- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT An important adaptation during colonization of land by plants is gravitropic growth of roots, which enabled roots to reach water and nutrients, and firmly anchor plants in the
ground. Here we provide insights into the evolution of an efficient root gravitropic mechanism in the seed plants. Architectural innovation, with gravity perception constrained in the root
tips along with a shootward transport route for the phytohormone auxin, appeared only upon the emergence of seed plants. Interspecies complementation and protein domain swapping revealed
functional innovations within the PIN family of auxin transporters leading to the evolution of gravitropism-specific PINs. The unique apical/shootward subcellular localization of PIN
proteins is the major evolutionary innovation that connected the anatomically separated sites of gravity perception and growth response via the mobile auxin signal. We conclude that the
crucial anatomical and functional components emerged hand-in-hand to facilitate the evolution of fast gravitropic response, which is one of the major adaptations of seed plants to dry land.
SIMILAR CONTENT BEING VIEWED BY OTHERS THE EVOLUTIONARY INNOVATION OF ROOT SUBERIN LAMELLAE CONTRIBUTED TO THE RISE OF SEED PLANTS Article 06 November 2023 SEEDLING ROOT SYSTEM ADAPTATION TO
WATER AVAILABILITY DURING MAIZE DOMESTICATION AND GLOBAL EXPANSION Article 22 May 2024 THE GENOME OF THE GLASSHOUSE PLANT NOBLE RHUBARB (_RHEUM NOBILE_) PROVIDES A WINDOW INTO ALPINE
ADAPTATION Article Open access 10 July 2023 INTRODUCTION Conquest of the land by plants marks one of the most important transition during evolution of life on Earth1,2,3,4. For plants to
thrive in this new environment, number of dramatic developmental adaptations occurred5; among them, the evolution of efficient root gravitropic response that allows roots to grow deep into
the soil. The early diverging land plants were non-vascular plants without true roots but with the root hair-like organ rhizoids, a structure, which helps plants to attach to the soil
surface as an early adaptation to the land environment6,7,8. The fossil evidence indicates that the true roots emerged in the vascular plants9, and in the flowering plants the root has
evolved into an organ to grow downwards along the gravity vector with two main purposes: anchoring in the soil and providing a source of water and nutrients for growth of the above-ground
parts of the plants10. Root gravitropism of flowering plants is well characterized and comprises three temporally and spatially distinct phases: gravity perception, transmission of the
gravitropic signal, and ultimately the growth response itself11,12,13,14. Unlike in green algae _Chara_, whose root hair-like structure rhizoids utilize the barium sulfate (BaSO4)
crystal-containing vacuoles as the gravity-perceiving organelles15, the gravity perception in flowering plant roots occurs by gravity-induced sedimentation of the dense starch-filled
amyloplasts within the specialized columella cells of the root apex. Gravity signal is further transmitted by the intercellular signal auxin with the aid of the auxin importers and exporters
from the AUX1/LAX and PIN protein families, respectively15,16,17,18,19,20. Gravity perception leads to the polarization of PIN transporters (PIN3 and PIN7) to the bottom side of columella
cells, thus driving the redirection of auxin flow downwards21,22,23. Along the lower root side, mediated by PIN2 protein, auxin is further translocated to the place of auxin response, the
elongation zone13,24,25,26,27,28,29,30. There in the root, unlike in shoots, where auxin promotes growth, auxin rapidly inhibits growth at the lower side and this asymmetry leads to the
downward root bending31,32,33,34,35. Notably, some findings suggest that, besides the major mechanism of gravity perception by the amyloplast sedimentation in the root cap, there is a
secondary, amyloplast-independent site of gravity sensing in the distal elongation zone of flowering plant roots36. Despite the profound importance of root gravitropism in plant growth and
adaption, most of the related works only focus on the flowering plants, especially the model plant _Arabidopsis thaliana_. The mechanism of root gravitropism has never been systematically
compared throughout the plant kingdom and its evolutionary origin remains unknown. Answering this fundamental question would reveal how, during plant evolutionary history, root evolved to be
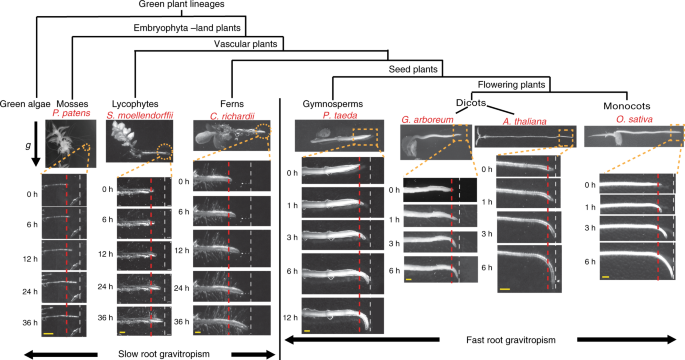
such an efficient device to respond to the Earth gravity. RESULTS SLOW AND FAST ROOT GRAVITROPISM DURING PLANT EVOLUTION To obtain a broad view of the evolutionary origin of root
gravitropism, we selected various plant species representing the lineages of mosses, lycophytes, ferns, gymnosperms, and flowering plants, including dicots and monocots, and analyzed their
root gravitropic response (Fig. 1). Mosses, including the model _Physcomitrella patens_, have rhizoids but no true roots37. After gravistimulation (90° reorientation), the rhizoid showed a
much slower gravitropism than the typical roots of flowering plants such as _A_. _thalian_a (Fig. 1 and Supplementary Fig. 1a). Lycophytes and ferns have a true root, but the model lycophyte
_Selaginella moellendorffii_ and the model fern _Ceratopteris richardii_ showed much slower gravitropism than the roots of the flowering plants _A_. _thaliana_, _Gossypium arboretum_, or
_Oryza sativa_ (Fig. 1 and Supplementary Fig. 1b, c). In contrast, the seed plant gymnosperm _Pinus taeda_ showed the fast root gravitropism comparable to that of the flowering plants and
much faster than that of the lycophyte _S_. _moellendorffii_ and the fern _C_. _richardii_ (Fig. 1). As the growth rates among these diverse plant roots are disparate (Supplementary Fig.
