- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The target of diastolic blood pressure (DBP) remains controversial in patients with coronary artery disease (CAD). We systematically searched PubMed/Medline and the Cochrane Central
database for randomized controlled trials (RCTs) assessing the efficacy and safety of reducing DBP in CAD patients from January 1965 to July 2017. Seven placebo-controlled RCTs enrolling
34,814 CAD patients who achieved DBP <80 mmHg were included in the drug-intervention group. The average achieved blood pressures (BPs) were 126.3/75.1 and 131.5/77.8 mmHg in the
drug-intervention and placebo-control groups, respectively. Drug intervention was associated with an 11% reduction in coronary revascularization and a 31% reduction in heart failure. In the
drug-intervention group, all-cause death, myocardial infarction, angina pectoris, and stroke were reduced with marginal significance, whereas hypotension was increased by 123%. A
meta-analysis of four RCTs, in which the achieved DBP was <75 mmHg, showed that the drug intervention was associated with a 22% reduction in heart failure. These results suggest that
reducing DBP to 80 mmHg or less would significantly reduce coronary revascularization and heart failure but at the expense of causing hypotension in CAD patients. Further trials are
warranted to prove this issue. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS INTENSIVE BLOOD PRESSURE-LOWERING TREATMENT TO
PREVENT CARDIOVASCULAR EVENTS IN PATIENTS WITH DIABETES: A SYSTEMATIC REVIEW AND META-ANALYSIS Article 23 April 2025 BLOOD PRESSURE AND MORTALITY AFTER PERCUTANEOUS CORONARY INTERVENTION: A
POPULATION-BASED COHORT STUDY Article Open access 17 February 2022 EFFECT OF INTENSIVE VERSUS STANDARD BLOOD PRESSURE CONTROL ON CARDIOVASCULAR OUTCOMES IN ADULT PATIENTS WITH HYPERTENSION:
A SYSTEMATIC REVIEW AND META-ANALYSIS Article 13 February 2025 INTRODUCTION It has been well established that reducing blood pressure (BP) can prevent cardiovascular events and death. A
meta-analysis of 61 prospective observational studies showed that BP was strongly and log-linearly associated with cardiovascular mortality down to 115/75 mmHg [1]. In Japanese people, BP is
related to the cumulative incidence of myocardial infarction down to 120/70 mmHg [2]. Importantly, BP reduction is associated with a reduced risk of cardiovascular events irrespective of
the class of antihypertensive drugs used, including angiotensin converting enzyme (ACE) inhibitors, calcium-channel blockers, diuretics, and β-blockers [3]. Thus, it is suggested that BP
lowering per se is important for preventing cardiovascular events. Current Japanese guidelines for hypertension management (JSH2014) recommend a BP target of <140/90 mmHg in patients with
coronary artery disease (CAD) and <130/80 mmHg in high-risk CAD patients if tolerable [4]. However, previous American and European hypertension guidelines have recommended a target of
<140/90 mmHg for patients with CAD [5, 6] because of a J-curve phenomenon; namely, a nonlinear relationship exists between BP and adverse outcomes with higher event rates at both very-low
and very-high BP [7, 8]. An observational study enrolling CAD patients showed that a systolic BP (SBP) of less than 120 mmHg and a diastolic BP (DBP) of less than 70 mmHg were associated
with an increased risk of cardiovascular disease [9]. An additional analysis of the Systolic Blood Pressure Intervention Trial demonstrated the presence of a J curve for DBP with a nadir of
approximately 70 mmHg based on the automated office BP measurement in hypertensive patients with or without cardiovascular disease; however, there was no J curve for SBP [10]. The most
recent 2017 ACC/AHA guidelines have lowered the BP target for CAD patients from 140/90 to 130/80 mmHg [11]. The following two studies may have an impact on the lowering of the SBP target:
first, the Systolic Blood Pressure Intervention Trial using the automated office BP measurement showed that intensive SBP reduction targeting 120 mmHg significantly reduced not only the
composite of myocardial infarction, other acute coronary syndromes, stroke, heart failure, and death from cardiovascular causes but also all-cause mortality, when compared with the standard
SBP reduction targeting 140 mmHg in patients with clinical/subclinical cardiovascular diseases other than stroke and in patients at a high risk for cardiovascular disease but without
diabetes [12]. Second, a large-scale meta-analysis enrolling 66,504 CAD patients demonstrated that an SBP reduction achieving SBP ≤ 130 mmHg was associated with greater reductions in heart
failure and stroke without the increase in all-cause death and cardiovascular death, when compared with the standard SBP reduction of SBP 136–140 mmHg [13]. However, only a few consistent
data are available regarding DBP targets based on randomized clinical trials (RCTs) in patients with CAD. Accordingly, we conducted searches of previous trials and performed a systematic
review and meta-analysis assessing the efficacy and safety of reducing DBP to <80 mmHg in CAD patients. METHODS ELIGIBILITY CRITERIA This systematic review and meta-analysis was conducted
according to the PRISMA statement [14]. We conducted a systematic search of MEDLINE and the Cochrane Central Register of Controlled Trials (CENTRAL) from 1965 to July 2017 using medical
subject headings and relevant text words for CAD and those for BP or DBP. The search criteria were fairly broad to avoid missing studies through a restricted search. We also checked the
reference lists of the original studies, meta-analyses, and review articles that were identified by the electronic searches to find other eligible trials. There was no language restriction
placed on the search. This analysis included RCTs conducted among patients with CAD (excluding the acute phase of myocardial infarction or heart failure) that [1] randomized patients to
antihypertensive drug or placebo arms; [2] reported long-term survival or cardiovascular outcomes for at least 1 year; [3] enrolled at least 500 patients to avoid bias associated with small
trials; [4] attained a DBP of <80 mmHg in the drug-intervention group. Studies reporting no significant difference in DBP between the two groups were excluded. DATA EXTRACTION, SYNTHESIS,
AND ANALYSIS Long-term efficacy and safety outcomes were evaluated. The efficacy outcomes were the following: all-cause mortality, cardiovascular mortality, myocardial infarction, angina
pectoris, revascularization, stroke, and heart failure. The safety outcome evaluated was hypotension, as reported, and compared between the two groups. For data synthesis and analysis, we
used the Cochrane Review Manager software, Review Manager version 5.3 (RevMan 5.3) (Nordic Cochrane Center, Copenhagen, Denmark). ASSESSMENT OF RISK OF BIAS IN THE INCLUDED STUDIES Two
review authors (RO and EK) independently assessed trial eligibility and trial-bias risk and extracted data. Disagreements were resolved by consensus. The bias risk of the trials was assessed
using the six domains of the ‘Risk of bias’ tool according to the method described in the Cochrane Handbook ‘Assessing Risk of Bias in Included Studies’
(http://methods.cochrane.org/bias/assessing-risk-bias-included-studies. Accessed July 11, 2017). STATISTICAL ANALYSIS Risk ratios (RRs) and 95% confidence intervals (CIs) were estimated for
each trial and each outcome. Random-effects models were used to pool RRs and 95% CI for study outcomes. We used the Cochrane chi-squared test and _I_2 statistics to test the heterogeneity
across the trials [15]. A chi-square value of less than 0.05 or an _I_2 value greater than 50% was regarded as indicating high heterogeneity. Publication bias was assessed using funnel
plots. Statistical analysis was performed using RevMan 5.3. ROLE OF FUNDING SOURCE This study was not funded; hence, no funding source played any role in study design; collection, analysis,
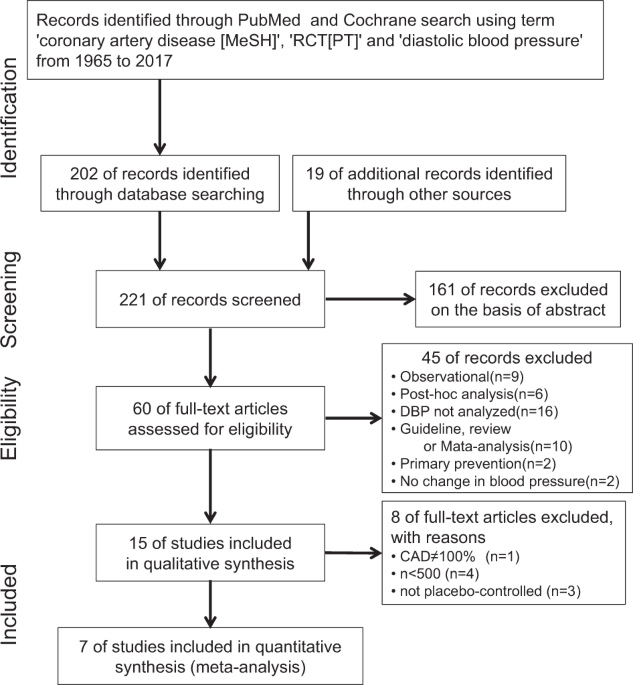
and interpretation of the data; or writing the paper. RESULTS TRIAL SELECTION AND STUDY CHARACTERISTICS We identified seven RCTs that fulfilled the inclusion criteria (Fig. 1)
[16,17,18,19,20,21,22]. The characteristics of included trials are summarized in Table 1. The drug-intervention group and the placebo-control group included 17,434 and 16,725 patients,
respectively. Achieved BPs were 126.3/75.1and 131.5/77.8 mmHg in the drug-intervention and placebo-control groups, respectively. Of note, none of the trials were designed to assign patients
to different target levels of SBP or DBP (e.g., DBP less than 80 mmHg). A summary of the risk of bias assessment of each trial is shown in Fig. 4. All seven RCTs were considered to have a
low risk of bias. EFFICACY OUTCOMES The drug-intervention group with an achieved DBP < 80 mmHg tended to have lower all-cause death (RR 0.94, 95% CI 0.86–1.02, _P_ = 0.12; Fig. 2a),
myocardial infarction (RR 0.87, 95% CI 0.74–1.03, _P_ = 0.10; Fig. 2c), angina pectoris (RR 0.88, 95% CI 0.76–1.02, _P_ = 0.08; Fig. 2d), and stroke (RR 0.87, 95% CI 0.73–1.06, _P_ = 0.16;
Fig. 2f) when compared with the placebo group, although the differences were not significant. No significant effect was found when comparing cardiovascular death in the drug-intervention
group with that in the placebo group (Fig. 2b). However, the intervention group was associated with a significant (11%) reduction in coronary revascularization (Fig. 2e) and a 31% reduction
in heart failure (Fig. 2g). There was a low level of heterogeneity for the effects of the intervention across eligible trials for all-cause death, cardiovascular death, stroke, and heart
failure; a modest level of heterogeneity for angina pectoris; and a high level of heterogeneity for myocardial infarction (_I_2 = 56%; Fig. 2c) and revascularization (_I_2 = 66%; Fig. 2e).
There was no clear evidence of publication bias (Supplementary Figure 1). Next, we performed an additional meta-analysis of four RCTs, CAMELOT-E [17], IMAGINE [19], PART-2 [20], and PEACE
[21], in which a DBP of <75 mmHg was attained in the drug-intervention group. When the achieved DBP was <75 mmHg, the drug intervention was associated with a 22% reduction in heart
failure when compared with the placebo group (Fig. 3g). There was no heterogeneity for the outcome of heart failure (_I_2 = 0%; Fig. 3g). In the drug-intervention group, a trend towards
reduced all-cause death was observed (Fig. 3a), but there were no significant reductions in cardiovascular death, myocardial infarction, angina pectoris, revascularization, and stroke (Fig.
3b–f). There was no clear evidence of publication bias (Supplementary Figure 2). SAFETY OUTCOME The drug-intervention group with an achieved DBP of 80 mmHg or less was associated with a 123%
increase in hypotension rate (Fig. 2h) when compared with the placebo-control group. There was high heterogeneity in the analysis (_I_2 = 73%; Fig. 2h), but bias was insignificant (Fig. 4
and Supplementary Tables 1–15). DISCUSSION The principal finding of the present study is that the achievement of a DBP of <80 mmHg in the drug-intervention group is associated with a
significant reduction in coronary revascularization and heart failure but does not increase all-cause death and cardiovascular death when the achieved SBP is ≤130 mmHg. A systematic review
of the effects of reducing SBP was conducted by Bangalore et al. including 15 RCTs involving CAD patients not having heart failure and acute myocardial infarction, who were randomized to
antihypertensive drugs or placebo [13]. The intensive SBP reduction with an achieved SBP of 131–135 mmHg was associated with a 15% decrease in heart failure and a 10% decrease in stroke but
at the expense of hypotension, when compared with the standard SBP reduction with an achieved SBP of 136–140 mmHg. Moreover, a more intensive SBP reduction achieving an SBP of ≤130 mmHg was
associated with greater reductions in heart failure and stroke (27 and 17%, respectively) as well as borderline reductions in myocardial infarction and angina pectoris, when compared with
the standard SBP reduction, while not affecting all-cause death and cardiovascular death. These findings might provide a rationale supporting the setting of an SBP target in CAD patients
down to <130 mmHg in the recent 2017 ACC/AHA guidelines [11]. However, this systematic review did not mention the effects of the achieved DBP ranges on the outcomes. Because the RCTs,
which investigated the effects of the achieved DBP ranges on the outcomes, were not found using a systematic search, we performed a systematic review of RCTs examining the effects of
reducing DBP in CAD patients who were randomized to antihypertensive drugs or placebo. Of note, all seven RCTs we included in our study are included in the meta-analysis by Bangalore et al.,
as mentioned above [13]. Indeed, the achieved SBP was 126.3 mmHg in the drug-intervention group and 131.5 mmHg in the placebo-control group in our study (Table 1). Our meta-analysis showed
that the achievement of a DBP < 80 mmHg was associated with an 11% reduction in coronary revascularization and a 31% reduction in heart failure (Fig. 2). We could not perform a
meta-analysis of the effects on renal failure because only the EUROPA trial reported the incidence of renal failure among seven RCTs [18]. An intervention group with a DBP < 75 mmHg,
including the four RCTs of CAMELOT-E [17], IMAGINE [19], PART-2 [20] and PEACE [21], was associated with a 22% reduction in heart failure and no increase in total death, cardiovascular
death, myocardial infarction, revascularization, or stroke (Fig. 3). Thus, it is important to note that controlling DBP to less than 80 or even 75 mmHg can prevent heart failure in patients
with CAD. The identified seven RCTs (DBP < 80 mmHg) used calcium-channel blockers and ACE inhibitors in the intervention group, and only ACE inhibitors were used in the four intensive
RCTs (DBP < 75 mmHg). This fact might be interesting given that calcium-channel blockers directly dilate the coronary artery while ACE inhibitors can improve autoregulation in the
coronary artery [23]. The J-curve phenomenon has been reported, especially in post-hoc analysis and observational studies. Coronary revascularization reduced the rate of primary outcome
(all-cause death, nonfatal myocardial infarction or nonfatal stroke) by half in the group achieving DBP < 60 mmHg in the INVEST study [24]. Another post-hoc analysis of the INVEST trial
showed that the relation between cardiovascular outcome and DBP was linear in patients with CAD and revascularization by coronary artery bypass grafting (CABG), and the lowest event rates
were observed in patients with a DBP of 125/55 mmHg [25]. The authors concluded that a more complete revascularization can attenuate hypoperfusion at a low DBP [25]. Therefore, it seems that
severe coronary lesions, which need revascularization, contribute to the increased rate of primary outcome at a low DBP. After total coronary revascularization, there is no reason to
hesitate to decrease DBP down to 80 mmHg. Indeed, in the Japan CREDO-Kyoto registry, a DBP ≤ 70 mmHg was not found to be an independent risk factor for cardiovascular death in patients with
CAD after revascularization [26]. A higher event rate was not observed in patients older than 75 years, even in those with a DBP < 60 mmHg [27]. Importantly, no RCT has been performed
that enables us to evaluate the effect of a DBP less than 70 mmHg in CAD patients. Further RCT studies targeting a DBP less than 70 mmHg are necessary. STUDY LIMITATIONS None of the trials
were designed to compare different DBP target levels. In seven RCTs, calcium antagonists and ACE inhibitors were used. Interestingly, only ACE inhibitors were used in trials that achieved
DBP levels <75 mmHg in the intervention group. Thus, we could not compare differences relating to the types of antihypertensive agents used in this meta-analysis. CONCLUSIONS The present
study showed that the achievement of a DBP < 80 mmHg significantly reduces coronary revascularization and heart failure but at the expense of causing hypotension in CAD patients with an
achieved SBP < 130 mmHg. We should not hesitate to target SBP down to 130 mmHg in patients with CAD when we observe DBP values of down to 80 mmHg, which are tolerable. Our results can
support the targeted DBP of 90 to 80 mmHg, which has been set in the newly published US guidelines [11]. REFERENCES * Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific
relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–13. Article Google
Scholar * Wakugami K, Iseki K, Kimura Y, Okumura K, Ikemiya Y, Muratani H, et al. Relationship between serum cholesterol and the risk of acute myocardial infarction in a screened cohort in
Okinawa, Japan. Jpn Circ J. 1998;62:7–14. Article CAS Google Scholar * Turnbull F. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of
prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–35. Article CAS Google Scholar * Shimamoto K, Ando K, Fujita T, Hasebe N, Higaki J, Horiuchi M, Japanese
Society of Hypertension Committee for Guidelines for the Management of Hypertension. et al. The Japanese Society of Hypertension Guidelines for the management of hypertension (JSH 2014).
Hypertens Res. 2014;37:253–390. Article Google Scholar * James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, Handler J, et al. 2014 evidence-based guideline for the
management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA. 2014;311:507–20. Article CAS Google Scholar *
Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Böhm M, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial
Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219. Article Google Scholar * Kang YY, Wang JG. The
J-curve phenomenon in hypertension. Pulse (Basel). 2016;4:49–60. Article Google Scholar * Tanna MS, Bangalore S. Antihypertensive therapy and the J-curve: fact or fiction? Curr Hypertens
Rep. 2015;17:6. Article Google Scholar * Vidal-Petiot E, Ford I, Greenlaw N, Ferrari R, Fox KM, Tardif JC, CLARIFY Investigators. et al. Cardiovascular event rates and mortality according
to achieved systolic and diastolic blood pressure in patients with stable coronary artery disease: an international cohort study. Lancet. 2016;388:2142–52. Article Google Scholar * Khan
NA, Rabkin SW, Zhao Y, McAlister FA, Park JE, Guan M, et al. Effect of lowering diastolic pressure in patients with and without cardiovascular disease: analysis of the SPRINT (Systolic Blood
Pressure Intervention Trial). Hypertension. 2018;71:840–7. Article CAS Google Scholar * Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, et al. 2017
ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the
American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–324. Article CAS Google Scholar * SPRINT Research Group,
Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103–16. Article
Google Scholar * Bangalore S, Kumar S, Volodarskiy A, Messerli FH. Blood pressure targets in patients with coronary artery disease: observations from traditional and Bayesian random
effects meta-analysis of randomised trials. Heart. 2013;99:601–13. Article Google Scholar * Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and
meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. Article Google Scholar * Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ.
2003;327:557–60. Article Google Scholar * Poole-Wilson PA, Lubsen J, Kirwan BA, van Dalen FJ, Wagener G, Danchin N, Coronary disease Trial Investigating Outcome with Nifedipine
gastrointestinal therapeutic system investigators. et al. Effect of long-acting nifedipine on mortality and cardiovascular morbidity in patients with stable angina requiring treatment
(ACTION trial): randomised controlled trial. Lancet. 2004;364:849–57. Article CAS Google Scholar * Nissen SE, Tuzcu EM, Libby P, Thompson PD, Ghali M, Garza D, CAMELOT Investigators. et
al. Effect of antihypertensive agents on cardiovascular events in patients with coronary disease and normal blood pressure: the CAMELOT study: a randomized controlled trial. JAMA.
2004;292:2217–25. Article CAS Google Scholar * Fox KM. Efficacy of perindopril in reduction of cardiovascular events among patients with stable coronary artery disease: randomised,
double-blind, placebo-controlled, multicentre trial (the EUROPA study). Lancet. 2003;362:782–8. Article CAS Google Scholar * Rouleau JL, Warnica WJ, Baillot R, Block PJ, Chocron S,
Johnstone D, IMAGINE (Ischemia Management with Accupril post-bypass Graft via Inhibition of the coNverting Enzyme) Investigators. et al. Effects of angiotensin-converting enzyme inhibition
in low-risk patients early after coronary artery bypass surgery. Circulation. 2008;117:24–31. Article CAS Google Scholar * MacMahon S, Sharpe N, Gamble G, Clague A, Mhurchu CN, Clark T,
et al. Randomized, placebo-controlled trial of the angiotensin-converting enzyme inhibitor, ramipril, in patients with coronary or other occlusive arterial disease. PART-2 Collaborative
Research Group. Prevention of Atherosclerosis with Ramipril. J Am Coll Cardiol. 2000;36:438–43. Article CAS Google Scholar * Braunwald E, Domanski MJ, Fowler SE, Geller NL, Gersh BJ, Hsia
J, PEACE Trial Investigators. et al. Angiotensin-converting-enzyme inhibition in stable coronary artery disease. N Engl J Med. 2004;351:2058–68. Article CAS Google Scholar * Pitt B,
Byington RP, Furberg CD, Hunninghake DB, Mancini GB, Miller ME, et al. Effect of amlodipine on the progression of atherosclerosis and the occurrence of clinical events. PREVENT
Investigators. Circulation. 2000;102:1503–10. Article CAS Google Scholar * Rouleau JR, Simard D, Blouin A, Kingma JG Jr. Angiotensin inhibition and coronary autoregulation in a canine
model of LV hypertrophy. Basic Res Cardiol. 2002;97:384–91. Article CAS Google Scholar * Messerli FH, Mancia G, Conti CR, Hewkin AC, Kupfer S, Champion A, et al. Dogma disputed: can
aggressively lowering blood pressure in hypertensive patients with coronary artery disease be dangerous? Ann Intern Med. 2006;144:884–93. Article Google Scholar * Denardo SJ, Gong Y,
Nichols WW, Hewkin AC, Kupfer S, Champion A, et al. Blood pressure and outcomes in very old hypertensive coronary artery disease patients: an INVEST substudy. Am J Med. 2010;123:719–26.
Article Google Scholar * Kai H, Ueno T, Kimura T, Adachi H, Furukawa Y, Kita T, Imaizumi T, CREDO-Kyoto Investigators. Low DBP may not be an independent risk for cardiovascular death in
revascularized coronary artery disease patients. J Hypertens. 2011;29:1889–96. Article CAS Google Scholar * Kai H, Kimura T, Fukuda K, Fukumoto Y, Kakuma T, Furukawa Y. Impact of low
diastolic blood pressure on risk of cardiovascular death in elderly patients with coronary artery disease after revascularization–The CREDO-Kyoto Registry Cohort-1. Circ J. 2016;80:1232–41.
Article CAS Google Scholar Download references AUTHOR INFORMATION Author notes * These authors contributed equally: Ryuji Okamoto, Eita Kumagai AUTHORS AND AFFILIATIONS * Department of
Cardiology and Nephrology, Mie University Graduate School of Medicine, 2-174 Edobashi, Tsu, Mie, 514-8507, Japan Ryuji Okamoto & Masaaki Ito * Division of Cardiovascular Medicine,
Department of Internal Medicine, Kurume University School of Medicine, 67 Asahi-machi, Kurume, Fukuoka, 830-0011, Japan Eita Kumagai & Yoshihiro Fukumoto * Department of Cardiology,
Kurume University Medical Center, 155-1 Kokubu-machi, Kurume, Fukuoka, 839-0863, Japan Hisashi Kai * Department of Advanced Cardiovascular Therapeutics, Nagoya University Graduate School of
Medicine, 65 Tsurumai, Showa-ku, Nagoya, 466-8550, Japan Rei Shibata * Department of Medicine and Clinical Science, Graduate School of Medical Sciences, Kyushu University, 3-1-1 Maidashi,
Higashi-ku, Fukuoka, 812-8582, Japan Toshio Ohtsubo * Department of Cardiovascular Medicine, Nagasaki University Graduate School of Biomedical Sciences, 1-7-1 Sakamoto, Nagasaki, 852-8501,
Japan Hiroaki Kawano * Divition of Nephrology and Hypertension, Yokohama City University Medical Center, 4-57 Urafune-cho, Minami-ku, Yokohama, 232-0024, Japan Akira Fujiwara * Department of
Preventive Medicine and Public Health, Fukuoka University, 8-19-1 Nanakuma, Jonan-ku, Fukuoka, Fukuoka, 814-0180, Japan Hisatomi Arima Authors * Ryuji Okamoto View author publications You
can also search for this author inPubMed Google Scholar * Eita Kumagai View author publications You can also search for this author inPubMed Google Scholar * Hisashi Kai View author
publications You can also search for this author inPubMed Google Scholar * Rei Shibata View author publications You can also search for this author inPubMed Google Scholar * Toshio Ohtsubo
View author publications You can also search for this author inPubMed Google Scholar * Hiroaki Kawano View author publications You can also search for this author inPubMed Google Scholar *
Akira Fujiwara View author publications You can also search for this author inPubMed Google Scholar * Masaaki Ito View author publications You can also search for this author inPubMed Google
Scholar * Yoshihiro Fukumoto View author publications You can also search for this author inPubMed Google Scholar * Hisatomi Arima View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Ryuji Okamoto. ETHICS DECLARATIONS CONFLICT OF INTEREST The Department of Cardiology and Nephrology, Mie University
Graduate School of Medicine, received research grants from Bristol-Myers Squibb, MSD K.K., Pfizer Japan Inc., Takeda Pharmaceutical Co., Ltd., Astellas Pharma Inc., Daiichi Sankyo
Pharmaceutical Co., Ltd., Genzyme Japan, Shionogi & Co., Ltd., Sumitomo Dainippon Pharma Co., Ltd., Mitsubishi Tanabe Corporation, Otsuka Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd.,
AstraZeneca K.K., and Boehringer Ingelheim Co., Ltd. Rei Shibata received honoraria from Medtoronic, Boehringer Ingelheim, Eli Lilly and Mitsubishi Tanabe Pharma. Toshio Ohtsubo received
lecture fees from Sanwa Kagaku Kenkyusho Co., Ltd. Hisashi Kai received lecture fees from Daiichi Sankyo Co Pharmaceutical Co., Ltd., Mitsubishi Tanabe Corporation, Shionogi & Co., Ltd.,
Sumitomo Dainippon Pharma Co., Ltd., and Takeda Pharmaceutical Co., Ltd. Masaaki Ito received lecture fees from Daiichi Sankyo Co Pharmaceutical Co., Ltd., Mitsubishi Tanabe Corporation,
Bayer Yakuhin, Ltd. and Takeda Pharmaceutical Co., Ltd. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 SUPPLEMENTARY FIGURE 2 SUPPLEMENTARY TABLES1-15 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Okamoto, R., Kumagai, E., Kai, H. _et al._ Effects of lowering diastolic blood pressure to <80 mmHg on cardiovascular mortality and events in patients with
coronary artery disease: a systematic review and meta-analysis. _Hypertens Res_ 42, 650–659 (2019). https://doi.org/10.1038/s41440-018-0189-z Download citation * Received: 17 August 2018 *
Revised: 07 October 2018 * Accepted: 09 October 2018 * Published: 05 April 2019 * Issue Date: May 2019 * DOI: https://doi.org/10.1038/s41440-018-0189-z SHARE THIS ARTICLE Anyone you share
the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative KEYWORDS * Coronary artery disease * diastolic blood pressure * hypertension * J curve * meta-analysis






