- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT All known riboswitches use their aptamer to senese one metabolite signal and their expression platform to regulate gene expression. Here, we characterize a SAM-I riboswitch
(SAM-I_Xcc_) from the _Xanthomonas campestris_ that regulates methionine synthesis via the _met_ operon. In vitro and in vivo experiments show that SAM-I_Xcc_ controls the _met_ operon
primarily at the translational level in response to cellular S-adenosylmethionine (SAM) levels. Biochemical and genetic data demonstrate that SAM-I_Xcc_ expression platform not only can
repress gene expression in response to SAM binding to SAM-I_Xcc_ aptamer but also can sense and bind uncharged initiator Met tRNA, resulting in the sequestering of the anti-Shine-Dalgarno
(SD) sequence and freeing the SD for translation initiation. These findings identify a SAM-I riboswitch with a dual functioning expression platform that regulates methionine synthesis
through a previously unrecognized mechanism and discover a natural tRNA-sensing RNA element. This SAM-I riboswitch appears to be highly conserved in _Xanthomonas_ species. SIMILAR CONTENT
BEING VIEWED BY OTHERS INSIGHTS INTO THE COTRANSCRIPTIONAL AND TRANSLATIONAL CONTROL MECHANISMS OF THE _ESCHERICHIA COLI TBPA_ THIAMIN PYROPHOSPHATE RIBOSWITCH Article Open access 17 October
2024 PROGRAMMABLE EUKARYOTIC PROTEIN SYNTHESIS WITH RNA SENSORS BY HARNESSING ADAR Article 27 October 2022 NA+ RIBOSWITCHES REGULATE GENES FOR DIVERSE PHYSIOLOGICAL PROCESSES IN BACTERIA
Article Open access 25 July 2022 INTRODUCTION Riboswitches are cis-acting regulatory mRNA elements that are usually located in the 5′ untranslated region (5′UTR) of a messenger RNA (mRNA)
and control gene expression by directly sensing small molecules1,2,3,4,5. Since their first discovery in 20021,6,7, riboswitches have become recognized as important and widespread regulators
of genes involved in many bacterial cellular processes8,9,10,11,12. Currently, almost 40 distinct classes of riboswitch have been identified13. A riboswitch typically consists of two
functional domains called the aptamer and the expression platform. The aptamer directly binds to a specific small molecule, and the expression platform undergoes structural changes in
response to the stabilization of the aptamer structure and then regulates gene expression8,9,10,11,12. The majority of riboswitches have been shown to specifically sense and bind small
molecules that include purines, amino acids, vitamins, co-factors, second messengers, and transfer RNA (tRNA)8,9,10,11,12,13. In this way, riboswitches can control a wide spectrum of
cellular processes including vitamin metabolism, nucleotide and amino acid biosynthesis, and sulfur metabolism8,9,10,11,12,13,14,15. Methionine (Met) is a unique proteinogenic amino acid
which plays acritical role in the initiation of translation and the precursor of the principal cellular methyl group donor _S_-adenosylmethionine (SAM)16. It has been shown in Gram-positive
bacteria that the key regulators of Met biosynthesis are the SAM-I14,15,17,18 and T-box8,11,12,19,20,21,22 riboswitches. The SAM-I (also called S-box) are a class of riboswitch that regulate
gene expression in response to SAM binding. In addition to modulating Met biosynthesis, SAM-I is also involved in cysteine biosynthesis, sulfur metabolism and SAM biosynthesis14,15,17,18.
In contrast, members of the T-box class of riboswitch monitor the aminoacylation status of specific tRNAs to induce the expression of regulated downstream gene(s), involved in the
biosynthesis of Met and other amino acids8,11,12,19,20,21,22. Interestingly, SAM-I and T-box riboswitches use opposite strategies to control Met biosynthesis: SAM-I uses a negative feedback
mechanism to turn off Met biosynthesis in response to increasing SAM concentration14,15,17,18, while T-box uses a positive feedback mechanism to turn on Met biosynthesis in response to the
accumulation of uncharged Met-tRNA8,11,12,19,20,21,22. The regulation of Met de novo biosynthesis in Gram-negative bacteria was discovered to be controlled by regulatory proteins23. In the
model organism _Escherichia coli_, MetR and MetJ have been demonstrated to be specifically involved in the control of Met biosynthesis. The MetR protein has been shown to act as a
transcriptional activator which uses homocysteine as an inducer23. In contrast, the MetJ has been demonstrated to function as a transcriptional repressor using SAM as co-repressor23. This
system of regulation in _E. coli_ appears to be conserved in a high proportion of Gram-negative bacteria including the _Xanthomonas_ genus24. Although potential riboswitches involved in the
regulation of Met biosynthesis genes have been proposed in Gram-negative bacteria3,24, none of them has been functionally characterized. T-box riboswitches have long been thought to exist
primarily in Gram-positive bacteria8,11,12,19,20,21,22,25. Recent work examining the regulation of Met biosynthesis in the phytopathogen _Xanthomonas campestris_ pv. _campestris_ (hereafter
_Xcc_) provided functional evidence of a Gram-negative bacterium utilizing a 5′UTR region to control the expression of the genes involved in the generation of Met26. As well as being a plant
pathogen of global concern, _Xcc_ is a model organism for molecular studies of plant-microbe interactions27. The mechanism by which this 5′UTR region exerts its regulatory action is
incompletely understood. Here, we provide evidence demonstrating that this 5′UTR region from _Xcc _encodes a functional SAM-I riboswitch. Genetic and biochemical studies confirm that
SAM-I_Xcc_ modulates _met_ operon expression primarily at the translational level. Further analysis reveals that the SAM-I riboswitch from _Xcc_ displays previously uncharacterized
regulatory actions associated with the SAM-I class where the expression platform shows dual functionality. We demonstrate that the expression platform of SAM-I_Xcc_ is involved in feedback
regulation of the _met _operon in response to Met availability. In addition, we demonstrate that the SAM-I_Xcc _expression platform also functions as a sensor monitoring uncharged initiator
Met-tRNA. The findings describe a structurally typical SAM-I riboswitch from _Xcc _with a previously uncharacterized mode of action. SAM-I_Xcc_ appears to be broadly distributed in
Gram-negative _Xanthomonas_ species bacteria and its expression platform represents a type of natural tRNA-sensing RNA elements. RESULTS SAM-I_XCC_ CONTROLS THE _MET_ OPERON PRIMARILY AT
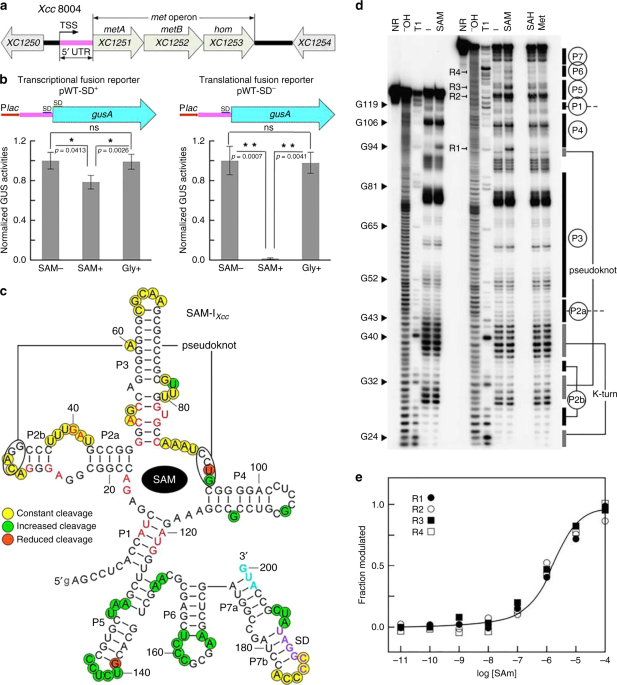
TRANSLATION Our previous work demonstrated that the _met_ operon is essential for Met de novo biosynthesis in _Xcc _strain 8004 and that a 5′UTR tightly regulates the operon in response to
cellular levels of Met26. Further sequence analysis of the 5′UTR revealed a putative 200-nucleotide (nt) SAM-I-like riboswitch (designated going forward as SAM-I_Xcc_) (Supplementary Fig.
1), similar to the SAM-I predicted previously28. The _met_ operon consists of three genes, i.e., _XC1251_ (_metA_), _XC1252_ (_metB_), and _XC1253_ (_hom_), which encod a homoserine
O-succinyltransferase, a cystathionine γ-synthase and a homoserine dehydrogenase, respectively (Fig. 1a). In addition to _XC1251_, _XC1889 _in the genome of _Xcc_ strain 8004 also encodes a
homoserine O-succinyltransferase29, whose promoter region does not contain sequences similar to SAM-I_Xcc_, suggesting that the expression of the two homoserine
O-succinyltransferase-encoding genes may be regulated by different modes. The predicted aptamer of SAM-I_Xcc_ displayed a 52% sequence similarity to the aptamer of _yitJ_ SAM-I from
_Bacillus subtilis_14. SAM-I_Xcc_ does not contain an Rho-independent transcription terminator. Given that all of the functionally characterized SAM-I riboswitches employ the Rho-independent
terminator to control gene expression at the transcriptional level14,15,16,18, we presume that SAM-I_Xcc _may use the translation attenuation mechanism to regulate gene expression, although
the possibility of using the Rho-dependent transcription termination mechanism can not be excluded. To examine the potential role of SAM-I_Xcc_ in gene regulation in reaction to cellular
levels of SAM, we used several reporter constructs carrying SAM-I_Xcc_ fused to the _gusA_ gene from _E. coli_ (Supplementary Fig. 2). Two SAM-I_Xcc_-_gusA_ fusion reporters were created to
monitor transcriptional (pWT-SD+) and translational (pWT-SD−) activity and introduced into the _Xcc_ 8004 wild-type strain (see Methods; Fig. 1b). It is known that bacteria can take up SAM
directly by a SAM-specific transporter30,31. The growth of _Xcc met_ operon inactivation mutant 1201PK2 (Supplementary Table 1), which is unable to synthesize Met and SAM26, could be
restored in the minimal medium MMX with addition of SAM (Supplementary Fig. 3), suggesting the presence of SAM transporter in _Xcc_. For the reporter strain _Xcc_ 8004/pWT-SD+, the GUS
activity was repressed by ~24% when grown in the medium supplemented with 300 µM SAM relative to medium with no SAM supplementation (Fig. 1b). However, GUS activity observed for the _Xcc_
8004/pWT-SD− strain was repressed by ~98% in the medium supplemented SAM relative to medium with no SAM supplementation (Fig. 1b). In addition, both reporter strains showed negligible change
in GUS activity when the medium was supplemented with an alternative amino acid, glycine, at a concentration of 300 µM (Fig. 1b).The data indicate that SAM-I_Xcc_ is specifically responsive
to the cellular levels of SAM and controls gene expression primarily at the translational level. In addition, the GUS activity of the transcriptional fusion reporter strain (8004/pWT-SD+)
shows a small but statistically significant reduction upon addition of SAM, suggesting that SAM-I_Xcc_ may also modulate gene expression weakly at the transcriptional level. However, we
cannot rule out that this reduction of GUS activity may be caused by an influence on mRNA stability induced by the binding of SAM to the aptamer or that this effect may be due to indirect
effects of SAM on transcription in general. To test whether the three consecutive hairpin structures (P5, P6, and P7) formed in the expression platform upon SAM binding to the aptamer
(Fig.1c) is involved in the reduction of GUS activity of the transcriptional fusion reporter strain in response to SAM addition, _gusA_ transcriptional fusion reporters carrying a series of
full-length or truncated expression platforms from SAM-I_Xcc_ were constructed (Supplementary Fig. 4) and their GUS activities were determined in the presence and absence of SAM. The result
showed that the three hairpins together and the combination of P5 and P6 or P6 and P7 hairpins can reduce the GUS activity although the efficiency is much lower than that of the _trp_
terminator (Supplementary Fig. 4). It is possible that these hairpins act as a transcription attenuator or serve as an RNase-binding target to recruit RNase which then degrades the mRNA, or
cause other indirect effects. To assess ligand binding in vitro, the predicted 200-nt SAM-I_Xcc_ (Fig. 1c) was subjected to in-line probing analysis as described in Methods32,33. Due to its
high G+C content, ligand-induced changes in spontaneous RNA cleavage of SAM-I_Xcc_ were only seen when the reaction temperature was above 37 °C (Supplementary Fig. 5). Upon PAGE separation,
the pattern of RNA cleavage products in the presence of SAM (Fig. 1d, Supplementary Figs. 6-9) was consistent with our predicted secondary structure model (Fig. 1c). More than 20 linkages
(Fig. 1c) exhibit increased strand scission in the concentration of 100 µM SAM, indicating that these nucleotides are structurally exposed during the SAM-binding induced reorganization.
Conversely, no structural modulation was evident upon the introduction of Met and _S_-adenosyl-L-homocysteine (SAH) up to a concentration of 1 mM, revealing great molecular discrimination of
SAM-I_Xcc_. In-line probing using a range of SAM concentrations (Supplementary Fig. 9) suggests a dissociation constant (_K_D) in one-to-one binding of ~2 µM for the 200-nt SAM-I_Xcc_ (Fig.
1e), which is an order of magnitude weaker than that of the 251-nt _yitJ_ SAM-I RNA (_K_D ~200 nM)14. Since modulation could only be seen in the in-line probing performed at a higher
temperature (37 °C) than that generally used (22 °C) (Supplementary Fig. 5), it is reasonable to believe that the low binding affinity of SAM-I_Xcc_ could be attributed to this. The SAM-I18
and SAM-IV34 riboswitches are known to possess similar SAM-binding core and can be distinguished by different architectural elements and nucleotide conservation patterns in many places34.
The overall architecture of the SAM-I_Xcc_ aptamer corresponds to SAM-I aptamer rather than SAM-IV aptamer, including a P4 hairpin in the core, a lack of an additional pseudoknot, a
kink-turn in the P2 stem and uridine residue at position 121 (Fig. 1c, Supplementary Fig. 10). Importantly, mutations of known conserved SAM-binding sites within SAM-I aptamer in SAM-I_Xcc_
resulted in the loss of SAM-responsive regulation in the SAM-I_Xcc_-_gusA_ fusion reporter strain (Supplementary Fig. 11). SAM-I_XCC_ SD SEQUENCE IS SEQUESTERED IN SAM-UNBOUND STATE It is
clear that SAM-I_Xcc_ is responsive to the cellular levels of SAM and appears to play an important role in inhibiting translation (Fig. 1). The potential mechanism by which SAM-I_Xcc_
inhibits translation likely involves SAM-mediated structural rearrangements that inhibit translation initiation. The in-line probing experiments revealed structural transitions seen by
SAM-I_Xcc_ in the presence and absence of SAM (Fig. 2a; Supplementary Figs. 6-9). The SAM-I_Xcc_ model developed from the in-line probing data suggests that in the SAM-bound state, the two
important RNA elements involved in translation initiation (the SD sequence and the start codon AUG) are sequestered within the expression platform’s hairpin structure (Fig. 2a; Supplementary
Fig. 10). In the SAM-unbound state, SAM-I_Xcc_ seems to fold in an alternative structure where the start codon AUG is exposed, but the SD sequence is still sequestered by the expression
platform’s short hairpin, implying that continued translation repression may occur (Fig. 2a). We attempted to validate this mechanism by constructing several SAM-I_Xcc _mutants that carry
disruptive changes in the expression platform in GUS reporter constructs (see Methods, Fig. 2b). As expected, the inhibition by SAM was completely abolished in the translational reporter
strain carryingthe construct with 9-nucleotide changes in the anti-AUG and anti-SD sequences (M1 + M2), which in theory disrupts the sequestration hairpin and releases both the SD and AUG
(Fig. 2c). The mutant (M2) exposing the SD only exhibited about 51% GUS reporter activity (Fig. 2c), indicating partial translation inhibition in the presence of SAM and suggesting the
independent sequestration of AUG. The construct (M1) exposing the start codon AUG showed about 50% GUS reporter activity (Fig. 2c), suggesting the independent sequestration of SD. These
resultsare consistent with the predicted structural model of SAM-I_Xcc _in the SAM-bound state but inconsistent with the model in the SAM-free state. As shown in Fig. 2a, the SD sequence is
still sequestered and thus translation repression should occur in the SAM-free state. However, in absence of SAM, the GUS activities produced by the reporter strains carryingthe wild-type
SAM-I_Xcc _construct (pWT-SD−) or the mutant construct (M2) exposing the SD are very similar (Fig. 2c), suggesting that the wild-type SAM-I_Xcc_ is fully switched on and both the SD and AUG
are accessible in absence of SAM. The simplest explanation for this is that the anti-SD hairpin may not exist in the SAM-free state. However, the RNase H cleavage experiments revealed that
in absence of SAM the anti-SD hairpin exists (Fig. 3b, and Supplementary Fig. 12). These results support the predicted SAM-I_Xcc _structure model and suggest that an additional factor(s) may
contribute to the accessibility of SD when the cellular SAM is deficient. SAM-I_XCC_ EXPRESSION PLATFORM CAN BIND UNCHARGED TRNAFMET In addition to SAM-I, the T-box riboswitches found in
various Gram-positive bacteria have been shown to regulate Met biosynthesis in response to the accumulation of uncharged Met-tRNA8,35,36. The homoserine O-acetyltransferase (encoded by
_XC1251_) is connected to _Xcc _Met metabolism pathway and, by extension, to uncharged/charged Met-tRNA (Supplementary Fig. 13). Therefore, it is conceivable that the additional factor that
might contribute to SAM-I_Xcc_ SD accessibility is Met-tRNA. If SAM-I_Xcc_ was to interact with Met-tRNA, it would likely use a different mechanism given that it does not have features
including the “specifier sequence” of a typical T-box riboswitch8 (Supplementary Fig. 14). To examine whether SAM-I_Xcc_ can interact with Met-tRNA, we employed the electrophoretic mobility
shift assay (EMSA). For these experiments, SAM-I_Xcc_ and uncharged Met-tRNA were examined in vitro (see Methods, Fig. 4a-e). SAM-I_Xcc_ was incubated with the three Met-tRNAs encoded in the
genome of _Xcc_ strain 8004 (Supplementary Fig. 15), i.e., the initiator Met-tRNA (tRNAfMet, encoded by _XC4339_), the elongator Met-tRNA tRNAMet1 (encoded by _XC4335_) or the tRNAMet2
(encoded by _XC4381_), respectively. Bands that correspond to the complex between tRNAfMet-SAM-I_Xcc_ were observed but no complex between SAM-I_Xcc_ and elongator Met-tRNA tRNAMet1 or
tRNAMet2 was seen (Fig. 4c), suggesting that SAM-I_Xcc_ can selectively bind with tRNAfMet. The specificity of the interaction between SAM-I_Xcc_ and tRNAfMet was comfirmed by competitive
EMSA (Supplementary Fig. 16). In addition to the three Met-tRNAs, the genome of _Xcc_ strain 8004 was predicted to encode 51 other tRNAs29 (Supplementary Table 3), nineteen of which were
subjected to EMSA to examine whether they can interact with SAM-I_Xcc_. The result showed that none of them could bind with SAM-I_Xcc_ (Supplementary Fig. 17), further supporting the
specificity of SAM-I_Xcc_-tRNAfMet interaction. In addition, the aptamer domain and the expression platform domain of SAM-I_Xcc_ were isolated and incubated independently with tRNAfMet (see
Methods). A band indicating a complex between the expression platform domain and tRNAfMet was detected but no band was seen when the aptamer domain and tRNAfMet were incubated together (Fig.
4c). The data indicate that tRNAfMet binds directly to the expression platform of SAM-I_Xcc_. Additional assays were carried out to examine the interaction of the expression platform with
variant tRNAfMet constructs (Fig. 4a). These variants mimicked charged tRNAfMet (M3′+C), had a deletion of the 3′CCA (Mdel), changed the 3′CCA to GGU, or carried a mutation of the anticodon
(Manti) in tRNAfMet. Severely weakened binding was seen between the expression platform and the charged tRNAfMet mimic (Fig. 4d), implying that SAM-I_Xcc_ is able to discriminate against the
charged tRNAfMet and selectively binds with the uncharged tRNAfMet. Interestingly, the binding was nearly abolished by the deletion of 3′CCA but not affected while 3′CCA was changed to GGU,
indicating that the 3′CCA nucleotide sequence itself is not important but the length of the 3′-end or the overall shape of tRNAfMet is important for the binding. The mutation in anticodon
did not affect its binding with the expression platform (Fig. 4d), suggesting that the anticodon is not important for the binding. These findings reveal that the tRNA recognizion mechanism
used by the expression platform of SAM-I_Xcc_ is different from previous characterized T-box8, in which base pairing interaction between the UGG in the T-box loop and the tRNA 3′-CCA end, as
well as between the codon in the specifier loop and its corresponding anticodon in the tRNA anticodon loop is essential for the recognizion8, but is consistent with the facts that neither
T-box loop nor specifier loopis presented in the expression platform of SAM-I_Xcc_ (Supplementary Fig. 14), and that the expression platform can not bind with the elongator Met-tRNAs
(tRNAMet1and tRNAMet2) (Fig. 4c), although their 3′-CCA end and anticodon are identical to that of the initiator Met-tRNA (tRNAfMet). Furthermore, deletion of the first nucleotide “U” at the
5′-end, substitution of the 73U74A with 73A74U, mutation in the D -loop or T-loop of the tRNAfMet severely reduced its binding ability with the expression platform (Supplementary Fig. 18),
indicating that these regions are important for the binding. To test whether the anti-SD sequence is the tRNAfMet binding site, a set of assays were carried out to examine the interaction
between tRNAfMet and the SAM-I_Xcc_ expression platform carrying a selection of modifications (M2-M6) (Fig. 4b). A complete loss of the binding between the tRNAfMet and the expression
platform was observed when multiple (M2, M4) or single nucleotide substitution (M6) were introduced into the anti-SD sequence, while the structural compensatory mutations (M7-9) did not
restore the binding ability (Fig. 4e). A single nucleotide substitution (M6) in the expression platform resulted in a complete loss of its binding ability towards the tRNAfMet (Fig. 4e),
suggesting that the binding between the tRNAfMet and the SAM-I_Xcc_ expression platform is not due to a gratuitous base-pairing. These data indicate that the anti-SD stem structure is not
important for the binding whereas the anti-SD sequence appears to be. In addition, disrupting the anti-SD stem by mutating the SD (M10) did not affect the binding (Supplementary Fig. 19).
Collectively, these data support the conclusion that the anti-SD sequence is the binding site of tRNAfMet. In addition, a mutation in the anti-AUG sequence (AUGGC, position 172-176), which
contains the only 3′CCA complemary sequence UGG in the expression platform, did not affect its binding with tRNAfMet (Supplementary Fig. 18), implying that the anti-AUG sequence is not the
binding site of tRNAfMet. This data further supports the conclusion that base pairing between the 3′CCA and the UGG is not important for the recongnition. Moreover, the binding of tRNAfMet
to SAM-I_Xcc_ was not affected by SAM in vitro (Supplementary Fig. 20), suggesting that the SAM induced structural reorganization of stems P1, P4-6, and P7a of SAM-I_Xcc_ (Fig. 4a) is
unlikely related with the binding, which is also consistent with the identification of a critical binding site (C181C182) in the P7b stem. TRNAFMET-BINDING DESTABILIZES ANTI-SD STEM AND
FREES THE SD The binding of tRNAfMet to the position C181C182 of SAM-I_Xcc_ in principle should disrupt the anti-SD stem (P7b) and release the SD. In order to validate this notion, we
carried out a selection of RNase H cleavage assays (see Methods)15. To achieve this the full-length SAM-I_Xcc_ was hybridized to a short (12 nt) DNA oligo complementary to the SD region
(Fig. 3a), followed by treatment with RNase H, which specifically cleaves the RNA:DNA heteroduplex (see Methods). As shown in Fig. 3b, the SD region became more available for the DNA oligo
to anneal when the uncharged tRNAfMet was present during the RNA refolding process, regardless of the presence or absence of SAM. This result supports the structural model in Fig. 2a and
provides direct evidence that the binding of tRNAfMet to SAM-I_Xcc_ frees the SD sequence. Addition of uncharged tRNAfMet did not affect the cleavage efficiency when a DNA oligo that
complementary to another region was used (Supplementary Fig. 12), demonstrating that the effect of tRNAfMet is site-specific. TRNAFMET-BINDING DEREPRESSES SAM’S INHIBITORY EFFECT IN VIVO
Whether the binding of tRNAfMet to SAM-I_Xcc _influences the expression of the _met_ operon in vivo was further investigated. A recombinant plasmid over-expressing tRNAfMet by the BAD
promoter was introduced into _Xcc_ strain to elevate the uncharged tRNAfMet level. Northern blotting analysis revealed that the levels of tRNAMet1, tRNAfMet, and a mutated tRNAfMet (73U74A →
73A74U, i.e., M7374 in Supplementary Fig. 18) in the over-expression strains were over 20-fold higher than that expressed from the chromosomal copy alone (Supplementary Fig. 21), and the
majority of the over-expressed tRNA was uncharged (Supplementary Fig. 22). When these strains were cultured in the minimal medium supplemented with 2.5 µM SAM, a concentration that can
completely inhibit the expression of the _met_ operon (Supplementary Fig. 23), a significantly increased expression of _XC1251_, the first downstream gene of SAM-I_Xcc_ in the _met_ operon
(Fig. 5a), was confirmed in the tRNAfMet over-expression strain relative to the normal strain by Western blotting (Fig. 5b). No obvious elevation of XC1251 level was observed in the strains
over-expressing tRNAMet1 and the mutated tRNAfMet (4339 M) that lost the binding ability to SAM-I_Xcc_ (Supplementary Fig. 18). Likewise, only over-expression of tRNAfMet led to an apparent
increase of the GUS activity in the _gusA_ translational fusion reporter strain when cultured in the minimal medium supplemented with 250 µM SAM, a concentration that can completely inhibit
the GUS activity of the translational reporter strain (Supplementary Fig. 23), compared to the normal expression of tRNAfMet, the over-expression of tRNAMet1 or over-expression of mutated
tRNAfMet (Fig. 5c). These findings suggest that over-expression of tRNAfMet can partially derepress SAM’s inhibitory effect in vivo, consistent with the in vitro observation that the
tRNAfMet can independently bind to SAM-I_Xcc_, resulting in the release of the SD for translational regulation. This tRNAfMet controlled regulation via its binding to SAM-I_Xcc_ also
finishes the last piece of the puzzle in the regulation process of SAM-I_Xcc_, that is, the uncharged tRNAfMet and SAM act independently on SAM-I_Xcc_ for genetic regulation (Fig. 6).
DISCUSSION Riboswitch-mediated gene regulation is one of the most direct and active feedback regulation systems found in bacteria2,12,37. The discovery of numerous riboswitch classes has
indicated these RNA molecules play critical roles in modulating many bacterial cellular processes including metabolism and virulence2,12,37. Riboswitch gene regulation is considered rapid
and responsive to changing environmental conditions when compared to protein-mediated regulation2,12,37. In this study, we characterized the riboswitch SAM-I_Xcc_ that regulates methionine
synthesis in the Gram-negative bacterial pathogen _Xcc_. We performed several in vitro and in vivo experiments showingthat SAM-I_Xcc_ controls the _met_ operon primarily by modulation of
translation in response to cellular levels of SAM. Through a series of biochemical and genetic assays we also demonstrate that the expression platform of SAM-I_Xcc_ is endowed with a dual
sensing ability. We specifically demonstrate that besides serving as a classic SAM-I expression platform, which undergo structural change to repress gene expression upon SAM binding to the
aptamer, the expression platform of SAM-I_Xcc _also has the unique ability to sense and bind with uncharged initiator Met-tRNA, allowing the platform itself to modulate translation
initiation. As far as we know, the expression platform of SAM-I_Xcc_ is the first riboswitch expression platform validated to have sensing function. In general, riboswitches are believed to
sense and respond to a single regulatory signal which allows the cell to effectively control gene expression in response to changes in the environment2,12,37. Riboswitches are usually unable
to carry out sophisticated genetic control because of simple in structure and mode of action. However, a few unusual riboswitches have been reported making sophisticated genetic decisions
by increasing structural complexity, such as tandem arrangement of two aptamers or two different classes of riboswitches1,7,38,39. Tandem riboswitches can sense two different small
molecules (e.g., SAM and AdoCbl)38 or two identical molecules (e.g., glycine, TPP, and AdoCbl)1,7,39. Similarly, some T-box riboswitches have also been identified to occur in tandem and thus
can bind two tRNA molecules40. Evidence presented here demonstrates that, unlike all known SAM-Iriboswitches, SAM-I_Xcc_, a structurally typical SAM-I riboswitch, appears to have the unique
ability to respond to two different types of signals, specifically a small molecule (SAM) and an RNA molecule (uncharged tRNAfMet) using a complex mechanism. The data presented indicates
that SAM-I_Xcc_ responds to intracellular concentrations of SAM and uncharged tRNAfMet. In order to accommodate these specific interactions, we believe that SAM-I_Xcc_ is able to switch
between four different states: ‘OFF’, ‘Partial ON 1’, ‘Partial ON 2’, and ‘ON’ (Fig. 6). It is possible that SAM-I_Xcc_ may have evolved in order to ensure Met supply in situations where the
intracellular concentrations of Met and SAM are not collinear. The observed structural and functional flexibility of SAM-I_Xcc_ could provide the bacterial cell with a survival advantage.
For example, in the situation when SAM cellular levels are high enough to stabilize an ‘OFF’ structure of SAM-I_Xcc_, but the Met level is not sufficient to maintain normal protein
synthesis, in this case, Met biosynthesis pathway can be partially activated by uncharged tRNAfMet binding directly to the expression platform (Fig. 6). Furthermore, by evaluating
intracellular Met status through the measure of two independent signals (SAM and uncharged tRNAfMet) allows SAM-I_Xcc_ more thorough sensing and modulating Met metabolism. In addition to
acting as a regulator in response to the binding of SAM to the aptamer, the expression platform of SAM-I_Xcc_ can directly and specifically recognize and bind uncharged tRNAfMet. Binding of
uncharged tRNAfMet to the expression platform of SAM-I_Xcc_ leads to sequestration of the anti-SD sequence, which frees the SD for translation initiation. The ability of SAM-I_Xcc_
expression platform to sense uncharged tRNAfMet is a previously unrecognized riboswitch trait. The only known tRNA-responsive riboswitches are the T-box family members, which share a highly
conserved T-box sequence (binding to the 3′CCA end of tRNA) and a specifier loop (binding to the anticodon of tRNA), and are restricted to Gram-positive bacteria8,11,22,41. As far as we
know, SAM-I_Xcc_ is the first RNA found to be capable of sensing tRNAs outside of T-box elements. SAM-I_Xcc_ displays no sequence and structure similarity to any known T-box riboswitches and
the anticodon of tRNAfMet is not important for SAM-I_Xcc_-tRNAfMet recognition, indicating that the tRNA recognition mechanism used by SAM-I_Xcc_ is different from that used by known T-box
riboswitches. For T-box, sequence complementary between tRNA 3′CCA end and the conserved UGG motif in the T-box loop, betweenthe anticodon of tRNA and the cognate codon in the specifier loop
of T-box, as well as the overall shape complementarity of both RNA binding partners have been shown to be essential for T-box-tRNA recognition22,41. It is possible that both sequence
complementarity and overall shape complementarity are essential for SAM-I_Xcc_-tRNAfMet recognition. Notably, unlike the other 19 aa-tRNAs, Met-tRNAs can be further divided into initiator
Met-tRNA (tRNAfMet) and elongator Met-tRNA (tRNAMet)42,43. Our data revealed that the expression platform of SAM-I_Xcc_ can selectively bind with tRNAfMet but not tRNAMet. Similarly, a
Met-RNA-specific T-box in _Staphylococcus aureus_ can also selectively bind to tRNAfMet but not tRNAMet20. How this T-box and SAM-I_Xcc_ distinguish the tRNAfMet from tRNAMet is an
attractive topic which remains to be further investigated. This work has demonstrated that the expression platform of SAM-I_Xcc_ possesses functional traits of both SAM-I and T-box
riboswitches described in Gram-positive bacteria. SAM-I_Xcc_ homologs exist and are highly conservedin sequence (more than 90% identity) and secondary structure (Supplementary Fig. 24) in
the 5′UTR of _metA_, a gene encoding the key Met biosynthesis enzyme homoserine O-acetyltransferase, in nearly all _Xanthomonas _species whose genomes have been sequenced26, indicating that
SAM-I_Xcc_ mechanism may be commonly used by _Xanthomonas_ species to control Met biosynthesis. Although there are a few single nucleotide changes in the base-pairing region of the
expression platform among different species (Supplementary Fig. 25a), it seems that these changes may not affect its function, since mutating the corresponding nucleotides in SAM-I_Xcc_ did
not affect its tRNAfMet-binding ability (Supplementary Fig. 25b). Moreover, inactivation of the _met_ operon in _Xcc_ resulted in Met auxotroph and significantly reduced virulence26,
suggesting that SAM-I_Xcc_ may be a potential target for controlling the diseases caused by _Xanthomonas_. METHODS BACTERIAL STRAINS AND PLASMIDS Bacterial strains and plasmids used in this
work are listed in Supplementary Table 1. _E. coli_ strains were grown routinely in LB medium at 37 °С. _Xcc_ strains were grown in the rich medium NYG44 or the minimal medium MMX45 at 28
°С. When required, growth media were supplemented with antibiotics at the following final concentrations: rifampicin-50 μg/ml, kanamycin-25 μg/ml, and tetracycline-5 μg/ml. PREPARATION OF
RNA MOLECULES RNA molecules were produced by in vitro transcription using the appropriate DNA templates and T7 RNA Polymerase (Roche Applied Science, Mannheim, Germany). The corresponding
DNA templates were prepared by PCR amplifying the genomic DNA of _Xcc_ strain 8004 using specific primers with the promoter sequence (TAATACGACTCACTATAGGG) for T7 RNA polymerase (T7 RNAP) at
the 5′-end (see Supplementary Table 2 for primer sequences). RNA molecules with a desired mutation were generated via primer-mediated apporach. In vitro transcription was carried out in 40
mM Tris-HCl (pH 8.0 at 23 °С), 6 mM MgCl2, 10 mM DDT and 2 mM spermidine at 37 °С for 4 h and the resulting transcripts were purified by gel extraction using an RNA gel extraction kit
(Shanghai solarbio Bioscience and Technology Co., LTD, Shanghai, China). Digoxigenin (DIG)-labeled RNAs were prepared by using DIG RNA labeling kit (Roche Applied Science, Mannheim, Germany)
according to the manufacturer’s protocols. To generate 32P-labeled RNAs, purified RNAs were dephosphorylated using alkaline phosphatase (New England Biolabs) and then 5′ radiolabeled using
[γ-32P] ATP (Applied Biosystems, USA) and T4 polynucleotide kinase (New England Biolabs), and the 5′-32P-labeled RNAs were isolated by denaturing 6% PAGE and recovered with an RNA gel
extraction kit (Shanghai solarbio Bioscience and Technology Co., LTD, Shanghai, China). IN-LINE PROBING REACTIONS Due to the high G+C content in the riboswitch, modified in-line probing
reactions (higher temperature) were conducted for 36 h at 37 °С, in addition to 22 °С, in mixtures containing 20 mM MgCl2, 100 mM KCl, and 50 mM Tris (pH 8.3 at 23 °С). For each probing
reaction, ~50 pM 5′-32P-labeled RNA was incubated with added compounds as indicated for each experiment. Partial alkaline digestion of RNA was performed by incubating ~1 nM 5′-32P-labeled
RNA (typically ~300 kcpm/μl) in a 20-μl mixture containing 50 mM Na2CO3 (pH 9.0 at 23 °С) and 1 mM EDTA at 90 °С for 5–10 min, followed by immediate cooling on ice.RNase T1 cleavage ladder
was created by incubating ~1 nM 5′-32P-labeled RNA (typically ~300 kcpm/μl) in a 20-μl mixture containing 3 M urea, 25 mM sodium citrate (pH 5.0 at 23 °С) and 2 units of RNase T1 at 55 °С
for ~15 min, followed by immediate cooling on ice. RNA cleavage products were separated by denaturing (8 M urea) 10% sequencing polyacrylamide gel (PAGE), which was dried and then visualized
using a Typhoon 9000 Phosphor Imager (GE Healthcare). The data on the gel were analyzed using ImageQuant software (Molecular Dynamics). The _K_D value for SAM-I_Xcc_ was determined by
performing in-line probing of the full-length RNA construct (aptamer + expression platform) and varying SAM concentration. Bands (R1-4 in Fig. 1d) undergoing SAM-mediated changes in
intensity were quantified, and the values were adjusted by subtracting background. The data were further normalized relative to the signal in a band that seems not undergo apparent
SAM-mediated changes. The resulting values, termed ‘fraction modulated’, were scaled from the minimum of 0 to the maximum of 1 and plotted versus the logarithm of the molar concentration of
ligand. The data were finally fit to a standard sigmoidal dose-response curve to obtain apparent _K_D value. _GUSA_ REPORTER CONSTRUCTS The experimental strategy and procedure for the
construction of SAM-I_Xcc_–_gusA_ fusion reporters were shown in Supplementary Fig. 2. The SD+-_gusA_ and SD−-_gusA_ DNA fragments were amplified by PCR using _E. coli_ k12 genomic DNA as
template46 and the primer pairs SD+-_gusA_-F/_gusA_-R and SD−-_gusA_-F/_gusA_-R (Supplementary Table 2), respectively. Wild-type SAM-I_Xcc_ DNA fragment from the _met_ operon transcription
start site to the translational start site (Supplementary Fig. 1) was amplified by PCR using _Xcc_ 8004 genomic DNA as template and the primer pairs Plac-SAM-I-F/SAM-I-R. Mutated SAM-I_Xcc
_DNA fragments were obtained by the similar PCR amplification procedure with specific primers (Supplementary Table 2). A 17-nt tag was designed in the primers to generate the overlap region
at the ends of _gusA_ and SAM-I_Xcc_ DNA fragments to allow them to be further amplified by fusion PCR47 to construct the SAM-I_Xcc_-SD+-_gusA_ and SAM-I_Xcc_-SD−-_gusA_ DNA fragments, which
were cloned into the vector pLAFR6 as an _Eco_RI-_Hin_dIII fragment to generate a transcriptional fusion (SD+) and translational fusion (SD−), respectively. GUS REPORTER ASSAYS The activity
of _β_-Glucuronidase (GUS) was determined as described by Jefferson _et al_.48. The _Xcc_ reporter strains were grown to mid-log phase (OD600 = 0.6–0.7) in 10 ml of the minimal medium MMX
with or without supplementation of SAM (to a desired final concentration) at 28 °С with shaking at 200 rpm. Cells were harvested by centrifugation for 10 min at 2.4×g and resuspended in
fresh MMX to a cell density of OD600 = 1.0. One microliter aliquot was transferred to a 1.5-ml EP tube, and cells were lysed by addition of 40 μl dimethylbenzene and vortexed vigorously for
1 min. Then 125 μl lysate was transferred to a new 1.5-ml EP tube, and 375 μl GUS reaction buffer [consisting of 50 mM sodium phosphate (pH 7.0), 10 mM 2-mercaptoethanol, 0.1% Triton X-100,
and 1 mM _ρ_-nitrophenyl-D− glucuronide] was added. Reactions occurred at 37 °С for 10 min and were terminated by addition of 200 μl of 2.5 M 2-amino-2-methylpropanediol. _ρ_-Nitrophenol
absorbance was measured at 415 nm using a UV–Vis spectrophotometer (UV-2400PC, Shimadzu, Japan). MMX medium alone was used as a blank. One unit of GUS activity was defined as 1 milligram
(mg) of _ρ_-nitrophenol released from _ρ_-nitrophenyl _β_-D-glucuronide per minute per ml of bacterial culture (cell density: OD600 = 1.0). The _ρ_-nitrophenol concentration (mg/ml) was
calculated according to the _ρ_-nitrophenol standard curve showing the absorbance at 415 nm of different concentrations (mg/ml) of _ρ_-nitrophenol. RNA GEL MOBILITY SHIFT ASSAYS DNA
templates for generating tRNAs [_XC4335_ (tRNAMet1), _XC4339_ (tRNAfMet), _XC4381_ (tRNAMet2), mutated _XC4339_ (M3′+C, Manti, Mdeland M3′CCA)] and riboswitch RNAs [SAM-I_Xcc_, the aptamer
of SAM-I_Xcc_, the expression platform of SAM-I_Xcc_, the mutated expression platforms (M2-9)] were produced by PCR amplification using _Xcc_ 8004 genomic DNA as template and the
corresponding primer pairs listed in Supplementary Table 2. DIG-labeled tRNAs and unlabeled riboswitch RNAs were prepared as described above. To examine the possible interaction between tRNA
and SAM-I_Xcc_ riboswitch (or components), the riboswitch RNA (final concentration: 10 μM) was mixed with the DIG-labeled tRNA (final concentration: 1 nM) in 20 μl of 500 mM Tris-HCl (pH
6.8) buffer for 30 min at 30 °C. Following that, the samples were electrophoresed in 10% native polyacrylamide gels at low voltage for certain time to allow the bands to be separated. The
gels were then transferred to a positively charged nylon membrane (Roche Applied Science, Mannheim, Germany), and bands were detected by using the DIG-Northern Starter Kit (Roche Applied
Science, Mannheim, Germany) and visualized with a ImageQuant LAS 500 imager (GE Healthcare). RNASE H CLEAVAGE EXPERIMENTS RNase H cleavage experiments were carried out as described
previously15 with minor modifications. DIG-labeled SAM-I_Xcc_ riboswitch RNAs and unlabeled tRNAfMet were prepared as described above. 10 μl of DIG-labeled riboswitch RNA (1 μM) was
incubated at 30 °C for 30 min in 500 mM Tris-HCl (pH 6.8) with or without the addition of unlabeled tRNAfMet (1 μM). Then 10 μl of antisense DNA oligos (100 μM) were added to each sample,
and incubated at 30 °C for 10 min. After that, 2 units of RNase H (New England Biolabs) were added to the samples to allow the digestion of DNA/RNA hybrids at 30 °C for 15 min. The products
were separated on 10% denaturing (8 M urea) polyacrylamide gel. For the following gel-processing, procedures similar to those in RNA gel mobility shift assays were used. Signals in the RNA
bands were quantified using ImageQuant Software (Molecular Dynamics). CONSTRUCTION OF MET-TRNA OVER-EXPRESSION STRAINS The _Xcc_ strains 1251-3 F/p4335, 8004/pWT-SD-/p4335, 1251-3 F/p4339,
8004/pWT-SD-/p4339, 1251-3 F/p4339M, and 8004/pWT-SD-/p4339M (Supplementary Table 1), which over-expressed the elongator Met-tRNA (tRNAMet), the initiator Met-tRNA (tRNAfMet), or the
tRNAfMet mutant (U73A74 → A73U74), were constructed by introducing the plasmid p4335, p4339, or p4339M into the _Xcc_ strains 1251-3 F and 8004 (Supplementary Table 1), respectively. The
plasmids p4335, p4339, and p4339M (Supplementary Table 1) were constructed by cloning the tRNAMet gene (_XC4335_), the tRNAfMet gene (_XC4339_), and the DNA fragment encoding the tRNAfMet
mutant (U73A74 → A73U74), which were obtained by PCR amplification using _Xcc_ 8004 genomic DNA as template and the primer sets OE4335F/OE4335R, OE4339F/OE4339R, and OE4339MF/OE4339MR
(Supplementary Table 2), into the expression vector pBBad (Supplementary Table 1), respectively. WESTERN BLOTTING _Xcc_ cells were cultivated in a desired condition, and then harvested by
centrifugation. After removing the supernatant, the cells were resuspended in PBS buffer and lysed by sonication. The cell lysates were boiled and separated using 12% Bis-Tris SDS-PAGE with
the Mini-Protean Tetra Electrophoresis System (Bio-Rad) and then electroblotted onto PVDF (0.45 μM, Merck Millipore) membrane with the Trans-Blot blotter (Bio-Rad). The membranes were
blocked with 5% Difco TM Skim Milk in TBST and probed with Anti-FLAG M2 monoclonal antibody (1:5000 dilution, Beyotime, China). Signals were detected with ahorseradish peroxidase-linked
anti-mouse secondary antibody (Beyotime, China) and SuperSignal West Pico PLUS Chemiluminescent Substrate (Thermo Fisher Scientific). Blots were imaged using the Amersham Imager 600 (GE
Healthcare). NORTHERN BLOTTING _Xcc_ strains were grown in a desired condition and harvested by centrifugation. Total RNA was isolated using the PureLink RNA Mini kit (Thermo Fisher
Scientific). Three microgram of total RNA were electrophoresed on a 6% denaturing (8 M urea) polyacrylamide gel and transferred to a positively charged nylon membrane (Roche Applied Science,
Mannheim, Germany). After UV-crosslinking, the membrane was hybridized at 68 °C for 8 h with a DIG-labeled RNA probe prepared by using a DIG RNA labeling kit (Roche Applied Science,
Mannheim, Germany). Signals were detected using the DIG-Northern Starter Kit (Roche Applied Science, Mannheim, Germany), visualized with the ImageQuant LAS 500 imager (GE Healthcare), and
quantified by using the GelQuant.NET software provided by biochemlabsolutions.com. STATISTICS AND REPRODUCIBILITY The experiments were not randomized and the investigators were not blinded
to allocation during experiments and outcome assessment. The Student’s two-tailed _t_-test was performed for comparison of means between two data points. The results presented are from a
representative experiment done in triplicate which was repeated at least three times with similar results. REPORTING SUMMARY Further information on research design is available in the Nature
Research Reporting Summary linked to this article. DATA AVAILABILITY The data that support the findings of this study are available from the corresponding authors upon request. The source
data underlying Fig. 1a, d, 2c, 3b, 4c, d, e, and 5b,c and Supplementary Figs 3, 4b, 5-9, 11, 12, 16–22, 23a, b and 25b are provided as a Source Data file. REFERENCES * Nahvi, A. et al.
Genetic control by a metabolite binding mRNA. _Chem. Biol._ 9, 1043–1049 (2002). Article CAS PubMed Google Scholar * Nudler, E. & Mironov, A. S. The riboswitch control of bacterial
metabolism. _Trends Biochem. Sci._ 29, 11–17 (2004). Article CAS PubMed Google Scholar * Vitreschak, A. G. et al. Riboswitches: the oldest mechanism for the regulation of gene
expression? _Trends Genet._ 20, 44–50 (2004). Article CAS PubMed Google Scholar * Tucker, B. J. & Breaker, R. R. Riboswitches as versatile gene control elements. _Curr. Opin. Struct.
Biol._ 15, 342–348 (2005). Article CAS PubMed Google Scholar * Batey, R. T. Structures of regulatory elements in mRNAs. _Curr. Opin. Struct. Biol._ 16, 299–306 (2006). Article CAS
PubMed Google Scholar * Mironov, A. S. et al. Sensing small molecules by nascent RNA: a mechanism to control transcription in bacteria. _Cell_ 111, 747–756 (2002). Article CAS PubMed
Google Scholar * Winkler, W. et al. Thiamine derivatives bind messenger RNAs directly to regulate bacterial gene expression. _Nature_ 419, 952–956 (2002). Article ADS CAS PubMed Google
Scholar * Gutiérrez-Preciado, A. et al. Biochemical features and functional implications of the RNA-based T-box regulatory mechanism. _Microbiol. Mol. Biol. Rev._ 73, 36–61 (2009). Article
PubMed PubMed Central CAS Google Scholar * Breaker, R. R. Prospects for riboswitch discovery and analysis. _Mol. Cell_ 43, 867–879 (2011). Article CAS PubMed PubMed Central Google
Scholar * Serganov, A. & Nudler, E. A decade of riboswitches. _Cell_ 152, 17–24 (2013). Article CAS PubMed PubMed Central Google Scholar * Henkin, T. M. The T box riboswitch: a
novel regulatory RNA that utilizes tRNA as its ligand. _Biochim. Biophys. Acta_ 959-963, 2014 (1839). Google Scholar * Sherwood, A. V. & Henkin, T. M. Riboswitch-mediated gene
regulation: novel RNA architectures dictate gene expression responses. _Annu. Rev. Microbiol._ 70, 361–374 (2016). Article CAS PubMed Google Scholar * McCown, P. J. W. et al. Riboswitch
diversity and distribution. _RNA_ 23, 995–1011 (2017). Article CAS PubMed PubMed Central Google Scholar * Winkler, W. C. et al. An mRNA structure that controls gene expression by
binding S-adenosylmethionine. _Nat. Struct. Biol._ 10, 701–707 (2003). Article CAS PubMed Google Scholar * Epshtein, V. et al. The riboswitch-mediated control of sulfur metabolism in
bacteria. _Proc. Natl Acad. Sci. USA_ 100,, 5052–5056 (2003). Article ADS CAS Google Scholar * Ferla, M. P. & Patrick, W. M. Bacterial methionine biosynthesis. _Microbiology_ 160,
1571–1584 (2014). Article CAS PubMed Google Scholar * McDaniel, B. A. et al. Transcription termination control of the S box system: direct measurement of S-adenosylmethionine by the
leader RNA. _Proc. Natl Acad. Sci. USA_ 100, 3083–3088 (2003). Article ADS CAS PubMed Google Scholar * Wang, J. X. & Breaker, R. R. Riboswitches that sense S-adenosylmethionine and
S-adenosylhomocysteine. _Biochem. Cell Biol._ 86, 157–168 (2008). Article CAS PubMed Google Scholar * André, G. et al. S-box and T-box riboswitches and antisense RNA control a sulfur
metabolic operon of _Clostridium acetobutylicum_. _Nucleic Acids Res._ 36, 5955–5969 (2008). Article PubMed PubMed Central CAS Google Scholar * Schoenfelder, S. M. et al. Methionine
biosynthesis in _Staphylococcus aureus_ is tightly controlled by a hierarchical network involving an initiator tRNA-specific T-box riboswitch. _PLoS Pathog._ 9, e1003606 (2013). Article CAS
PubMed PubMed Central Google Scholar * Grundy, F. J. & Henkin, T. M. tRNA as a positive regulator of transcription antitermination in _B_. _subtilis_. _Cell_ 74, 475–482 (1993).
Article CAS PubMed Google Scholar * Zhang, J. & Ferré-D’Amaré, A. R. Structure and mechanism of the T-box riboswitches. _Wiley Interdiscip. Rev. RNA_ 6, 419–433 (2015). Article CAS
PubMed PubMed Central Google Scholar * Weissbach, H. & Brot, N. Regulation of methionine synthesis in _Escherichia coli_. _Mol. Microbiol._ 5, 1593–1597 (1991). Article CAS PubMed
Google Scholar * Leyn, S. A. et al. Comparative genomics of transcriptional regulation of methionine metabolism in proteobacteria. _PLoS ONE_ 9, e113714 (2014). Article ADS PubMed
PubMed Central CAS Google Scholar * Vitreschak, A. G. et al. Comparative genomic analysis of T-box regulatory systems in bacteria. _RNA_ 14, 717–735 (2008). Article CAS PubMed PubMed
Central Google Scholar * Zhang, J. L. et al. The Gram-negative phytopathogen _Xanthomonas campestris_ pv. _campestris_ employs a 5′UTR as a feedback controller to regulate methionine
biosynthesis. _Microbiology_ 164, 1146–1155 (2018). Article CAS PubMed PubMed Central Google Scholar * Mansfield, J. et al. Top 10 plant pathogenic bacteria in molecular plant
pathology. _Mol. Plant Pathol._ 13, 614–629 (2012). Article PubMed PubMed Central Google Scholar * Barrick, J. E. & Breaker, R. R. The distributions, mechanisms, and structures of
metabolite-binding riboswitches. _Genome Biol._ 8, R239 (2007). Article PubMed PubMed Central CAS Google Scholar * Qian, W. et al. Comparative and functional genomic analyses of the
pathogenicity of phytopathogen _Xanthomonas campestris_ pv. _campestris_. _Genome Res._ 15, 757–767 (2005). Article CAS PubMed PubMed Central Google Scholar * Tucker, A. M. et al.
S-adenosylmethionine transport in _Rickettsia prowazekii_. _J. Bacteriol._ 185, 3031–3035 (2003). Article CAS PubMed PubMed Central Google Scholar * Binet, R. et al. Identification and
characterization of the _Chlamydia trachomatis_ L2 S-adenosylmethionine transporter. _mBio_ 2, e00051–11 (2011). Article PubMed PubMed Central CAS Google Scholar * Soukup, G. A. &
Breaker, R. R. Relationship between internucleotide linkage geometry and the stability of RNA. _RNA_ 5, 1308–1325 (1999). Article CAS PubMed PubMed Central Google Scholar * Regulski, E.
E. & Breaker, R. R. In-line probing analysis of riboswitches. _Methods Mol. Biol._ 419, 53–67 (2008). Article CAS PubMed Google Scholar * Weinberg, Z. et al. The aptamer core of
SAM-IV riboswitches mimics the ligand-binding site of SAM-I riboswitches. _RNA_ 14, 822–828 (2008). Article CAS PubMed PubMed Central Google Scholar * Grundy, F. J. & Henkin, T. M.
Conservation of a transcription antitermination mechanism in aminoacyl-tRNA synthetase and amino acid biosynthesis genes in gram-positive bacteria. _J. Mol. Biol._ 235, 798–804 (1994).
Article CAS PubMed Google Scholar * Henkin, T. M. tRNA-directed transcription antitermination. _Mol. Microbiol._ 13, 381–387 (1994). Article CAS PubMed Google Scholar * Breaker, R.
R. Riboswitches and the RNA world. _Cold Spring Harb. Perspect. Biol._ 4, a003566 (2012). Article PubMed PubMed Central CAS Google Scholar * Sudarsan, N. et al. Tandem riboswitch
architectures exhibit complex gene control functions. _Science_ 314, 300–304 (2006). Article ADS CAS PubMed Google Scholar * Nahvi, A., Barrick, J. E. & Breaker, R. R. Coenzyme B12
riboswitches are widespread genetic control elements in prokaryotes. _Nucleic Acids Res._ 32, 143–150 (2004). Article CAS PubMed PubMed Central Google Scholar * Gutierrez-Preciado, A.
et al. New insights into regulation of the tryptophan biosynthetic operon in Gram-positive bacteria. _Trends Genet._ 21, 432–436 (2005). Article CAS PubMed Google Scholar * Zhang, J.
& Ferré-D’Amaré, A. R. Co-crystal structure of a T-box riboswitch stem I domain in complex with its cognate tRNA. _Nature_ 500, 363 (2013). Article ADS CAS PubMed Google Scholar *
Dube, S. et al. Nucleotide sequence of N-formyl-methionyl-transfer RNA. _Nature_ 218, 232 (1968). Article ADS CAS PubMed Google Scholar * Samhita, L. et al. Unconventional initiator
tRNAs sustain _Escherichia coli_. _Proc. Natl Acad. Sci. USA_ 109, 13058–13063 (2012). Article ADS CAS PubMed Google Scholar * Daniels, M. et al. Cloning of genes involved in
pathogenicity of _Xanthomonas campestris_ pv. _campestris_ using the broad host range cosmid pLAFR1. _EMBO J._ 3, 3323–3328 (1984). Article CAS PubMed PubMed Central Google Scholar *
Daniels, M. J. et al. Isolation of mutants of _Xanthomonas campestris_ pv. _campestris_ showing altered pathogenicity. _J. Gen. Microbiol._ 130, 2447–2455 (1984). Google Scholar *
Platteeuw, C. et al. Use of the _Escherichia coli_ beta-glucuronidase (_gusA_) gene as a reporter gene for analyzing promoters in lactic acid bacteria. _Appl. Environ. Microbiol._ 60,
587–593 (1994). Article CAS PubMed PubMed Central Google Scholar * Kuwayama, H. et al. PCR-mediated generation of a gene disruption construct without the use of DNA ligase and plasmid
vectors. _Nucleic Acids Res._ 30, e2–e2 (2002). Article PubMed PubMed Central Google Scholar * Jefferson, R. A. et al. beta-Glucuronidase from _Escherichia coli_ as a gene-fusion marker.
_Proc. Natl Acad. Sci. USA._ 83, 8447–8451 (1986). Article ADS CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank professor Zihe Rao (Tsinghua University, Beijing,
China) for providing manual DNA sequencing advice for in-line probing analysis, professor Guoliang Xu (Shanghai Institute of Biochemistry and Cell Biology, CAS, Shanghai, China) for
offering space and equipment for in-line probing experiments, and professor Sanshu Li (School of Biomedical Science, Huaqiao University, Quanzhou, China) for helpful discussions and
constructive suggestions. This work was supported by the National Natural Science Foundation of China (31470237, 91859104, 81861138004, 21673050), the 973 Program of the Ministry of Science
and Technology of China (2015CB150600), the Ba Gui Scholar Program of Guangxi Zhuang Autonomous Region of China (2014A002), and the Outstanding Clinical Discipline Project of Shanghai Pudong
(PWYgy2018-08). AUTHOR INFORMATION Author notes * Jian-Ling Zhang Present address: School of Public Health, Zunyi Medical University, 563000, Zunyi, Guizhou, China * These authors
contributed equally: Dong-Jie Tang, Xinyu Du, Qiang Shi, Jian-Ling Zhang. AUTHORS AND AFFILIATIONS * State Key Laboratory for Conservation and Utilization of Subtropical Agro-bioresources,
College of Life Science and Technology, Guangxi University, Guangxi, China Dong-Jie Tang, Jian-Ling Zhang, Yuan-Ping He, Yan-Miao Chen, Zhenhua Ming, Dan Wang, Wan-Ying Zhong, Yu-Wei Liang,
Jin-Yang Liu & Ji-Liang Tang * Shanghai Key Laboratory of Medical Epigenetics, the International Co-laboratory of Medical Epigenetics and Metabolism, Ministry of Science and Technology,
Institutes of Biomedical Sciences, Fudan University, Shanghai, China Xinyu Du & Hongzhou Gu * Center for Medical Research and Innovation, Shanghai Pudong Hospital, Fudan University
Pudong Medical Center, Shanghai, China Xinyu Du, Jian-Ming Huang & Hongzhou Gu * Endoscopy Center, Zhongshan Hospital of Fudan University, Shanghai, China Qiang Shi, Yun-Shi Zhong &
Hongzhou Gu * National Biofilms Innovation Centre (NBIC), Biological Sciences, University of Southampton, University Road, Southampton, SO17 1BJ, UK Shi-Qi An Authors * Dong-Jie Tang View
author publications You can also search for this author inPubMed Google Scholar * Xinyu Du View author publications You can also search for this author inPubMed Google Scholar * Qiang Shi
View author publications You can also search for this author inPubMed Google Scholar * Jian-Ling Zhang View author publications You can also search for this author inPubMed Google Scholar *
Yuan-Ping He View author publications You can also search for this author inPubMed Google Scholar * Yan-Miao Chen View author publications You can also search for this author inPubMed Google
Scholar * Zhenhua Ming View author publications You can also search for this author inPubMed Google Scholar * Dan Wang View author publications You can also search for this author inPubMed
Google Scholar * Wan-Ying Zhong View author publications You can also search for this author inPubMed Google Scholar * Yu-Wei Liang View author publications You can also search for this
author inPubMed Google Scholar * Jin-Yang Liu View author publications You can also search for this author inPubMed Google Scholar * Jian-Ming Huang View author publications You can also
search for this author inPubMed Google Scholar * Yun-Shi Zhong View author publications You can also search for this author inPubMed Google Scholar * Shi-Qi An View author publications You
can also search for this author inPubMed Google Scholar * Hongzhou Gu View author publications You can also search for this author inPubMed Google Scholar * Ji-Liang Tang View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.-L.T., H.G., and D.-J.T. designed the experiments. H.G. and D.-J.T. constructed the structure model
of SAM-I_Xcc_. D.W. and W.-Y.Z. constructed some -_gusA_ reporters and performed some GUS assay experiments. H.G., X.D., Q.S., J.H., and Y.Z. performed in-line probing analysis and
interpreted the data. Y.-P.H. performed Western blotting analysis. J.-Y.L. performed Northern blotting analysis. Z.M., Y.-M.C., and Y.-W.L. contributed to -_gusA_ reporter construction and
GUS assays. D.-J.T. and J.-L.Z. performed all other experiments, including RNA preparations, generation ofSAM-I_Xcc_ and tRNAfMet mutations, electrophoretic mobility shift assays, -_gusA_
reporter constructions and GUS assays. S.-Q.A. interpreted the data and revised the manuscript. J.-L.T., H.G., and D.-J.T. wrote the manuscript. CORRESPONDING AUTHORS Correspondence to
Hongzhou Gu or Ji-Liang Tang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PEER REVIEW INFORMATION _Nature Communications_
thanks the anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available. PUBLISHER’S NOTE Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION PEER REVIEW FILE REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS
AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in
any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Tang, DJ., Du, X.,
Shi, Q. _et al._ A SAM-I riboswitch with the ability to sense and respond to uncharged initiator tRNA. _Nat Commun_ 11, 2794 (2020). https://doi.org/10.1038/s41467-020-16417-z Download
citation * Received: 02 June 2019 * Accepted: 29 April 2020 * Published: 03 June 2020 * DOI: https://doi.org/10.1038/s41467-020-16417-z SHARE THIS ARTICLE Anyone you share the following link
with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative




![[withdrawn] po18 8ex, mr john wells and mrs susie wells: environmental permit application advertisement](https://www.gov.uk/assets/static/govuk-opengraph-image-03837e1cec82f217cf32514635a13c879b8c400ae3b1c207c5744411658c7635.png)