2a), to exclude the effect of the growth rate during the evaluation of root gravitropism, we evaluated the vertical growth index (VGI)38 on roots with the same root elongation (~2 mm) after
the gravistimulation. The results further confirmed the much slower root gravitropism of non-seed plants as compared with that of the seed plants (Supplementary Fig. 2b, c). This notable
difference in the gravitropic efficiency suggest that there are two mechanistically distinct root gravitropic responses: the slow, less efficient gravitropism of basal vascular plant species
and the fast root gravitropism, which might have originated in the most recent common ancestor of the gymnosperms and flowering plants after divergence of these seed plants from the basal
vascular plant lineages. ORIGIN OF ROOT APEX-EXCLUSIVE GRAVITY PERCEPTION To determine whether the root architectural innovation may have facilitated the fast root gravitropism in seed
plants during evolution, we analyzed the root structures of the representative plant species with a focus on localization of starch-containing amyloplasts (Fig. 2a), which act as the
statoliths for the gravity perception in the root of flowering plants39 (Supplementary Fig. 3a). Lugol’s staining for starch granule location of the rhizoids of the moss _P_. _patent_
revealed that they were devoid of amyloplasts (Fig. 2b). In the most primitive living vascular plants, the lycophyte _S_. _moellendorffii_, amyloplasts have evolved and were found in the
root but, interestingly, these starch-filled cells were distributed not within but above the root apex (Supplementary Fig. 4a). In the root of the fern _C_. _richardii_, the amyloplasts were
present both above and within the root apex (Supplementary Fig. 4b). Only in seed plant, the gymnosperm _P_. _taeda_, the amyploplasts were specifically localized within the root apex
(Supplementary Fig. 4c), which is the same as the pattern of amyloplast accumulation in the roots of the flowering plants, the dicots _A_. _thaliana_ and _G_. _arboretum_, and the monocot
_O_. _sativa_ (Supplementary Fig. 4d–f). These results suggest that the amyloplast localization specifically confined to the root apex might have originated in the common ancestor of seed
plants only after their divergence from the fern lineage. To further confirm these results, we performed the modified pseudo-Schiff-propidium iodide staining (mPS-PI) to observe detailed
root structure and starch granule localization in these representative plant species. In the lycophyte _S_. _moellendorffii_, the starch granules (amyloplasts) mainly localized at the two
lateral sides of the root above the apex and surrounded by the epidermal cells, but they were absent in both the root apex and the vascular bundle located in the middle of the root (Fig.
2c). In the root of the fern _C_. _richardii_, the localization of starch granules above the root apex was similar to that observed in _S_. _moellendorffii_, but they were also present in
the root apex below the apical cell, a single large pyramidal and quiescent center (QC)-like cell (yellow arrow in Fig. 2d). Correlating with the observation of fast root gravitropism (Fig.
1a), the starch granules in the gymnosperm _P_. _taeda_ specifically accumulated within the root apex below the QC (Fig. 2e), which is similar to the localization pattern observed in the
flowering plants _A_. _thaliana_, _G_. _arboretum_, and _O_. _sativa_ (Fig. 2f–h). In addition, we examined whether the amyloplasts in these basal vascular plant roots served as the
gravity-perceiving statoliths of the flowering plants. Notably, the amyloplast sedimentation analysis revealed that in contrast with the amyloplasts in the root cap of _A_. _thaliana_, which
were mainly located at the basal ends of the cells and showed fast sedimentation after the 180° reorientation, the amyloplasts in the roots of the fern _C_. _richardii_ and lycophyte _S_.
_moellendorffii_ showed a random localization in the root cells and failed to sediment after the 180° reorientation (Supplementary Fig. 5a–f). These results strongly indicates that, unlike
in flowering plant roots, the gravity-sensing machinery with the amyloplast sedimentation along the gravity vector did not evolve in roots of these basal vascular plants. All the results
above show that the root architectural innovation, in particular root apex-specific amyloplast localization spatially separated from the elongation zone, coincides with the advancement of
the fast root gravitropism in seed plants. It suggests that this particular arrangement of gravity perception and growth control has been selected as a strategy for efficient root
gravitropism during plant evolution. FAST ROOT GRAVITROPISM-SPECIFIC PIN2 OF _ARABIDOPSIS_ In _Arabidopsis_, the directional auxin flow from the apex to the elongation zone is driven by PIN2
auxin transporter that is localized at the shootward sides of root epidermal cells20,29,30. PIN2 plays a pivotal role in fast root gravitropism of flowering plant _Arabidopsis_, as
disruption of PIN2 blocks the gravity-induced asymmetric auxin redistribution and result in the defective root gravitropism28 (Fig. 3a and Supplementary Fig. 3b, c). There are eight _PIN_
genes in _A. thaliana_ that can be divided into three lineages based on their lengths of hydrophilic loop (HL) and subcellular localizations40,41,42: the canonical, plasma membrane
(PM)-localized PINs (PIN1, PIN2, PIN3, PIN4, and PIN7), the endoplasmic reticulum (ER)-localized PIN5 and PIN8, and PIN6 with dual PM and ER localization40,41,42. To determine which of the
PINs can mediate the fast root gravitropism, we used the _Arabidopsis PIN2_ promoter to drive the expression of the seven _PINs_ in a loss-of-function _pin2_ mutant (Fig. 3a–d). The
non-canonical _PIN6_ and _PIN5_ were not able to rescue the _pin2_ mutant (Fig. 3c, d), and also of the canonical _PINs_ only _PIN2_ was able to complement the defective root gravitropism
phenotype of _pin2_ (Fig. 3b). These results were confirmed by quantification of root gravitropism using the VGI (Supplementary Fig. 6a, b), confirming that only PIN2 can mediate fast root
gravitropism in _Arabidopsis_. EVOLUTION OF PIN2 FUNCTIONALITY IN FAST ROOT GRAVITROPISM Next, we wanted to know when this PIN2-specific function arose during plant evolution. First, to
obtain a broad view of the evolution of PIN2 during the green plant diversification, we used the full-length protein sequence of _Arabidopsis_ PIN2 as a query in searches against the
available databases for 14 species representing the green algae, the most primitive living land plant marchantiophyta (liverworts), mosses, lycophytes, ferns, gymnosperms, and flowering
plants (Supplementary Fig. 7). Then we aligned these PIN protein sequences and constructed a phylogenetic tree (Supplementary Fig. 8). According to the comprehensive PIN phylogeny by Bennett
et al.42, the PIN2 proteins were only present in the flowering plants, which is congruent with our phylogenetic tree. However, it leaves open whether there are PIN proteins in gymnosperms
that are functionally similar to the flowering plant PIN2 in root gravitropism. So to test when the PIN2-specific functionality in root gravitropism has evolved, we performed interspecies
genetic complementation experiments with _PIN_ genes from various representative plant lineages expressed in _Arabidopsis pin2_ mutant under the control of the _Arabidopsis PIN2_ promoter.
