs to augment rice yield. SIMILAR CONTENT BEING VIEWED BY OTHERS
NATURAL VARIATION IN _OSMYB8_ CONFERS DIURNAL FLORET OPENING TIME DIVERGENCE BETWEEN _INDICA_ AND _JAPONICA_ SUBSPECIES Article Open access 13 March 2024 WILD RICE _GL12_ SYNERGISTICALLY
IMPROVES GRAIN LENGTH AND SALT TOLERANCE IN CULTIVATED RICE Article Open access 01 November 2024 GENETIC BASIS AND ADAPTATION TRAJECTORY OF SOYBEAN FROM ITS TEMPERATE ORIGIN TO TROPICS
Article Open access 14 September 2021 INTRODUCTION The world’s population is expected to increase by 35% over the next 30 years, so crop production must also increase to meet the growing
demand1. Rice is a staple food for half of the world’s population. Although rice-yield potential has increased considerably over the past five decades, mainly through the utilization of
semi-dwarf varieties and heterosis2, it has stagnated worldwide in recent years3. Asian rice cultivars belong mostly to two subspecies, _O. sativa_ L. ssp. _japonica_ and _indica_, which
bear distinct morphological and physiological features4 and show drastically different lifespans, with _indica_ showing early senescence5. Compared with _japonica_, _indica_ exhibits earlier
senescence in both whole plants and leaf organs, resulting in a rapid life cycle6. _Indica_ rice is thought to have evolved under _r_-selection in the tropical zone, emphasizing a rapid
cycling life history strategy with a trade-off between rapid reproduction and parental survivorship. The accelerated senescence along with the short lifespan of _indica_ rice increases its
reproductive output, providing an adaptive strategy in the given environments7. However, in temperate and subtropics rice fields, where human population density is high, early senescence of
rice cultivars is undesirable because it often results in shorter times for grain filling and poor productivity. Although these two rice subspecies have been used extensively for rice
breeding in East, South, and Southeast Asia to improve rice yield and other agronomic traits8, the effect of senescence has rarely been considered during the breeding process. Senescence,
the final stage of development, is an active phase of orderly degradation and remobilization processes involving a series of changes at the cellular, tissue, organ, and organism levels9,10.
Senescence affects grain yield and quality in crop species9,11. Heritable delays in senescence extend the photosynthetic period and are thus important considerations for improving crop
yield9,12. Indeed, progressive increases in grain yield in maize and sorghum positively correlate with impaired chlorophyll catabolism, so-called stay-green traits9,12. For example, _sgr_
mutants in rice and _Arabidopsis_ exhibit a stay-green phenotype, due to defects in the chlorophyll-degrading enzyme Mg++ dechelatase, resulting in a stay-green phenotype9,13,14. However,
rice _ossgr_ mutants did not maintain photosynthetic capability during the grain-filling period and did not show a yield advantage, indicating that they are nonfunctional _stay-green_
mutants13,15. Chlorophyll breakdown pathways operating during leaf senescence have been functionally characterized by studying loss-of-function mutants in rice10. In addition, a recent
genome wide association study with natural variations of rice uncovered new alleles for chlorophyll content and stay-green traits in _japonica_ and _indica_ rice cultivars16. Here, QTL
mapping was used to determine the genetic factors responsible for the differential senescence patterns and lifespans between _indica_ and _japonica-_type cultivars, and to identify promoter
polymorphisms in the _OsSGR_ gene. Promoter variations in _indica_ lead to higher and earlier induction of _OsSGR_, resulting in accelerated senescence and shorter lifespans. Evolutionary
and population genetic analyses show the _indica_-type promoter was acquired from the progenitor subspecies _O. nivara_ during its evolution. Lastly, _japonica OsSGR_ alleles introgressed
into elite _indica-_type cultivars show delayed senescence, longer maintenance of photosynthetic competence, and improved grain yield. These results highlight that _OsSGR_ promoter
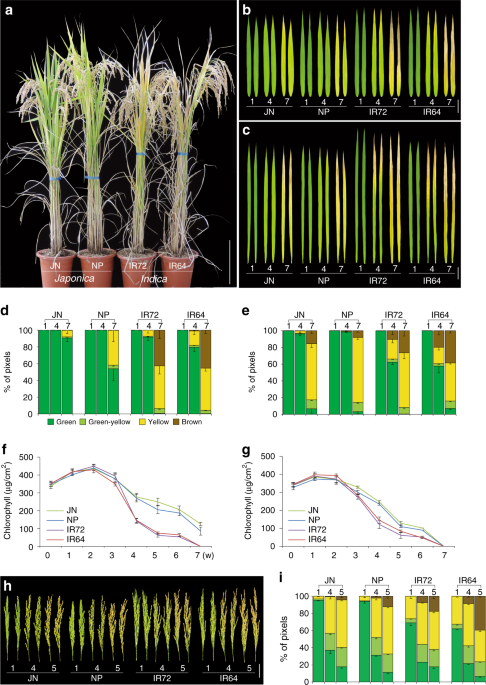
variations can be utilized to improve rice productivity. RESULTS AGE-ASSOCIATED SENESCENCE IN _JAPONICA_ AND _INDICA_ CULTIVARS The senescence phenotypes of two _japonica-_type cultivars,
Junam (JN) and Nampyeong (NP), and two _indica_-type cultivars, IR72 and IR64, were examined. These cultivars have similar heading dates (Supplementary Fig. 1), and thus reproductive
differences are not likely to explain any differences in senescence. The plants were grown in a natural rice field in the National Institute of Crop Science, Korea (35.3° N; 128.5° E), and
senescence was monitored by leaf color changes, for instance from green to yellow due to the loss of chlorophyll, and from yellow to brown due partly to oxidation of phenolic compounds in
dying cells10. The two cultivar types showed clear differences in senescence patterns for whole plants and leaves (Fig. 1a–c), as previously reported5. The senescence patterns of individual
leaves and panicles were then quantitatively analyzed (Fig. 1b–i). The senescence patterns of the last two leaves are known to critically influence grain yield in rice17. Interestingly,
these two leaves have distinct senescence-related transcriptome patterns, and show distinct contributions to grain filling18. Age-dependent changes of leaf color (Fig. 1b, c), as well as
quantification of leaf color changes (Fig. 1d, e) and total chlorophyll levels (Fig. 1f, g), revealed faster senescence in both flag and second upper leaves of the two _indica-_type rice
cultivars compared with those of the two _japonica_ cultivars. In addition, the second upper leaves showed faster senescence than the flag leaf in both _indica_ and _japonica_ cultivars. The
panicle is the last organ to develop in the aboveground part of the rice, therefore panicle senescence associated with grain maturation determines the aboveground lifespan of whole plants.
The panicles of the two _indica_ cultivars showed earlier senescence than those of the two _japonica_ cultivars (Fig. 1h), as quantified by colourimetric assays (Fig. 1i). QTL MAPPING AND
MAP-BASED CLONING OF _OSSGR_ To determine the genetic loci responsible for the different senescence patterns between the two subspecies, QTL mapping was performed with F2:3 populations
derived from a cross between the _indica_ cultivar IR72 and the _japonica_ cultivar JN. Leaf senescence phenotypes in the F2:3 population showed a continuous variation, exhibiting the
characteristics of quantitative inheritance (Supplementary Fig. 2a, b). The senescence-associated QTL of the flag and second upper leaves mapped separately, consistent with their different
senescence schedules (Fig. 1b–g). The senescence differences between the two parental cultivars were governed by multiple loci (Fig. 2a, b; Supplementary Table 1). Three loci above the 95%
confidence cutoff were shared by the flag and second upper leaves in the two cultivars, whereas three others distinctly controlled senescence in the flag and second upper leaves. The genetic
locus on chromosome 9 with the highest LOD score was examined to identify the gene(s) responsible for the differential senescence phenotypes. The locus was associated with early senescence
of both the flag and second upper leaves, suggesting that it influences senescence of the whole plant rather than a specific leaf. 6,349 BC5F2 lines derived from a cross between _japonica_
JN and _indica_ IR72 were generated and used for fine mapping of the early-senescence locus, with molecular markers developed for the _indica_ and _japonica_ subspecies (Supplementary Table
2). The locus for the _indica-_type early leaf senescence was detected between markers C9–10 and C9–12, which spans ~26 kb of chromosome 9 (Fig. 2c; Supplementary Fig. 3). This region was
examined for known coding sequence(s) that could underlie early leaf senescence, revealing _OsSGR_ (LOC_Os09g36200), _Harpin-induced protein 1_ (LOC_Os09g36210), and _OsPRR95_
(LOC_Os09g36220). _OsSGR_ encodes a Mg++-dechelatase that is involved in chlorophyll degradation, which promotes degradation of the light-harvesting complexes and senescence of leaves and
whole plants13,15,19,20. _OsPRR95_, a two-component signaling gene21, is a homolog of _Arabidopsis PRR9_, which controls age-dependent leaf senescence22, and _Harpin-induced protein 1_ has
been implicated in stress responses such as disease resistance23. Activation-tagging lines for _OsSGR_ and _OsPRR95_ were isolated from an activation-tagging library generated in a
_japonica_ cultivar Dongjin, and their senescence patterns were examined. Leaf and whole-plant senescence were unaltered in activation lines of _OsPRR95_ compared to controls (Supplementary
Fig. 4), but were accelerated in two activation lines of _OsSGR_ (Supplementary Fig. 5a–g). _OsNAP_24, a senescence marker gene, was more highly expressed in these mutants than in WT
(Supplementary Fig. 5d). In addition, chlorophyll contents in a whole plant and leaves were maintained higher in _ossgr_ mutants of _japonica_ cultivars (Supplementary Fig. 6a–f), consistent
with previous reports13,15,25. Leaf senescence, as probed by _OsNAP_ expression, proceeded in these mutants but appeared to be partially delayed compared to wild-type plants. Chlorophyll
loss in panicles was also accelerated in _OsSGR_-activation lines, and delayed in _ossgr_ mutants (Supplementary Figs. 5h, i and 6g, h). These results suggest that, among the three genes in
the 26-kb region, _OsSGR_ is responsible for early senescence of _indica_ rice plants, leading to the rapid life cycle. Activation of _OsSGR_ led to reduced grain yield with poor agronomic
traits (Supplementary Fig. 5j–n), but _ossgr_ mutants did not show any yield advantage (Supplementary Fig. 6i–m). To verify that the _indica_ allele of _OsSGR_ is required for its
early-senescence phenotype, _OsSGR_ knockout (KO) mutants were generated in the _indica_ cultivar Kasalath via CRISPR/Cas9 genome editing (Supplementary Fig. 7a). The KO _indica_ plants
showed delayed loss of chlorophyll in whole plants, leaves, and panicles (Fig. 2d–h; Supplementary Fig. 7b). Furthermore, RNAi-mediated silencing of _OsSGR_ in the _indica_ cultivar led to
delayed chlorophyll loss (Supplementary Fig. 8). Leaf senescence, as probed by _OsNAP_ expression, also proceeded in these mutants but appeared to be partially delayed compared with their
parental lines (Supplementary Figs. 7c and 8e). These observations suggest that the _indica_ allele of _OsSGR_ contributes to its relatively early senescence, short lifespan, and rapid life
cycle. To determine how the _indica_ allele of _OsSGR_ leads to earlier senescence, genomic polymorphisms between the _OsSGR_ alleles of the parental mapping cultivars JN (_japonica_) and
IR72 (_indica_) were examined. There were four nonsynonymous SNPs in the coding region, and 13 SNPs and 3 indels in the promoter region (Supplementary Fig. 9). As _OsSGR_ encodes a
Mg++-dechelatase enzyme that converts chlorophyll a to pheophytin a19, the amino acid changes in the coding region of _OsSGR_ were first tested for their effect on senescence. Transgenic
_japonica_ JN rice plants that ubiquitously overexpressed either the _indica_ or _japonica OsSGR_ allele were generated and found to display similar early leaf senescence phenotypes
(Supplementary Fig. 10), suggesting that the SNPs in the coding region of the two alleles are not responsible for the early _indica-_type leaf senescence (Supplementary Fig. 10c, d). In
addition, the two alleles displayed similar chlorophyll-degradation activities in vitro (Supplementary Fig. 11a) and _in planta_ (Supplementary Fig. 11b). The _ossgr_ allele did not show any
chlorophyll breakdown activity (Supplementary Fig. 11a, b). _OSSGR_ PROMOTER POLYMORPHISMS ARE ASSOCIATED WITH SENESCENCE _OsSGR_ expression levels during senescence were examined to
determine whether the _OsSGR_ promoter polymorphisms contributed to the differential senescence (Supplementary Fig. 9). _OsSGR_ was induced in an age-dependent manner during senescence in
both _japonica_ cultivars and both _indica_ cultivars (Fig. 3a). Importantly, _OsSGR_ expression was higher and earlier in the two _indica_ cultivars than in the two _japonica_ cultivars
(Fig. 3a). Furthermore, a transient assay in rice protoplasts revealed that _OsSGR_ promoter segments from the _indica_ cultivar IR72 had 3.1-fold higher activity than those from the
_japonica_ cultivar JN (Supplementary Fig. 12). This result suggests that higher expression of _OsSGR_ in _indica_ rice is responsible for its early-senescence phenotype. To further
investigate the role of _OsSGR_ promoter polymorphisms in senescence, an association analysis was performed between the _OsSGR_ promoter region and leaf senescence phenotypes in 105 rice
accessions belonging to five _O. sativa_ subgroups26 (Supplementary Fig. 13 and Supplementary Data 1). Sequencing of the _OsSGR_ promoters of these accessions identified 21 polymorphic sites
that establish eight haplotypes (Fig. 3b). Each haplotype showed distinct chlorophyll levels, with haplotype 1 (_indica_) showing substantially lower levels than haplotype 8 (_japonica_)
(Fig. 3c). A comparison of chlorophyll levels between the two groups of accessions with and without the polymorphism at each site showed that 16 of the 21 polymorphic sites were
significantly associated with chlorophyll content (Fig. 3d). Furthermore, among the 16 sites, 15 were associated with a difference in chlorophyll content between _indica_ and _japonica_ rice
plants. Together, these data strongly suggest that _OsSGR_ promoter polymorphisms broadly influence senescence, lifespan, and life cycle in various rice accessions. Phylogenetic analysis of
the _OsSGR_ promoter sequences in the 105 rice accessions with wild rice sequences indicated that _indica_ groups with _O. nivara_, whereas _japonica_ groups with _O. rufipogon_ (Fig. 3e).
_O. nivara_ is considered to be the progenitor of _indica_, whereas _O. rufipogon_ is regarded to be the progenitor of _japonica_26. Phylogenetic analysis of an expanded set of wild and
domesticated rice samples further supported this relationship (Supplementary Fig. 14). A group of _O. rufipogon_-like wild rice is considered to be a sister group to all domesticated rice
and its progenitor wild rice (Supplementary Fig. 14)27. Therefore, the _indica_-type promoter variations may be derived from _japonica_ or from the progenitor of _O. rufipogon_. Efficient
seed production requires coincidence of panicle development with leaf senescence that leads to generation of the nutritional resources for grain filling. The _indica-_type promoter of
_OsSGR_ could have played a critical role during the evolution and domestication of _indica_ type for a rapid life cycle, through concomitant control of leaf and panicle senescence. There
was no evidence of a selective sweep in the region surrounding _OsSGR_ (Supplementary Fig. 15), suggesting the promoter variations of _OsSGR_ in _indica_ rice may have existed in _O. nivara_
and were later inherited into _indica_. INTROGRESSION OF _JAPONICA OSSGR_ ALLELE INTO _INDICA_ Three elite rice cultivars that are bred in Korea (Milyang21 and Milyang23) and in IRRI (IR72)
have _indica OsSGR_ promoter alleles with haplotype 1 (Supplementary Table 3a). When grown in a conventional paddy rice field in the National Institute of Crop Science, Korea (35.3° N;
128.5° E), grain yields per plant in the year 2018 were 35.9 (IR72), 29.0 (Milyang21), and 29.1 g plant−1 (Milyang23) (Supplementary Table 3b). We hypothesized that these elite cultivars
undergo undesirable early senescence due to the presence of the _indica OsSGR_ alleles, decreasing the yield due to a reduced grain-filling period. This hypothesis predicts that replacing
the _indica OsSGR_ alleles in these cultivars with a _japonica OsSGR_ allele would increase the yield and grain filling. To test this hypothesis, near isogenic lines (NILs) of these lines
harboring a _japonica OsSGR_ allele were generated by crossing them with JN or Saeilmi (haplotype 8). The three NILs harboring the _japonica OsSGR_ allele (IR72-NIL, Milyang21-NIL, and
Milyang23-NIL) all showed delayed senescence (Fig. 4a–c, g–i; Supplementary Fig. 16a, b) and the expression of _OsSGR_ was reduced in IR72-NIL compared to IR72 (Fig. 4j). The lower
expression of _OsNAP_ in IR72-NIL also supported delayed senescence (Supplementary Fig. 17a). To evaluate photosynthetic competence in NILs, the net CO2 assimilation rates and Fv/Fm ratios
representing the photosynthetic ability in the flag leaves were measured (Fig. 4k; Supplementary Fig. 17b, c). Three NILs of the _indica_ background harboring _japonica OsSGR_ allele
displayed extended photosynthetic competence with higher chlorophyll contents (Fig. 4g, i, k; Supplementary Fig. 17b, c). The relative growth rates (RGR) quantifies plant growth speed and is
considered to be a reliable standard for estimating plant productivity28,29. It is calculated as the dry mass increment per aboveground biomass at a given time point. RGRs of IR72-NIL are
shown in Supplementary Fig. 17d. The RGR of all the rice plants we examined declined continuously during the grain-filling stage. However, IR72-NIL harboring _japonica OsSGR_ allele
maintained higher RGR, especially between 5 and 7 weeks after flowering, than the parental IR72 (Supplementary Fig. 17d), indicating the contribution of the _japonica OsSGR_ allele in higher
biomass productivity. On the other hand, JN-NIL, generated by replacing the _japonica OsSGR_ allele (haplotype 8) in JN with the _indica OsSGR_ allele in IR72 (haplotype 1), showed earlier
and higher expression of _OsSGR_ and _OsNAP_ during the senescence period along with earlier senescence phenotypes across whole plants, flag leaves, and panicles (Fig. 4d–h; Supplementary
Fig. 17e–g). The photosynthetic activity of JN-NIL was lower than JN during the grain-filling period (Supplementary Fig. 17h, i). JN-NIL harboring the _indica OsSGR_ allele showed relatively
faster decline of the RGR value than its parental cultivar (Supplementary Fig. 17j), indicating the negative effect of the _indica OsSGR_ allele in biomass productivity in our field
condition. All the NILs with the _japonica OsSGR_ allele showed higher grain yields. The grain yields of the IR72-NIL, Milyang21-NIL, and Milyang23-NIL were 39.6, 32.7, and 32.6 g per plant,
which corresponds to increases of 10.6, 12.7, and 12.0%, respectively, compared with their parental cultivars (Fig. 4l; Supplementary Table 3b). The increased grain yield was positively
correlated grain-filling rate (Fig. 4m; Supplementary Table 3b) during the grain-filling stage. In contrast, JN-NIL with the _indica OsSGR_ allele showed lower grain yields with 27.6 g per
plant, which corresponds to decrease of 10.1% (Fig. 4l; Supplementary Table 3b). Furthermore, the grain-filling rate was reduced by 6.5% in JN-NIL plants (Fig. 4m; Supplementary Table 3b)
compared with its parental JN plants. The grain yields of the all NIL lines were further confirmed through a large-scale field trial in the following year. Their grain yields and the
senescence phenotypes in the field are shown in Supplementary Fig. 18. DISCUSSION Rice is a daily staple food crop for over 10 billion people, and improving crop productivity is an important
task to meet future demands of the growing world population1. Senescence is an active phase that involves well-orchestrated degradation and remobilization processes that affect crop
productivity and quality9. Here, we explore the differential senescence patterns of two rice subspecies, _japonica_ and _indica_, as a rice-breeding trait. The two rice subspecies show
different senescence patterns and lifespans, which are regulated through quantitative inheritance (Figs. 1, 2a–b; Supplementary Fig. 2). In this study, promoter variations in the _OsSGR_
gene are uncovered as one of the main genetic mechanisms underlying differential senescence between the two subspecies (Fig. 2c; Supplementary Fig. 9). Allelic polymorphisms in the _OsSGR_
promoter in _indica_ cultivars lead to higher and earlier expression of _OsSGR_ and thereby to their early-senescence phenotype (Fig. 3a), affecting the senescence and lifespan traits
broadly in various rice accessions (Fig. 3b–d). When we analyzed the binding motifs for transcriptional factors in the 2-kb _OsSGR_ promoter region using NEW PLACE (A Database of Plant
Cis-acting Regulatory DNA element; https://www.dna.affrc.go.jp/PLACE/?action=newplace), we found that the _japonica_ and _indica_ promoters contained same numbers (13) of WRKY-biding motifs
and no NAC-binding motif. However, there is a distinctive difference in the Dof-binding motifs between the two promoters. There are 14 Dof protein-binding motifs (consensus AAAG sequences or
its reversibly complementary sequence, CTTT30) in the _japonica_ promoter. On the other hand, in the _indica_ promoter, there is a new Dof protein-binding motif formed by insertion of
AAAAGCTC (position −1, 377; Supplementary Fig. 9). This region with the new Dof-binding motif is tightly associated with low levels of chlorophyll contents. We anticipate that this new
Dof-binding motif, and the respective Dof transcription factor may together lead to the _indica-_type phenotype of chlorophyll loss. Elucidating the molecular mechanism regulating the early
and higher induction of _OsSGR_ should be a key future effort. Population genetic and evolutionary analyses revealed that _indica_ and _japonica_ alleles originate from _O. nivara_ and _O.
rufipogon_ rice, respectively (Fig. 3e; Supplementary Fig. 14), indicating that the _indica_-type promoter of _OsSGR_ had a critical role during the evolution and domestication of _indica_
rice for a rapid life cycle. We propose that a critical event in the evolution of the _indica_ subspecies as a rapid cycling rice is acquisitions of variations in the promoter, rather than
the coding sequence, of the chlorophyll-degrading _OsSGR_ gene. Senescence, which determines lifespan, is a fundamental question in evolutionary ecology. Here, we provide a crucial
evolutionary mechanism of the senescence and death for the _r_-selection life history in rice plants. The nature of _OsSGR_ as a nonfunctional stay-green gene has been well-characterized
previously13,15. _OsSGR_ encodes the enzyme Mg++-dechelatase in the chlorophyll-degradation pathway, and is not a regulatory gene19. We conducted chlorophyll-degradation activity assays in
vitro and in planta to analyze the biochemical activity of the proteins derived from various _OsSGR_ alleles (Supplementary Fig. 11a, b). The results showed that the protein from the _ossgr_
knockout mutant allele showed a negligible enzyme activity toward chlorophyll degradation. Accordingly, _ossgr_ mutant plants maintain a high level of chlorophyll content in senescent
leaves but without comparable maintenance of net photosynthesis15, which is a characteristic feature of nonfunctional stay-green mutants. However, the _ossgr_ knockout mutation leads to
delay in some aspects of functional senescence upon a prolonged growth; Fv/Fm value was higher in 30 days after heading in the mutant plants than in wild-type plants15. This previous
observation is consistent with the expression pattern of _OsNAP_24, a senescence marker gene, in _ossgr_ mutant plants. Expression of _OsNAP_ is increased in both wild-type and _ossgr_
mutant plants during the grain-filling stage, indicating that _ossgr_ mutant plants undergo senescence despite of their stay-green phenotype. However, the expression of _OsNAP_ was lower in
_ossgr_ knockout mutants than in wild-type plants, indicating a partial delay of a functional senescence in terms of _OsNAP_ expression (Supplementary Fig. 6d, 7c) in addition to the Fv/Fm
values15. Here, we showed that rice grain yield can be increased by replacing the _indica_ allele of _OsSGR_ with the _japonica_ allele. As _OsSGR_ is a nonfunctional stay-green gene in
terms of net photosynthesis, it seems paradoxical to increase rice yield via _OsSGR_. It was the difference in the promoter region of the _indica_ and _japonica_ alleles of the _OsSGR_ gene
that led to yield increase in the NILs we generated. Unlike the _ossgr_ knockout mutation with no enzyme activity of OsSGR, the proteins from _japonica_ and _indica_ alleles showed
comparable enzyme activity (Supplementary Fig. 11). However, the promoter regions of the two alleles of _OsSGR_ are diverged (Supplementary Fig. 9). Compared with the promoter of the
_japonica_ allele, the promoter of the _indica_ allele led to earlier and higher induction of _OsSGR_ (Fig. 3a) and to earlier loss of chlorophyll (Fig. 1) with concomitant reduction of
photosynthesis (Fig. 4k) and grain yield (Fig. 4l). The senescence response in rice plants with the _indica_ allele is similar to that in the activation-tagging lines (Supplementary Fig. 5)
with the _japonica_ alleles, where _OsSGR_ expression is increased at a later stage with lower level of chlorophyll and reduced grain yield (Supplementary Fig. 5). In generating the NIL
lines with increased yield, we replaced the _indica_ allele with the _japonica_ allele, so that the induction of _OsSGR_ is slower than the parental lines with the _indica_ allele (Fig. 4j).
This leads to slower loss of chlorophyll (Fig. 4g. i), higher photosynthesis (Fig. 4k), and increased yield (Fig. 4l) compared with their parental lines. Thus, the senescence response of
rice plants with the _japonica_ allele with slower induction of _OsSGR_ became comparable with a functional stay-green phenotype, unlike the _ossgr_ knockout mutant which showed
nonfunctional stay-green phenotype with no yield advantage13,15. As the heading date of these lines are same, our results show that a senescence period with extended photosynthetic
competence can lead to higher productivity. In agreement with this notion, a recent report showed that an extended photosynthetic competence during senescence stage leads to increased
harvest index in SGM-3 mutant of upland rice variety Nagina 22; SGM-3 was suggested to be a “novel and functional stay green mutant”31. Lifespan indicates the maximal life expectancy from
the seed to seed and the length of time that plants live or expected to live32. In this regard, the lifespan of rice plants is the duration time from seed germination to the panicle
senescence and death associated with grain maturation. In analyzing senescence processes of rice plants in our experiments, we chose two _japonica_ and _indica_ rice cultivars, which showed
the same heading date (Supplementary Fig. 1) to avoid an influence of the differences in reproductive timing on senescence processes and the related lifespan. Despite of the same heading
dates, the panicles of the two _indica_ cultivars showed earlier senescence than those of the two _japonica_ cultivars (Fig. 1h), as quantified by colorimetric assays (Fig. 1i). Thus, the
lifespans of these rice cultivars were largely related to senescence process of the panicles after heading, which is controlled by the differential expression levels and induction kinetics
of _OsSGR_ (Fig. 4c, f, h, j). Optimal lifespans and senescence patterns of rice varieties have been selected to maximize rice productivity and/or economic income based on their cultivating
climates and cropping systems. In the single-cropping region, including most of _japonica_ cultivation areas, the balance of grain-filling period and rate is an important agricultural trait
for maximum crop production. Thus, the lower transcript levels of _OsSGR_ in _japonica_ lead to lower chlorophyll degradation and extended photosynthesis capacity, leading to improved
productivity with extended grain-filling period and a balanced nutrient remobilization rate in the single-crop system of _japonica_-cultivating areas. On the contrary, most of
_indica_-cultivation areas have double- or triple-cropping systems. Therefore, cultivars with a short lifespan and earlier senescence associated with rapid grain-filling rate in a short
grain-filling period should be favored for total maximum output for a given year. For this reason, the promoter of _OsSGR_ in _indica_ was naturally selected for faster and higher expression
of _OsSGR_. In fact, promoter variation leads to seasonally higher expression of the _OsSGR_ gene in the _indica_ subspecies, which in turn triggers earlier senescence of leaves and
panicles leading to a rapid life cycle. Thus, the seasonal induction kinetics of the _OsSGR_ promoter variations and the related lifespan variations are key ecological and agronomic traits
in rice evolution. On the other hand, natural selection on the coding region of _OsSGR_ may have been unfavorable during domestication as the continual impairment of enzyme activity might
have not been beneficial, or might even be detrimental for crop productivity, as observed in the nonfunctional _ossgr_ mutants, or in the _OsSGR_-overexpression lines with early senescence
without an adaptive advantage. This work shows that introgression of the _japonica OsSGR_ allele into elite _indica_-type cultivars delays senescence, thereby further increasing grain
filling and yield in the already high-yielding cultivars in the single-cropping systems in Korean rice field. Thus, utilization of the naturally occurring _OsSGR_ alleles provides a
beneficial breeding strategy in rice. METHODS PLANT MATERIALS For QTL analysis and NIL development, we used three _indica-_type cultivars, IR72, Milyang21 (M21), and Milyang23 (M23), and two
_japonica-_type cultivars, Junam (JN) and Saeilmi, showing early- and late-senescence phenotype, respectively. A F2:3 populations (141 lines) derived from a cross between IR72 and JN were
used to identify leaf senescence phenotype. IR72-NIL (harboring _japonica_-type _OsSGR_ allele), M21-NIL, and M23-NIL plants were generated by backcrossing the IR72 × JN, M21 × JN, and M23 ×
Saeilmi and further four times with its recurrent parents, IR72, M21, and M23, respectively. JN-NIL (harboring _indica-_type _OsSGR_ allele) plants were generated by backcrossing the JN ×
IR72 line and further six times with JN. For haplotype and phylogenetic analysis, the 105 cultivated rice varieties (Supplementary Data 1) including the 30 _indica_, 41 _temperate japonica_,
16 _javanica_ (_tropical japonica_), 8 _aromatic_, and 10 _aus_, were obtained from a collection of National Agrobiodiversity Center, RDA, Republic of Korea. Two _O. rufipogon_ and two _O.
nivara_ accessions were obtained from _Oryzabase_ and NIAS Genebank, respectively. Accessions used in this study were cultivated in the paddy field located at National Institute of Crop
science, RDA (Miryang; 35.3° N; 128.5° E) in 2017 and 2018. QTL ANALYSIS AND GENE CLONING OF THE LOCUS ON CHROMOSOME 9 The construction of genetic linkage map and QTL analysis were carried
out based on the genotypes and leaf chlorophyll contents of F2:3 populations derived from a cross between IR72 and JN using the QTL IciMapping program by inclusive composite interval mapping
(ICIM) with 1000 permutations (Supplementary Table 4 and Supplementary Data 2)33,34. The QTL locus on chromosome 9 was first delimited to a genomic interval between the markers Chr09-99 and
Chr09-107 on Chromosome 9 (Supplementary Data 2). To narrow down of locus, one line was selected from F2:3 population showing early-senescence phenotype and containing the genomic interval
between the markers Chr09-98 and Chr09-108. Furthermore, we crossed this line four times with the _japonica-_type JN as a recurrent and the resultant BC4F1 plants were selfed to obtain BC4F6
population. The BC4F6 population with 250 lines was screened using seven interval markers (Supplementary Table 2). The chlorophyll contents of leaves were investigated as the senescence
phenotype. For the gene cloning, two of BC4F6 lines were chosen and cross these two lines with JN again. To obtain enough recombinants for fine mapping, BC5F2 with 6349 plants were screened
using four flanking markers (RM24674, C9-5, C9-13, and C-17). Then new markers were developed to genotype those recombinants. Finally, the locus on chromosome 9 was delimited to a 26-kb
genomic interval between the markers C9–10 and C9–12. For progeny test, we tested the genotype and chlorophyll content of leaf of thirteen recombinant lines to confirm the fine-mapping
results using primers listed in Supplementary Table 2. DETERMINATION OF LEAF SENESCENCE Two methods were adopted to evaluate the leaf senescence during grain-filling stage. The chlorophyll
contents were estimated once a week by measuring the middle parts of the flag or second upper leaves using a CCM-300 chlorophyll meter (Opti-Sciences, Hudson, NH). To quantify the
progressive leaf and panicle color modification from green to green-yellow, to yellow, and to brown, the Automated Colourimetric Assay (ACA) described in the previous report was used35.
After scanning of detached leaves or panicles, background removed images of single leaf or panicle was prepared by using ImageJ. After that, the percentage of colors (green, green-yellow,
yellow, and brown) within the extracted images in a pixel-wise manner by using _R_ implemented ACA software was calculated. MG++-DECHELATASE ASSAY OF OSSGR To compare the biochemical
activities of _indica_ (IR72)-OsSGR _japonica_ (JN)-OsSGR and _ossgr-_type proteins, we examined the Mg++-dechelating activity of three mature OsSGR proteins prepared by a wheat-germ
protein-expression system, as previously described19. Recombinant OsSGR proteins were synthesized with an in vitro transcription/translation system (TNT SP6 High-Yield Wheat Germ Protein
Expression System; Promega). Transit peptide was removed, and a FLAG-tag was introduced at the C-terminus of the OsSGR proteins. First, the DNA fragments (5′-TCC CCA CCG CGC GAT AAG CTT GAC
TAC AAA GAC GAT GACGAC AAG TGA AAA CGA ATT CGA GCT-3′, the underlined section is _Hind_III site) were cloned into the pF3A WG (BYDV) Flexi vector (Promega) using an In-Fusion cloning system
(Clontech Laboratories) to introduce _Hind_III site. Then, the _OsSGR_ DNA fragments were amplified using the primer pair OsSGR-WG-F and OsSGR-WG-R (Supplementary Table 5) and cloned into
the pF3A WG _Hind_III site using an In-Fusion cloning system. Plasmid DNA was purified with the PureYield Plasmid Miniprep System (Promega). The recombinant proteins were produced according
to the manufacture’s protocol. The reaction mixture (12.5 µL) and the buffer (12.5 µL) containing 100 mM Tris-HCl (pH 7.5), 200 mM NaCl, and 0.1% polysorbate 20, and 0.5 nmol of chlorophyll
_a_ and incubated at 25 °C in the dark for 60 min. After incubation, 200 µL of acetone was added, and the pigments were analyzed with HPLC as previously described14. The elution profiles
were determined with a fluorescence detector monitoring 680-nm fluorescence with 410-nm excitation (RF-20A; Shimadzu). RNA ISOLATION AND QUANTIFICATION OF RT-PCR ANALYSIS The total RNA was
extracted from various rice tissues with WelPrep total RNA isolation reagent (WELGENE, Republic of Korea), according to the manufacturer’s instructions, and treated with RNase-free DNase I
(Takara Bio, Shiga, Japan) to prevent genomic DNA contamination. First-strand cDNA was synthesized from 2 µg of total RNA in a 25-µL reaction mixture with using the ImProm II Reverse
Transcriptase system kit (Promega, Madison, WI), followed by quantitative real-time PCR (qRT-PCR) analysis to determine gene expression levels (Bio-Rad, CFX96 Touch Real-Time PCR Detection
System, USA) using a SYBR premix ExTaq kit (Takara Bio, Shiga, Japan). The gene expressions were normalized using the rice _ubiquitin_ gene (LOC_Os06g46770) as an internal control. Changes
in expression were calculated via the ΔΔCt method. Primers for PCR are listed in Supplementary Table 5. GENERATION OF TRANSGENIC PLANTS To make _OsSGR-_overexpression construct, the
full-length cDNA sequences were amplified from the flag leaves of IR72 (_indica_) and JN (_japonica_) with primer pair OxF and OxR (Supplementary Table 5). The PCR products were inserted
into pGA3426, driven by maize _Ubiquitin_ (_Ubi_) promoter36. JN was used for producing transgenic plants by _Agrobacterium_-mediated co-cultivation37. For the construction of _OsSGR_ RNAi
vector, pANDA gateway vector38 was used for cloning the C-terminal regions using primer sets listed in Supplementary Table 5. To generate the Cas9-targeting construct for _OsSGR_, two
gene-specific spacer sequences listed in Supplementary Table 5 were cloned into the entry vectors, and then cloned into destination vectors containing the Cas9 expression cassette
pH-Ubi-cas9-7, of which the Cas9 coding sequence was codon-optimized for expression in rice and was driven by the maize _Ubi_ promoter39. These two constructs were transformed into _Oryza
sativa_ cv. Kasalath40. Transgenic plants were grown in the paddy field located at Daegu Gyeongbuk Institute of Science and Technology (Daegu; 35.8° N; 127.6° E) in the year 2017 and 2018.
ISOLATION OF T-DNA MUTANTS Putative _OsSGR_ and _OsPRR95_ mutant lines in _japonica_ cultivar Dongjin were isolated from rice flanking sequence-tag database41,42. For genotyping, two
gene-specific primers and one T-DNA-specific primer were used. Transcript levels of _OsSGR_ or _OsPRR95_ were determined by qRT-PCR, using cDNA prepared from 15- and 100-DAG (Days after
germination) leaves from WT, _OsSGR-D1_ (line 3A-01206), _OsSGR-D2_ (line 3A-00334), _OsPRR95-D1_ (5A-00143), and _OsPRR95-D2_ (3A-13152). Primers for genotyping and qRT-PCR are listed in
Supplementary Table 5. TOBACCO-INFILTRATION ASSAY The pCAMBIA1302 vector used for expression of _indica_ (IR72)-, _japonica_ (JN)- and _ossgr_-type _OsSGR_. cDNAs were amplified by PCR with
primers in Supplementary Table 5. After sequencing, PCR products were cloned in pCAMBIA1302 using _Nco_I/_Spe_I restriction sites. The resulting constructs and empty vectors were introduced
into _A. tumefaciens_ GV3101. Tobacco-infiltration assay was conducted as described previously with minor modifications43. Cultures for inoculation were prepared by harvesting _A.
tumefaciens_ (OD600:0.4), and then resuspended the harvested cells in infiltration buffer containing 10 mM MES, pH 5.5; 10 mM MgSO4 and 100 μM acetosyringone. Infiltration with syringe was
performed by infiltrating the prepared _Agrobacterium_ into 4-weeks-old _Nicotiana benthamiana_ leaves. Photos were taken 4 days after infiltration. TRANSIENT EXPRESSION ASSAY IN RICE
PROTOPLAST To generate reporter vectors, the promoters of _OsSGR_ (_japonica_: JN and _indica_: IR72) were amplified with the _OsSGR_ promoter primer set (Supplementary Table 5). The primers
used were as follows: _OsSGR_-pro-F1 and _OsSGR_-pro-R1 for #1 (−2000 to −1 and 5′UTR of _japonica_ promoter) and #2 (−2008 to −1 and 5′UTR of _indica_ promoter_)_. For luciferase fusion
with _OsSGR_ promoter, the PCR products were ligated into pGreenII 800-Luc after restriction enzyme treatment. All PCR products were sequenced to confirm the nucleotide sequences. The maize
_Ubi_ promoter: _β-glucuronidase_ (_ZmUbi: GUS_) construct was used as an internal control44. Protoplasts were isolated from rice root-derived callus suspension (Oc) cells according to the
reported method44, but with minor modifications. Briefly, the Oc suspension solution was collected by centrifugation, and the supernatant was removed. The cells were then incubated in enzyme
solution (2% cellulose RS, 1% macerozyme, 0.4 M mannitol, 0.1% MES, pH 5.7, and 0.1% CaCl2) for 4 h with gentle shaking. After washing with equal volume of KMC solution (117 mM KCl, 82 mM
MgCl2, and 85 mM CaCl2), harvested protoplasts were resuspended in MMG solution (0.4 M mannitol, 15 mM MgCl2, and 4 mM MES, pH 5.7) to achieve a density of 3 × 106 cells mL−1, as quantified
with a hemocytometer. For transient expression assays, isolated protoplasts were cotransfected with _OsSGR_ promoter reporter constructs using a polyethylene glycol-calcium-mediated
method45. _ZmUbi: GUS_ was included in each sample as an internal control. Transfected protoplasts were incubated in incubation solution (0.5 M mannitol, 20 mM KCl, 4 mM MES, pH 5.7) for 5 h
and then harvested. The harvested protoplasts were resuspended in lysis buffer and used for Luciferase and GUS assays. Luciferase assays were performed using the Luciferase assay system
(Promega), and GUS assays were performed by previously described methods46. The fluorescence generated by Luciferase and GUS activity was measured by the VICTOR2 1420 multilabel counter
(PerkinElmer Life Sciences). In each sample, the measured Luciferase activity was divided by the GUS activity to normalize the data for variation in experimental conditions, and all
transient expression experiments were repeated three times with similar results. HAPLOTYPE ANALYSIS AND SNP ANALYSIS 2-kb promoter regions of 105 accessions (Supplementary Data 1) were
amplified by PCR using primers listed in Supplementary Table 5. The PCR products were sequenced and used for haplotype analysis. The sequences from accessions were aligned using MEGA6.047. A
phylogenetic tree was constructed using the neighbor-joining method in MEGA6.0. Chlorophyll contents were also measured in flag leaves at 6 weeks after heading date using these accessions
grown in paddy fields. ASSOCIATION OF POLYMORPHIC SITES WITH CHLOROPHYLL CONTENTS For each polymorphic site in the _OsSGR_ promoter, we divided the accessions into two groups, one with the
same DNA base and the other with the different base compared with the reference sequence of _japonica_ Nipponbare (IRGSP-1.0). Next, we compared the chlorophyll contents between the two
groups using Student’s _t_ test. For multiple testing correction of the measured _T_ value for each site, an empirical null distribution of _T_ values was estimated by performing random
permutations of the accessions 1000 times. The adjusted _P_-value was then calculated by applying the two-tailed test to the measured _T_ value for each site using the distribution.
PHYLOGENY AND SELECTIVE SWEEP ANALYSIS The whole genome sequencing dataset of Huang et al.27 were reanalyzed to examine the phylogeny of the _OsSGR_ region in an expanded set of wild and
domesticated rice sample. Raw sequencing reads were downloaded from the National Center for Biotechnology Information website under bioproject ID numbers PRJEB2052, PRJEB2578, and PRJEB2829,
for a total of 1477 rice samples. Raw sequencing reads were trimmed using trimmomatic ver. 0.3648, and realigned to the reference Nipponbare genome (MSU7/IRGSP-1.0) using BWA-MEM ver.
0.7.1549. Because the sequencing depth was very shallow (~1 to 2×) for each sample, we used genotype probability and likelihood for all downstream analysis to incorporate the uncertainty
associated with the low coverage dataset. The programs ANGSD ver. 0.91350 and ngsTools51 were used while using values from Choi and Purugganan52 during parameter specifications. Phylogenetic
tree of the _OsSGR_ region was reconstructed by estimating the genotype probabilities of 10-kb upstream and downstream of the _OsSGR_ gene. The genotype probabilities were then used to
estimate a pairwise genetic distance matrix53, which were then used to build a neighbor-joining tree with FastME ver. 2.1.554. Evidence of a domestication-related selective sweep was
examined by estimating the ratio of wild to domesticated rice polymorphism levels (πW/πD). Because domestication-mediated selection would reduce the level of polymorphism in only the
domesticated but not the wild rice, πW/πD values will be elevated for genomic regions that have undergone a domestication-related selective sweep27. ANGSD was used to estimate the level of
polymorphism in 20 kb non-overlapping windows for each wild and domesticated rice subpopulation. The level of polymorphism for each rice subpopulation (_aus_, _indica_, and _japonica_) was
compared to the Or-II group of wild rice, which was previously determined to be the most genetically diverged wild rice group from all domesticated rice subpopulations27,52. A window was
assumed to be significant if the πW/πD value was greater than the 1% empirical distribution of πW/πD values. MEASUREMENT OF FV/FM RATIO The _Fv_/_Fm_ ratio was measured using a plant
efficiency analyzer (PEA) (Hansatech, Norfolk, UK) following the manufacturer’s instructions. After the dark adaptation of the middle part of each flag leaves for 20 min, the _Fv_/_Fm_ ratio
was recorded in the paddy field. More than four experimental replicates were conducted. MEASUREMENT OF NET CO2 ASSIMILATION RATE Plants were transferred from rice paddy field to glasshouse
after heading and adapted in the glasshouse for 2 weeks to examine CO2 assimilation rate. CO2 assimilation rate was measured with portable gas exchange system LI-6850 (LI-COR Inc., Lincoln,
NE, USA) following saturating irradiance of 1500 μmol m−2 s−1, CO2 concentration of 400 ppm, relative humidity of 60%, fan speed of 10,000 rpm, and flow rate of 600 μmol s–1 between 11 am to
2 pm. MEASUREMENT OF RELATIVE GROWTH RATE Three plants of each genotypes were carefully sampled and washed to remove the roots and soil. Total dry weight of each plant was measured after
drying the plant in an oven at 70 °C to a constant weight. RGR is calculated according to the following formula28,29: RGR = (lnW2 − lnW1)/(_t_2 − _t_1) where: ln = natural log, W1 = dry
weight of plant at time t1 (in grams), W2 = dry weight of plant at time _t_2, _t_1 = time one (in days), _t_2 = time 2, respectively and is expressed as g g−1 day−1. DATA AVAILABILITY All
data and analysis needed to understand and evaluates the conclusions in the paper are present in the paper or Supplementary Materials. The source data underlying Figs. 1d–g, i, 2f–h, 3a, c
and 4g–m and Supplementary Figs. 1, 2a, b, 3, 4b, 5b–d, g, 5i–n, 6c, d, f, h–m, 7b, c, 8b, d, e, g, i, 10b, e–i, 11a, 16c–g and 17a–j are provided as a Source Data file. REFERENCES * Wing,
R. A., Purugganan, M. D. & Zhang, Q. The rice genome revolution: from an ancient grain to green super rice. _Nat. Rev. Genet._ 19, 505–517 (2018). Article CAS PubMed Google Scholar *
Huang, X. et al. Genomic architecture of heterosis for yield traits in rice. _Nature_ 537, 629–633 (2016). Article ADS CAS PubMed Google Scholar * Ray, D. K., Ramankutty, N., Mueller,
N. D., West, P. C. & Foley, J. A. Recent patterns of crop yield growth and stagnation. _Nat. Commun._ 3, 1293 (2012). Article ADS PubMed CAS Google Scholar * Hu, C. et al. Metabolic
variation between _japonica_ and _indica_ rice cultivars as revealed by non-targeted metabolomics. _Sci. Rep._ 4, 50–67 (2014). Google Scholar * Abdelkhalik, A. F., Shishido, R., Nomura,
K. & Ikehashi, H. QTL-based analysis of leaf senescence in an indica/japonica hybrid in rice (Oryza sativa L.). _Theor. Appl Genet_ 110, 1226–1235 (2005). Article CAS PubMed Google
Scholar * Yoshida, S. _Fundamentals of Rice Crop Science_, Chapter 1 (International Rice Research Institute, Los Baños, 1981). * Bonser, S. P. & Ladd, B. The evolution of competitive
strategies in annual plants. _Plant Ecol._ 212, 1441–1449 (2011). Article Google Scholar * Guo, J. et al. Overcoming inter-subspecific hybrid sterility in rice by developing
_indica_‐compatible _japonica_ lines. _Sci. Rep._ 6, 26878 (2016). Article ADS CAS PubMed PubMed Central Google Scholar * Gregersen, P. L., Culetic, A., Boschian, L. & Krupinska,
K. Plant senescence and crop productivity. _Plant Mol. Biol._ 82, 603–622 (2013). Article CAS PubMed Google Scholar * Leng, Y., Ye, G. & Zeng, D. Genetic dissection of leaf
senescence in rice. _Int J. Mol. Sci._ 18, 2686 (2017). Article PubMed Central CAS Google Scholar * Gan, S. Concepts and types of senescence in plants. _Methods Mol. Biol._ 1744, 3–8
(2018). Article CAS PubMed Google Scholar * Thomas, H. & Ougham, H. The stay-green trait. _J. Exp. Bot._ 65, 3889–3900 (2014). Article CAS PubMed Google Scholar * Park, S. Y. et
al. The senescence-induced staygreen protein regulates chlorophyll degradation. _Plant Cell_ 19, 1649–1664 (2007). Article CAS PubMed PubMed Central Google Scholar * Kuai, B., Chen, J.
& Hörtensteiner, S. The biochemistry and molecular biology of chlorophyll breakdown. _J. Exp. Bot._ 69, 751–767 (2018). Article CAS PubMed Google Scholar * Jiang, H. et al. Molecular
cloning and function analysis of the _stay green_ gene in rice. _Plant J._ 52, 197–209 (2007). Article CAS PubMed Google Scholar * Zhao, Y. et al. New alleles for chlorophyll content
and stay-green traits revealed by a genome wide association study in rice (Oryza sativa). _Sci. Rep._ 9, 2541 (2019). Article ADS PubMed PubMed Central CAS Google Scholar * Zhang, C.
F., Peng, S. B. & Laza, R. C. Senescence of top three leaves in field-grown rice plants. _J. Plant Nutr._ 26, 2453–2468 (2003). Article CAS Google Scholar * Lee, S. et al. Molecular
bases for differential aging programs between flag and second leaves during grain-filling in rice. _Sci. Rep._ 7, 8792 (2017). Article ADS PubMed PubMed Central CAS Google Scholar *
Shimoda, Y., Ito, H. & Tanaka, A. Arabidopsis STAY-GREEN, Mendel’s green cotyledon gene, encodes magnesium-dechelatase. _Plant Cell_ 28, 2147–2160 (2016). Article CAS PubMed PubMed
Central Google Scholar * Ren, G. et al. Identification of a novel chloroplast protein AtNYE1 regulating chlorophyll degradation during leaf senescence in _Arabidopsis_. _Plant Physiol._
144, 1429–1441 (2007). Article ADS CAS PubMed PubMed Central Google Scholar * Zhao, J. et al. _OsELF3-1_, an ortholog of Arabidopsis _EARLY FLOWERING 3_, regulates rice circadian
rhythm and photoperiodic flowering. _PLoS ONE_ 7, e43705 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Kim, H. et al. Circadian control of _ORE1_ by PRR9 positively
regulates leaf senescence in _Arabidopsis_. _Proc. Natl Acad. Sci. USA_ 115, 8448–8453 (2018). Article CAS PubMed PubMed Central Google Scholar * Zhang, C. et al. Harpin-induced
expression and transgenic overexpression of the phloem protein gene _AtPP2-A1_in Arabidopsis repress phloem feeding of the green peach aphid _Myzus persicae_. _BMC Plant Biol._ 11, 11
(2011). Article CAS PubMed PubMed Central Google Scholar * Liang, C. et al. OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly
targeting senescence-associated genes in rice. _Proc. Natl Acad. Sci. USA_ 111, 10013–10018 (2014). Article ADS CAS PubMed PubMed Central Google Scholar * Sato, Y., Morita, R.,
Nishimura, M., Yamaguchi, H. & Kusaba, M. Mendel’s green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway. _Proc. Natl Acad. Sci. USA_ 104, 14169–14174
(2007). Article ADS CAS PubMed PubMed Central Google Scholar * Garris, A. J., Tai, T. H., Coburn, J., Kresovich, S. & McCouch, S. Genetic structure and diversity in _Oryza sativa_
L. _Genetics_ 169, 1631–1638 (2005). Article CAS PubMed PubMed Central Google Scholar * Huang, X. et al. A map of rice genome variation reveals the origin of cultivated rice. _Nature_
490, 497–501 (2012). Article ADS CAS PubMed PubMed Central Google Scholar * Al-Tamimi, N. et al. Salinity tolerance loci revealed in rice using high-throughput non-invasive
phenotyping. _Nat. Commun._ 7, 13342 (2016). Article ADS PubMed PubMed Central Google Scholar * Rajput, A., Rajput, S. S. & Jha, G. Physiological parameters leaf area index, crop
growth rate, relative growth rate and net assimilation rate of different varieties of rice grown under different planting geometries and depths in SRI. _Int. J. Pure Appl. Biosci._ 5,
362–367 (2017). Article Google Scholar * Noguero, M., Atif, R. M., Ochatt, S. & Thompson, R. D. The role of the DNA-binding One Zinc Finger (DOF) transcription factor family in plants.
_Plant Sci._ 209, 32–45 (2013). Article CAS PubMed Google Scholar * Ramkumar, M. et al. A novel stay-green mutant of rice with delayed leaf senescence and better harvest index confers
drought tolerance. _Plants_ 8, 375 (2019). Article CAS PubMed Central Google Scholar * Thomas, H. Senescence, ageing and death of the whole plant. _N. Phytol._ 197, 696–711 (2013).
Article Google Scholar * Kim, T. H. et al. Drought-tolerant QTL qVDT11 leads to stable tiller formation under drought stress conditions in rice. _Plant Sci._ 256, 131–138 (2017). Article
CAS PubMed Google Scholar * Furuta, T., Ashikari, M., Jena, K. K., Doi, K. & Reuscher, S. Adapting genotyping-by-sequencing for rice F2 populations. _G3 (Bethesda)_ 7, 881–893 (2017).
Article CAS Google Scholar * Bresson, J., Bieker, S., Riester, L., Doll, J. & Zentgraf, U. A guideline for leaf senescence analyses: from quantification to physiological and
molecular investigations. _J. Exp. Bot._ 69, 769–786 (2018). Article CAS PubMed Google Scholar * Kim, S. R., Lee, D. Y., Yang, J. I., Moon, S. & An, G. Cloning vectors for rice. _J.
Plant Biol._ 52, 73–78 (2009). Article CAS Google Scholar * Lee, S., Jeon, J. S., Jung, K. H. & An, G. Binary vector for efficient transformation of rice. _J. Plant Biol._ 42, 310–316
(1999). Article CAS Google Scholar * Miki, D. & Shimamoto, K. Simple RNAi vectors for stable and transient suppression of gene function in rice. _Plant Cell Physiol._ 45, 490–495
(2004). Article CAS PubMed Google Scholar * Miao, J. et al. Targeted mutagenesis in rice using CRISPR-Cas system. _Cell Res._ 23, 1233–1236 (2013). Article CAS PubMed PubMed Central
Google Scholar * Saika, H. & Toki, S. Mature seed-derived callus of the model _indica_ rice variety Kasalath is highly competent in _Agrobacterium_-mediated transformation. _Plant Cell
Rep._ 29, 1351–1364 (2010). Article CAS PubMed PubMed Central Google Scholar * Jeon, J. S. et al. T-DNA insertional mutagenesis for functional genomics in rice. _Plant J._ 22, 561–570
(2000). Article CAS PubMed Google Scholar * Jeong, D. H. et al. Generation of a flanking sequence-tag database for activation-tagging lines in japonica rice. _Plant J._ 45, 123–132
(2006). Article CAS PubMed Google Scholar * Leuzinger, K. et al. Efficient agroinfiltration of plants for high-level transient expression of recombinant proteins. _J. Vis. Exp._ 77,
e50521 (2013). Google Scholar * Cho, J. I. et al. Role of the rice hexokinases OsHXK5 and OsHXK6 as glucose sensors. _Plant Physiol._ 149, 745–759 (2009). Article CAS PubMed PubMed
Central Google Scholar * Choi, S. C. et al. Trithorax group protein _Oryza sativa_ Trithorax1 controls flowering time in rice via interaction with early heading date3. _Plant Physiol._
164, 1326–1337 (2014). Article CAS PubMed PubMed Central Google Scholar * Jefferson, R. A., Kavanagh, T. A. & Bevan, M. W. GUS fusions: b-glucuronidase as a sensitive and versatile
gene fusion marker in higher plants. _EMBO J._ 20, 3901–3907 (1987). Article Google Scholar * Tamura, K., Stecher, G., Peterson, D., Filipski, A. & Kumar, S. MEGA6: molecular
evolutionary genetics analysis version 6.0. _Mol. Biol. Evol._ 30, 2725–2729 (2013). Article CAS PubMed PubMed Central Google Scholar * Bolger, A. M., Lohse, M. & Usadel, B.
Trimmomatic: a flexible trimmer for Illumina sequence data. _Bioinformatics_ 30, 2114–2120 (2014). Article CAS PubMed PubMed Central Google Scholar * Li, H. Aligning sequence reads,
clone sequences and assembly contigs with BWA-MEM. _arXiv_ 1303, 3997 (2013). ADS Google Scholar * Korneliussen, T. S., Albrechtsen, A. & Nielsen, R. ANGSD: analysis of next generation
sequencing data. _BMC Bioinforma._ 15, 356 (2014). Article Google Scholar * Fumagalli, M., Vieira, F. G., Linderoth, T. & Nielsen, R. ngsTools: methods for population genetics
analyses from next-generation sequencing data. _Bioinformatics_ 30, 1486–1487 (2014). Article CAS PubMed PubMed Central Google Scholar * Choi, J. Y. & Purugganan, M. D. Multiple
origin but single domestication led to _Oryza sativa_. _G3 (Bethesda)_ 8, 797–803 (2018). Article CAS Google Scholar * Vieira, F. G., Lassalle, F., Korneliussen, T. S. & Fumagalli, M.
Improving the estimation of genetic distances from next-generation sequencing data. _Biol. J. Linn. Soc._ 117, 139–149 (2016). Article Google Scholar * Lefort, V., Desper, R. &
Gascuel, O. FastME 2.0: a comprehensive, accurate, and fast distance-based phylogeny inference program. _Mol. Biol. Evol._ 32, 2798–2800 (2015). Article CAS PubMed PubMed Central Google
Scholar Download references ACKNOWLEDGEMENTS We thank Dr. Gi-Gyeong Park and Kyungsook An for taking care of the transgenic rice plants; Nam-Chon Paek for sharing _ossgr1_ seeds; Makoto
Kusaba for _ossgr2_ seeds. We thank Life Science Editors for editorial assistance. This research was supported by the Institute for Basic Science (IBS-R013-D1) from the Ministry of Science,
ICT & Future Planning, and by the Research Program for Agricultural Science and Technology Development (Project No. PJ01099902) Rural Development Administration. AUTHOR INFORMATION
Author notes * These authors contributed equally: Dongjin Shin, Sichul Lee, Tae-Heon Kim AUTHORS AND AFFILIATIONS * Department of Southern Area Crop Science, National Institute of Crop
Science (NICS), RDA, Miryang, Republic of Korea Dongjin Shin, Tae-Heon Kim, Jong-Hee Lee, Ji Yoon Lee, Jun-Hyeon Cho & You-Chun Song * Center for Plant Aging Research, Institute for
Basic Science (IBS), Daegu, Republic of Korea Sichul Lee, Joonheum Park, Jinwon Lee, Ji-Hwan Park, Daehee Hwang & Hong Gil Nam * Crop Biotech Institute and Graduate School of
Biotechnology, Kyung Hee University, Yongin, Republic of Korea Lae-Hyeon Cho, Dae-Woo Lee, Jong-Seong Jeon & Gynheung An * Center for Genomics and Systems Biology, Department of Biology,
New York University, New York, NY, USA Jae Young Choi & Michael D. Purugganan * Department of New Biology, DGIST, Daegu, Republic of Korea Wonhee Lee, Daehee Hwang & Hong Gil Nam *
Institute of Low Temperature Science, Hokkaido University, Sapporo, Japan Hisashi Ito & Ayumi Tanaka * Department of Biology, Sunchon National University, Sunchon, Republic of Korea Dae
Heon Kim Authors * Dongjin Shin View author publications You can also search for this author inPubMed Google Scholar * Sichul Lee View author publications You can also search for this author
inPubMed Google Scholar * Tae-Heon Kim View author publications You can also search for this author inPubMed Google Scholar * Jong-Hee Lee View author publications You can also search for
this author inPubMed Google Scholar * Joonheum Park View author publications You can also search for this author inPubMed Google Scholar * Jinwon Lee View author publications You can also
search for this author inPubMed Google Scholar * Ji Yoon Lee View author publications You can also search for this author inPubMed Google Scholar * Lae-Hyeon Cho View author publications You
can also search for this author inPubMed Google Scholar * Jae Young Choi View author publications You can also search for this author inPubMed Google Scholar * Wonhee Lee View author
publications You can also search for this author inPubMed Google Scholar * Ji-Hwan Park View author publications You can also search for this author inPubMed Google Scholar * Dae-Woo Lee
View author publications You can also search for this author inPubMed Google Scholar * Hisashi Ito View author publications You can also search for this author inPubMed Google Scholar * Dae
Heon Kim View author publications You can also search for this author inPubMed Google Scholar * Ayumi Tanaka View author publications You can also search for this author inPubMed Google
Scholar * Jun-Hyeon Cho View author publications You can also search for this author inPubMed Google Scholar * You-Chun Song View author publications You can also search for this author
inPubMed Google Scholar * Daehee Hwang View author publications You can also search for this author inPubMed Google Scholar * Michael D. Purugganan View author publications You can also
search for this author inPubMed Google Scholar * Jong-Seong Jeon View author publications You can also search for this author inPubMed Google Scholar * Gynheung An View author publications
You can also search for this author inPubMed Google Scholar * Hong Gil Nam View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS H.G.N. and S.L.
conceived and supervised the project. H.G.N., S.L., D.S., and J.H.L. designed the experiments. D.S., T.H.K., J.Y. L., J.H.C., Y.C.S., and J.H.L. developed the NILs. D.S., S.L., J.P., and
T.H.K. conducted the QTL analysis and field experiments. G.A., L.H.C., and J.L. provided the T-DNA mutants and generated the transgenic plants. D.W.L., J.L., J.S.J., and D.H.K. performed the
protoplast experiments. W.L., J.H.P., D.H., J.Y.C., S.L., and M.D.P. performed the bioinformatics analysis. H.I., A.T., and J.W.L. conducted the biochemical assay and in planta infiltration
assay of rice OsSGR. D.S., S.L., T.H.K., J.H.L., and H.G.N. analyzed the data and wrote the paper. All the authors discussed the results and contributed to the paper. CORRESPONDING AUTHORS
Correspondence to Sichul Lee or Hong Gil Nam. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature
Communications_ thanks the anonymous reviewers for their contribution to the peer review of this work. Peer review reports are available. PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION PEER REVIEW FILE SUPPORTING INFORMATION DESCRIPTION OF ADDITIONAL
SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 SUPPLEMENTARY DATA 2 SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Shin, D., Lee, S., Kim, TH. _et al._ Natural variations at the _Stay-Green_ gene
promoter control lifespan and yield in rice cultivars. _Nat Commun_ 11, 2819 (2020). https://doi.org/10.1038/s41467-020-16573-2 Download citation * Received: 14 April 2019 * Accepted: 06 May
2020 * Published: 04 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16573-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable
link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative