- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Participants in the 100,000 Genomes Project (100kGP) could consent to receive additional finding (AF) results, individual variants relating to genes associated with susceptibility
to cancer and familial hypercholesterolemia (FH). In the study reported here, qualitative interviews were used to explore the experiences of National Health Service (NHS) professionals from
across England who were tasked with returning over 80,000 “no AF” results and 700 positive AF results to 100kGP participants. Interviews were conducted with 45 professionals from a range of
backgrounds, including Genetic Counsellors, Clinical Geneticists, FH Clinical Nurse Specialists and Clinical Scientists. Interviews were analysed using a codebook thematic analysis approach.
Returning AF results has been a significant endeavour, with challenges for pathways, administrative processes and clinical and laboratory time when the capacity of NHS services is already
stretched. Professionals discussed going “above and beyond” to prioritise patient care through pathway design, additional clinics, overtime, longer appointments and provision of follow-up
appointments. Professionals also described facing practical and emotional challenges when returning AFs. Benefits for patients from receiving AFs in the 100kGP were highlighted and
professionals were generally positive about offering clinically actionable AFs within routine NHS clinical care. Professionals were, however, cautious around the implementation of AFs into
routine care and felt more research and discussion was needed to determine which AFs to offer, approaches to consent and communication of results, costs and the potential strain on NHS
capacity and resources. Further consultation is required with careful review of pathways and resources before offering AFs in clinical practice. SIMILAR CONTENT BEING VIEWED BY OTHERS
GENOMIC HEALTH DATA GENERATION IN THE UK: A 360 VIEW Article Open access 19 October 2021 MY RESEARCH RESULTS: A PROGRAM TO FACILITATE RETURN OF CLINICALLY ACTIONABLE GENOMIC RESEARCH
FINDINGS Article 04 October 2021 ELICITING PARENTAL PREFERENCES AND VALUES FOR THE RETURN OF ADDITIONAL FINDINGS FROM GENOMIC SEQUENCING Article Open access 14 February 2024 INTRODUCTION
Genome sequencing (GS) is transforming modern healthcare by improving the diagnostic yield of rare disease and providing information on cause, prognosis, and therapeutic impact for some
cancers. When GS is performed there is an opportunity to look for health related “additional findings” (AFs), also called “secondary findings”, that are unrelated to the patient’s primary
indication for GS testing. The goal of offering AFs is to identify a possible increased risk for conditions that the individual may not be aware of that will allow patients to be proactive
in reducing risks and sharing information with family members. There is, however, ongoing discussion around whether and in what circumstances AFs should be offered, and how to offer AFs in a
way that balances benefits and minimises potential harms [1,2,3,4,5]. Considerations include the potential burden of unwanted information and the clinical value of the information,
especially in the absence of a relevant family history [1,2,3,4,5]. Consensus from professional bodies is lacking. Current American College of Medical Genetics and Genomics (ACMG) guidelines
recommend returning pathogenic and likely pathogenic variants in 73 genes [6], while guidelines from other countries are more conservative [4, 7, 8]. Moreover, while previous research shows
patients, clinicians and the public support reporting medically actionable AFs [9,10,11] and a recent systematic review found no evidence of negative psychological impacts on patients [12],
reported processes for consent and return of results and the type of AF reported vary widely [5]. In England, GS has been offered in routine care since 2020 through the National Health
Service (NHS) Genomic Medicine Service [13]. AFs are not routinely offered to patients who have GS as more evidence is needed to guide whether and how AFs should be offered. The NHS Genomic
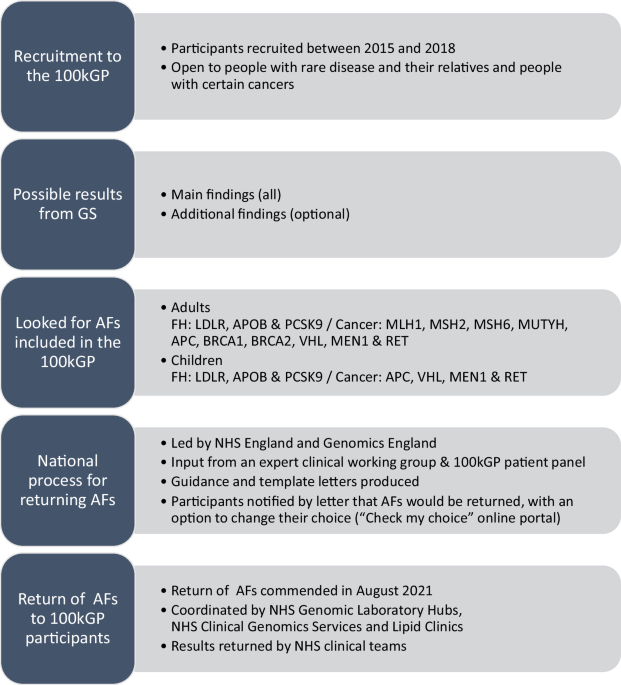
Medicine Service was largely informed by the 100,000 Genomes Project (100kGP) [14, 15]. Between 2015 and 2018 over 85,000 patients with cancer or rare disease, and their relatives, were
recruited to the 100kGP (Fig. 1). All participants consented to receive main findings relating to their cancer or rare disease and had the option to consent to receive clinically actionable
AFs for 13 genes associated with an increased risk of some cancers or familial hypercholesterolaemia (FH) [16]. AFs for children were a subset of seven of these 13 genes, with adult-onset
conditions omitted. Consent was taken by professionals from a range of backgrounds, including genetic counsellors and research nurses, who had undergone 100kGP consent training [17]. Main
findings were returned to 100kGP participants by the referring NHS clinical teams as results became available. After main findings were returned, a unified national process was established
to return AFs to 100kGP participants. Over 90% of 100kGP participants had opted to receive AFs and more than 700 positive AFs (PAF) results and 80,000 no AF (NAF) results have been returned
through NHS pathways. Recent research in one English region has shown that the identification of patients with PAF results has enabled appropriate clinical interventions [18]. Here we have
used qualitative interviews with professionals to explore their experiences of returning AFs and gather their views on offering AFs in routine care. This study is part of a broader
evaluation of the clinical, behavioural, psychological and economic impacts of returning AFs to 100kGP participants. Findings relating to patient experiences and to costs will be reported
separately. METHODS STUDY DESIGN Qualitative semi-structured interviews were used to facilitate an in-depth exploration of professionals’ views and experiences. SETTING The setting is the
return of AFs from the 100kGP in England. Starting in August 2021, AFs were released by Genomics England to local services in batches that were several weeks apart. National guidance and
template letters were produced to guide local processes for returning AFs. All NAF results were sent out by letter. The suggested pathway for PAF results was to send a notification letter
that explains a PAF has been found with an invitation to a clinical appointment where the condition will be disclosed. The suggested maximum time between notification and disclosure was six
weeks. Standard NHS pathways for ongoing care were then followed, including recommendations for risk management and cascade testing. SAMPLING AND RECRUITMENT Professionals from NHS Trusts,
Genomics England and NHS England involved in planning, overseeing and delivering the return of AF results to 100kGP participants were purposefully sampled to include participants from
different geographic locations and professional backgrounds. Potential participants were identified by the research team and invited to take part via email. INTERVIEWS Interview topic guides
explored: (1) Views on offering AFs to 100kGP participants, (2) Local pathways, processes and challenges, (3) Experiences of returning AFs and (4) Views on offering AFs in routine practice
(Supplementary Materials). Interviews were conducted via telephone or video call. DATA ANALYSIS Interviews were digitally recorded, transcribed verbatim and pseudonymised. Data were analysed
using the principles of thematic analysis [19] with a team-based codebook approach [20]. NVivo version 13 (QSR International Ltd) facilitated coding. Inductive and deductive codes were used
to develop the codebook [21]. BSS drafted an initial codebook based on study aims and topic guides (deductive codes). The draft codebook was inductively revised by BSS, JG and MH who
independently coded three transcripts and added additional codes (inductive codes). The final codebook was then used to code all transcripts. Additional inductive codes were added throughout
the coding process. RESULTS PARTICIPANT CHARACTERISTICS Of 65 professionals invited by email to participate, six did not respond, 14 declined and 45 participated (response rate: 69%).
Interviews were conducted between May and October 2022 (seven by telephone, 38 by video call), by BSS (_n_ = 8), JG (_n_ = 10) and MH (_n_ = 27) and lasted between 26 and 70 min (median = 43
min). Participants included genetic counsellors (53%), clinical geneticists (20%), specialist FH nurses (16%) and clinical scientists (7%) working across England (Table 1). The majority of
participants had direct experience of returning PAFs to patients (32/45), with others involved in administration, coordination or laboratory work. INTERVIEW FINDINGS Findings are described
within three overarching themes: * 1. Pathways and processes for returning AFs from the 100kGP * 2. Experiences of returning AFs from the 100kGP * 3. Views on offering AFs in routine care
PATHWAYS AND PROCESSES FOR RETURNING AFS FROM THE 100KGP VARIATION IN PATHWAYS AND PROCESSES Care pathways and template letters were adapted from the national guidance by some services (Fig.
2). One key difference between local pathways for returning PAFs was the approach for disclosing the condition. Some teams disclosed the condition in a notification letter (FH only) or in a
notification telephone call (cancer or FH), while others waited until the subsequent clinical appointment (cancer or FH). Disclosing the condition in the notification letter or telephone
call was chosen to minimise anxiety while patients waited for their clinical appointment (Table 2: Q1). The types of clinicians involved in returning AFs varied between local services (Fig.
2). Sending out NAF results letters was managed by administrative teams. Some professionals reported receiving patient queries about their NAF result letter and some services had assigned a
genetic counsellor to provide support and answer questions from participants with NAF results by telephone (Table 2: Q2). GOING ABOVE AND BEYOND TO PRIORITISE PATIENT CARE Many professionals
described how their teams went “above and beyond” to prioritise patient care, highlighting that “flexibility” and “working around the patient” were key to constructing local pathways (Table
2: Q3). Approaches included: pathway planning to minimise delays between notification letter/telephone call and clinic appointment, additional clinics (including evenings or weekends),
telephone helplines, development of guidance for counselling, longer appointments and overtime (paid and unpaid). Many highlighted the value of close team working to effectively return AFs.
Regular team meetings were valued as a space to “troubleshoot any problems, if we’ve had any issues disclosing the result or if it’s been a particularly psychosocially challenging
consultation then that’s the space really where we can share thoughts, concerns and ideas” (HP04 – Genetic Counsellor). Successfully establishing pathways and addressing challenges that
would allow teams to provide the best possible care for patients gave professionals a sense of pride (Table 2: Q4). PRACTICAL CHALLENGES The sheer number of results to be returned meant that
“one of the biggest challenges was just the huge amount of admin involved” (HP45 – Clinical Scientist). Clinical scientists noted challenges arising from the time required for variant
interpretation and the lack of standardised software across different laboratories. While some professionals felt their team had sufficient capacity, many commented that clinical and
laboratory services were already strained due to COVID-19 (Table 2: Q5). It was also noted that returning AFs within the set timeframes was “something additional” that impacted existing
workloads and the delivery of routine care (Table 2: Q6). Some participants added that “these patients get pushed in front of others” (HP16 – Genetic Counsellor), increasing routine waiting
times. Conversely, one FH nurse noted that COVID-19 had reduced referrals for FH testing, which meant that patients identified through the 100kGP “filled a gap” and were not a burden on
capacity. The time between recruitment and returning AFs meant that local knowledge and experience of the 100kGP was reduced because the dedicated “infrastructure and staff had moved away”
(HP37 – Clinical Scientist). Other issues added to demands on capacity and resources. For example, initiating the return of AFs highlighted that not all 100kGP participants had received
their main findings due to gaps in coordination and communication (Table 2: Q7). Some patients already knew of their increased risk for the condition identified as a PAF, because the PAF was
the same as their main finding or because the condition had already been identified through standard clinical pathways (Table 2: Q8). Finally, returning results in batches that did not
include all family members could generate anxiety for some patients and required additional administrative and clinical time (Table 2: Q9). EXPERIENCES OF RETURNING AFS FROM THE 100KGP
RETURNING AFS TO 100KGP PARTICIPANTS WAS VIEWED POSITIVELY Benefits of returning AFs to 100kGP participants primarily centred on the included conditions being recognised as actionable with
clear clinical benefits for patients (Table 3: Q1). For cancer, the value of facilitating access to screening and early diagnosis was frequently noted (Table 3: Q2). For FH, the importance
of identifying patients who can have a “simple treatment” to reduce their risk of cardiovascular disease (Table 3: Q3) made FH a “good example of minimal psychological harm – massive
benefit” (HP06 – FH Clinical Nurse Specialist). Another frequently noted benefit was cascade testing to identify at risk family members. Professionals who had returned results to 100kGP
participants described psychosocial benefits for patients, as some patients felt “empowered” to make choices about their healthcare (Table 3: Q4) and some patients expressed relief that
their family history of cancer or FH now made sense. RETURNING AFS FELT “OUT OF THE BLUE” FOR PATIENTS AND PROFESSIONALS Professionals reported that the three to six years between consent
and results meant that many patients had either forgotten or had limited recall of the consent discussion (Table 3: Q5). There were also misunderstandings about what conditions had been
looked for (Table 3: Q6). Consequently, AF results came “out of the blue” or were “quite a shock” and often meant patients “were quite anxious… not just for themselves but also for the wider
family” (HP08 – Genetic Counsellor). The disclosure appointment was sometimes described as “overwhelming” for patients, particularly for cancer AFs. Follow-up appointments allowed patients
to “regroup” and “digest it in a calmer way” (Table 3: Q7). Professionals described supporting patients to enable a good understanding of their risk and management options, which allowed
them to feel “back in control” (HP09 – Clinical Geneticist). As many patients were previously unknown to the clinical service, professionals had not met the patient before or did not have
access to their clinical notes (Table 3: Q8). Several professionals commented that their usual approach to supporting patients through genetic testing that involved pre- then post-test
counselling was reversed which “felt all a little bit, I don’t know, out of the blue for me and for them” (HP04 – Genetic Counsellor). Discussions that usually occur during pre-test
counselling, such as describing test implications and taking a family history, were incorporated into the results appointment. Some noted that when patients have timely pre-test counselling
it is clear why a genetic test is indicated and “expectations are managed much better” (HP34 – Genetic Counsellor). In addition, without the opportunity to build rapport through pre-test
counselling, it could be difficult to gauge the patient’s emotional response to the AF results (Table 3: Q9). COMPLEX AND CHALLENGING CLINICAL SCENARIOS Several clinical scenarios arose that
professionals found practically and emotionally challenging. Many highlighted the difficulty of interpreting the risk for a participant with a PAF when there was no family history (Table 3:
Q10). Professionals described balancing “finding something and then being able to offer patients screening which would be a good thing versus the worry that might cause when there’s no
family history” (HP44 – Genetic Counsellor). In addition, explaining the uncertainty around the level of risk for the patient and their family members was described as “challenging” or
“tricky” (Table 3: Q11). Several professionals also noted the “clinical quandary” of dealing with AF results released for 100kGP participants who were deceased with no recorded next of kin
or relatives said they did not want the results (Table 3: Q12). Knowing they had information of value that they were unable to share left professionals unsure of next steps and concerned
that they could not discharge their responsibilities. Emotionally challenging scenarios included sharing cancer AF results with participants who had developed cancer after consenting for the
100kGP or with participants who were currently pregnant. These situations were distressing for patients and their families, and professionals described feeling frustrated and upset
themselves. VIEWS ON OFFERING AFS IN ROUTINE CARE CAUTIOUS OPTIMISM FOR OFFERING AFS MORE WIDELY Professionals generally felt that including AFs routinely when GS was offered would be a
“positive step” and described a range of potential benefits but also highlighted multiple practical and resource challenges to overcome (Table 4: Q1). Accordingly, many professionals felt
that further evidence was needed. For example, establishing a list of clinically actionable AFs that aligns with the availability of NHS resources to action them, and more evidence to
accurately interpret risks when there is no family history of the condition. Many also noted that further consultation with stakeholders was needed to inform the individual specific
decisions about whether AFs should be included routinely as well as how, when and to whom they should be offered. One professional with experience of bringing new genomic tests into clinical
practice noted the importance of gauging acceptability amongst a wide range of key stakeholders, including clinicians, clinical scientists, patients and the public because “people that are
part of a research project have a very different view to people who might just be coming as part of a clinical service” (HP41 – Service manager). Benefits for offering AFs routinely included
providing patients with clinically actionable findings, screening, information for management, cascade testing, earlier detection and treatment and insights into gene penetrance. The most
common concern was whether the NHS had the capacity and resources to manage the additional workload for clinicians, clinical scientists and administrative staff. Many professionals felt the
NHS is already “stretched,” “swamped,” and “not set up to deliver on this scale” and questioned if it would be feasible to add AFs: “who’s going to fund it and who’s going to see them” (HP22
– FH Clinical Nurse Specialist). A linked concern was how to provide the necessary psychological support for patients (Table 4: Q2). The issue of costs relative to benefits was raised and
concerns around equity of access to genetic testing were also discussed. Some professionals noted the inequity of offering tests to patients without a family history ahead of patients with
concerns around their family history who fall short of current eligibility criteria which requires a strong family history of cancer (Table 4: Q3). Several professionals also noted the
“ethical tensions” around offering AFs to paediatric patients around what to report, holding the information, the timing of the disclosure for actionable variants in adult-onset genes and
distress for parents unable to access some tests for their child until adulthood (Table 4: Q4). CAREFUL CURATION OF THE SPECIFIC AFS TO OFFER IS NEEDED All professionals felt that if AFs
were to be offered routinely, then the list of genes and variants would need to be carefully curated to include only actionable findings with a clear pathway for surveillance and risk
management (Table 4: Q5). For example, some clinicians worried about including genes where screening is not universally available across the UK (e.g., _TP53_). Several professionals also
strongly felt that “ambiguous results”, such as genetic alterations in low penetrance genes and variants of uncertain significance, should not be offered (Table 4: Q6). Many professionals
thought that the 13 genes offered in the 100kGP would be a good starting point. Most felt the current ACMG list was too extensive, holding concerns that not all ACMG genes were truly
actionable within the NHS and that the “infrastructure to deal with it” (HP28 – Clinical Scientist) is lacking. APPROACHES TO TAKING CONSENT FOR AFS NEED CAREFUL CONSIDERATION Professionals
discussed possible consent processes for offering AFs in clinical practice. Many professionals felt that consent should be specific to AFs, rather than how it was offered in the 100kGP, with
a “tick-box” added onto an existing consent form. Some participants acknowledged that a more in-depth consent process would be ideal, but it may not be feasible due to limited resources.
Offering AFs alongside GS in acute care settings, such as rapid sequencing for acutely unwell children, was flagged as requiring more thought as parents may not be able to fully attend to
the implications. A two-step consent conversation was viewed positively to separate decisions about clinically indicated testing and AFs, to allow patients to “deal with what they need to
deal with and when they’re ready they can think about anything further” (HP16 – Genetic Counsellor). Approaches where patients “opt out” of receiving AFs were not thought to be appropriate.
While many felt that appropriately trained professionals from a range of backgrounds could offer and consent for AFs, returning results needs to involve specialists in the condition or
clinical genetics (genetic counsellors and clinical geneticists) experienced in explaining next steps for management and referrals (Table 4: Q7). While some professionals felt strongly that
returning AFs should remain with genetics teams who are “much more used to dealing with families,” others felt that the shift to embed mainstreaming and the value of multidisciplinary team
(MDT) working meant that integrating the return of AFs across relevant health disciplines would “make the most sense”. MDT working requires coordinated care pathways to allow mainstream
teams to access support from genetics when needed (Table 4: Q8). DISCUSSION The return of AFs through NHS clinical care pathways from a research project with the expansive scale of the
100kGP provides a unique opportunity for insight for offering GS in both research and clinical settings. Interview findings have been considered against the wider literature and the
identified lessons are summarised in Fig. 3. Patient-centred care has been prioritised throughout the process of returning AFs. Practical challenges primarily related to the large number of
AFs to be returned when clinical and laboratory services are already stretched. Some teams struggled with the additional workload and reported impacts on routine patient care. These findings
align with previous studies highlighting the potential for tension between research and clinical practice [22, 23], including the challenges of disclosing research results when there are
limitations in infrastructure and staffing [23]. Timelines of several years between consent and return of results brought multiple challenges including patients’ poor recall of consent,
results coming “out of the blue”, unclear responsibilities when a participant was deceased and emotionally charged interactions when patients were pregnant or had already developed the
condition identified as a PAF. In another study, qualitative interviews with 100kGP participants with a PAF found that some had incomplete recall or misunderstandings about consent and most
were surprised or shocked to receive their PAF result [24]. In addition, earlier research looking at consent experiences in the 100kGP found that some participants had misunderstood or could
not remember whether they had opted to receive AFs [25, 26]. Research exploring the lessons from returning AFs in eMERGE also highlight similar challenges for consent and return of results
and minimising the time between testing and reporting was suggested [27] (Fig. 3: Lesson 1A). If timelines for returning results are not clear at the outset, which was the case for the
100kGP, pathways for ongoing communication with participants are essential (Fig. 1: Lesson 1B). Guidance for professionals is needed that provides clarity on their responsibilities for
deceased patients or those who do not want the result (Fig. 3: Lesson 2B). The potential for emotional burden for professionals also needs to be addressed, with time to process the
experience and space for reflective practice [28]. Several participants noted that offering AFs in the 100kGP differed from the traditional genetic counselling model of pre- and post-test
counselling appointments conducted by the same professional and closely spaced in time. For 100kGP participants who had consented to receive AFs several years ago, AF results were often
unexpected, and results were rarely returned by the clinician who had recruited them. This experience aligns with a “genome first” approach to results disclosure where research participants
are referred to clinical services after GS testing has been performed [29, 30]. Professionals in our study described how they supported patients with this model of care by adapting the
traditional content of disclosure appointments to include elements of pre-test counselling, such as gathering family history information. They also emphasised the importance of follow-up
appointments which allowed patients time to process information and return with further questions (Fig. 3: Lesson 1C). When AF results are returned after a long time or by clinicians not
known to a patient, context specific guidance is needed for genome first counselling that considers adapting risk assessments, counselling content and information materials [30] (Fig. 3:
Lesson 2D). Returning AFs from the 100kGP will inform NHS practice. Professionals noted potential benefits of offering a curated list of medically actionable AFs (Fig. 3: Lesson 3A), but
also highlighted the need for further research evidence and consultation with key stakeholders (e.g. patients, clinicians, clinical scientists and policy makers) around what AFs to offer and
to whom, processes for consent and communicating results, provision of sufficient staff to support patients and potential strain on resources in an already stretched NHS. Our findings echo
previous research conducted with professionals involved in 100kGP recruitment who supported disclosing a limited number of highly predictive and medically actionable AFs and raised concerns
around how capacity and funding would be provided [2, 3] (Fig. 3: Lesson 3B). Our findings also align with ongoing discussions about the need to determine best practice for consent with
consideration for options such as “broad consent” [27] or “dynamic consent” that allow for changes over time [10] or “two step” approaches where the offer of AFs is made after diagnostic
testing [31]. Appropriate pathways for communicating results must also be addressed, including: whether to return AFs separately to main findings and consideration for how to support people
with NAF results. In this study, some teams had a genetic counsellor available to discuss queries about NAF results by phone, other strategies to consider include developing resources such
as websites or videos to answer frequently asked questions [32] (Fig. 3: Lesson 1E). Considerations for offering AFs must be set against the challenges the NHS faces in offering GS
generally, with recent research highlighting the lack of a trained and available workforce of clinicians and scientists, and the need for improved digital infrastructure [33]. Mainstreaming
GS requires new pathway development for consent and results and consideration for the roles of mainstream and genetics professionals. Establishing clear pathways and good communication
between mainstream and genetics teams will be essential (Fig. 3: Lesson 2C). Notably, the type of staff involved in returning PAF results differed between services and professionals
emphasised the value of MDT working. The role of lipid clinics in returning FH AFs highlights the value of condition specific approaches to returning AFs, with established pathways deployed
to support patients (Fig. 3: Lesson 3D). Our findings also demonstrate the need for flexibility in future guidance to allow local adaptation to suit existing infrastructure, care pathways
and skill sets, with minimum standards defined (Fig. 3: Lesson 2A). Reaching agreement on what constitutes “best practice” in genomic healthcare and how this is applied in clinical practice
by local teams can be challenging, especially as new demands must be applied within existing contexts [34,35,36]. Previous research addressing the role of ambivalence has highlighted the
importance of open discussions and additional voices, including those of patients and the public, to help interpret and inform local practices [34]. As such, broad consultation will be a
crucial next step in decision making about offering AFs in clinical practice. STRENGTHS AND LIMITATIONS A key strength was that participants were recruited across England, had a range of
professional backgrounds and differing roles in returning AFs. As interviews took place during the process of returning AFs, we have captured experiences in real time, however this may have
limited time for reflection. This was a relatively small qualitative study within the specific setting of the 100kGP and NHS clinical practice which may not be generalisable to other
settings. The small sample size prevented sub-group comparisons. Professionals chose to take part, potentially introducing self-selection bias as they may hold differing views and
experiences to the professionals who choose not to take part. Additionally, the researchers naturally and unintentionally may introduce their own inherent bias from their experience in
genomics, personal beliefs and cultural backgrounds, however the researchers engaged in reflexivity to help reduce such bias [37]. CONCLUSIONS As considerations are made about incorporating
AFs into routine care when GS is offered, our findings provide valuable information for the design and delivery of care pathways offering AFs in research and clinical settings. Prior to
routine implementation in the NHS, further consideration is required around which AFs to offer, the consent process, approaches to communicating results, and managing the increased demand on
NHS laboratory and clinical services. Future guidance will benefit from the flexibility to allow local adaptations to existing pathways, infrastructure and roles. This study also highlights
the need for tailored support for patients receiving unexpected results and the importance of timely results. DATA AVAILABILITY The data that support the findings of this study are
available from the corresponding author (MH) upon reasonable request and where participant consent has been given. REFERENCES * Christensen KD, Green RC. How could disclosing incidental
information from whole-genome sequencing affect patient behavior? Per Med. 2013;10. https://doi.org/10.2217/pme. * Ormondroyd E, Mackley MP, Blair E, Craft J, Knight JC, Taylor JC, et al.
Not pathogenic until proven otherwise”: perspectives of UK clinical genomics professionals toward secondary findings in context of a Genomic Medicine Multidisciplinary Team and the 100,000
Genomes Project. Genet Med. 2018;20:320–8. Article PubMed Google Scholar * Sanderson SC, Hill M, Patch C, Searle B, Lewis C, Chitty LS. Delivering genome sequencing in clinical practice:
an interview study with healthcare professionals involved in the 100 000 Genomes Project. BMJ Open. 2019;9:e029699. Article PubMed PubMed Central Google Scholar * de Wert G, Dondorp W,
Clarke A, Dequeker EMC, Cordier C, Deans Z, et al. Opportunistic genomic screening. Recommendations of the European Society of Human Genetics. Eur J Hum Genet. 2021;29:365–77. Article
PubMed Google Scholar * Sapp JC, Facio FM, Cooper D, Lewis KL, Modlin E, van der Wees P, et al. A systematic literature review of disclosure practices and reported outcomes for medically
actionable genomic secondary findings. Genet Med. 2021;23:2260–9. Article PubMed PubMed Central Google Scholar * Miller DT, Lee K, Gordon AS, Amendola LM, Adelman K, Bale SJ, et al.
Recommendations for reporting of secondary findings in clinical exome and genome sequencing, 2021 update: a policy statement of the American College of Medical Genetics and Genomics (ACMG).
Genet Med. 2021;23:1391–8. Article PubMed Google Scholar * van El CG, Cornel MC, Borry P, Hastings RJ, Fellmann F, Hodgson SV, et al. Whole-genome sequencing in health care.
Recommendations of the European Society of Human Genetics. Eur J Hum Genet. 2013;21:S1–5. PubMed PubMed Central Google Scholar * Boycott K, Hartley T, Adam S, Bernier F, Chong K,
Fernandez BA, et al. The clinical application of genome-wide sequencing for monogenic diseases in Canada: Position Statement of the Canadian College of Medical Geneticists. J Med Genet.
2015;52:431–7. Article PubMed CAS Google Scholar * Mackley MP, Fletcher B, Parker M, Watkins H, Ormondroyd E. Stakeholder views on secondary findings in whole-genome and whole-exome
sequencing: a systematic review of quantitative and qualitative studies. Genet Med. 2017;19:283–93. Article PubMed Google Scholar * Vears DF, Minion JT, Roberts SJ, Cummings J, Machirori
M, Blell M, et al. Return of individual research results from genomic research: A systematic review of stakeholder perspectives. PLoS One. 2021;16:e0258646. Article PubMed PubMed Central
CAS Google Scholar * Middleton A, Morley KI, Bragin E, Firth HV, Hurles ME, Wright CF, et al. Attitudes of nearly 7000 health professionals, genomic researchers and publics toward the
return of incidental results from sequencing research. Eur J Hum Genet. 2016;24:21–9. Article PubMed Google Scholar * Mitchell LA, Jivani K, Young MA, Jacobs C, Willis AM. Systematic
review of the uptake and outcomes from returning secondary findings to adult participants in research genomic testing. J Genet Couns. 2024. https://doi.org/10.1002/jgc4.1865. * Department of
Health and Social Care. Genome UK: the future of healthcare. 2020. Available from; https://www.gov.uk/government/publications/genome-uk-the-future-of-healthcare. Accessed July 2024. *
Genomics England. The 100,000 genomes project protocol. Third edition. London: Genomics England Ltd.; 2017. * Turnbull C, Scott RH, Thomas E, Jones L, Murugaesu N, Pretty FB, et al. The 100
000 Genomes Project: bringing whole genome sequencing to the NHS. BMJ. 2018;361:k1687. * Genomics England. The 100,000 Genomes Project 2022. Available from:
https://www.genomicsengland.co.uk/the-100000-genomes-project/. Accessed July 2024. * Sanderson SC, Lewis C, Patch C, Hill M, Bitner-Glindzicz M, Chitty LS. Opening the “black box” of
informed consent appointments for genome sequencing: a multisite observational study. Genet Med. 2019;21:1083–91. Article PubMed Google Scholar * Nolan J, Buchanan J, Taylor J, Almeida J,
Bedenham T, Blair E, et al. Secondary (additional) findings from the 100,000 Genomes Project: disease manifestation, healthcare outcomes and costs of disclosure. Genet Med. 2023;26:101051.
* Braun V, Clarke V. Thematic analysis: A practical guide. SAGE Publications Ltd; 2021. Google Scholar * MacQueen KM, McLellan E, Kay K, Milstein B. Codebook Development for Team-Based
Qualitative Analysis. Field Methods. 1998;10:31–6. * Roberts K, Dowell A, Nie J-B. Attempting rigour and replicability in thematic analysis of qualitative research data; a case study of
codebook development. BMC Med Res Methodol. 2019;19:66. Article PubMed PubMed Central Google Scholar * Dheensa S, Samuel G, Lucassen AM, Farsides B. Towards a national genomics medicine
service: the challenges facing clinical-research hybrid practices and the case of the 100 000 genomes project. J Med Ethics. 2018;44:397–403. Article PubMed Google Scholar * Halverson
CME, Bland HT, Leppig KA, Marasa M, Myers M, Rasouly HM, et al. Ethical conflicts in translational genetic research: lessons learned from the eMERGE-III experience. Genet Med.
2020;22:1667–72. Article PubMed PubMed Central Google Scholar * Nolan JJ, Forrest J, Ormondroyd E. Additional findings from the 100,000 Genomes Project: A qualitative study of recipient
perspectives. Genet Med. 2024;26:101103. Article PubMed CAS Google Scholar * Ballard LM, Horton RH, Dheensa S, Fenwick A, Lucassen AM. Exploring broad consent in the context of the
100,000 Genomes Project: a mixed methods study. Eur J Hum Genet. 2020;28:732–41. Article PubMed PubMed Central Google Scholar * Lewis C, Sanderson S, Hill M, Patch C, Searle B, Hunter A,
et al. Parents’ motivations, concerns and understanding of genome sequencing: a qualitative interview study. Eur J Hum Genet. 2020;28:874–84. Article PubMed PubMed Central Google Scholar
* Clayton EW, Smith ME, Anderson KC, Chung WK, Connolly JJ, Fullerton SM, et al. Studying the impact of translational genomic research: Lessons from eMERGE. Am J Hum Genet.
2023;110:1021–33. Article PubMed PubMed Central CAS Google Scholar * Francis L, Robertson N. Healthcare practitioners’ experiences of breaking bad news: A critical interpretative meta
synthesis. Patient Educ Couns 2023;107:107574. * Schwartz MLB, McCormick CZ, Lazzeri AL, Lindbuchler DM, Hallquist MLG, Manickam K, et al. A model for genome-first care: returning secondary
genomic findings to participants and their healthcare providers in a large research cohort. Am J Hum Genet. 2018;103:328–37. Article PubMed PubMed Central CAS Google Scholar * Schwartz
MLB, Buchanan AH, Hallquist MLG, Haggerty CM, Sturm AC. Genetic counseling for patients with positive genomic screening results: Considerations for when the genetic test comes first. J Genet
Couns. 2021;30:634–44. Article PubMed PubMed Central Google Scholar * Bouffler SE, Lee L, Lynch F, Martyn M, Lynch E, Macciocca I, et al. Two-step offer and return of multiple types of
additional genomic findings to families after ultrarapid trio genomic testing in the acute care setting: a study protocol. BMJ Open. 2023;13:e072999. Article PubMed PubMed Central Google
Scholar * Finn KS, Lynch J, Aufox S, Bland HT, Chung W, Halverson C, et al. Returning negative results from large-scale genomic screening: Experiences from the eMERGE III network. Am J Med
Genet A. 2021;185:508–16. Article PubMed Google Scholar * Friedrich B, Vindrola-Padros C, Lucassen AM, Patch C, Clarke A, Lakhanpaul M, et al. “A very big challenge”: a qualitative study
to explore the early barriers and enablers to implementing a national genomic medicine service in England. Front Genet. 2023;14:1282034. * Kuiper JML, Borry P, Vears DF, Van Esch H, Cornel
MC, Van Hoyweghen I. Dealing with ambivalence in the practice of advanced genetic healthcare: towards an ethical choreography. Eur J Hum Genet. 2023;31:1387–92. Article PubMed Google
Scholar * Vrijenhoek T, Tonisson N, Kääriäinen H, Leitsalu L, Rigter T. Clinical genetics in transition-a comparison of genetic services in Estonia, Finland, and the Netherlands. J
Community Genet. 2021;12:277–90. Article PubMed PubMed Central CAS Google Scholar * Vears DF, Sénécal K, Borry P. Reporting practices for unsolicited and secondary findings from
next-generation sequencing technologies: Perspectives of laboratory personnel. Hum Mutat. 2017;38:905–11. Article PubMed Google Scholar * Berger R. Now I see it, now I don’t: researcher’s
position and reflexivity in qualitative research. Qual Res. 2013;15:219–34. Article Google Scholar Download references ACKNOWLEDGEMENTS We are very grateful to all of the participants who
took part in this study. FUNDING This study was funded by NHS England. The funder had no role in considering the study design or in the collection, analysis, and interpretation of data, the
writing of the report, or the decision to submit the article for publication. All research at Great Ormond Street Hospital NHS Foundation Trust and UCL Great Ormond Street Institute of
Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The NIHR Great Ormond Street Hospital (GOSH) Biomedical Research Centre (BRC) also part
funds Melissa Hill, Michelle Peter, and Lyn Chitty. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
AUTHOR INFORMATION Author notes * These authors contributed equally: Bethany Stafford-Smith, Jana Gurasashvili. AUTHORS AND AFFILIATIONS * NHS North Thames Genomic Laboratory Hub, Great
Ormond Street Hospital for Children NHS Foundation Trust, London, UK Bethany Stafford-Smith, Jana Gurasashvili, Michelle Peter, Morgan Daniel, Lyn S. Chitty & Melissa Hill * Genetics and
Genomic Medicine, UCL Great Ormond Street Institute of Child Health, London, UK Bethany Stafford-Smith, Jana Gurasashvili, Michelle Peter, Morgan Daniel, Lyn S. Chitty & Melissa Hill *
Sheffield Clinical Genetics Service, Sheffield Children’s NHS Foundation Trust, Sheffield, UK Meena Balasubramanian * Division of Clinical Medicine, University of Sheffield, Sheffield, UK
Meena Balasubramanian * Clinical Genetics, St Michael’s Hospital Bristol, University Hospitals Bristol NHS Foundation Trust, Bristol, UK Lucy Bownass * Northern Genetics Service, Newcastle
Hospitals NHS Foundation Trust, Newcastle, UK Paul Brennan * Peninsula Clinical Genetics Service, Royal Devon University Healthcare NHS Foundation Trust, Exeter, UK Ruth Cleaver * North West
Thames Regional Genetics Service, Northwick Park and St Mark’s Hospital, London, UK Virginia Clowes & Bianca DeSouza * Wessex Clinical Genetics Service, Princess Anne Hospital,
Southampton, UK Philandra Costello * Liverpool Centre for Genomic Medicine, Liverpool Women’s NHS Foundation Trust, Liverpool, UK Louise Dubois * Department of Clinical Genetics, Nottingham
University Hospitals NHS Trust, Nottingham, UK Rachel Harrison * Oxford Centre for Genomic Medicine, ACE building, Nuffield Orthopaedic Centre, Oxford, UK Lara Hawkes * Manchester Centre for
Genomic Medicine, St Mary’s Hospital, Manchester University NHS Foundation Trust, Manchester, UK Elizabeth A. Jones * Division of Evolution, Infection and Genomics, School of Biological
Sciences, Faculty of Biology, Medicine and Health, University of Manchester, Manchester, UK Elizabeth A. Jones * Yorkshire Regional Genetics Service, Chapel Allerton Hospital, Leeds, UK
Alison Kraus * Medical Genetics, Clinical Developmental Sciences, St. George’s University of London, London, UK Meriel McEntagart * Clinical Genetics Unit, Birmingham Women’s and Children’s
NHS Foundation Trust, Birmingham, UK Suresh Somarathi * Clinical Genetics, East Anglian Medical Genetics Service, Cambridge, UK Amy Taylor * Department of Clinical Genetics, Guy’s and St
Thomas’ Hospitals NHS Trust, London, UK Vishakha Tripathi Authors * Bethany Stafford-Smith View author publications You can also search for this author inPubMed Google Scholar * Jana
Gurasashvili View author publications You can also search for this author inPubMed Google Scholar * Michelle Peter View author publications You can also search for this author inPubMed
Google Scholar * Morgan Daniel View author publications You can also search for this author inPubMed Google Scholar * Meena Balasubramanian View author publications You can also search for
this author inPubMed Google Scholar * Lucy Bownass View author publications You can also search for this author inPubMed Google Scholar * Paul Brennan View author publications You can also
search for this author inPubMed Google Scholar * Ruth Cleaver View author publications You can also search for this author inPubMed Google Scholar * Virginia Clowes View author publications
You can also search for this author inPubMed Google Scholar * Philandra Costello View author publications You can also search for this author inPubMed Google Scholar * Bianca DeSouza View
author publications You can also search for this author inPubMed Google Scholar * Louise Dubois View author publications You can also search for this author inPubMed Google Scholar * Rachel
Harrison View author publications You can also search for this author inPubMed Google Scholar * Lara Hawkes View author publications You can also search for this author inPubMed Google
Scholar * Elizabeth A. Jones View author publications You can also search for this author inPubMed Google Scholar * Alison Kraus View author publications You can also search for this author
inPubMed Google Scholar * Meriel McEntagart View author publications You can also search for this author inPubMed Google Scholar * Suresh Somarathi View author publications You can also
search for this author inPubMed Google Scholar * Amy Taylor View author publications You can also search for this author inPubMed Google Scholar * Vishakha Tripathi View author publications
You can also search for this author inPubMed Google Scholar * Lyn S. Chitty View author publications You can also search for this author inPubMed Google Scholar * Melissa Hill View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS MH and LSC conceived the study. MH, LSC, BSS and JG contributed to study design and development of
study materials. MB, LB, PB, RC, VC, PC, BD, LB, RH, LH, EAJ, AK, MM, SS, AT and VT supported recruitment of participants to the study. BSS, JG and MH performed the data analysis with input
from MP and MD. The manuscript was first drafted by BSS, JG and MH. All authors contributed to manuscript revision and read and approved the final version of the manuscript before
submission. CORRESPONDING AUTHOR Correspondence to Melissa Hill. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICS Ethics approval was obtained from
the West Midlands – Edgbaston NHS Research Ethics Committee (15/WM/0258). ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION ADDITIONAL FINDINGS STUDY – TOPIC GUIDE FOR HEALTH PROFESSIONAL INTERVIEWS RIGHTS AND PERMISSIONS OPEN ACCESS This
article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as
you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party
material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s
Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Stafford-Smith, B., Gurasashvili, J., Peter,
M. _et al._ “I’m quite proud of how we’ve handled it”: health professionals’ experiences of returning additional findings from the 100,000 genomes project. _Eur J Hum Genet_ (2024).
https://doi.org/10.1038/s41431-024-01716-6 Download citation * Received: 02 May 2024 * Revised: 24 September 2024 * Accepted: 14 October 2024 * Published: 05 November 2024 * DOI:
https://doi.org/10.1038/s41431-024-01716-6 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative