- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Despite much progress in the comprehension of the complex process of somatic cell reprogramming, many questions regarding the molecular mechanism of regulation remain to be
answered. At present, the knowledge on the negative regulation of reprogramming process is indeed poor in contrary to the identification of positive regulators. Here we report for the first
time that ubiquitin-specific protease 26 negatively regulates somatic cell-reprogramming process by stabilizing chromobox (CBX)-containing proteins CBX4 and CBX6 of polycomb-repressive
complex 1 through the removal of K48-linked polyubiquitination. Thus, accumulated CBX4 and CBX6 repress the expression of pluripotency genes, such as _Sox2_ and _Nanog_, through PRC1
complexes to ubiquitinate histone H2A at their promoters. In all, our findings have revealed an essential role for ubiquitin-specific protease 26 in cellular reprogramming through
polycomb-repressive complex 1. SIMILAR CONTENT BEING VIEWED BY OTHERS THE DEUBIQUITINASE USP9X REGULATES PRC2-MEDIATED CHROMATIN REPROGRAMMING DURING MOUSE DEVELOPMENT Article Open access 25
March 2021 A CHROMATIN-FOCUSED CRISPR SCREEN IDENTIFIES USP22 AS A BARRIER TO SOMATIC CELL REPROGRAMMING Article Open access 18 March 2025 THE NURD COMPLEX COOPERATES WITH SALL4 TO
ORCHESTRATE REPROGRAMMING Article Open access 18 May 2023 INTRODUCTION Somatic cells can be reprogrammed by the transduction of four key transcription factors (_Oct4_, _Sox2_, _Klf4_, and
_cMyc_) to give rise to induced pluripotent stem cells (iPSCs)1,2,3.With a view to utilizing iPSCs for the studies involving regenerative medicine and drug screening, many researchers have
focused on the dissection of the molecular mechanisms of cellular reprogramming4. However, this reprogramming process is inefficient and variable. Therefore, understanding other factors
particularly negative regulators of cellular reprogramming should be instrumental for developing a more reliable and accelerated process for obtaining iPSCs. In the last few years, many
epigenetic factors have been identified to play critical roles and reprogramming somatic cells into a pluripotent state5,6,7. For instance, histone methyltransferase and demethylase, such as
Wrd5, SUV39H1/2, Setdb1, Utx, Jmjd3, and Dot1L, either positively or negatively regulate the kinetics and efficiency of cellular reprogramming6,7,8,9,10. Despite theoretical role of PRC1 in
the repression of specific genes in differentiation11, the precise mechanisms that control the dynamic of the various protein subunits of the PRC1 complexes during cellular reprogramming
are poorly understood. The core proteins of PRC1 complex comprises RING1A or RING1B with one of six polycomb group RING finger (PCGF) proteins, which can bind to RING1A or RING1B within the
E3 catalytic unit of the PRC1 complex12. On the basis of the composition of various protein subunits, these PRC1 complexes are classified as PRC1.1–PRC1.6 families13. PRC1 complexes can
ubiquitinate histone 2A lysine 119 (H2AK119), repressing cell lineage-specific or pluripotency gene transcription14, 15. The canonical PRC1 variants (PRC1.2 and PRC1.4) contain PCGF2 and
PCGF4, respectively, RING1A/B, polyhomeotic (PHC) and chromobox-containing (CBX) proteins, such as CBX2, CBX4, CBX6, or CBX7, and can specifically recognize H3K27me3 on H3 histone12. The
dynamic interchange of PRC1.2 and PRC1.4 subunits modulates the balance between self-renewal and lineage commitment in embryonic stem cells (ESCs)16. In this study, we report that the
post-translational regulation of PRC1 components CBX4 and CBX6 by ubiquitination is critical for reprogramming. Importantly, our systematic investigation demonstrates that deubiquitinase
ubiquitin-specific protease 26 (USP26) acts as a potent negative regulator in the process of somatic cell reprogramming into iPSCs by stabilizing CBX4 and CBX6 through the USP26-mediated
removal of K48-linked ubiquitination. We further show that the ectopic expression of _Usp26_ blocks reprogramming by repression of pluripotency genes, such as _Sox2_ and _Nanog_, mediated
through CBX4 and CBX6 accumulation. By contrast, knockdown of _Usp26_ enhances the efficiency of reprogramming by reactivating _Sox2_ and _Nanog_ through degradation of CBX4 and CBX6. To our
knowledge, this is the first demonstration of the coordination of the USP26-CBX4/CBX6 axis in the negative regulation of somatic cell reprogramming, thus providing new insights into
molecular mechanisms by which USP26 regulates the specific components of PRC1 complexes. RESULTS FUNCTIONAL ROLE OF USP26 IN CELLULAR REPROGRAMMING Recent studies show that the ubiquitin
(Ub)–proteasome system regulates stem cell pluripotency and cellular reprogramming by ubiquitination-mediated degradation of key pluripotency factors17, but the function and mechanisms of
many USP proteins in cellular reprogramming remain poorly understood. To define the potential functions of the USP family proteins in cellular reprogramming, we screened USP family members,
using short hairpin RNA (shRNA) knockdown, based on their ability to increase or inhibit the reprogramming efficiency of mouse embryonic fibroblast (MEF) into iPSCs. For this purpose, shRNAs
for 48 mouse _Usp_ family members or reference shRNA from Dharmacon mouse shRNA library (with a GFP expression cassette in the vector) were selected based on their ability to knockdown
their corresponding genes. We used at least two individual shRNAs of each gene with high knockdown efficiency (>70%) for screening. Dox-inducible Oct4-Sox2-Klf4-cMyc (OSKM) transgenic
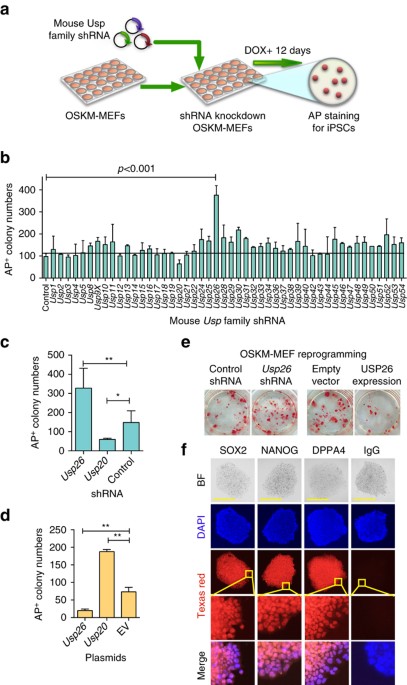
MEFs were transduced with individual lentiviral shRNAs specific for USP gene. The reprogramming efficiency was determined by alkaline phosphatase (AP) staining after doxycycline (Dox)
induction for 12 days (Fig. 1a). AP staining showed that knockdown of _Usp26_ in MEFs (Supplementary Fig. 1a) generated ~400 iPSC colonies, which was approximately fourfold higher than MEFs
transduced with control shRNA (Fig. 1b, c). Conversely, iPSC colony numbers decreased upon _Usp20_ knockdown (Fig. 1c). The knockdown of other Usp family member proteins, such as USP30 and
USP52, was also found to exert effects on reprogramming efficiency, albeit at much lesser extent (less than twofold). In contrast, the overexpression of _Usp26_ decreased the number of AP+
colonies (Fig. 1d, e), while overexpression of _Usp20_ increased the number of AP+ colonies (Fig. 1d). The previous study has shown that Usp20 increases the expression of HIF1A18, 19, which
is reported as a metabolic switch for an early stage of iPSC20. _Usp26_ shRNA transduction efficiency was characterized by shRNA GFP expression in iPSC colonies (Supplementary Fig. 1d) and
by quantitative polymerase chain reaction (qPCR) of _Usp26_ (Supplementary Fig. 1a). The reprogramming efficiency of USP26 was also evaluated by NANOG immunofluorescent staining
(Supplementary Fig. 1b, c). The role of USP26 in somatic cell reprogramming was further confirmed by the development of teratomas, containing several tissues, such as adipose tissue, neural
rosette-like tissue, and gut-like epithelial tissue (Supplementary Fig. 1e) and by protein immunostaining of pluripotency markers, such as OCT4, NANOG, and DPPA4 (Fig. 1f). Cumulatively, our
data suggest that USP26 plays an important role in cellular reprogramming into a pluripotent state. USP26 EXPRESSION PROMOTES ESC DIFFERENTIATION To study the function of USP26 in
pluripotency, we compared mRNA and protein levels of USP26 in MEFs, ESCs, and iPSCs. _Usp26_ mRNA and protein levels in MEFs were higher than those in iPSCs or ESCs (Fig. 2a, b). As
expected, _Nanog_, a known pluripotency gene, increased dramatically during Dox-induced OSKM MEF reprogramming and decreased upon removal of leukemia inhibitory factor (LIF) during retinoic
acid (RA)-induced ESC differentiation (Fig. 2c, d). However, _Usp26_ mRNA gradually decreased during Dox-induced OSKM-mediated MEF reprogramming and increased during ESC differentiation
(Fig. 2c, d). Similarly, USP26 protein levels increased from day 0 to day 6 upon addition of RA (Fig. 2e). Furthermore, infection of ESCs with GFP-tagged mouse (m) USP26 led to ESC
differentiation in an RA-independent manner (Fig. 2f). To further understand the role of USP26 in ES cells, we generated _Usp26_ knockout ESCs using a CRISPR/Cas9 technology. Since the
expression level of USP26 is low in ESCs, we tested the knockout efficiency of _Usp26_ using MEFs (Supplementary Fig. 2c, d). Wild-type (WT) ESCs began differentiating within 2 days after RA
stimulation, whereas _Usp26_ knockout ESCs maintained ESC morphology even after incubation with RA for 6 days (Fig. 2g) and began differentiating on day 8 (Supplementary Fig. 2a). However,
protein levels of pluripotency and Polycomb markers, such as NANOG and CBX7, in _Usp26_ knockout cells, remain similar during a 6-day RA induction of ESCs (Supplementary Fig. 2b). These
results suggest that USP26 expression promotes ESC differentiation. USP26 INHIBITS EXPRESSION OF PLURIPOTENCY CORE GENES To study the effects of _Usp26_ knockdown on gene expression during
MEF reprogramming, we performed qPCR and measured the expression levels of 90 genes, which have been reported to be involved in reprogramming (Supplementary Data 1). Our results showed that
after _Usp26_ knockdown during Dox-induced OSKM-mediated MEF reprogramming led to increased mRNA levels of _Sox2_ and _Nanog_, but not _Oct4_, compared with control (Fig. 3a). Overexpression
of _Usp26_ inhibited _Sox2_ and _Nanog_ gene expression during Dox-induced OSKM-mediated MEF reprogramming (Fig. 3b). These data suggest that the expression of _Sox2_ and _Nanog_ gene is
regulated by _Usp26_ during Dox-induced OSKM-mediated MEF reprogramming. Overexpression of _Usp26_ in ESCs led to decreased mRNA levels of _Sox2_, _Nanog_, and _Oct4_ compared with control
cells (Fig. 3c). This inhibition of pluripotent gene expression began as early as 2 days after induction of ESC differentiation without RA. These results suggest that USP26 regulates the
expression of pluripotency genes during cellular reprogramming to a pluripotent state and ESC differentiation. We also found significant decrease in the gene expression of _Cbx7_, a Polycomb
protein, upon _Usp26_-induced ESC differentiation (Fig. 3c). Upon Dox-induced OSKM-mediated MEF reprogramming, _Cbx7_ gene expression increased when _Usp26_ was knocked down (Fig. 3a) and
decreased when _Usp26_ was overexpressed (Fig. 3b). In addition, based on qPCR analysis (Supplementary Table 1), _Cbx7_, _Nanog_, and _So_ _x2_ were identified as the most significantly
changed genes in MEFs during reprogramming with _Usp26_ knockdown. Overall, these data suggest that the PRC1 components are potential targets of USP26. USP26 BINDS TO CBX4 AND CBX6 IN
RING1A-INDEPENDENT MANNER To determine whether USP26 targets subunits of the PRC1 complexes, we performed immunoprecipitation (IP) experiments to screen for protein–protein interactions.
Hemagglutinin (HA)-tagged PRC1 components, including RING1A, RING1B, RYBP, PCGF1, PCGF2, BM1, CBX4, CBX6, CBX7, and CBX2, were transfected into 293T cells along with FLAG-tagged human (h)
USP26. Components of the PRC1.2 complex including RING1A, PCGF2, CBX4, CBX6, and CBX7 interacted with USP26, but components of other PRC1 families, such as RING1B (all PRC1s), RYBP (all
PRC1s), PCGF1 (PRC1.1), BMI1 (PRC1.4; Fig. 4a) as well as KDM2B (PRC1.1) and PHC1 (PRC1.4; Supplementary Fig. 3a), did not interact with USP26. Components of the PRC2 complex EZH1, EED, and
EZH2 were also tested, but no interactions were observed (Supplementary Fig. 3a). Since the undifferentiated ESCs expressed CBX7 but no USP26, endogenous interactions between USP26 and CBX4
or CBX6, but not CBX7, were only observed in differentiating ESCs (Fig. 4b). To determine whether USP26 interacts with the PRC1 components RING1A, CBX4, and CBX6 independent of each other,
we generated RING1A, CBX4, or CBX6 knockout 293T cells using CRISPR/Cas9 technology (Supplementary Fig. 3c, d). IP experiments showed binding of FLAG-USP26 and CBX4 or CBX6 in RING1A KO 293T
cells (Fig. 4c), suggesting that USP26 and CBX4 or CBX6 interactions are RING1A-independent. However, in CBX4 or CBX6 knockout 293T cells, USP26 did not interact with RING1A, suggesting
that USP26 and RING1A interactions are dependent on the presence of CBX4 or CBX6 (Fig. 4c). These protein–protein interactions in RING1A KO, CBX4 KO, or CBX6 KO 293T cells were further
demonstrated using HA-tagged PRC1 proteins and FLAG-tagged USP26 (Supplementary Fig. 3b, e, f). Taken together, these results suggest that USP26 physically binds to components of the PRC1
complex, including RING1A, CBX4, and CBX6, and the interaction between USP26 and CBX4 or CBX6 is independent of the presence of RING1A. USP26 STABILIZES CBX4 AND CBX6 To further identify
molecular mechanisms of how USP26 regulates CBX4 and CBX6, we measured _Cbx4_ and _Cbx6_ mRNA and protein levels in MEFs with or without _Usp26_ overexpression. Although _Usp26_ ectopic
expression in MEFs did not change _Cbx4_ or _Cbx6_ mRNA levels (Fig. 5a and Supplementary Fig. 4a), we found that _Usp26_ expression resulted in increased CBX4 and CBX6 protein levels (Fig.
5b and Supplementary Fig. 4b), thus suggesting that _Usp26_ regulates CBX4 and CBX6 in a post-transcriptional manner. Furthermore, with increasing expression of Dox-inducible USP26 in 293T
cells (Supplementary Fig. 4c) and in ES cells (Fig. 5c and Supplementary Fig. 4d), protein levels of CBX4 and CBX6 were also increased. Additionally, CBX4 and CBX6 protein levels were also
significantly increased upon addition of the proteasome inhibitors, MG132 or Lactacystin in 293T cells (Supplementary Fig. 4e). In MEF cells, which express USP26 endogenously, we also found
that proteasome inhibitors have less effect on CBX4 and CBX6 accumulation (Supplementary Fig. 4f), USP26 stabilize CBX4 and CBX6 by reducing proteasomal degradations. Protein modifications
by K48-linked poly-Ub chains are well-established signals for recognition and initiation of degradation by the 26S proteasome complexes. Therefore, we analyzed K6-linked, K11-linked,
K27-linked, K29-linked, K33-linked, K48-linked, and K63-linked polyubiquitination of CBX4 and CBX6 by IP (Supplementary Fig. 4g). We found that both CBX4 and CBX6 were modified by K48-linked
polyubiquitination, which could be removed by USP26 (Fig. 5d). We also generated a catalytically inactive USP26 mutant by replacing the active-site cysteine with a serine residue (C304S),
and a deletion mutant by deleting the conserved domain (Δ295-312). Neither of these USP26 mutants could remove K48-linked polyubiquitination of CBX4 or CBX6 (Fig. 5e), indicating that the
deubiquitinase activity of USP26 is required for the regulation of CBX4 or CBX6 protein stability. ACCUMULATED CBX4 AND CBX6 INHIBIT PLURIPOTENT GENES Next, we sought to determine whether
increased CBX4 and CBX6 levels affect pluripotent genes. ChIP–qPCR of the promoters of _Sox2_, _Nanog_, _Oct4_, and _Cbx7_ in _Usp26_-differentiated ESCs on day 6 revealed binding of USP26,
CBX4, CBX6, H2A-ubi1, and H3K27me3 to the _Sox2_, _Nanog_, and _Cbx7_ promoters (Fig. 6a), suggesting that promoter-binding of these components led to a decrease in pluripotency gene
expression (Fig. 3c). Similarly, during RA-induced ESC differentiation, USP26, CBX4, CBX6, H2A-ubi1, and H3K27me3 also bound to the _Sox2_, _Nanog_, and _Cbx7_ promoters (Supplementary Fig.
5a). However, only H3K27me3 occupied the promoter of _Oct4_ (Fig. 6a and Supplementary Fig. 5a). In addition, the core components of PRC1.2, RING1A, and PCGF2 were also localized to _Sox2_,
_Nanog_, and _Cbx7_ promoters on day 0 and day 6 of ESC differentiation (Supplementary Fig. 5a). This suggests that RING1A and PCGF2 heterodimers form stable complexes at the _Sox2_,
_Nanog_, and _Cbx7_ promoters during pluripotency maintenance and differentiation. Upon ESC differentiation, other components, such as USP26, CBX4, and CBX6, may bind to these core RING1A
and PCGF2 heterodimers to constitute the PRC1.2 complex, which can specifically recognize H2A-ubi1 and H3K27me3 to repress _Sox2_, _Nanog_, and _Cbx7_ gene transcription12, 21. Conversely,
during _Usp26_-induced ESC differentiation and RA-induced ESC differentiation, the _Cbx4_ and _Cbx6_ promoters bound less H2A-ubi1 and H3K27me3, which was associated with less CBX7 binding
(Fig. 6b) and less RING1A and PCGF2 binding (Supplementary Fig. 5a). Increased _Cbx4_ and _Cbx6_ mRNAs were also observed in _Usp26_-induced or RA-induced ESC differentiation (Supplementary
Fig. 5b). CBX4 was also previously reported to silence _Cbx7_, which, in turn, may relieve _Cbx4_ and _Cbx6_ gene repression22, suggesting that CBX4 and CBX6 may form an amplification loop
to permit their continuous expression during ESC differentiation. This result suggests that the increased _Usp26_ expression during ESC differentiation may lead to increased CBX4 and CBX6
protein expression not only by CBX4 and CBX6 protein stabilization but also indirectly by reversing the repression of their gene expression. We further confirmed that CBX4 and CBX6 repressed
the promoter activities of _Sox2_, _Nanog_, and _Cbx7_ using a luciferase assay (Fig. 6c). Furthermore, we analyzed _Sox2, Nanog_, and _Cbx7_ promoters in _Cbx4_ and/or _Cbx6_ knockdown ES
cells. ChIP–qPCR analyses revealed the recruitment of Usp26 and H2A-ubi1 to these promoters (Supplementary Fig. 5e), suggesting the repression of _Sox2, Nanog_, and _Cbx7_ expression by
Usp26. These results suggest that _Cbx4_ or _Cbx6_ alone is sufficient for downregulating _Sox2, Nanog_, or _Cbx7_ expression. To understand how _Usp26_ expression is regulated, we assessed
the promoter-binding activity of _Usp26_ by PRC1 subunits using the ChIP–qPCR assay during RA-induced ESC differentiation, and observed that PRC1 components PCGF2 and _Cbx7_ occupation as
well as H2A-ubi1 modifications decreased significantly in _Usp26_ promoter (Supplementary Fig. 5d), thus indicating that CBX7 containing PRC1 complex is a potential repressor of _Usp26_. To
study whether USP26 can regulate CBX7 directly or through interactions with CBX4 and CBX6, we performed luciferase assay and found that overexpression of USP26 alone could not inhibit
promoter activities of these pluripotency genes. However, when USP26 was expressed with either Cbx4 or CBX6, significantly decreased promoter activities of _Sox2, Nanog_, and _Cbx7_ were
observed (Supplementary Fig. 5g), suggesting that USP26 interacts with _Cbx4_ or Cbx6 alone or both in _Cbx7_ promoter for CBX7 suppression. Taken together, these results provide molecular
mechanisms of Usp26-mediated increased CBX4 and CBX6 protein stability for suppressing pluripotent gene expression. ECTOPIC EXPRESSION OF CBX4 AND CBX6 BLOCKS REPROGRAMMING To determine how
Polycomb proteins affect MEF reprogramming, we used shRNA to knockdown _Cbx4_, _Cbx6_, _Cbx7_, and _Ring1a_ in MEFs. After 12 days of Dox treatment, knockdown of _Cbx4_ or _Cbx6_, but not
_Cbx7_ or _Ring1a_, led to an increased number of AP+ colonies compared with the control shRNA group (Fig. 7a). These results, showing increased number of AP+ colonies after _Cbx4_ or _Cbx6_
knockdown, were similar to the increased number of AP+ colonies after _Usp26_ knockdown (Fig. 1c), suggesting that Cbx4 or Cbx6 inhibits cellular reprogramming. _Sox2_ and _Nanog_ mRNA
levels, but not _Oct4_, dramatically increased in _Cbx4_ or _Cbx6_ knockdown cells (Fig. 7b), similar to the increased _Sox2_ and _Nanog_ mRNA levels in _Usp26_ knockdown cells (Fig. 3a). To
determine the reprogramming efficiency of _Usp26_ knockdown alone, and with different combinations of _Usp26_ with _Cbx4_ and/or _Cbx6_ shRNAs, we performed similar experiments and found no
significant differences in reprogramming efficiency between _Usp26_ knockdown alone and different combined knockdown of _Usp26, Cbx4_, and _Cbx6_. Thus, our data indicate that CBX4 and
_Cbx6_ are downstream genes of _Usp26_. Furthermore, _Cbx4_ or CBX6 alone could inhibit increased reprogramming efficiency mediated by Usp26 shRNA (Supplementary Fig. 6b). We also showed
that _Cbx4_ and _Cbx6_ could promote ESC differentiation in _Usp26_ knockout cells (Supplementary Fig. 6c), similar to results obtained by overexpressing _Usp26_ (Fig. 2g). Overall, these
results indicate that USP26 regulates CBX4 and CBX6 protein abundance through its deubiquitination activity during ESC differentiation, the increased amounts of CBX4 and CBX6 inhibits _Sox2_
and _Nanog_ expression, thus blocking cellular reprogramming and reducing pluripotency. DISCUSSION The development of strategies to more efficiently reprogram somatic cells into a
pluripotent state has broader applicability for disease modeling and tissue regeneration. The epigenetic modifier PRC1 is directly involved in regulating gene expression. The components of
PRC1 complexes are more diverse than PRC2 complexes, which primarily function as H3K27-specific histone methyltransferases. PRC1 complexes mediate the monoubiquitination of histone H2A to
repress transcriptional elongation for maintaining ES pluripotency23, 24. While protein ubiquitination and the Ub proteasome system are known to be important in maintaining pluripotency17,
much less is known about the regulatory role of deubiquitination of proteins in somatic cell reprogramming. In this respect, several members of the DUB subfamily have been shown to be
involved in the maintenance of ESC pluripotency. For instance, USP family member USP28 regulates the stability of Myc protein, which is a key factor for somatic cell reprogramming25. Another
USP family member, USP22, acts as a transcriptional repressor of the _Sox2_ locus during ESC differentiation, implicating its role in adult somatic stem cell potency26. Furthermore, the USP
family member USP16 can remove Ub from H2AK119 and is required for ESC differentiation14, 27. In addition, USP7 and USP11 can regulate the ubiquitination status of PCGF2 and BMI1 in primary
human fibroblast cells, leading to derepression of _INK4A_ 28. Although these studies link USP proteins to sustain pluripotency, but the role of USPs in the regulation of the components of
PRC1 complexes during cellular reprogramming has never been clearly elucidated. Using screening of shRNA specific for the DUB subfamily USPs, we identified a novel cellular reprogramming
repressor gene _Usp26_, which blocked somatic cell reprogramming into iPSCs and promoted differentiation by stabilizing PRC1 complexes. A major component of the canonical PRC1 complexes is
CBX7, which is present within the complex while ESCs are in a pluripotent state16, 29. Our results demonstrate that USP26 can diminish the expression of the _Cbx7_ gene and inhibit cellular
reprogramming. Furthermore, the expression of CBX7 protein did not increase during ESC differentiation in contrary to the increase in CBX4 and CBX6 protein expression. Our finding is
concordant with the notion that the binding of different components of the PRC1 complex acts as a switch between maintenance of pluripotency and differentiation30. In fact, a previous report
revealed that CBX4 acted to repress the expression of _Cbx7_ 22. In line with this observation, we further demonstrated that CBX4 and CBX6 can bind to the promoter of _Cbx7_. Conversely,
lesser extent of CBX7 protein binding to the promoters of _Cbx4_ and _Cbx6_ is observed during _Usp26_-mediated ESC differentiation and RA-induced ESC differentiation. We further identified
that USP26 directly bound to CBX4 and CBX6 proteins and other components of the PRC1.2 complex, including RING1A, PCGF2, and CBX7. However, the interaction of USP26 with CBX4 and CBX6 was
independent of RING1A. In differentiated cells, USP26 removed K48-linked Ub chains from CBX4 and CBX6, thus stabilizing the proteins and preventing their degradation. Henceforth, the
accumulation of CBX4 and CBX6 proteins increased pluripotent gene (_Sox2_ and _Nanog_) and PRC1 gene (_Cbx7_) promoter occupancy and decreased promoter activity. The knockdown of _Cbx4_ or
_Cbx6_ during OSKM-induced MEF reprogramming led to increased numbers of AP+ colonies and increased pluripotent gene (_Sox2_ and _Nanog_) expression, which is consistent with the improved
reprogramming efficiency obtained with the knockdown of _Usp26_. However, _Usp26_ shRNA combined with _Cbx4_ or _Cbx6_ shRNA in OSKM-induced reprogrammed MEFs did not further increase
numbers of AP+ colonies, suggesting that USP26 regulates somatic cell reprogramming through the control of CBX4 and CBX6 protein abundance, which in turn inhibit cellular reprogramming. The
knockdown of _Usp26_ does not further increase _Oct4_ gene expression during OSKM-induced MEF reprogramming, but the ectopic expression of _Usp26_ in ESCs results in the reduction of the
expression of all three pluripotency genes, suggesting that a low level of _Oct4_ expression is sufficient to maintain ESC in a stable pluripotent state31. Correlative with the _Oct4_ gene
expression data, occupancy at the _Oct4_ promoter by USP26, CBX4, CBX6, and H2A-ubi1 was low even during USP26-mediated ESC differentiation. However, H3K27me3 levels dramatically increased
at the _Oct4_ promoter, which was similar to previously published reports32, 33. While our data show that USP26 does not directly bind to proteins of other PRC1 complex components, such as
RING1B, RYBP, PCGF1, BMI1, KDM2B, and PHC1, these complexes may still bind to the _Oct4_ promoter to induce USP26-independent _Oct4_ gene expression. Therefore, the increased H3K27me3 levels
at the _Oct4_ promoter might be due to USP26-independent recruitment of PRC2. In summary, our study identifies an important role for USP26 in cellular reprogramming by stabilizing two core
components CBX4 and CBX6 of the PRC1.2 complex. Mechanistically, the availability of the accumulated CBX4 and CBX6 facilitates their binding to the promoters of the pluripotent genes and may
lead to the observed inhibition of pluripotent gene expression as well as the switching of the different components of PRC1.2. As a broader perspective, the greater understanding of the
regulation of these transcription factors and epigenetic modifiers in cellular reprogramming is warranted for the rapid and consistent iPSC generation. METHODS MEF CELL ISOLATION Animal
experiments were performed in accordance with an approved protocol from the Institutional Animal Care and Use committee (Houston Methodist Research Institute). Kill a pregnant OSKM mouse on
13 or 14 day post coitum by cervical dislocation. Dissect out the uterine horns and separate embryos, and then dissect head and red organs, wash in phosphate-buffered saline (PBS), and
finely mince the tissue with a sterile razor blade until it becomes possible to pipette. Trypsinize each embryo with 1 ml of 0.05% trypsin/EDTA (Gibco, Invitrogen) for 15 min at 37 °C. Plate
cells from three embryos in each T175 flask for 24 h (P0). Expand P0 cells till P2 or P3, and then cells were frozen for future usage. CELL CULTURE Human embryonic kidney (HEK) 293T cells
were obtained from ATCC and maintained in Dulbecco’s modified Eagle’s medium (DMEM; Hyclone) with 10% fetal bovine serum (Valley Biomedical) and 1% antibiotic–antimycotic solution (Gibco).
ESCs were maintained in ES culture medium (DMEM supplemented with 10% Knockout serum, 2 mM L-glutamine, 100 μM non-essential amino acids (Gibco), 0.1 mM ß-mercaptoethanol, and 50 ng ml−1
LIF). For Usp26-induced differentiation, ES cells were transduced with lentiviruses expressing Dox-inducible GFP-tagged Usp26 or empty vector (Day −3). After ESCs formed colonies, 0.1 µg
ml−1 Dox was added for pre-selection (Day −1). Day 0 was defined as the day when pre-selected GFP-positive colonies were cultured in iSF1 medium with high-dose Dox (2 µg ml−1), and
individual colonies were tracked and taken pictures on days 1, 2, 3, and 4. For RA-induced differentiation, _Usp26_ KO, _Cbx4_ KO, _Cbx6_ KO, or WT ES cells were cultured in ES
differentiation medium (DMEM supplemented with 10% Knockout serum, 2 mM L-glutamine, 100 μM non-essential amino acids (Gibco), and 0.1 mM ß-mercaptoethanol) with 1 μM RA. Individual colonies
were tracked and photographed over 8 days. GENERATION AND REPROGRAMMING EFFICIENCY EVALUATION OF IPSCS Mouse iPSCs were generated from MEFs with some minor modifications2. Tet-O-inducible
OSKM MEFs were used to generate iPSCs. OSKM transgenic MEF cells were transduced with GIPZ lentivirus-based shRNAs targeting USP family members, _Cbx4, Cbx6_, or _Cbx4_, and _Cbx6_. These
cells were selected with 2 µg ml−1 puromycin for 2 days. Before reprogramming, 3 × 105 feeder cells (irradiated MEFs) per well were seeded into six-well plates previously coated with 0.1%
gelatin. Thousand puromycin-selected OSKM transgenic MEF cells were reseeded onto feeder cells in six-well plates. The next day, modified iSF1 medium (DMEM supplemented with 10% Knockout
serum, 2 mM L-glutamine, 100 μM non-essential amino acids (Gibco), 0.1 mM ß-mercaptoethanol, 50 ng ml−1 LIF, 5 μg ml−1 CHIR99021, and 2.5 μg/ml PD0325901) containing 2 µg ml−1 Dox was added
and replenished every day. The efficiency of iPSC formation was calculated based on the number of AP+ iPSC colonies. The colonies were stained for AP activity on day 12 with AP detection Red
Substrate kit (Vector). TERATOMA ASSAY iPSCs (1 × 106) were injected subcutaneously into the skin on the dorsal rear flank of five severe combined immunodeficiency (SCID) mice (6 week,
female). Four weeks after injection, mice were killed and tumors were removed and fixed in formalin for 24 h. These tumors were imbedded in paraffin, sectioned, and stained with hematoxylin
and eosin for histological analysis. IMMUNOSTAINING To fully characterize iPSCs, these cells were fixed with methanol for 20 min at −20 °C, and nonspecific receptors were blocked with 10%
normal goat serum. Cells in culture plates or chamber slides were fixed for 20 min at −20 °C with methanol, and nonspecific receptors were blocked with 10% normal goat serum. Oct4, SSEA1,
and Nanog were stained with specific antibodies (anti-Oct4, 1:500, Santa Cruz Biotechnology SC-5279; anti-Nanog, 1:500, Santa Cruz Biotechnology SC-293121; and anti-Dppa4, 1:500, R&D
AF3674; anti-SSEA1, 1:1000, Invitrogen MA1-022), followed by goat anti-mouse antibody-conjugated Texas Red (1:2000, Invitrogen T-862). Nuclei were stained with 4,6-diamidino-2-phenylindole
(DAPI; Invitrogen). Immunofluorescence staining was visualized, and cells were photographed with an Olympus 1X71S1F fluorescence microscope. QPCR AND CHIP–PCR Total RNA was isolated using an
RNeasy Mini Kit (Qiagen). Reverse transcription was performed using SuperScript III reverse transcriptase (Invitrogen). Real-time PCR (qPCR) was performed using a StepOneTM Real-Time PCR
Systems and Power SYBR® PCR Master Mix (Life Technologies). qPCR primers are listed in Supplementary Table 1. Mouse ESCs were grown to 80–90% confluence and were chemically crosslinked by
the addition of fresh formaldehyde solution (37%) to a final concentration of 1% for 10 min at room temperature. Cells were rinsed twice with cold PBS followed by addition of 2 M glycine to
stop crosslinking and were collected using a silicon scraper. Cells were lysed and sonicated to solubilize and shear crosslinked DNA, with a minor modification. Briefly, we used an
ultrasonic liquid processor (Misonix) and sonicated at an amplification of 4 for 12 × 10 s pulses (30 s pause between pulses) at 4 °C. The resulting whole-cell extract was pre-cleared with
50 μl protein A/G beads, 10 μl IgG, 10 μl 5% bovine serum albumin (BSA), and 5 μg of sheared salmon sperm DNA for each sample. After centrifugation, 20% of the supernatant was incubated
overnight at 4 °C with 30 μl of Protein A/G agarose beads and 3 μg of the appropriate antibodies, 1 μl BSA (5%), and 25 μg of sheared salmon sperm DNA. Beads were washed four times with ChIP
buffer (0.1% SDS, 1% Triton X-100, 2 mM EDTA (pH 8.0), 150 mM NaCl, and 20 mM Tris-HCl (pH 8.0)) and once with tris-EDTA (TE) containing 1 mM dithiothreitol (DTT). Bound complexes were
eluted from the beads, and crosslinking was reversed by overnight incubation at 65 °C in reverse crosslink buffer (1% SDS, 100 mM NaHCO3, 1 μg ml−1 RNase A, and 500 mM NaCl). Whole-cell
extract DNA was also treated for reverse crosslinking. Immunoprecipitated DNA and whole-cell extract DNA were purified by Zymoclean PCR purification kit (Zymo). ChIP–PCR primers are listed
in Supplementary Table 1. Antibodies used for ChIP–PCR: anti-USP26, 1:100, Abcam ab101650; anti-RING1A, 1:100, Cell Signaling 13069; anti-CBX4, 1:100, Santa Cruz Biotechnology sc-130822;
anti-CBX6, 1:100, Santa Cruz Biotechnology sc-86355; anti-CBX7, 1:100, Santa Cruz Biotechnology sc-376274; anti-H2Aui1, 1:100, Cell Signaling 8240; anti-H3K27me3, 1:200, Abcam ab6002;
anti-H3K4me3, 1:200, Abcam ab8580; anti-PCGF2, 1:100, Santa Cruz Biotechnology sc-130415. IP AND WESTERN BLOTTING For IP, whole-cell extracts were prepared after transfection or stimulation
with appropriate ligands, followed by incubation overnight with the appropriate antibodies plus protein A/G beads (Pierce). Beads were washed five times with low-salt lysis buffer, and
immunoprecipitates were eluted with 4× SDS loading buffer and resolved by SDS-PAGE. Proteins were transferred to nitrocellulose membranes (Bio-Rad) followed by further incubation with the
appropriate antibodies. Luminata Crescendo Western HRP substrate (Millipore) was used for protein detection. For endogenous IP, mouse ESC nuclear extracts (150 μl for each IP) were
immunoprecipitated with appropriate antibodies (3 μg for each IP) followed by western blotting or mass spectrometry analysis. As controls, either rabbit or mouse IgG antibodies were used.
For interaction studies, 293T cells were co-transfected with plasmids encoding various potential USP26 proteins in different combinations using the Lipofectamine 2000 (Invitrogen) method. At
48 h after transfection, cells were lysed in cell lysis buffer (50 mM Tris-HCl (pH 8.0), 150 mM NaCl, 1 mM EDTA, 1% Nonidet P-40, 2 mM MgCl2, 8 U benzonase, and 10% glycerol with protease
inhibitor mixture) for 30 min, followed by IP of cell lysates with anti-FLAG M2 antibody (1:2000, Sigma F3165). Next, immunocomplex pulled down by anti-FLAG M2 antibody was subjected to
western blotting with anti-RING1A, CBX4, CBX6, CBX7, and PCGF2 antibodies. As controls, whole-cell extracts were fractionated by SDS-PAGE, followed by immunoblotting with specific or
anti-FLAG antibodies. Antibodies used for western blotting, anti-USP26, 1:1000, Abcam ab101650; anti-RING1A, 1:1000, Cell Signaling 13069; anti-CBX4, 1:1000, Santa Cruz Biotechnology
sc-130822; anti-CBX6, 1:1000, Santa Cruz Biotechnology sc-86355; anti-CBX7, 1:1000, Santa Cruz Biotechnology sc-376274; anti-PCGF2, 1:1000, Santa Cruz Biotechnology sc-130415; anti-H2A,
1:1000, Abcam ab18255; anti-Ub, 1:1000, Santa Cruz Biotechnology sc-271289. Original blots were provided in Supplementary Fig. 7. PLASMIDS CONSTRUCTS Full-length mouse _Usp26_, _Cbx4_, and
_Cbx6_ cDNA were obtained from MEF cDNA by two-step PCR and cloned into pcDNA3.1 with HA tag sequence. A similar strategy was used to clone human USP26. The C-terminal GFP-tagged m_Usp26_,
m_Cbx4_, and m_Cbx6_ cassettes were amplified by overlapping PCR and cloned into plTet-O lentiviral vector through BP and LR reactions of Gateway cloning system (Invitrogen).
plTet-O-mUsp26-GFP, plTet-O-mCbx4-GFP, plTet-O-mCbx6-GFP, pcDNA-HA-hUSP26, and pcDNA-HA-mUsp26 were sequenced to verify the correct DNA sequence and their open reading frames. LENTIVIRUS
PRODUCTION AND TRANSDUCTION One day before transfection, HEK293T cells were seeded at 50% confluency in 15 cm dishes. Cells were transfected the next day at 80–90% confluency. For each
flask, 20 μg of plasmid containing the vector of interest, 10 μg of VSV-G, and 15 μg of Δ8.9 were transfected using calcium transfection methods. Five hours (h) after transfection, the media
was changed. Virus supernatant was harvested at 48 h post transfection, filtered with a 0.45 μm polyvinylidene fluroride (PVDF) filter (Millipore), and centrifuged at 25,000 × g for 2 h.
Lentiviral pellet was resuspended with 1 ml target cell medium. MEFs were cultured in DMEM supplemented with 10% Knockout serum, 2 mM L-_glutamine_, 100 μM non-essential amino acids (Gibco),
and ß-Mercaptoethanol, and passaged every other day at a 1:4 ratios. Cells were transduced with lentivirus via spinfection in six-well plates. One thousand cells in 2 ml of media
supplemented with 8 mg ml−1 polybrene (Sigma) were added to each well, supplemented with 10 μl lentiviral supernatant. Medium was refreshed on day 2, and cells were passaged every other day
starting on day 4 after replating. CRISPR KNOCKOUT Short guide RNAs (sgRNAs) were designed using the CRISPRtool (http://crispr.mit.edu) to minimize potential off-target effects. sgRNA
sequences and genomic primers are listed in Supplementary Table 1. Corresponding oligonucleotides were ordered (IDT) and subcloned into the LentiCRISPR plasmid (Addgene), expressing a human
codon-optimized SpCas9, guide RNA, and puromycin expression plasmid, following a previously published protocol34. Specific sgRNA lentiviruses were packaged as described in the Methods
section. One milliliter lentiviral supernatant was added into 1 × 106 293T cells or 1 × 105 MEFs in six-well plates. After 48 h, 2 or 4 µM puromycin was used for selection.
Puromycin-selected 293T or MEF cells (Fig. 4c and Supplementary Fig. 3b–e) or MEFs (Supplementary Fig. 2c, d) were expanded for western blot assay. TIDE ASSAY Genomic regions surrounding
sgRNA-targeted sites were amplified by PCR. TIDE assay35 primers are listed in Supplementary Table 1. PCR products were purified using the Zymoclean™ Gel DNA Recovery Kits (Zymo) and
sequenced. Sequencing results were analyzed with TIDE web tool (https://tide.nki.nl). DEUBIQUITINASE ACTIVITY ASSAY FLAG-USP26 (1 mM) proteins were incubated with 100 ng of poly-linked Ub
chains (K48 or K29, BIOMOL) for 3 h at 37 °C in 50 mM Tris (pH 8.0) and 1 mM DTT, separated by SDS-PAGE, and analyzed by anti-Ub immunoblotting (1:1000 dilution; Santa Cruz Biotechnology).
LUCIFERASE ASSAY 293T cells (8 × 104 cells) were transduced with reporter (pLenti-Luc-_OCT4_, _SOX2_, _NANOG_, or _CBX7_) lentiviral supernatants and then transfected with 100 ng HA-USP26,
HA-CBX4, or HA-CBX6. Cell lysate was prepared following harvesting cells 24 h after transfection, and reporter activity was measured with the Dual Luciferase Assay (Promega). STATISTICAL
ANALYSIS Significant differences between groups were assessed with two-way ANOVA test or two-tailed Student’s _t_-test. Values of _p_ < 0.05 were considered statistically significant.
DATA AVAILABILITY The data sets generated during and analyzed during the current study are available from the corresponding author on reasonable request. REFERENCES * Takahashi, K. &
Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. _Cell_ 126, 663–676 (2006). Article CAS PubMed Google Scholar *
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. _Cell_ 131, 861–872 (2007). Article CAS PubMed Google Scholar * Jaenisch, R.
& Young, R. Stem cells, the molecular circuitry of pluripotency and nuclear reprogramming. _Cell_ 132, 567–582 (2008). Article CAS PubMed PubMed Central Google Scholar * Vierbuchen,
T. & Wernig, M. Molecular roadblocks for cellular reprogramming. _Mol. Cell_ 47, 827–838 (2012). Article CAS PubMed Google Scholar * Wen, L. & Tang, F. C. Charting a map through
the cellular reprogramming landscape. _Cell Stem Cell_ 16, 215–216 (2015). Article CAS PubMed Google Scholar * Nashun, B., Hill, P. W. & Hajkova, P. Reprogramming of cell fate:
epigenetic memory and the erasure of memories past. _EMBO J._ 34, 1296–1308 (2015). Article CAS PubMed PubMed Central Google Scholar * Takahashi, K. & Yamanaka, S. A developmental
framework for induced pluripotency. _Development_ 142, 3274–3285 (2015). Article CAS PubMed Google Scholar * Zhao, W. et al. Jmjd3 inhibits reprogramming by upregulating expression of
INK4a/Arf and targeting PHF20 for ubiquitination. _Cell_ 152, 1037–1050 (2013). Article CAS PubMed PubMed Central Google Scholar * Fragola, G. et al. Cell reprogramming requires
silencing of a core subset of polycomb targets. _PLoS Genet._ 9, e1003292 (2013). Article CAS PubMed PubMed Central Google Scholar * Pereira, C. F. et al. ESCs require PRC2 to direct
the successful reprogramming of differentiated cells toward pluripotency. _Cell Stem Cell_ 6, 547–556 (2010). Article CAS PubMed Google Scholar * Margueron, R. & Reinberg, D. The
Polycomb complex PRC2 and its mark in life. _Nature_ 469, 343–349 (2011). Article ADS CAS PubMed PubMed Central Google Scholar * Blackledge, N. P. et al. Variant PRC1 complex-dependent
H2A ubiquitylation drives PRC2 recruitment and polycomb domain formation. _Cell_ 157, 1445–1459 (2014). Article CAS PubMed PubMed Central Google Scholar * Gao, Z. et al. PCGF homologs,
CBX proteins, and RYBP define functionally distinct PRC1 family complexes. _Mol. Cell_ 45, 344–356 (2012). Article CAS PubMed PubMed Central Google Scholar * Adorno, M. et al. Usp16
contributes to somatic stem-cell defects in Down’s syndrome. _Nature_ 501, 380–384 (2013). Article ADS CAS PubMed PubMed Central Google Scholar * Endoh, M. et al. Histone H2A
mono-ubiquitination is a crucial step to mediate PRC1-dependent repression of developmental genes to maintain ES cell identity. _PLoS Genet._ 8, e1002774 (2012). Article CAS PubMed PubMed
Central Google Scholar * O’Loghlen, A. et al. MicroRNA regulation of Cbx7 mediates a switch of polycomb orthologs during ESC differentiation. _Cell Stem Cell_ 10, 33–46 (2012). Article
PubMed PubMed Central Google Scholar * Buckley, S. M. et al. Regulation of pluripotency and cellular reprogramming by the ubiquitin-proteasome system. _Cell Stem Cell_ 11, 783–798 (2012).
Article CAS PubMed PubMed Central Google Scholar * Li, Z., Wang, D., Messing, E. M. & Wu, G. VHL protein-interacting deubiquitinating enzyme 2 deubiquitinates and stabilizes
HIF-1alpha. _EMBO Rep._ 6, 373–378 (2005). Article CAS PubMed PubMed Central Google Scholar * Bett, J. S. et al. The P-body component USP52/PAN2 is a novel regulator of HIF1A mRNA
stability. _Biochem. J._ 451, 185–194 (2013). Article CAS PubMed PubMed Central Google Scholar * Mathieu, J. et al. Hypoxia-inducible factors have distinct and stage-specific roles
during reprogramming of human cells to pluripotency. _Cell Stem Cell_ 14, 592–605 (2014). Article CAS PubMed PubMed Central Google Scholar * Cooper, S. et al. Targeting polycomb to
pericentric heterochromatin in embryonic stem cells reveals a role for H2AK119u1 in PRC2 recruitment. _Cell Rep._ 7, 1456–1470 (2014). Article CAS PubMed PubMed Central Google Scholar *
Luis, N. M., Morey, L., Di Croce, L. & Benitah, S. A. Polycomb in stem cells: PRC1 branches out. _Cell Stem Cell_ 11, 16–21 (2012). Article CAS PubMed Google Scholar * Stock, J. K.
et al. Ring1-mediated ubiquitination of H2A restrains poised RNA polymerase II at bivalent genes in mouse ES cells. _Nat. Cell Biol._ 9, 1428–1435 (2007). Article CAS PubMed Google
Scholar * van der Stoop, P. et al. Ubiquitin E3 ligase Ring1b/Rnf2 of polycomb repressive complex 1 contributes to stable maintenance of mouse embryonic stem cells. _PLoS ONE_ 3, e2235
(2008). Article ADS PubMed PubMed Central Google Scholar * Popov, N. et al. The ubiquitin-specific protease USP28 is required for MYC stability. _Nat. Cell Biol._ 9, 765–774 (2007).
Article CAS PubMed Google Scholar * Sussman, R. T. et al. The epigenetic modifier ubiquitin-specific protease 22 (USP22) regulates embryonic stem cell differentiation via transcriptional
repression of sex-determining region Y-box 2 (SOX2). _J. Biol. Chem._ 288, 24234–24246 (2013). Article CAS PubMed PubMed Central Google Scholar * Yang, W. et al. The histone H2A
deubiquitinase Usp16 regulates embryonic stem cell gene expression and lineage commitment. _Nat. Commun._ 5, 3818 (2014). CAS PubMed PubMed Central Google Scholar * Maertens, G. N., El
Messaoudi-Aubert, S., Elderkin, S., Hiom, K. & Peters, G. Ubiquitin-specific proteases 7 and 11 modulate Polycomb regulation of the INK4a tumour suppressor. _EMBO J._ 29, 2553–2565
(2010). Article CAS PubMed PubMed Central Google Scholar * Morey, L. et al. Nonoverlapping functions of the Polycomb group Cbx family of proteins in embryonic stem cells. _Cell Stem
Cell_ 10, 47–62 (2012). Article CAS PubMed Google Scholar * Sauvageau, M. & Sauvageau, G. Polycomb group proteins: multi-faceted regulators of somatic stem cells and cancer. _Cell
Stem Cell_ 7, 299–313 (2010). Article CAS PubMed PubMed Central Google Scholar * Karwacki-Neisius, V. et al. Reduced Oct4 expression directs a robust pluripotent state with distinct
signaling activity and increased enhancer occupancy by Oct4 and Nanog. _Cell Stem Cell_ 12, 531–545 (2013). Article CAS PubMed PubMed Central Google Scholar * Mikkelsen, T. S. et al.
Dissecting direct reprogramming through integrative genomic analysis. _Nature_ 454, 49–55 (2008). Article ADS CAS PubMed PubMed Central Google Scholar * Pinter, S. F. et al. Spreading
of X chromosome inactivation via a hierarchy of defined Polycomb stations. _Genome Res._ 22, 1864–1876 (2012). Article CAS PubMed PubMed Central Google Scholar * Ran, F. A. et al.
Genome engineering using the CRISPR-Cas9 system. _Nat. Protoc._ 8, 2281–2308 (2013). Article CAS PubMed PubMed Central Google Scholar * Brinkman, E. K., Chen, T., Amendola, M. & van
Steensel, B. Easy quantitative assessment of genome editing by sequence trace decomposition. _Nucleic Acids Res._ 42, e168–e168 (2014). Article PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS This work was in part supported by grants from the NCI, NIH (R01CA090327 and R01CA101795), Cancer Prevention and Research Institute of Texas (CPRIT),
National Natural Science Foundation of China (No. 81572766), and Dottie and Jimmy C. Adair Myelodysplastic Syndrome Research and Treatment Fund. W.L. was supported in part by Xiangya
Hospital, Xiangya School of Medicine, Central South University, China. We would like to thank Dr. Jana Burchfield for critical reading of this manuscript. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Center for Inflammation and Epigenetics, Houston Methodist Research Institute, Houston, TX, 77030, USA Bo Ning, Wei Zhao, Chen Qian, Pinghua Liu, Qingtian Li, Wenyuan Li &
Rong-Fu Wang * Key Laboratory for Stem Cells and Tissue Engineering, Ministry of Education, Sun Yat-sen University, Guangzhou, 510080, China Wei Zhao * Xiangya Hospital, Xiangya School of
Medicine, Central South University, Changsha, 410008, China Wenyuan Li * Department of Microbiology and Immunology, Weill Cornell Medical College, Cornell University, New York, NY, 10065,
USA Rong-Fu Wang Authors * Bo Ning View author publications You can also search for this author inPubMed Google Scholar * Wei Zhao View author publications You can also search for this
author inPubMed Google Scholar * Chen Qian View author publications You can also search for this author inPubMed Google Scholar * Pinghua Liu View author publications You can also search for
this author inPubMed Google Scholar * Qingtian Li View author publications You can also search for this author inPubMed Google Scholar * Wenyuan Li View author publications You can also
search for this author inPubMed Google Scholar * Rong-Fu Wang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Conceptualization: B.N. and
R.-F.W.; methodology: W.Z., C.Q., Q.L., P.L., and W.L.; writing—original draft: B.N.; writing—review and editing: R.-F.W.; funding acquisition: R.-F.W. CORRESPONDING AUTHOR Correspondence to
Rong-Fu Wang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing financial interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY INFORMATION SUPPLEMENTARY DATA 1 PEER REVIEW FILE
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Ning, B., Zhao, W., Qian, C. _et al._ USP26 functions as a negative regulator of cellular reprogramming by stabilising PRC1 complex components. _Nat Commun_ 8, 349 (2017).
https://doi.org/10.1038/s41467-017-00301-4 Download citation * Received: 27 May 2016 * Accepted: 20 June 2017 * Published: 24 August 2017 * DOI: https://doi.org/10.1038/s41467-017-00301-4
SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative