- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
Download PDF Article Open access Published: 31 July 2020 Rare heterozygous GDF6 variants in patients with renal anomalies Helge Martens ORCID: orcid.org/0000-0002-7294-34761, Imke Hennies2,
Maike Getwan3,4, Anne Christians1, Anna-Carina Weiss5, Frank Brand1, Ann Christin Gjerstad6, Arne Christians7, Zoran Gucev8, Robert Geffers9, Tomáš Seeman10, Andreas Kispert5, Velibor Tasic
ORCID: orcid.org/0000-0002-3377-12458, Anna Bjerre6, Soeren S. Lienkamp3,4, Dieter Haffner ORCID: orcid.org/0000-0002-9601-78132 na1 & …Ruthild G. Weber ORCID:
orcid.org/0000-0001-6610-10801 na1 Show authors European Journal of Human Genetics volume 28, pages 1681–1693 (2020)Cite this article
3267 Accesses
7 Citations
4 Altmetric
Metrics details
Subjects DevelopmentGenetics researchMedical geneticsMutation AbstractAlthough over 50 genes are known to cause renal malformation if mutated, the underlying genetic basis, most easily identified in syndromic cases, remains unsolved in most patients. In search
of novel causative genes, whole-exome sequencing in a patient with renal, i.e., crossed fused renal ectopia, and extrarenal, i.e., skeletal, eye, and ear, malformations yielded a rare
heterozygous variant in the GDF6 gene encoding growth differentiation factor 6, a member of the BMP family of ligands. Previously, GDF6 variants were reported to cause pleiotropic defects
including skeletal, e.g., vertebral, carpal, tarsal fusions, and ocular, e.g., microphthalmia and coloboma, phenotypes. To assess the role of GDF6 in the pathogenesis of renal malformation,
we performed targeted sequencing in 193 further patients identifying rare GDF6 variants in two cases with kidney hypodysplasia and extrarenal manifestations. During development, gdf6 was
expressed in the pronephric tubule of Xenopus laevis, and Gdf6 expression was observed in the ureteric tree of the murine kidney by RNA in situ hybridization. CRISPR/Cas9-derived knockout of
Gdf6 attenuated migration of murine IMCD3 cells, an effect rescued by expression of wild-type but not mutant GDF6, indicating affected variant function regarding a fundamental developmental
process. Knockdown of gdf6 in Xenopus laevis resulted in impaired pronephros development. Altogether, we identified rare heterozygous GDF6 variants in 1.6% of all renal anomaly patients and
5.4% of renal anomaly patients additionally manifesting skeletal, ocular, or auricular abnormalities, adding renal hypodysplasia and fusion to the phenotype spectrum of GDF6 variant
carriers and suggesting an involvement of GDF6 in nephrogenesis.
Similar content being viewed by others Haploinsufficiency of SF3B2 causes craniofacial microsomia Article Open access 03August 2021 Heterozygous variants in the teashirt zinc finger homeobox 3 (TSHZ3) gene in human congenital anomalies of the kidney and urinary tract Article Open access 17 October 2024
Expanding the phenotypic spectrum and clinical severity associated with WLS gene Article 28 April 2023 Introduction
Structural defects of the kidney range from renal agenesis, hypoplasia, and dysplasia to duplication and fusion phenotypes, such as horseshoe kidneys and crossed fused renal ectopia. The
latter is a rare form of renal anomaly where two fused kidneys come to lie on the same side of the spine, each with their own ureter, one of which crossing the midline to enter the bladder
on the contralateral side. As other renal anomalies are also frequently associated with malformations of the urinary tract, such as ureteropelvic or ureterovesical junction obstruction with
hydroureter, or vesicoureteral reflux (VUR), the term congenital anomalies of the kidney and urinary tract (CAKUT) has been coined to subsume these abnormalities. Taken together, CAKUT
phenotypes account for 15–30% of all prenatally detected congenital malformations [1], and cause around 40% of cases with end-stage kidney disease in children and adolescents [2], thus
representing a significant health burden. In around 85% of patients, CAKUT occur sporadically, while in the remaining 15% of cases familial occurrence is observed. CAKUT may occur in
isolation or be part of a mild or complex syndromal disease. Since over 500 syndromes have been associated with CAKUT [3], it is not surprising that one-third of patients are additionally
affected by extrarenal manifestations [4], and that around 20% of patients may have a genetic disorder that is not detected based on standard clinical evaluation [5]. Although over 50 genes
are known to cause CAKUT in humans if mutated [6, 7], <20% of CAKUT manifestations can be explained by aberrations in these genes [8, 9], indicating a high genetic heterogeneity underlying
these defects and making clear the need to identify new genes associated with renal development and malformation. However, the identification of causative genetic variants in cohorts of
sporadic CAKUT patients and CAKUT families is hampered by variable expressivity, meaning that individuals harboring the same variant may have very different phenotypes within the broad CAKUT
spectrum, and by incomplete penetrance implying that variant carriers may exist that are not affected by a CAKUT phenotype at all.
In the last few years, whole-exome sequencing (WES) using next generation sequencing (NGS) techniques was successfully applied to the study of germline variation underlying human CAKUT [10].
Thereby, novel CAKUT-associated genes were identified e.g., by using a linkage-based strategy in large CAKUT families [11, 12], a double hit-based strategy in smaller CAKUT families [13],
an overlapping strategy in a cohort of patients with similar phenotypes [14], and a trio-based de novo strategy in patients with sporadic CAKUT [15, 16]. In addition, WES has improved the
diagnostic yield of genetic CAKUT causes, particularly in syndromic cases [9].
Here, in an effort to identify new genes associated with renal malformation in humans, we used a WES approach to determine the genetic variation underlying renal anomalies in an index
patient with syndromic CAKUT. By a targeted mutational screen in a cohort of 193 further patients with renal anomalies, expression analyses, and functional studies in a cellular system
modified by CRISPR/Cas9 genome engineering and an animal model, we suggest that the candidate gene identified in the index patient, GDF6, plays a role in kidney development and
malformation.
Subjects and methodsPatientsThis study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Boards of Hannover Medical School, Hannover, Germany, Oslo University Hospital, Oslo,
Norway, and University Children’s Hospital, Skopje, North Macedonia. Each family provided informed consent for participation in the study. A total of 194 patients with renal anomalies
comprising 122 males and 72 females with a mean age of 10 years (range 1–35 years) were analyzed. Renal phenotypes of all 194 patients are listed in Supplementary Table 1. Rare heterozygous
GDF6 variants predicted to be disease causing were detected in families F006, H435, and N038.
Family F006The index patient, F006.II.1, born as the second daughter of non-consanguineous German parents is now 3 years old. After birth, renal ultrasound was notable for left-sided crossed fused
renal ectopia (Fig. 1a). Voiding cystourethrography revealed two megaureters, both with orthotopic ostia in the bladder, one connected to the superior pelvis, and the other to the inferior
pelvis of the left-sided fused kidneys (Fig. 1b), and grade-IV VUR in both ureters. Recurrent urinary tract infections were diagnosed. The patient also showed a left-convex torsion
scoliosis, malformations of multiple vertebral bodies of the cervical and thoracic spine including butterfly and fused vertebrae, and a missing fifth sacral vertebral body and coccyx (Fig.
1c, d). A tethered cord was diagnosed because of a low standing conus medullaris, and detethering surgery was performed. The patient also presented with anal atresia and a rectovestibular
fistula, which was surgically corrected. By echocardiography, two small muscular ventricular septal defects and a patent foramen ovale were diagnosed. Ophthalmologic examination revealed
anisometropia with hyperopia, astigmatism, amblyopia, suspected microphthalmia, corneal opacities, and a best-corrected visual acuity of 0.16 in the left eye and of 1.0 in the right eye. In
addition, the patient presented with a left-sided auricle dysplasia and aplasia of the external auditory canal, while the cochleae and the semicircular canals were unremarkable on both sides
according to cranial MRI. Neurological examination was normal and no developmental or intellectual deficits were observed. Her 6-year-old sister and 36-year-old mother presented with
right-sided preauricular pits, while renal ultrasound examinations revealed no abnormalities. Her father was clinically unremarkable and renal sonography was normal.
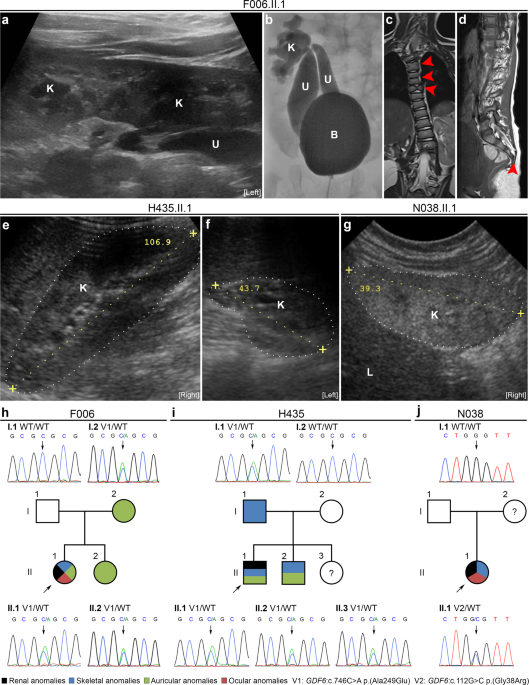
Fig. 1: WES andtargeted sequencing identified heterozygous rare GDF6 variants in three of 194 patients with renal anomalies (1.6%), and in three of 56 patients with renal plus skeletal, ocular or auricular
anomalies (5.4%).
a Renal ultrasound of index patient F006.II.1 was notable for left-sided crossed fused kidney (K) ectopia with megaureters (U). b Voiding cystourethrography of patient F006.II.1 revealed
grade-IV vesicoureteral reflux into two megaureters (U) with orthotopic ostia in the bladder (B) and connected to the superior or inferior dilated kidney (K) pelvis of the fused kidneys. c,
d MRI of the spine of patient F006.II.1 showing malformations of cervical and thoracic vertebral bodies, e.g., fused and butterfly vertebrae (arrows) (c) and missing fifth sacral vertebral
body and coccyx (arrow) (d). e, f Renal ultrasound of patient H435.II.1 at the age of 11 years was unremarkable on the right side (e), while the left kidney was hypodysplastic (f) and
nonfunctional on DMSA scan. g Renal ultrasound of patient N038.II.1 at age 6 months showing renal hypodysplasia, as indicated by reduced size, hyperechogenicity, and reduced corticomedullary
differentiation of the right kidney depicted. The left kidney was also hypodysplastic (not shown), and the patient required kidney transplantation at 5 years of age. h–j Pedigrees of
families F006 (h), H435 (i), and N038 (j) with colored shading indicating phenotypical overlap with respect to renal, skeletal, auricular, and ocular anomalies in individuals with rare GDF6
variants. The GDF6 mutational status (V1: c.746C>A p.(Ala249Glu), V2: c.112G>C p.(Gly38Arg), WT: wild-type) is indicated, and corresponding electropherograms are shown for all analyzed
family members (no DNA sample was available from individual N038.I.2). Clinical and radiological information was not available from individuals H435.II.3 (1 year of age) or N038.I.2. L,
liver; the kidneys are marked by dotted lines (e–g).
Full size imageFamily H435Twelve-year-old patient H435.II.1 was born as the second son of non-consanguineous Macedonian parents. After birth, the boy presented with a right-sided normal kidney and a left-sided
hypodysplastic kidney, a diagnosis confirmed by ultrasound at age 11 years (Fig. 1e, f). The left kidney could not be visualized by a radionuclide scan using DMSA indicating that it is not
functional. In addition, the patient showed a mild torsion scoliosis. The philtrum appeared shorter than usual, a high-arched palate and malocclusion due to prognathism were noted. His ears
were of normal size but had a slight lop deformity. Except for the presence of a short frenulum, external genitalia were unremarkable. No developmental or intellectual deficits were noted.
His 15-year-old brother also had high-arched palate and lop ears, whereby particularly the right ear was smaller and showed a poorly developed antihelix. A triangular-shaped chin was
observed. Neurological examination was normal and no developmental or intellectual deficits were diagnosed. His 1-year-old sister was clinically unremarkable, but renal ultrasound or other
examinations were not performed. His father presented with short stature. Renal sonography of both parents was normal.
Family N038Fourteen-year-old patient N038.II.1 is the only daughter of an African mother and a Caucasian father. She was prenatally diagnosed with oligohydramnios and small kidneys. Renal ultrasound at
age 6 months showed bilateral renal hypodysplasia as indicated by reduced size, hyperechogenicity, and reduced corticomedullary differentiation of the kidneys (right kidney shown in Fig.
1g). After birth, she additionally presented with macrocephaly, high-arched palate, and short narrow palpebral fissures. She required kidney transplantation at the age of 5 years and was
re-transplanted at 9 years of age due to chronic rejection and noncompliance with drug treatment. At 14 years, she has poor kidney function and several comorbidities such as obesity and
hypertension.
AnimalsHusbandry and treatment of Xenopus laevis were approved by the Regierungspräsidium Freiburg, Germany. Mice were kept in accordance with the National Institutes of Health guidelines for the
care and use of laboratory animals. All experiments on mice were approved by the Ethics Board of the Lower Saxony State Office for Consumer Protection and Food Safety. Murine embryos for
gene expression analysis were derived from matings of wild-type mice with NMRI background. For timed pregnancies, vaginal plugs were checked in the morning after mating, and noon was defined
as embryonic day (E) 0.5. Embryos were dissected in phosphate-buffered saline (PBS; Merck, Darmstadt, Germany) and fixed in 4% paraformaldehyde in PBS followed by dehydration using
increasing methanol concentrations, i.e., incubation in 25%, 50%, and 75% methanol for 1 h each. Fixed embryos were stored in 100% methanol at −20 °C prior to in situ hybridization
analyses.
WES and targeted GDF6 sequencingWES was performed on whole-blood DNA of one patient–sibling–parents index family, 30 additional patients with renal malformations, and 74 control individuals using the SureSelectXT Human All
Exon V4 target enrichment kit (Agilent, Santa Clara, CA, USA) on a HiSeq 2000 (Illumina, San Diego, CA, USA) sequencer or the SureSelectXT Human All Exon V5+UTRs target enrichment kit
(Agilent) on a HiSeq 2500 (Illumina) sequencer. All samples were sequenced to a mean target coverage of >50×. Sequencing data were aligned to the human reference genome (hg19) using the
Biomedical Genomics Workbench (Qiagen, Hilden, Germany). WES data of the index family were annotated and prioritized using Ingenuity Variant Analysis (Qiagen) and our in-house NGS data
analysis workflow as described in “Results” and summarized in Supplementary Tables 2 and 3. Using conventional chain termination protocols and a 3130XL Genetic Analyzer (Thermo Fisher
Scientific, Waltham, MA, USA), all coding exons and adjacent intronic regions of GDF6 were analyzed for sequence variants in 163 further patients with kidney anomalies, selected GDF6
variants identified by WES were verified, and familial segregation analysis was done (oligonucleotide sequences are given in Supplementary Table 4). Nucleotide numbering of the identified
variants reflects the nucleotide position in the coding sequence of human GDF6 mRNA (https://www.ncbi.nlm.nih.gov/nuccore/NM_001001557.4) (Supplementary Fig. 1).
Immunohistochemistry, RNAin situ hybridization, CRISPR/Cas9 genome engineering and cellular assays, knockdown and rescue experiments in Xenopus laevis
Procedures are described in “Supplementary materials” (including Supplementary Tables 5–8).
Statistical analysisStatistical analysis was done using MATLAB and Statistics Toolbox Release 2018b (The MathWorks, Inc., Natick, MA, USA). Student’s t-test or Fisher’s exact test were used, as applicable, and
p values are indicated (*p < 0.05, **p < 0.01, and ***p < 0.001).
ResultsUsing WES, a rare heterozygous GDF6 missense variant, c.746C>A p.(Ala249Glu), was detected in the index patientUnder the assumption that NGS techniques are particularly successful in identifying the genetic cause in patients with syndromic CAKUT, we applied WES to whole-blood DNA of female patient
F006.II.1 with a renal malformation (i.e., crossed fused renal ectopia) as well as skeletal (e.g., scoliosis, fused and butterfly vertebrae) (Fig. 1a–d), auricular (i.e., auricle dysplasia
and aplasia of the external auditory canal), ocular (e.g., anisometropia), and other extrarenal anomalies, and of her mother, father, and sister who were not affected by renal anomalies. As
no rare de novo, homozygous, or compound-heterozygous variants predicted to be disease causing could be detected in the high-quality exome data of patient F006.II.1, variants were
prioritized using the strategy summarized in Supplementary Table 2. Prioritization of high-quality variants of patient F006.II.1 by seriousness, rareness, exclusiveness, and localization in
genes (n = 207) reported to be mutated in at least one patient with syndromic CAKUT according to our in-house gene list yielded five rare (minor allele frequency (MAF) ≤ 1%) non-silent
variants not present in controls and predicted to be disease causing by at least one prediction tool (MutationTaster, SIFT, or PolyPhen-2) (Supplementary Tables 2 and 3). One of these five
variants, the GDF6 variant c.746C>A p.(Ala249Glu), was presumed causative in patient F006.II.1 because it is reported to be disease causing by the HGMD Professional (v2018.2; Qiagen) and
ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) databases in patients with skeletal and ocular anomalies [17,18,19,20,21,22] matching the patient’s extrarenal phenotype (Supplementary Table
9). By direct sequencing, the GDF6 c.746C>A variant was confirmed to be heterozygous in the patient as well as in her sister and mother, both affected by the same mild auricular anomaly
(Fig. 1h). The c.746C>A variant is located in the second exon of GDF6 and occurs with a MAF of 0.001948 in the global population cohort and a MAF of 0.003783 in the non-Finnish European
cohort of the Genome Aggregation Database (gnomAD v2.1.1) (Table 1). The amino acid alanine at position 249 is evolutionary conserved and located in the propeptide/prodomain of GDF6
(Supplementary Fig. 1). Based on the ACMG/AMP 2015 guidelines [23], we classified the c.746C>A variant as “pathogenic” (Table 1).
Table 1 Rare heterozygous variants in the GDF6 genepredicted to be “deleterious” identified in 3 of 194 patients with renal anomalies.Full size tableHeterozygous GDF6 missense variants were detected in a total of three of 194 patients (1.6%)
with renal malformations and three of 56 patients (5.4%) with renal plus skeletal, ocular or auricular anomalies
Having identified a GDF6 variant previously associated with skeletal and ocular anomalies in a patient presenting with these phenotypes and additionally with a renal malformation, we
explored the frequency of rare GDF6 variants in a cohort of patients with renal anomalies. A total of 193 additional cases were either analyzed by WES (30 patients) or targeted sequencing of
the GDF6 gene (163 patients). By targeted sequencing, we identified the GDF6 variant c.746C>A in an additional patient, H435.II.1 from North Macedonia, presenting with a hypodysplastic left
kidney (Fig. 1f, i) that was nonfunctional on DMSA scan, mild skeletal, and auricular phenotypes, i.e., torsion scoliosis, high-arched palate, and lop ears (Table 1). The GDF6 c.746C>A
variant was detected in a heterozygous state in the patient, his father who presented with short stature, his brother who had a high-arched palate and lop ears, and a 1-year-old sister who
was not further examined (Fig. 1i). Another rare heterozygous GDF6 missense variant, c.112G>C p.(Gly38Arg), was detected by targeted sequencing in female patient N038.II.1 presenting with
bilateral renal hypodysplasia (Fig. 1g) requiring kidney transplantation at age 5 years in addition to skeletal, i.e., macrocephaly and high-arched palate, and ocular, i.e., short narrow
palpebral fissures, phenotypes (Fig. 1j and Table 1). As the c.112G>C variant was not inherited from her Caucasian father, it may be a de novo variant or have been inherited from her African
mother (Fig. 1j) from whom no DNA sample was available for clarification. Located in the first exon of GDF6 (Supplementary Fig. 1), the c.112G>C variant occurs with a MAF of 0.000656 in the
global population cohort, a MAF of 0.007016 in the African population cohort, and is not present in the non-Finnish European cohort of the gnomAD database v2.1.1 (Table 1). While the
c.112G>C variant was of “uncertain significance” based on the ACMG/AMP 2015 guidelines [23], the prediction tools SIFT and PolyPhen-2 rated it as “damaging” or “possibly damaging” (Table 1).
The amino acid glycine at position 38 is evolutionary highly conserved. In total, we detected rare heterozygous GDF6 missense variants predicted to be disease causing in 3 of 194 families
with renal anomalies (1.6%). Clinical or radiological reevaluation of the skeleton, eye or ear revealed skeletal, ocular, or auricular abnormalities in 56 of the 194 patients with renal
anomalies analyzed. As the three GDF6 variant carriers were among these patients, 3 of 56 patients (5.4%) with renal plus skeletal, ocular, or auricular features carried GDF6 variants with a
disease-causing prediction, a significant frequency increase compared with that in renal anomaly patients without abnormalities of the skeleton, eye, or ear (0/138, p = 0.0231, Fisher’s
exact test).
GDF6 is expressed in the infant human kidney, and in the developing murine urogenital system and pronephros of Xenopus laevisHaving detected rare GDF6 variants in patients with renal anomalies, we went on to explore a possible role for GDF6 in kidney development by determining the expression pattern of GDF6 in the
infant human kidney as well as in Mus musculus and Xenopus laevis during development. GDF6 protein expression was detected in the human infant kidney, most prominently in proximal tubules,
by immunohistochemistry (Fig. 2a). During lower vertebrate development, expression of gdf6 was observed in the area of the pronephros, the embryonic kidney of Xenopus laevis, with particular
enhancement in the tissue just adjacent to the pronephric tubule at stage 38 by whole-mount RNA in situ hybridization. Expression at this stage was also detected around the eye vesicle,
branchial arches, notochord, and neural tube of Xenopus laevis embryos (Fig. 2b). During murine urogenital system development, Gdf6 mRNA was present in the ureteric tips at E11.5 shortly
after onset of metanephros development. At E13.5, expression of Gdf6 in the kidney reached its peak and was found in all compartments, i.e., the ureter and ureteric tips, of the developing
ureteric tree. After onset of collecting duct differentiation at E14.5, Gdf6 transcript levels decreased in the kidney, and renal expression was barely detectable at E18.5. At E11.5, Gdf6
was also detectable in the mesothelial lining of the abdominal cavity and weakly in the epithelium of the urogenital sinus, which develops into the bladder. Expression in the bladder
urothelium vanished after E14.5. Gdf6 mRNA was also found in the nephric duct or differentiated vas deferens of male embryos at all analyzed stages (Fig. 2c, data partly not shown).
Fig.2: GDF6 is expressed in the infant kidney, during pronephros development in Xenopus laevis and murine urinary tract development.
a By immunohistochemical detection of GDF6 and the marker proteins aquaporin-1 (AQP1, straight and convoluted proximal tubule and thin descending limb of loop of Henle), aquaporin-2 (AQP2,
collecting duct), and Tamm-Horsfall protein (THP, thick ascending limb of loop of Henle and distal tubule) in a normal human infant kidney section, GDF6 localized most prominently to
proximal tubules. b By whole-mount RNA in situ hybridization in Xenopus laevis at stage 38, gdf6 was expressed in the tissue surrounding the pronephric tubule (arrow, enlarged image) as well
as in the developing eye, branchial arches, notochord, and neural tube. c RNA in situ hybridization analysis on sagittal sections of the murine kidney and bladder from E11.5 to E18.5. Gdf6
expression was present in all compartments of the developing ureteric tree, including the ureteric tips (ut) and the ureter (u), at E11.5 to E14.5, dropped after onset of collecting duct
differentiation, and was barely detectable at E18.5 (p pelvis, c cortex). In the lower urogenital tract, Gdf6 expression was found in the mesothelial lining (m) of the abdominal cavity at
E11.5, in the undifferentiated urothelium of the urogenital sinus (us) and the bladder (bl) until E14.5, and in the nephric duct (nd) of male embryos at all analyzed stages (data partly not
shown). Sections from three independent murine specimens were analyzed.
Full size imageKnockout of Gdf6 in murine inner medullary collecting duct (mIMCD3) cells impairs cell migration, aneffect rescued by expression of wild-type not mutant GDF6 in Gdf6−/− mIMCD3 cells
Next, we generated an in vitro test system to determine whether GDF6 impacts cell migration, a central process in development, and to assess whether the identified GDF6 variants affect this
function. Using CRISPR/Cas9 technology, a single guide RNA targeting the first coding exon of Gdf6 was applied to knockout Gdf6 in mIMCD3 cells. Two Gdf6−/− mIMCD3 cell clones with
frameshift variants predicted to result in premature stop codons and nonfunctional proteins were identified, i.e., clone 32 harboring the homozygous Gdf6 variant c.377_378delCA
p.(Ser126Cysfs*2), and clone 34 containing the biallelic Gdf6 variants c.373_376delAAGT p.(Lys125Glnfs*9) and c.377_378delCA p.(Ser126Cysfs*2) (Supplementary Fig. 2). A mIMCD3 cell clone
with no mutational event in Gdf6 (clone 2, Gdf6+/+) was also identified and used as a control (Supplementary Fig. 2). No differences in cell viability were observed when comparing mIMCD3
cells and Gdf6+/+ mIMCD3 cell clone 2 with the Gdf6−/− mIMCD3 cell clones 32 or 34 using a cell viability assay (Supplementary Fig. 3).
A time series analyzing mIMCD3 cell migration in a wound healing assay resulted in a reduction of the cell-free area by 50% after 8 h (Supplementary Fig. 4). Therefore, in subsequent
analyses the cell-free areas were determined and compared at 0 h and 8 h. While migration of mIMCD3 cells and Gdf6+/+ mIMCD3 cell clone 2 did not differ significantly, migration of Gdf6−/−
mIMCD3 cell clones 32 and 34 was significantly decreased compared with Gdf6+/+ mIMCD3 cell clone 2 (p = 0.002 and p = 0.005, respectively; Fig. 3a), thereby providing evidence that Gdf6
knockout impacts cell migration. Migration was also significantly impaired in Gdf6+/− mIMCD3 cell clone 30 versus Gdf6+/+ mIMCD3 cell clone 2 (p = 0.041; Supplementary Fig. 5), indicating an
effect on migration of heterozygously mutated cells, a cellular model for patients with heterozygous GDF6 variants. A significant increase in migration of Gdf6−/− mIMCD3 cell clone 32
stably transfected with a wild-type GDF6 expression construct was detected compared with Gdf6−/− mIMCD3 cell clone 32 transfected with empty vector (p = 0.007; Fig. 3b and Supplementary Fig.
6), showing that re-expression of human wild-type GDF6 can rescue the reduced migration. In contrast, migration of Gdf6−/− mIMCD3 cell clone 32 transfected with constructs expressing GDF6
variants c.112G>C or c.746C>A versus GDF6 wild-type was significantly reduced (p = 0.003 and p = 0.008, respectively; Fig. 3b and Supplementary Fig. 6), demonstrating that the identified
GDF6 variants are not functional in this assay.
Fig. 3: Knockout of Gdf6 impacts migration of murine IMCD3 cells, an effect partially reversed by expression of wild-type not mutant GDF6.a Relative to mIMCD3 cells, migration of Gdf6−/− mIMCD3 cells (clones 32 and 34) was significantly decreased compared with Gdf6+/+ mIMCD3 cell clone 2. b The effect of Gdf6 knockout in
mIMCD3 cell clone 32 expressing empty vector (vector control) relative to mIMCD3 cells was partially rescued by stable expression of human wild-type (WT) GDF6 in mIMCD3 cell clone 32.
Conversely, relative cell migration of Gdf6−/− mIMCD3 cell clone 32 stably expressing GDF6 c.112G>C or c.746C>A variants was significantly reduced compared with Gdf6−/− mIMCD3 cell clone 32
stably expressing wild-type GDF6. All results are mean ± SD from three independent experiments. Scale bar: 150 µm. N.s. not significant; **p < 0.01; ***p < 0.001.
Full sizeimageMorpholino (MO) knockdown of gdf6 in Xenopus laevis impairs pronephros development
To explore a possible role of gdf6 in renal development in vivo, we performed gdf6 knockdown unilaterally using a specific gdf6 antisense MO in Xenopus laevis tadpoles, and analyzed the
developing pronephros, constituting the embryonic kidney in lower vertebrates, at stage 39 (Fig. 4). The pronephric area, calculated as log2 ratio of the injected and uninjected side of the
tadpole, was significantly reduced after gdf6 MO injection compared with control MO injection (p = 7.6 × 10−13; Fig. 4). This effect was partially rescued by co-injection of GDF6 mRNA with
gdf6 MO that increased the pronephros area significantly compared with injecting gdf6 MO alone (p = 0.035; Fig. 4). Thereby, we demonstrate a role for gdf6 in pronephric tubule development
in Xenopus laevis.
Fig. 4: Morpholino (MO) knockdown of gdf6 in Xenopus laevis impairs pronephros development.Xenopus laevis stage (st.) 39 tadpoles were unilaterally injected with a control MO or a translation-blocking gdf6 MO with or without co-injection of GDF6 mRNA, and stained with
fluorescein-labeled lectin to visualize the pronephros. The ratios of the pronephros areas of the injected (inj.) and the uninjected (uninj.) side of the embryo were calculated and log2
transformed. Knockdown with the gdf6 MO led to a significantly reduced pronephros area. This effect was significantly rescued by the co-injection of GDF6 mRNA. Results are mean ± SEM from n
embryos analyzed in four independent experiments. N.s. not significant; *p < 0.05; ***p < 0.001.
Full size imageDiscussionIn the present study systematically investigating a role of GDF6 in renal anomalies, we identified rare heterozygous GDF6 variants in 1.6% of patients with kidney malformations. Initially,
we found GDF6 to be associated with renal malformation by an unbiased screen for germline variation in a patient with renal as well as skeletal, auricular, ocular, and other anomalies. This
patient had been chosen for WES analysis because NGS technologies have been particularly successful in identifying causative genes in syndromic CAKUT [9]. Subsequently, we detected two
further renal anomaly patients with rare heterozygous GDF6 variants among the 193 patients additionally analyzed. In line with the extrarenal manifestations of our three renal anomaly
patients carrying GDF6 variants, variants in GDF6 have previously been reported in patients with skeletal phenotypes, i.e., (i) Klippel–Feil syndrome (KFS) with vertebral segmentation
defects frequently associated with scoliosis, rib abnormalities, and Sprengel’s deformity now known as KFS1 [17, 18], (ii) Chiari malformations [17, 24], and (iii) multiple synostoses
syndrome including carpal and tarsal fusions [25,26,27], as well as ocular phenotypes, i.e., (i) microphthalmia [20,21,22], (ii) coloboma [18, 28], (iii) Leber congenital amaurosis or
juvenile retinitis pigmentosa [19], and (iv) glaucoma [29]. By adding three patients with renal anomalies to the two patients previously reported [17, 18], a total of 5 of 86 (5.8%)
individuals carrying rare GDF6 variants described here and in the literature (Supplementary Table 9) are known to be affected by congenital kidney malformations. This finding suggests that
renal sonography is warranted in patients carrying rare GDF6 variants irrespective of the other abnormalities or disorders they may present with. Vice versa, GDF6 mutational analysis may be
advisable in patients with renal anomalies, in particular in those cases additionally affected by the skeletal and ocular phenotypes previously associated with rare GDF6 variants and also
present in the renal anomaly patients with GDF6 variants of this study. Accordingly, the percentage of rare GDF6 variants in patients with renal plus bone, eye, or ear abnormalities was
5.4%, significantly higher than in renal anomaly patients without these extrarenal manifestations. In this context, it is notable that we detected one GDF6 variant, the missense variant
c.746C>A, recurrently in renal anomaly patients, resulting in a significant frequency increase compared with the cohort of the 1000 Genomes Project. Previously, the GDF6 c.746C>A variant was
reported in patients with KFS-like skeletal anomalies [17, 18, 20], Chiari malformation [24], micro- or anophthalmia [20,21,22], and Leber congenital amaurosis or juvenile retinitis
pigmentosa [19] (Supplementary Table 9), demonstrating that it can be associated with a spectrum of different phenotypes. In line with these findings, we detected skeletal anomalies in four
of seven GDF6 c
.746C>A variant carriers from two families, and ocular anomalies in one carrier. Similar to kidney malformations that were present in two of the seven GDF6 c.746C>A variant carriers here,
penetrance is reduced for these abnormalities. Reduced penetrance is a known feature associated with renal anomalies. This is exemplified by a family with Stickler syndrome and a
heterozygous nonsense variant in the BMP4 gene encoding bone morphogenetic protein 4, a ligand related to GDF6, with renal dysplasia in only one of five family members carrying the BMP4
variant [30].
GDF6 encodes growth differentiation factor 6, a member of the BMP family within the transforming growth factor beta (TGF-β) superfamily of ligands that utilize type I and type II
transmembrane serine–threonine kinase receptors [31]. Therefore, in GDF6 variant carriers reduced penetrance for renal malformations may be explained by the complexity of TGF-β signaling
comprising numerous ligands (around 30), receptors, and downstream interacting proteins [31], suggesting some redundancy. The GDF6 amino acid sequence consists of three domains. These are a
signal peptide, a propeptide/prodomain, and a mature receptor-binding carboxy-terminal TGF-domain that is highly conserved between species, i.e., it is more than 90% identical in Xenopus
laevis and mouse [32]. Interestingly, 74% of the GDF6 variants detected in patients with different phenotypes (Supplementary Table 9), including the recurrent c.746C>A variant and the
c.112G>C variant identified in renal anomaly patients here, affect amino acids located in the prodomain of the GDF6 sequence. Although it is less conserved, the prodomain of TGF-β
superfamily ligands is known to regulate the synthesis, extracellular localization, and activity of these proteins [33]. The prodomain of BMP4, another member of the BMP family of ligands,
for instance, is necessary to generate stable BMP4/7 heterodimers with enhanced bioactivity in vivo [34]. Therefore, it is not unexpected that although the c.746C>A variant affects an amino
acid located in the prodomain of GDF6, the activity of variant GDF6 was significantly decreased in a SOX9-reporter luciferase assay [18], and amounts of variant pre-pro-protein and mature
ligand were reduced in the media of transfected cells [19] compared with wild-type. Furthermore, we show here that impaired cell migration of murine Gdf6 knockout cells from the renal inner
medullary collecting duct is rescued by expression of wild-type GDF6 but not of c.746C>A and c.112G>C variants, indicating that both are hypomorphic variants with respect to cell movements,
a fundamental process in development.
BMP signaling is highly implicated in embryogenesis including nephrogenesis and development [35,36,37,38]. CAKUT phenotypes were observed with high penetrance in mice carrying a heterozygous
or homozygous knockout for genes encoding BMP ligands such as BMP4 [39], BMP7 [40], and GDF11 [41]. Pleiotropic defects have been described as a result of Gdf6 knockout, knockdown, or
variation in mice, zebrafish, or Xenopus laevis including defects in joint, ligament, and cartilage formation causing carpal and tarsal fusions and coronal craniosynostosis [42], altered
tail tendon fascicles [43], shorter lengths of digits and dermal flat bones in the skull [44], microphthalmia, anophthalmia, and coloboma [20, 45]. Whether urogenital tract anomalies exist
has not previously been examined in these animal models. Based on the results from our expression and functional studies and the finding that other BMP ligands, such as BMP4, regulate the
budding site and elongation of the developing mouse ureter [39], we propose that the rare GDF6 variants detected in five renal anomaly patients here and previously [17, 18] may be causally
related to their kidney malformation. This proposal is in line with the spectrum of renal abnormalities seen in GDF6 variant carriers that includes renal agenesis [17] and renal
hypodysplasia (two cases here), two anomalies also found in patients carrying BMP4 variants [46]. Kidney agenesis or hypoplasia are potentially caused by defects in ureteric budding and
branching of the ureteric tree induced by abberant BMP signaling as detected in Bmp4 heterozygous knockout mice [39]. It is quite conceivable that such budding and branching defects giving
rise to missing or small dysplastic kidneys also occur in human carriers of rare GDF6 variants because the developing ureteric tree expresses Gdf6, and migration of collecting duct cells
that originate from the ureteric tree is impaired by Gdf6 knockout, as shown here. Similarly, pronephros size was reduced by gdf6 knockdown in Xenopus laevis. We and others additionally
observed kidney abnormalities involving renal fusions, i.e., a horseshoe kidney [18] and a crossed fused renal ectopia (one case here), in patients with rare GDF6 variants, similar to the
skeletal fusions seen (Supplementary Table 9). These data imply that BMP signaling is also linked to skeletal and renal fusions, similar to observations in a conditional Bmp4 knockout mouse
in which reduced BMP signaling resulted in hindlimb fusion [47]. According to an established view, renal fusions result as a consequence of abnormal renal ascent during embryogenesis [48],
possibly connecting GDF6 to the migration of the kidneys as also suggested by our cell migration studies.
In summary, we identified rare heterozygous GDF6 variants in 1.6% of all patients of our renal anomaly cohort, and in 5.4% of those patients additionally manifesting skeletal, ocular, or
auricular abnormalities suggesting that GDF6 is associated with human kidney malformations. The phenotype spectrum identified in renal anomaly patients with GDF6 variants ranged from
hypodysplasia to fusion. Furthermore, Gdf6 expression in the murine developing ureteric tree, diminished migration of murine Gdf6−/− collecting duct cells and impaired pronephros development
after gdf6 knockdown in Xenopus laevis may support a role of GDF6 in kidney development.
References Queisser-Luft A, Stolz G, Wiesel A, Schlaefer K, Spranger J. Malformations in newborn: results based on 30,940 infants and fetuses from the Mainz congenital birth defect
monitoring system (1990–1998). Arch Gynecol Obstet. 2002;266:163–7.
Article CAS PubMed Google Scholar
Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27:363–73.
Article PubMed Google Scholar
Limwongse C. Syndromes and malformations of the urinary tract. In: Avner E, Harmon W, Niaudet P, Yoshikawa N, editors. Pediatric nephrology. Berlin, Heidelberg, Germany: Springer; 2009. p.
121–56.
Chapter Google Scholar
Stoll C, Dott B, Alembik Y, Roth MP. Associated nonurinary congenital anomalies among infants with congenital anomalies of kidney and urinary tract (CAKUT). Eur J Med Genet. 2014;57:322–8.
Article PubMed Google Scholar
Sanna-Cherchi S, Westland R, Ghiggeri GM, Gharavi AG. Genetic basis of human congenital anomalies of the kidney and urinary tract. J Clin Invest. 2018;128:4–15.
Article PubMed PubMed Central Google Scholar
Kosfeld A, Martens H, Hennies I, Haffner D, Weber RG. Kongenitale Anomalien der Nieren und ableitenden Harnwege (CAKUT). Med Genet. 2018;30:448–60.
CAS Google Scholar
van der Ven AT, Vivante A, Hildebrandt F. Novel insights into the pathogenesis of monogenic congenital anomalies of the kidney and urinary tract. J Am Soc Nephrol. 2018;29:36–50.
Article PubMed Google Scholar
Heidet L, Moriniere V, Henry C, De Tomasi L, Reilly ML, Humbert C, et al. Targeted exome sequencing identifies PBX1 as involved in monogenic congenital anomalies of the kidney and urinary
tract. J Am Soc Nephrol. 2017;28:2901–14.
Article CAS PubMed PubMed Central Google Scholar
van der Ven AT, Connaughton DM, Ityel H, Mann N, Nakayama M, Chen J, et al. Whole-exome sequencing identifies causative mutations in families with congenital anomalies of the kidney and
urinary tract. J Am Soc Nephrol. 2018;29:2348–61.
Article PubMed PubMed Central Google Scholar
Nigam A, Knoers N, Renkema KY. Impact of next generation sequencing on our understanding of CAKUT. Semin Cell Dev Biol. 2019;91:104–10.
Article CAS PubMed Google Scholar
Vivante A, Kleppa MJ, Schulz J, Kohl S, Sharma A, Chen J, et al. Mutations in TBX18 cause dominant urinary tract malformations via transcriptional dysregulation of ureter development. Am J
Hum Genet. 2015;97:291–301.
Article CAS PubMed PubMed Central Google Scholar
Brophy PD, Rasmussen M, Parida M, Bonde G, Darbro BW, Hong X, et al. A gene implicated in activation of retinoic acid receptor targets is a novel renal agenesis gene in humans. Genetics.
2017;207:215–28.
Article CAS PubMed PubMed Central Google Scholar
Humbert C, Silbermann F, Morar B, Parisot M, Zarhrate M, Masson C, et al. Integrin alpha 8 recessive mutations are responsible for bilateral renal agenesis in humans. Am J Hum Genet.
2014;94:288–94.
Article CAS PubMed PubMed Central Google Scholar
De Tomasi L, David P, Humbert C, Silbermann F, Arrondel C, Tores F, et al. Mutations in GREB1L cause bilateral kidney agenesis in humans and mice. Am J Hum Genet. 2017;101:803–14.
Article PubMed PubMed Central CAS Google Scholar
Kosfeld A, Kreuzer M, Daniel C, Brand F, Schafer AK, Chadt A, et al. Whole-exome sequencing identifies mutations of TBC1D1 encoding a Rab-GTPase-activating protein in patients with
congenital anomalies of the kidneys and urinary tract (CAKUT). Hum Genet. 2016;135:69–87.
Article CAS PubMed Google Scholar
Kosfeld A, Brand F, Weiss AC, Kreuzer M, Goerk M, Martens H, et al. Mutations in the leukemia inhibitory factor receptor (LIFR) gene and Lifr deficiency cause urinary tract malformations.
Hum Mol Genet. 2017;26:1716–31.
Article CAS PubMed Google Scholar
Tassabehji M, Fang ZM, Hilton EN, McGaughran J, Zhao Z, Bock CEd, et al. Mutations in GDF6 are associated with vertebral segmentation defects in Klippel–Feil syndrome. Hum Mutat.
2008;29:1017–27.
Article CAS PubMed Google Scholar
Asai-Coakwell M, French CR, Ye M, Garcha K, Bigot K, Perera AG, et al. Incomplete penetrance and phenotypic variability characterize Gdf6-attributable oculo-skeletal phenotypes. Hum Mol
Genet. 2009;18:1110–21.
Article CAS PubMed Google Scholar
Asai-Coakwell M, March L, Dai XH, Duval M, Lopez I, French CR, et al. Contribution of growth differentiation factor 6-dependent cell survival to early-onset retinal dystrophies. Hum Mol
Genet. 2013;22:1432–42.
Article CAS PubMed Google Scholar
den Hollander AI, Biyanwila J, Kovach P, Bardakjian T, Traboulsi EI, Ragge NK, et al. Genetic defects of GDF6 in the zebrafish out of sight mutant and in human eye developmental anomalies.
BMC Genet. 2010;11:102.
Article CAS Google Scholar
Gonzalez-Rodriguez J, Pelcastre EL, Tovilla-Canales JL, Garcia-Ortiz JE, Amato-Almanza M, Villanueva-Mendoza C, et al. Mutational screening of CHX10, GDF6, OTX2, RAX and SOX2 genes in 50
unrelated microphthalmia-anophthalmia-coloboma (MAC) spectrum cases. Br J Ophthalmol. 2010;94:1100–4.
Article CAS PubMed Google Scholar
Slavotinek AM, Garcia ST, Chandratillake G, Bardakjian T, Ullah E, Wu D, et al. Exome sequencing in 32 patients with anophthalmia/microphthalmia and developmental eye defects. Clin Genet.
2015;88:468–73.
Article CAS PubMed PubMed Central Google Scholar
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American
College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Article PubMed PubMed Central Google Scholar
Markunas CA, Soldano K, Dunlap K, Cope H, Asiimwe E, Stajich J, et al. Stratified whole genome linkage analysis of Chiari type I malformation implicates known Klippel-Feil syndrome genes as
putative disease candidates. PLoS ONE. 2013;8:e61521.
Article CAS PubMed PubMed Central Google Scholar
Drage Berentsen R, Haukanes BI, Juliusson PB, Rosendahl K, Houge G. A novel GDF6 mutation in a family with multiple synostoses syndrome without hearing loss. Mol Syndromol. 2019;9:228–34.
Article PubMed CAS Google Scholar
Terhal PA, Verbeek NE, Knoers N, Nievelstein R, van den Ouweland A, Sakkers RJ, et al. Further delineation of the GDF6 related multiple synostoses syndrome. Am J Med Genet A. 2018;176:225–9.
Article CAS PubMed Google Scholar
Wang J, Yu T, Wang Z, Ohte S, Yao R-E, Zheng Z, et al. A new subtype of multiple synostoses syndrome is caused by a mutation in GDF6 that decreases its sensitivity to noggin and enhances its
potency as a BMP signal. J Bone Min Res. 2016;31:882–9.
Article CAS Google Scholar
Chassaing N, Causse A, Vigouroux A, Delahaye A, Alessandri J-L, Boespflug-Tanguy O, et al. Molecular findings and clinical data in a cohort of 150 patients with anophthalmia/microphthalmia.
Clin Genet. 2014;86:326–34.
Article CAS PubMed Google Scholar
Huang X, Xiao X, Jia X, Li S, Li M, Guo X, et al. Mutation analysis of the genes associated with anterior segment dysgenesis, microcornea and microphthalmia in 257 patients with glaucoma.
Int J Mol Med. 2015;36:1111–7.
Article CAS PubMed Google Scholar
Nixon TRW, Richards A, Towns LK, Fuller G, Abbs S, Alexander P, et al. Bone morphogenetic protein 4 (BMP4) loss-of-function variant associated with autosomal dominant Stickler syndrome and
renal dysplasia. Eur J Hum Genet. 2019;27:369–77.
Article CAS PubMed Google Scholar
De Caestecker M. The transforming growth factor-b: superfamily of receptors. Cytokine Growth Factor Rev. 2004;15:1–11.
Article CAS PubMed Google Scholar
Chang C, Hemmati-Brivanlou A. Xenopus GDF6, a new antagonist of noggin and a partner of BMPs. Development. 1999;126:3347–57.
Article CAS PubMed Google Scholar
Harrison CA, Al-Musawi SL, Walton KL. Prodomains regulate the synthesis, extracellular localisation and activity of TGF-β superfamily ligands. Growth Factors. 2011;29:174–86.
Article CAS PubMed Google Scholar
Neugebauer JM, Kwon S, Kim HS, Donley N, Tilak A, Sopory S, et al. The prodomain of BMP4 is necessary and sufficient to generate stable BMP4/7 heterodimers with enhanced bioactivity in vivo.
Proc Natl Acad Sci USA. 2015;112:E2307–16.
Article CAS PubMed PubMed Central Google Scholar
Cain JE, Hartwig S, Bertram JF, Rosenblum ND. Bone morphogenetic protein signaling in the developing kidney: present and future. Differentiation. 2008;76:831–42.
Article CAS PubMed Google Scholar
Nishinakamura R, Sakaguchi M. BMP signaling and its modifiers in kidney development. Pediatr Nephrol. 2014;29:681–6.
Article PubMed Google Scholar
Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, et al. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis. 2014;1:87–105.
Article CAS PubMed PubMed Central Google Scholar
Mamo TM, Wittern AB, Kleppa M-J, Bohnenpoll T, Weiss A-C, Kispert A. BMP4 uses several different effector pathways to regulate proliferation and differentiation in the epithelial and
mesenchymal tissue compartments of the developing mouse ureter. Hum Mol Genet. 2017;26:3553–63.
Article CAS PubMed Google Scholar
Miyazaki Y, Oshima K, Fogo A, Hogan BL, Ichikawa I. Bone morphogenetic protein 4 regulates the budding site and elongation of the mouse ureter. J Clin Invest. 2000;105:863–73.
Article CAS PubMed PubMed Central Google Scholar
Dudley AT, Lyons KM, Robertson EJ. A requirement for bone morphogenetic protein-7 during development of the mammalian kidney and eye. Genes Dev. 1995;9:2795–807.
Article CAS PubMed Google Scholar
Esquela AF, Lee SJ. Regulation of metanephric kidney development by growth/differentiation factor 11. Dev Biol. 2003;257:356–70.
Article CAS PubMed Google Scholar
Settle SH, Rountree RB, Sinha A, Thacker A, Higgins K, Kingsley DM. Multiple joint and skeletal patterning defects caused by single and double mutations in the mouse Gdf6 and Gdf5 genes. Dev
Biol. 2003;254:116–30.
Article CAS PubMed Google Scholar
Mikic B, Rossmeier K, Bierwert L. Identification of a tendon phenotype in GDF6 deficient mice. Anat Rec. 2009;292:396–400.
Article Google Scholar
Indjeian VB, Kingman GA, Jones FC, Guenther CA, Grimwood J, Schmutz J, et al. Evolving new skeletal traits by cis-regulatory changes in bone morphogenetic proteins. Cell. 2016;164:45–56.
Article CAS PubMed PubMed Central Google Scholar
Asai-Coakwell M, French CR, Berry KM, Ye M, Koss R, Somerville M, et al. GDF6, a novel locus for a spectrum of ocular developmental anomalies. Am J Hum Genet. 2007;80:306–15.
Article CAS PubMed Google Scholar
Weber S, Taylor JC, Winyard P, Baker KF, Sullivan-Brown J, Schild R, et al. SIX2 and BMP4 mutations associate with anomalous kidney development. J Am Soc Nephrol. 2008;19:891–903.
Article CAS PubMed PubMed Central Google Scholar
Suzuki K, Adachi Y, Numata T, Nakada S, Yanagita M, Nakagata N, et al. Reduced BMP signaling results in hindlimb fusion with lethal pelvic/urogenital organ aplasia: a new mouse model of
sirenomelia. PLoS ONE. 2012;7:e43453.
Article CAS PubMed PubMed Central Google Scholar
Taghavi K, Kirkpatrick J, Mirjalili SA. The horseshoe kidney: Surgical anatomy and embryology. J Pediatr Urol. 2016;12:275–80.
Article CAS PubMed Google Scholar
Download references
AcknowledgementsWe deeply appreciate the participation of patients and families in this study. We wish to thank Achim Gossler and Michael Klintschar, Hannover Medical School, Hannover, Germany for providing
mIMCD3 cells and human infant kidney sections, respectively. The Xenopus laevis gdf6 construct for whole-mount RNA in situ hybridization was kindly provided by Alexandra Schambony,
Friedrich-Alexander University Erlangen-Nuremberg, Erlangen, Germany. We wish to acknowledge the assistance of the Cell Sorting Core Facility of Hannover Medical School. Open Access Funding
provided by Projekt DEAL.
FundingThis research was supported by grants from the Else Kröner-Fresenius-Stiftung to RGW (2014_A234), the Deutsche Forschungsgemeinschaft to AnC (KO5614/2-1) and SSL (LI1817/2-1), the Swiss
National Science Foundation (SNF) to SSL (NCCR Kidney.CH), and the Hochschulinterne Leistungsförderung (HiLF) program of Hannover Medical School to FB. The Cell Sorting Core Facility of
Hannover Medical School is supported by the Braukmann-Wittenberg-Herz-Stiftung and the Deutsche Forschungsgemeinschaft.
Author informationAuthor notesThese authors contributed equally: Dieter Haffner, Ruthild G. Weber
Authors and Affiliations Department of Human Genetics, Hannover Medical School, 30625, Hannover, Germany
Helge Martens, Anne Christians, Frank Brand & Ruthild G. Weber
Department of Pediatric Kidney, Liver and Metabolic Diseases, Hannover Medical School, 30625, Hannover, Germany
Imke Hennies & Dieter Haffner
Department of Medicine, Renal Division, University Medical Center Freiburg, Faculty of Medicine, University of Freiburg, 79110, Freiburg, Germany
Maike Getwan & Soeren S. Lienkamp
Institute of Anatomy and Zurich Center for Integrative Human Physiology (ZIHP), University of Zurich, 8057, Zurich, Switzerland
Maike Getwan & Soeren S. Lienkamp
Institute of Molecular Biology, Hannover Medical School, 30625, Hannover, Germany
Anna-Carina Weiss & Andreas Kispert
Division of Paediatric and Adolescent Medicine, Oslo University Hospital, 0424, Oslo, Norway
Ann Christin Gjerstad & Anna Bjerre
Department of Neuropathology, Institute of Pathology, Hannover Medical School, 30625, Hannover, Germany
Arne Christians
Medical Faculty Skopje, University Children’s Hospital, 1000, Skopje, North Macedonia
Zoran Gucev & Velibor Tasic
Genome Analytics Research Group, Helmholtz Centre for Infection Research, 38124, Braunschweig, Germany
Robert Geffers
Department of Paediatrics and Transplantation Center, University Hospital Motol, Second Faculty of Medicine, Charles University, 150 06, Prague, Czech Republic
Tomáš Seeman
AuthorsHelge MartensView author publications You can also search for this author inPubMed Google Scholar
Imke HenniesView author publications You can also search for this author inPubMed Google Scholar
Maike GetwanView author publications You can also search for this author inPubMed Google Scholar
Anne ChristiansView author publications You can also search for this author inPubMed Google Scholar
Anna-Carina WeissView author publications You can also search for this author inPubMed Google Scholar
Frank BrandView author publications You can also search for this author inPubMed Google Scholar
Ann Christin GjerstadView author publications You can also search for this author inPubMed Google Scholar
Arne ChristiansView author publications You can also search for this author inPubMed Google Scholar
Zoran GucevView author publications You can also search for this author inPubMed Google Scholar
Robert GeffersView author publications You can also search for this author inPubMed Google Scholar
Tomáš SeemanView author publications You can also search for this author inPubMed Google Scholar
Andreas KispertView author publications You can also search for this author inPubMed Google Scholar
Velibor TasicView author publications You can also search for this author inPubMed Google Scholar
Anna BjerreView author publications You can also search for this author inPubMed Google Scholar
Soeren S. LienkampView author publications You can also search for this author inPubMed Google Scholar
Dieter HaffnerView author publications You can also search for this author inPubMed Google Scholar
Ruthild G. WeberView author publications You can also search for this author inPubMed Google Scholar
ContributionsHM, AnC, FB, DH, and RGW designed research; HM analyzed WES data, performed and provided figures of all experiments except RNA in situ hybridization and Xenopus studies, compiled literature
data; HM, AnC, FB, and RGW analyzed these data; MG and SSL performed, analyzed, and provided figures of Xenopus studies; ACW and AK performed, analyzed, and provided figures of RNA in situ
hybridization on murine tissue; IH, ACG, ZG, TS, VT, AB, and DH performed clinical examinations, contributed patient materials, data, and clinical expertise; ArC provided technical
expertise; RG generated WES data; HM, AnC, FB, DH, and RGW wrote the manuscript; all authors reviewed and revised the manuscript.
Corresponding author Correspondence to Ruthild G. Weber.
Ethics declarations Conflict of interestThe authors declare that they have no conflict of interest.
Additional informationPublisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary informationSupplementarymaterialSupplementary Table 3Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or
other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in
the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the
copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
Reprints and permissions
About this articleCite this article Martens, H., Hennies, I., Getwan, M. et al. Rare heterozygous GDF6 variants in patients with renal anomalies. Eur J Hum Genet 28, 1681–1693 (2020).
https://doi.org/10.1038/s41431-020-0678-9
Download citation
Received: 20 March 2020
Revised: 05 June 2020
Accepted: 15 June 2020
Published: 31 July 2020
Issue Date: December 2020
DOI: https://doi.org/10.1038/s41431-020-0678-9
Share this article Anyone you share the following link with will be able to read this content:
Get shareable link Sorry, a shareable link is not currently available for this article.
Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative
This article is cited by Heterozygous variants in the DVL2 interaction region of DACT1 cause CAKUT and features of Townes–Brocks syndrome 2 Anne ChristiansEsra KesdirenRuthild G. Weber Human
Genetics (2023)







.jpg)
