- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
We designed dielectric gels, a new type of polymer-based dielectric material. By using solvents with high dielectric constants, the gels achieve a unique combination of ultra-high dielectric
constant, low elastic modulus, and excellent transparency, which are extremely challenging or impossible to realize with traditional polymer dielectrics. The gels exhibit high
stretchability (stretch of approximately 10) and low mechanical hysteresis. We demonstrated the use of the dielectric gels by fabricating a bioinspired tunable lens, the focal length of
which can be adjusted by varying the applied voltage. We believe that the dielectric gels, as a new type of polymer dielectric, offer new opportunities for soft robotics, sensors,
electronics, optics, and biomimetics.
As soft and flexible electrical insulators, polymer dielectrics have enabled diverse modern technologies, including electric power systems1, flexible electronics2, non-volatile memory
devices3, electrocaloric cooling4, and soft robotics5,6,7, benefiting from their diverse functionality. Deformable polymer dielectrics can express mechanical motions in response to
electrical stimulation: when a dielectric membrane is subjected to a voltage across its thickness, the membrane suffers an electrostatic force, the so-called Maxwell stress, and thus
squeezes in the thickness direction and expands in the area direction. Such dielectrics behave as actuators, as demonstrated by ferroelectric polymers8, liquid crystal elastomers9,
electrostrictive polymers10, and dielectric elastomers5. However, the high voltage needed for actuation and the poor mechanical reliability hinder developments in practical applications.
Improving the dielectric constant and lowering the elastic modulus of the polymer dielectrics are efficient ways of lowering the actuating voltage11,12. Conductive particles or high-k
ceramics have been filled into elastomers to increase their dielectric constants, but these rigid fillers have also dramatically increased the elastic modulus and reduced the
extensibility13,14,15,16. Another approach is to use fluids as fillers; liquid–metal microdroplets have been integrated into elastomer substrates, which increased the dielectric constant by
over 400% and avoided the internal compliance mismatch of rigid fillers17.
However, polymer dielectrics with optical transmittance functionality are emerging materials with practical significance in next-generation flexible displays and flexible touchscreen panels.
By fabricating graphene interlayers to form polymer/graphene/polymer structures, Kim et al. prepared flexible and transparent dielectric films with a high dielectric constant (ε) of 51 and
a transmittance of ~90%18. Park et al. used ultralong metal nanofibers as fillers to increase ε and obtained flexible and transparent dielectric cellulose nanofiber films (ε above 9.2 with a
high transmittance of 90%)19.
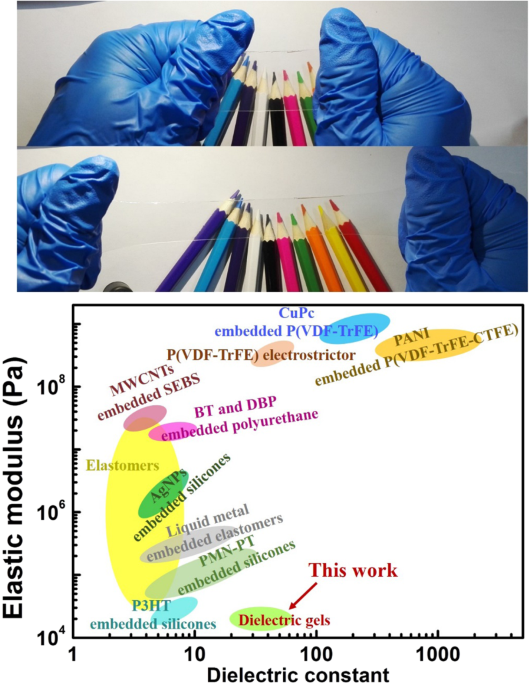
Herein, we introduce a new type of polymer dielectric, dielectric gels. The new materials achieve a unique combination of ultra-high ε (30–50), low elastic modulus (from 20 to 60 KPa), and
excellent transparency (~99%). A gel is a polymer composite with a three-dimensional polymer network that contains a large amount of solvent20,21. Gels are present as solid-state soft
materials. We designed dielectric gels by using solvents with ultra-high ε and a polymer network that matched well with the solvents. Dielectric gels offer new opportunities for soft
robotics, sensors, electronics, optics, and biomimetics.
We mixed appropriate amounts of 4-acryloylmorpholine (ACMO, monomer), N,N′-methylenebis(acrylamide) (MBA, cross-linker), and 1-hydroxycyclohexyl phenyl ketone (photoinitiator 184) with
solvents to form gelation precursor solutions. Unless otherwise stated, the solvent content was fixed at 50 wt%. The precursor solution was poured into a transparent glass mold with a
silicone spacer and release films. After irradiation with ultraviolet light (365 nm, 400 W power) for 5 min, the dielectric gel was formed. We controlled the thickness of the dielectric gel
by adjusting the thickness of the silicone spacer. Dielectric gels for electroactive actuation and tunable lens experiments were synthesized by a similar photocuring process. The solvent
(PC:EC = 1:4) content was fixed at 20 wt%, the volume ratio of 4-acryloylmorpholine (ACMO) and 2-ethylhexyl acrylate (2-EHA) monomers was fixed at 1:1, and the molar ratios of the
photoinitiator and monomer and the cross-linker and monomer were fixed at 1% and 0.1%, respectively.
Mechanical tests were performed on an electronic tensile machine (CMT6503, MTS) with a 500 N load cell. The gels were cut into dumbbell shapes (testing dimensions of 12.0 × 2.0 × 2.0 mm3)
for the tests.
Dielectric tests were performed on a broadband dielectric/impedance spectrometer (Novocontrol GmbH). The gels were 1-mm thick, the testing cupper electrodes were 30 mm in diameter, and the
testing Vrms (Volt root mean square) was set at 1 V. The gel samples were treated without metal sputtering on their surfaces.
The transparency tests were performed on a ultraviolet–visible spectrophotometer (UV–vis) spectrophotometer (PE Lambda950, Instrument Analysis Center of Xi’an Jiaotong University). The gels
for the tests were 1-mm thick.
The lenses were fabricated from two gel membranes with silicone oil sealed between them. The membranes were equally biaxially pre-stretched by λ = 2.5 with an original thickness h = 1 mm.
We chose propylene carbonate (PC) and ethylene carbonate (EC) as solvents for the dielectric gels (Fig. 1a) because they possess ultra-high ε (65 for PC and 90 for EC)22, good chemical
stability, low vapor pressure, and low toxicity23. They are widely used as electrolyte solvents in lithium-ion batteries. The melting points of PC and EC are −48.8 °C and 36.4 °C,
respectively24. Although EC is icy at room temperature, it can be mixed with PC to form a homogeneous solution. The mixed solution is liquid at room temperature and has a higher ε than PC.
We mixed appropriate amounts of 4-acryloylmorpholine (ACMO, monomer), N,N′-methylenebis (acrylamide) (MBA, cross-linker), and 1-hydroxycyclohexyl phenyl ketone (photoinitiator 184) with the
solvents to form gelation precursor solutions (Fig. 1b). Unless otherwise stated, the solvent content was fixed at 50 wt%. The precursor solution was poured into a transparent glass mold
with a silicone spacer and release films. After irradiation with ultraviolet light (365 nm, 400 W power) for 5 min, the dielectric gel was formed (Fig. 1c, d).
a Schematic of a dielectric gel. Both the polymer chain and the solvents in the gel system are dielectric materials. b Ingredients: monomer, 4-acryloylmorpholine (ACMO); cross-linker
N,N′-methylenebis (acrylamide) (MBA); photoinitiator, 1-hydroxycyclohexyl phenyl ketone; and solvents, propylene carbonate (PC) and ethylene carbonate (EC). c Fabrication process of a
dielectric gel. The gel was synthesized in minutes by photoinitiated polymerization. d Photographs of the dielectric gels demonstrating good stretchability and high transparency. The upper
photograph is the gel without stretching and the lower photograph is the stretched gel
The gels were then cut into dumbbell shapes with dimensions 12.0 × 2.0 × 2.0 mm3. Mechanical tests were performed on an electronic tensile machine with a 500 N load cell. The stretching rate
was set at 100 mm min−1 during both loading and unloading. The gels possess a low elastic modulus (tens of KPa) and good stretchability (with elongation at break ranging from ~200 to
~1100%), as shown in Fig. 2a, b. The cross-linker content of a gel greatly affects its mechanical properties: as the cross-linker content increases, the elastic modulus of a gel increases
and the elongation at break decreases. A poly(4-acryloylmorpholine) gel with a cross-linker content of 0.15% was chosen for the cyclic loading–unloading tests (Fig. 2c). The initial loading
cycle was set with a strain of 100%. After the loading–unloading cycle, the sample was immediately reloaded to 300% strain and then to 500% strain. The gel showed negligible mechanical
hysteresis at each strain cycle, and it fully recovered its original length after unloading. A long-term cyclic loading–unloading test was also performed, in which the strain was set to
400%; the 100th cycle curve almost coincided with that of the initial cycle, and the elastic modulus remained unchanged (Figure S1).
The polymer and solvent contents of the testing gels are fixed at 50 wt% each. a Stress–strain curves of dielectric gels with different cross-linker contents (molar ratios to monomer, as
labeled). Each gel was stretched until rupture. b Elastic modulus (calculated from the stress–strain curves at a strain of 10%) and strain at break, plotted against the cross-linker content.
c Cyclic loading of a dielectric gel (cross-linker content of 0.15%). The initial loading cycle was set to 100% strain. After the loading–unloading cycle, the sample was immediately
reloaded to 300% strain for the second cycle and then 500% strain for the third cycle. d Plot of the dielectric constants versus testing frequency for the dielectric gels with PC and EC/PC
solvents; the ratio represents the volume ratio. e Plot of the dielectric dissipation factor versus testing frequency for the dielectric gels with PC and EC/PC solvents. f Transmittance test
of a 1-mm-thick dielectric gel in the visible range
Dielectric properties were tested on a broadband dielectric/impedance spectrometer. The testing samples were 1-mm thick, and the testing copper electrodes were 30 mm in diameter. The gel
samples were soft enough to be in good contact with the electrodes. The testing Vrms was set at 1 V. Figure 2d, e shows the dielectric properties of gels with solvents of different EC/PC
volumetric ratios. The gels exhibit a high ε (30~50) over a broad frequency range (1 K–10 MHz). The solvent composition greatly affects the ε of the gels. A higher EC content results in a
higher ε. As the frequency increases, the ε decreases slightly, as shown in Fig. 2d. This may be because the switching of the small-molecule dipoles is unable to match the switching of the
electric field at high frequencies. The dielectric loss (loss tangent) of the gels was maintained at