The evolutionary most primitive _PIN_ gene known to date from the basal Streptophyte green alga _Klebsormidium flaccidum_ (_KfPIN_) was unable to rescue the defects in root gravitropism in
the _pin2_ mutant (Fig. 3e), although it is a functional auxin transporter (Skokan et al., submitted). Similarly, the single canonical _PIN_ (_MpPINZ_) found in the _Marchantia
polymorpha_42,43, a probable representative of the earliest diverging land plants6, also failed to complement the defective _pin2_ root gravitropism (Fig. 3f). Representative canonical PINs
from the non-vascular plant, the moss _P_. _patens_ (i.e., _PpPINA_ and _PpPINB_ from clade 6), the basal vascular plants, the lycophyte _S_. _moellendorffii_ (i.e., _SmPINR_ and _SmPINU_
from clade 6), and the fern _C_. _richardii_ (i.e., _CrPINJ_ and _CrPINN_ from clade 7), all failed to replace the fast root gravitropism function of _Arabidopsis AtPIN2_ (Fig. 3g–i and
Supplementary Fig. 9). In the basal seed plant gymnosperm _P_. _taeda_ (Pt), we identified five _PIN_ genes, distributed in the five clades of the PIN phylogeny42, but domain prediction
clearly indicated that the PtPINF protein is not complete. Therefore, we cloned the four _PIN_ genes from the other four clades to perform the interspecies complementation experiments. In
contrast to other _PIN_ genes from the _P_. _taeda_, only two, _PtPINH_ and _PtPING_, were able to rescue the defective root gravitropism phenotype of the _Arabidopsis pin2_ mutant (Fig.
3j). The quantitative PCR analysis revealed that the two _PIN_ genes of _P_. _taeda_, _PtPING_ and _PtPINH_, were strongly expressed in the root tip as compared with shoot and the other part
of the root (Supplementary Fig. 10a–c), thus resembling the expression pattern of _PIN2_ in the flowering plant root. The auxin transport assay with 3H-labeled indoleacetic acid (3H-IAA)
showed efficient shootward auxin transport from the root tip of _P_. _taeda_ that was sensitive to the N-1-naphthylphthalamic acid (NPA), an established inhibitor of auxin transport44
(Supplementary Fig. 10d, e). The shootward auxin transport efficiency in the root of fern _C_. _richardii_ is much lower than that in the _P_. _taeda_ and largely NPA-insensitive
(Supplementary Fig. 10e). These results suggest that the efficient shootward auxin transport along with the required functional PIN proteins for fast root gravitropism have originated in the
seed plants after the divergence from the basal vascular plant lineages. Notably, in flowering plants, such as _Arabidopsis_ and _O_. _sativa_, there is only one _PIN2_ gene, whereas there
are two _PIN_ genes in gymnosperm _P_. _taeda_ and _P_. _abies_ with the functional equivalent to the _Arabidopsis PIN2_, suggesting that a duplication event of the _PING/H_ progenitor
occurred during the evolution of gymnosperms. Heterologous expression of the _PIN2_ gene from the flowering plant _G_. _arboretum_ (_GaPIN2_) in _Arabidopsis_ successfully complemented the
_Arabidopsis pin2_ mutant phenotype, indicating that this protein is functionally equivalent to _Arabidopsis AtPIN2_ (Fig. 3k–m). A recent report showed that the monocot rice _PIN2_ gene
(_OsPIN2_) also could rescue the _Arabidopsis pin2_ mutant phenotype45. These successful interspecies complementation experiments imply that the unique PING/H and PIN2 with fast gravitropic
function appeared and evolved in gymnosperm and flowering plant lineages since the separation of the seed plants from the vascular plants. ORIGIN OF APICAL PIN LOCALIZATION FOR SHOOTWARD
AUXIN FLOW We hypothesized that the shootward subcellular localization of the PIN proteins was the innovation leading to fast gravitropism. To confirm this, we analyzed the root epidermal
cell localization of a series of PIN-GFP fusion proteins driven by the _Arabidopsis_ native _PIN2_ promoter. In contrast to the _Arabidopsis_ AtPIN2-GFP fusion protein, which is
predominantly localized at the shootward side of the epidermal cells (Fig. 3n, o), the green alga KfPIN-GFP showed non-polar and also lateral localization in these cells (Fig. 3n, p). The
marchantia MpPINZ-GFP, the moss PpPINA-GFP and PpPINB-GFP, and the lycophyte SmPINR-GFP fusion proteins showed non-polar localization at the PM of the _Arabidopsis_ root epidermal cells and
occasionally aggregated granules of these PIN proteins in the cytoplasm were also observed (Fig. 3q–s and Supplementary Fig. 11). Interestingly, the PpPINA and PpPINB proteins showed obvious
polar cellular localization in the moss _P_. _patens_ rhizoid43 (Supplementary Fig. 12) but they did not acquire the specific ability to localize at the shootward side of cells. The fern
CrPINJ-GFP fusion protein showed the predominately bipolar localization in _Arabidopsis_ root epidermal cells (Fig. 3n, t). The gymnosperm PtPINI-GFP proteins showed bipolar and strong
lateral localization in the _Arabidopsis_ root epidermal cells (Fig. 3u), whereas most of the PtPINE-GFP fusion proteins showed rootward/bipolar localization (Fig. 3v). Only the gymnosperm
proteins PtPINH-GFP and PtPING-GFP were predominantly localized at the shootward side of the root epidermal cells (Fig. 3w, x), which correlates with their ability to complement the
_Arabidopsis pin2_ mutant phenotype (Fig. 3j), and efficient shootward auxin transport in the root tip of _P_. _taeda_ (Supplementary Fig. 10d, e). Moreover, the amino acid sequence
alignments revealed that the key phosphorylation sites of _Arabidopsis_ PIN2 (AtPIN2), which were identified in its central HL and critical for PIN2 shootward subcellular localization and
its function in root gravitropism46, have been evolutionarily conserved in other flowering plant PIN2 and gymnosperm PING/H (Supplementary Fig. 13). This is consistent with the shootward
localization and conserved function of these PIN2-like proteins in root gravitropism as revealed by the successful interspecies complementation experiments. Our results indicate that during
the evolution of land plants, the specific shootward cellular localization of PIN2 appeared together with the efficient shootward auxin transport and fast gravitropic response was among the
crucial innovations, which endowed PIN2 with its specific ability to mediate this process in seed plants. FUNCTIONAL INNOVATIONS OF PIN2 DURING PLANT EVOLUTION Our analysis revealed that the
shootward localized PIN protein, which mediates fast root gravitropism, evolved only in seed plants. However, it is still unclear what functional innovations at the sequence level were
important for the unique PIN2 function in fast root gravitropism. The intergenic domain swapping experiments combined with interspecies complementation experiments showed that when the
central HL of the green alga KfPIN was replaced by the HL of the _Arabidopsis_ AtPIN2 (Fig. 4a, upper panel), the hybrid PIN protein (denoted as X1) still failed to complement the
_Arabidopsis pin2_ mutant root gravitropism phenotype (Fig. 4a, middle panel). Consistent with this, the hybrid PIN (X1-GFP) also showed abnormal cellular localization (shootward/bipolar
localization) in _Arabidopsis_ root epidermal cells (Fig. 4a, lower panel), suggesting that the transmembrane domains (TMDs) of PIN also contribute to the regulation of the PIN2 polar
localization and its function in root gravitropism (Fig. 4h). However, when the central HL of MpPINZ from the primitive living land plant was replaced by the central HL of AtPIN2, this
hybrid PIN (denoted as X2) X2-GFP fusion protein was predominantly localized at the shootward side of _Arabidopsis_ root epidermal cells and was able to rescue the defective root
gravitropism phenotype of the _Arabidopsis pin2_ mutant (Fig. 4b). These results, combined with the observation of the X1-GFP protein property (Fig. 4a), strongly suggests that a functional
innovation in the TMDs of the PIN2 predecessor occurred in the common ancestor of land plants after their divergence from the green alga lineage. Moreover, when we replaced the central HL of
the lycophyte SmPINR or the fern CrPINJ with the _Arabidopsis_ AtPIN2 central HL (Fig. 4c, upper panel), both hybrid PINs (denoted as X3 and X4, respectively), similar to _marchantiophyta_
hybrid X2 also showed shootward localization in _Arabidopsis_ epidermal cells and were able to rescue the impaired gravitropism phenotype of _pin2_ (Fig. 4c, middle/lower panels). These
results suggest that after the first functional innovation of the ancestral PIN protein in the early diverging land plants, the function of the canonical PIN TMDs in root gravitropism has
been evolutionarily conserved during the evolution of the vascular plants after they split from the bryophyte lineages. We postulated that another functional innovation of PIN2 occurred in
its central HL in the most recent common ancestor of the seed plants (gymnosperms and flowering plants), because the gymnosperm PtPING-GFP and flowering plant AtPIN2-GFP fusion proteins were
able to rescue the _pin2_ mutant phenotype, and these proteins predominantly localized at the shootward side of the _Arabidopsis_ root epidermal cells (Fig. 4d), indicating that not only
functional TMDs but also a functional HL, both contributing to their polar localization and fast root gravitropism, were acquired by these PIN2 proteins. To further test our hypothesis that
the functional PIN2 central HL in root gravitropism originated in seed plants after the divergence from the fern lineage, we fused the central HL of PtPING from the gymnosperm _P_. _taeda_
with the TMDs of the fern protein CrPINJ, and investigated its function in root gravitropism (Fig. 4e, upper panel). This hybrid PIN (denoted as X5) was able to complement the defective root
gravitropism phenotype of the _Arabidopsis pin2_ mutant and also showed the shootward localization in root epidermal cells (Fig. 4e–g, middle/lower panels). These results further confirmed
that the PIN shootward localization was the crucial functional innovation enabling PIN2 to mediate fast root gravitropism in flowering plants. It also shows that this occurred through in two
steps during plant evolution: (i) early (at the onset of land plants) functional innovations in the TMD and (ii) later innovations (after the divergence of the seed plants) in the central
HL. DISCUSSION Our systematic comparison of the root bending dynamics in different species revealed at least two distinct modes of root gravitropic response in the plant kingdom: (i) slow,
rudimentary response of early diverging vascular plant lineages (lycophytes and ferns) and (ii) much more effective, faster gravitropic bending of roots from seed plants (gymnosperm and
flowering plants). This difference in the gravitropic functionality may be related to the independent evolutionary origin of roots in these plant groups47 and also correlates with the
anatomical innovations of root architecture, in particular the presence of gravity-sensing statoliths exclusively confined to the root apex, which we detected only in seed plants. As a
consequence, the apex-specific place of gravity perception in the root cap has evolved to be separated from the place of the growth response necessitating a new signaling mechanism between
these tissues (Fig. 5). This has been enabled by the evolution of a new type of PIN auxin transporter, which might be driven by the positive natural selection (Supplementary Fig. 14) and was
able to localize to the shootward side of root epidermal cells. This functional innovations occurred first at the onset of the land plants in the PIN2 TMDs and later with advancement of the
seed plants in its central HL. These sequence changes resulted in the exclusively shootward, subcellular localization of PIN2 to establish a new, shootward auxin transport flow connecting
the place of gravity perception in the root cap and growth regulation in the elongation zone (Fig. 5). Our genetic complementation experiments suggested that the PING/H in gymnosperm and the
PIN2 in flowering plants evolved the similar biological property in mediating the fast root gravitropism, but it is still unresolved whether this is resulted from the convergence evolution
of PIN2 and PING/PINH or because they originated from a shared descent. The protein-level phylogenetic analysis with PIN sequences from hundreds of representative plant species showed that
the PING/PINH and PIN2 can be grouped in the same clade42, supporting that gymnosperm PING/PINH are the co-orthologs of flowering plant PIN2 and they may have originated in the most recent
common ancestor of the seed plants. However, the nucleotide-level phylogenetic analysis of the PIN family and modular structure analysis of the HL suggested that the function of PINH/PING
and PIN2 in root gravitropism evolved by convergence in gymnosperms and flowering plants42. In the flowering plant _Arabidopsis_, besides the AtPIN2, the AtPIN3, and AtPIN7 from the PIN3
clade (Supplementary Fig. 8), which are localized at the bottom side of the gravity-sensing statocytes, are also involved in the root gravitropism. Following a gravitropic stimulus, AtPIN3
and AtPIN7 rapidly relocalize laterally within the first few minutes to facilitate the asymmetrical auxin redistribution between the upper and lower parts of the root21,22 (Fig. 5).
Interestingly, the recent protein-level phylogenetic analysis revealed that the seed plant gymnosperm PINE can be grouped in the same clade with the PIN342. Moreover, according to the
phylogenetic tree, the PINE/PIN3 clade is clearly absent in the non-seed plant species. These results are congruent with our observation that the fast root gravitropism has evolved in the
seed plants rather than in the non-seed plants, which strongly suggests that also this PIN clade (PINE/PIN3) evolved to facilitate the fast root gravitropism of the seed plants after their
divergence from the fern lineage. Notably, in some of the monocots (e.g., _O_. _sativa_ and _Z_. _mays_), the PIN3 clade is missing as well (Supplementary Fig. 8). Given that the number of
PIN family members in monocot dramatically expanded (Supplementary Fig. 7b), some of the other monocot PIN clades presumably replaced the PIN3 function in root gravitropism during the
evolution. The seed plants, which may have evolved in the late Devonian around 370 million years ago and represents a remarkable life-history transition for photosynthetic organism,
underwent dramatic evolutionary radiations and became the dominant group of vascular plants in most habitats. Compared with their predecessors, the seed plants evolved numerous
characteristics to facilitate their adaption, such as seed organs, which allowed them to break their dependence on water for reproduction and embryo development5,48,49,50. Our work
demonstrates how root anatomical innovation, combined with the evolution of _PIN_ auxin transporters, led to the evolution of the seed plant root to become a delicate and efficient organ to
mediate fast gravitropism, which might have facilitated their adaption to the new environment along with numerous other evolved traits (e.g., hydrotropism and other growth behaviors).
METHODS SEARCH FOR PIN FAMILY MEMBERS The PIN coding sequences (CDS) in the following plants were identified by using the _A_. _thaliana_ PIN2 protein sequence as query in BLAST searches
against Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html#!search?show=BLAST): _M_. _polymorpha_, _P_. _patens_, _S_. _moellendorffii_, _A_. _thaliana_, _O_. _sativa_, _and Zea mays_.
The CDS sequences of _MvPIN_ in _Mesostigma viride_ and _KfPIN_ in _K. flaccidum_ (UTEX strain #321; GenBank number: KJ466099) were obtained from the unpublished transcriptome database
provided by E. D. Cooper and C. F. Delwiche. The complementary DNA sequence of _C_. _richardii_ PIN was obtained from the transcriptome sequences of _C_. _richardii_ (Jody Banks unpublished
data). The PIN sequences of _Cystopteris fragilis_ was identified from the 1KP project database (https://db.cngb.org/onekp/). The PIN sequences of _P_. _abies_ and _P_. _taeda_ were
identified from the Spruce Genome Project database (http://congenie.org/start). The PIN CDS of _Amborella trichopoda_ was identified from the _Amborella_ database
(http://amborella.huck.psu.edu/wwwblast). The accession numbers/IDs of the identified _PIN_ genes are given in Supplementary Table 1. The accession numbers/IDs of the PIN proteins in _G_.
_arboretum_ can be found in Zhang et al.51. EVOLUTIONARY ANALYSIS _PIN_ genes were translated into protein sequences and subsequently aligned with ClustalX52. Neighbor-joining (NJ) and
maximum-parsimony (MP) phylogenetic analyses were conducted with MEGA 753. Maximum-likelihood (ML) phylogenetic analysis was conducted with PhyML v3.054. NJ analysis was performed using the
protein Poisson distances and the pairwise deletion of gap sites. The default parameters were used for MP analysis. The best-fitting substitution model for the ML analysis was selected with
the jModelTest2 program55. For each of three phylogenetic analyses, 1000 bootstrap replicates were performed to evaluate the reliability of the phylogenetic trees. PLANT MATERIALS AND GROWTH
CONDITIONS Protonemal tissue of the moss _P_. _patens_ was subcultured several times for a minimum of 7 days on cellophane-covered plates with BCD medium containing 5 mM ammonium tartrate
and 0.8% agar. Growth conditions were as follows: 24 °C in a long-day light regime, light intensity 55 µmol m−2 s−1. _K_. _flaccidum_ plants were grown on solid or in liquid M-medium56 with
no sucrose added. The growth conditions were the same as those for _P_. _patens_. Unless stated otherwise, other plant species were grown vertically in Petri dishes on 0.5× Murashige and
Skoog (MS) medium (pH 5.9) containing 1% sucrose and 0.8% agar, at 18 °C under a long-day light regime (light intensity: 250 µmol m−2 s−1). _A_. _thaliana_, _P_. _taeda_, and _S_.
_moellendorffii_ were grown at 22 °C, whereas _C_. _richardii_, _G_. _arboreum_, and _O_. _sativa_ were grown at 30 °C. The _Arabidopsis_ loss-of-function mutant _pin2_ and the starchless
mutant _pgm-1_ were previously described39,57. The VGI of _Arabidopsis_ root was measured as previously described38. For microscopic analyses of gravitropism, seedlings grown in Petri dishes
containing 0.5× MS medium were gravi-stimulated by rotating the stage 90° for the specified amount of time before imaging. Bending angles were measured by ImageJ for more than 60 seedlings
per genotype (NIH; http://rsb.info.nih.gov/ij). SHOOTWARD AUXIN TRANSPORT ASSAY The seedlings were placed on new 0.5× MS plates. Three seedlings for each plant species or treatment with
three replicates. Fifteen microliters of 3H-IAA was added into 10 mL of 0.5× MS medium with 1.25% agar to make a final 5 µM 3H-IAA and then incubated at 65 °C. Five miroliters of 3H-IAA
droplet was placed on the root apex for 6 h in the dark. N-1-Naphthylphthalamic acid (NPA; 10 µM) was used as a control. There independent experiments were carried out with a similar
significant results. VECTOR CONSTRUCTION AND COMPLEMENTATION ANALYSIS To generate plasmids for genetic complementation analysis, _PIN_ CDS from different plant species and 1.4 kb _PIN2_
promoter were separately cloned into the Gateway entry vector pDONR221 and pPONRP4P1r vector by BP reaction, and then they were fused and cloned into Gateway destination vector pB7m24GW.3 by
LR reaction. To construct the PIN-GFP fusion proteins, GFP was fused in-frame to the central HL of various PIN open reading frames by performing overlapping PCR and the PCR products were
then cloned into the Gateway vector pB7m24GW.3 containing the _Arabidopsis PIN2_ promoter as described above. The primers used to generate these constructs are detailed in Supplementary Data
1. Transgenic _Arabidopsis_ plants were generated using the floral dip method and selected on solid, half-strength MS medium containing 15 mg/mL of Basta (Glufosinate). STARCH STAINING
_Arabidopsis_ roots (7 days old) were dipped in Lugol’s staining solution (Sigma-Aldrich) for 5 min, washed with distilled water, and then observed under a differential interference contrast
microscope (Leica DMRE). The starch granules and cell walls in _Arabidopsis_ root tips (7 days old) were stained using the mPS-PI method and imaged with a confocal microscope as previously
described58. In brief, whole seedlings were fixed in 50% methanol/10% acetic acid at 4 °C for up to 24 h. The tissue was rinsed briefly with ddH2O and incubated in 1% periodic acid at room
temperature for 40 min. The tissue was then rinsed twice with ddH2O and incubated in Schiff reagent with PI (100 mM sodium metabisulphite, 0.15 N HCl, and 100 mg/mL PI) for 2 h until the
plants were visibly stained. More than three samples were transferred onto microscope slides and covered with chloral hydrate solution (4 g chloral hydrate, 1 mL glycerol, and 2 mL water).
The slides were kept overnight at room temperature, after which excess chloral hydrate was removed. The seedlings were mounted in Hoyer’s solution (30 g gum arabic, 200 g chloral hydrate, 20
g glycerol, and 50 mL water). The slides were left undisturbed for at least 3 days before observation (excitation 488 nm and emission 520–720 nm). REPORTING SUMMARY Further information on
research design is available in the Nature Research Reporting Summary linked to this article. DATA AVAILABILITY The data that support the findings of this study are available from the
corresponding author upon reasonable request. The source data underlying graphs and gels are provided as a Source Data file. REFERENCES * Rensing, S. A. Great moments in evolution: the
conquest of land by plants. _Curr. Opin. Plant. Biol._ 42, 49–54 (2018). Article CAS Google Scholar * Bowman, J. L. Walkabout on the long branches of plant evolution. _Curr. Opin. Plant.
Biol._ 16, 70–77 (2013). Article Google Scholar * Delwiche, C. F. & Cooper, E. D. The evolutionary origin of a terrestrial flora. _Curr. Biol._ 25, 899–910 (2015). Article Google
Scholar * Nishiyama, T. et al. Comparative genomics of _Physcomitrella patens_ gametophytic transcriptome and _Arabidopsis thaliana_: implication for land plant evolution. _Proc. Natl Acad.
Sci. USA_ 100, 8007–8012 (2003). Article ADS CAS Google Scholar * Langdale, J. A. & Harrison, C. J. in _Fusco AMAG. Evolving Pathways: Key Themes in Evolutionary Developmental
Biology_ p. 299–315 (Cambridge Univ. Press, Cambridge, MA, 2008). * Bowman, J. L. et al. Insights into land plant evolution garnered from the _Marchantia polymorpha_ genome. _Cell_ 171,
287–304 (2017). Article CAS Google Scholar * Delwiche, C. F., Goodman, C. A. & Chang, C. Land plant model systems branch out. _Cell_ 171, 265–266 (2017). Article CAS Google Scholar
* Xu, B. et al. Contribution of NAC transcription factors to plant adaptation to land. _Science_ 343, 1505–1508 (2014). Article ADS CAS Google Scholar * Kenrick, P. &
Strullu-Derrien, C. The origin and early evolution of roots. _Plant Physiol._ 166, 570–580 (2014). Article Google Scholar * Seago, J. L. & Fernando, D. D. Anatomical aspects of
angiosperm root evolution. _Ann. Bot._ 112, 223–238 (2013). Article CAS Google Scholar * Morita, M. T. & Tasaka, M. Gravity sensing and signaling. _Curr. Opin. Plant. Biol._ 7,
712–718 (2004). Article CAS Google Scholar * Sato, E. M. et al. New insights into root gravitropic signaling. _J. Exp. Bot._ 66, 2155–2165 (2015). Article CAS Google Scholar * Swarup,
R. et al. Root gravitropism requires lateral root cap and epidermal cells for transport and response to a mobile auxin signal. _Nat. Cell Biol._ 7, 1057–1065 (2005). Article CAS Google
Scholar * Gray, W. M. Hormonal regulation of plant growth and development. _PLoS Biol._ 2, e311 (2004). Article Google Scholar * Limbach, C., Hauslage, J., Schäfer, C. & Braun, M. How
to activate a plant gravireceptor. Early mechanisms of gravity sensing studied in characean rhizoids during parabolic flights. _Plant Physiol._ 139, 1030–1040 (2005). Article CAS Google
Scholar * Swarup, R. et al. Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the _Arabidopsis_ root apex. _Genes Dev._ 15,
2648–2653 (2001). Article CAS Google Scholar * Adamowski, M. & Friml, J. PIN-dependent auxin transport: action, regulation, and evolution. _Plant Cell_ 27, 20–32 (2015). Article CAS
Google Scholar * Bennett, M. J. et al. _Arabidopsis AUX1_ gene: a permease-like regulator of root gravitropism. _Science_ 273, 948–950 (1996). Article ADS CAS Google Scholar *
Petrášek, J. et al. PIN proteins are auxin efflux catalysts determining both quantity and direction of auxin flow in plants. _Science_ 312, 914–918 (2006). Article ADS Google Scholar *
Wiśniewska, J. et al. Polar PIN localization directs auxin flow in plants. _Science_ 312, 883 (2006). Article Google Scholar * Friml, J. et al. Lateral relocation of auxin efflux regulator
PIN3 mediates tropism in _Arabidopsis_. _Nature_ 415, 806–809 (2002). Article ADS Google Scholar * Kleine-Vehn, J. et al. Gravity-induced PIN transcytosis for polarization of auxin
fluxes in gravity-sensing root cells. _Proc. Natl_ _Acad. Sci. USA_ 107, 22344–22349 (2010). Article ADS CAS Google Scholar * Rakusová, H. et al. Termination of shoot gravitropic
responses by auxin feedback on PIN3 polarity. _Curr. Biol._ 26, 3026–3032 (2016). Article Google Scholar * Blakeslee, J. J., Peer, W. A. & Murphy, A. S. Auxin transport. _Curr. Opin.
Plant. Biol._ 8, 494–500 (2005). Article CAS Google Scholar * Péret, B. et al. AUX/LAX genes encode a family of auxin influx transporters that perform distinct functions during
_Arabidopsis_ development. _Plant Cell_ 24, 2874–2885 (2012). Article Google Scholar * Baldwin, K. L., Strohm, A. K. & Masson, P. H. Gravity sensing and signal transduction in vascular
plant primary roots. _Am. J. Bot._ 100, 126–142 (2013). Article CAS Google Scholar * Band, L. R. et al. Systems analysis of auxin transport in the _Arabidopsis_ root apex. _Plant Cell_
26, 862–875 (2014). Article CAS Google Scholar * Luschnig, C., Gaxiola, R. A., Grisafi, P. & Fink, G. R. EIR1, a root-specific protein involved in auxin transport, is required for
gravitropism in _Arabidopsis thaliana_. _Genes Dev._ 12, 2175–2187 (1998). Article CAS Google Scholar * Baster, P. et al. SCFTIR1/AFB-auxin signaling regulates PIN vacuolar trafficking
and auxin fluxes during root gravitropism. _EMBO J._ 32, 260–274 (2013). Article CAS Google Scholar * Abas, L. et al. Intracellular trafficking and proteolysis of the _Arabidopsis_
auxin-efflux facilitator PIN2 are involved in root gravitropism. _Nat. Cell Biol._ 8, 249–256 (2006). Article CAS Google Scholar * Mutte, S. K. et al. Origin and evolution of the nuclear
auxin response system. _eLife_ 7, e33399 (2018). Article Google Scholar * Lincoln, C., Britton, J. H. & Estelle, M. Growth and development of the _axr1_ mutants of _Arabidopsis_.
_Plant Cell_ 2, 1071–1080 (1990). CAS PubMed PubMed Central Google Scholar * Fendrych, M. et al. Rapid and reversible root growth inhibition by TIR1 auxin signaling. _Nat. Plants_ 4,
453–459 (2018). Article CAS Google Scholar * Fendrych, M., Leung, J. & Friml, J. TIR1/AFB-Aux/IAA auxin perception mediates rapid cell wall acidification and growth of _Arabidopsis_
hypocotyls. _eLife_ 5, e19048 (2016). Article Google Scholar * Ren, H. & Gray, W. M. SAUR proteins as effectors of hormonal and environmental signals in plant growth. _Mol. Plant_ 8,
1153–1164 (2015). Article CAS Google Scholar * Chen, R., Guan, C., Boonsirichai, K. & Masson, P. H. Complex physiological and molecular processes underlying root gravitropism. _Plant
Mol. Biol._ 49, 305–317 (2002). Article CAS Google Scholar * Jones, V. A. S. & Dolan, L. The evolution of root hairs and rhizoids. _Ann. Bot._ 110, 205–212 (2012). Article CAS
Google Scholar * Grabov, A. et al. Morphometric analysis of root shape. _New Phytol._ 165, 641–652 (2005). Article CAS Google Scholar * Wolverton, C., Paya, A. M. & Toska, J. Root
cap angle and gravitropic response rate are uncoupled in the _Arabidopsis pgm-1_ mutant. _Plant Physiol._ 141, 373–382 (2011). Article CAS Google Scholar * Viaene, T., Delwiche, C. F.,
Rensing, S. A. & Friml, J. Origin and evolution of PIN auxin transporters in the green lineage. _Trends Plant. Sci._ 18, 5–10 (2013). Article CAS Google Scholar * Křeček, P. et al.
The PINFORMED (PIN) protein family of auxin transporters. _Genome Biol._ 10, 249 (2009). Article Google Scholar * Bennett, T. et al. Paralogous radiations of PIN proteins with multiple
origins of noncanonical PIN structure. _Mol. Biol. Evol._ 31, 2042–2060 (2014). Article CAS Google Scholar * Viaene, T. et al. Directional auxin transport mechanisms in early diverging
land plants. _Curr. Biol._ 24, 2786–2791 (2014). Article CAS Google Scholar * Blakeslee, J. J. et al. Interactions among PIN-FORMED and P-glycoprotein auxin transporters in _Arabidopsis_.
_Plant Cell_ 19, 131–147 (2007). Article CAS Google Scholar * Wang, L. et al. LARGE ROOT ANGLE1, encoding OsPIN2, is involved in root system architecture in rice. _J. Exp. Bot._ 69,
385–397 (2018). Article CAS Google Scholar * Dhonukshe, P. et al. Plasma membrane bound AGC3 kinases phosphorylate PIN auxin carriers at TPRXS (N/S) motifs to direct apical PIN recycling.
_Development_ 137, 3245–3255 (2010). Article CAS Google Scholar * Hetherington, A. J. & Dolan, L. Stepwise and independent origins of roots among land plants. _Nature_ 561, 235–238
(2018). Article ADS CAS Google Scholar * Linkies, A., Gräber, K., Knight, C. & Leubner-Metzger, G. The evolution of seeds. _New Phytol._ 186, 817–831 (2010). Article CAS Google
Scholar * Kenrick, P. & Crane, P. R. The origin and early evolution of plants on land. _Nature_ 389, 33–39 (1997). Article ADS CAS Google Scholar * Barrett, S. C. H. The evolution
of plant sexual diversity. _Nat. Rev. Genet._ 3, 274–284 (2002). Article CAS Google Scholar * Zhang, Y. et al. A genome-scale analysis of the PIN gene family reveals its functions in
cotton fiber development. _Front. Plant Sci._ 8, 467 (2017). Google Scholar * Thompson, J. D. et al. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment
aided by quality analysis tools. _Nucleic Acids Res._ 25, 4876–4882 (1997). Article ADS CAS Google Scholar * Kumar, S., Stecher, G. & Tamura, K. MEGA7: molecular evolutionary
genetics analysis version 7.0 for bigger datasets. _Mol. Biol. Evol._ 33, 1870–1874 (2016). Article CAS Google Scholar * Guindon, S. & Gascuel, O. A simple, fast, and accurate
algorithm to estimate large phylogenies by maximum likelihood. _Syst. Biol._ 52, 696–704 (2003). Article Google Scholar * Darriba, D., Taboada, G. L., Doallo, R. & Posada, D.
jModelTest 2: more models, new heuristics and parallel computing. _Nat. Methods_ 9, 772 (2012). Article CAS Google Scholar * Bécard, G. & Fortin, J. A. Early events of
vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. _New Phytol._ 108, 211–218 (1988). Article Google Scholar * Müller, A. et al. AtPIN2 defines a locus of
_Arabidopsis_ for root gravitropism control. _EMBO J._ 17, 6903–6911 (1998). Article Google Scholar * Truernit, E. et al. High-resolution whole-mount imaging of three-dimensional tissue
organization and gene expression enables the study of Phloem development and structure in _Arabidopsis_. _Plant Cell_ 20, 1494–1503 (2008). Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS We thank J. Banks for providing _C_. _richardii_ spores and PIN sequences, and valuable comments on the manuscript; K. Wang for seeds of _Gossypium arboreum_ and _O.
sativa_. We also thank E. Medvecká for the KfPIN-GFP fusion protein construct; E. D. Cooper and C. F. Delwiche for the MvPIN sequence and the KfPIN. The research leading to these results has
received funding from the European Research Council under the European Union’s Horizon 2020 research and innovation Programme (ERC grant agreement number 742985), Austrian Science Fund
(FWF, grant number I 3630-B25) and IST Fellow program. AUTHOR INFORMATION Author notes * These authors contributed equally: Yuzhou Zhang and Guanghui Xiao. AUTHORS AND AFFILIATIONS *
Institute of Science and Technology (IST) Austria, 3400, Klosterneuburg, Austria Yuzhou Zhang, Xixi Zhang & Jiří Friml * College of Life Sciences, Shaanxi Normal University, 710119,
Xi’an, China Guanghui Xiao & Xiaojuan Wang * College of Life Sciences, Northwest University, 710069, Xi’an, China Xiaojuan Wang Authors * Yuzhou Zhang View author publications You can
also search for this author inPubMed Google Scholar * Guanghui Xiao View author publications You can also search for this author inPubMed Google Scholar * Xiaojuan Wang View author
publications You can also search for this author inPubMed Google Scholar * Xixi Zhang View author publications You can also search for this author inPubMed Google Scholar * Jiří Friml View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.F. and Y.Z. conceived the research and designed the experiments. Y.Z. and X.Z. performed the
experiments. G.X. and X.W. performed the bioinformatics analysis. J.F. and Y.Z. wrote the manuscript, and G.X. and X.W. edited the manuscripts. All authors contributed to the manuscript and
discussed the results extensively. CORRESPONDING AUTHOR Correspondence to Jiří Friml. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PEER REVIEW INFORMATION: _Nature Communications_ thanks Tom Bennett and other anonymous reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports
are available. PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY INFORMATION PEER REVIEW FILE DESCRIPTION OF ADDITIONAL SUPPLEMENTARY FILES SUPPLEMENTARY DATA 1 REPORTING SUMMARY SOURCE DATA SOURCE DATA RIGHTS AND PERMISSIONS OPEN ACCESS
This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as
long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third
party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the
article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright
holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Zhang, Y., Xiao, G., Wang, X. _et
al._ Evolution of fast root gravitropism in seed plants. _Nat Commun_ 10, 3480 (2019). https://doi.org/10.1038/s41467-019-11471-8 Download citation * Received: 11 February 2019 * Accepted:
05 July 2019 * Published: 02 August 2019 * DOI: https://doi.org/10.1038/s41467-019-11471-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative