- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
You have full access to this article via your institution. Download PDF INTESTINAL STROMAL CELLS PLAY KEY ROLES IN THE REGULATION OF INTESTINAL STEM CELL BIOLOGY, INJURY-INDUCED INTESTINAL
EPITHELIAL REGENERATION, AND TUMORIGENESIS. ROULIS AND COLLEAGUES PRESENT EVIDENCE INDICATING THAT PGE 2SECRETED FROM PTGS2-EXPRESSING FIBROBLASTS INDUCES EXPANSION OF SCA-1 +RESERVE
STEM-LIKE CELLS AND ACCELERATES INTESTINAL ADENOMA FORMATION VIA AN EP4-YAP1 PATHWAY. Colorectal cancer (CRC) is the third most common malignancy and the second leading cause of cancer
deaths in the United States. Epidemiologic and experimental evidence strongly implicates chronic inflammation as a risk factor for multiple types of cancers, including CRC. Moreover,
epidemiologic and clinical evidence that nonsteroidal anti-inflammatory drugs, including selective inhibitors of cyclooxygenase-2 (COX-2, also known as prostaglandin-endoperoxide synthases
2, PTGS2), suppress CRC formation and growth supports the hypothesis that COX-2 promotes CRC progression. COX enzymes convert arachidonic acid into prostanoids, including prostaglandins
(PGs) such as PGE2, PGD2, PGF2α, PGI2 and thromboxane A2 (TxA2) via specific PG synthases. Among prostanoids, PGE2 is clearly the most abundant in human CRC and only PGE2 and PGI2 levels are
elevated in human CRC specimens as compared to matched normal tissues.1 Moreover, multiple lines of in vivo evidence demonstrate that only PGE2 accelerates intestinal tumor formation and
growth.1 The roles of other PGs in CRC remain unclear and controversial.1 PGE2 exerts its effects by binding to EP receptors (EP1, EP2, EP3, and EP4) that belong to the family of G
protein-coupled receptors. Similarly, animal studies demonstrate that PGE2 promotes intestinal tumorigenesis via EP2 and EP4, but not EP3.1 The role of EP1 in CRC remains unclear.1 Although
COX-2 is predominantly expressed in stromal cells such as macrophages, (myo)fibroblasts, and endothelial cells in human and rodent intestinal tumors, it was unclear which COX-2-expressing
cells contribute to CRC. A recent study showed that macrophage-specific overexpression of COX-2 promoted colon tumor burden in _Apc__Min/+_ mice, a mouse model of CRC.2 In a recent _Nature_
paper, Roulis et al. reported for the first time that fibroblast-specific deletion of _Cox-2_ attenuated intestinal tumor formation but did not affect tumor size in two mouse models of CRC,
_Apc__Min/+_ mice and azoxymethane-treated mice.3 Moreover, they confirmed that fibroblast-specific overexpression of Cox-2 accelerated tumor formation in _Apc__Min/+_ mice. Their results
demonstrate that COX-2-expressing fibroblasts contribute to CRC initiation. In addition, they found that intestinal fibroblasts are a predominant source of COX-2 in the intestine under
normal physiological conditions. It is unclear whether COX-2 is also mainly expressed in intestinal tumor-associated fibroblasts. Intestinal stem cells (ISCs) are responsible for homeostatic
epithelial renewal and injury-induced intestinal epithelial regeneration. Active Lgr5+ ISCs are responsible for homeostatic intestinal epithelial renewal, whereas surviving Lgr5+ ISCs,
reserve ISCs such as Bmi1+ cells, and other mature cells are responsible for intestinal epithelial regeneration during injury and repair.4 Understanding how the ISC niche is regulated during
injury and repair may provide new therapeutic approaches for human intestinal disorders. Although the stromal compartment plays a key role in the regulation of ISC niches, it is not clear
which types of stromal cells produce different juxtracrine factors that are required for maintenance of ISC niches. Virshup’s group has identified intestinal myofibroblasts that produce Wnts
and RSPO3, involved in the regulation of ISCs in vivo.5 The results reported in this recent _Nature_ paper showed for the first time that PGE2 secreted from fibroblasts induced expansion of
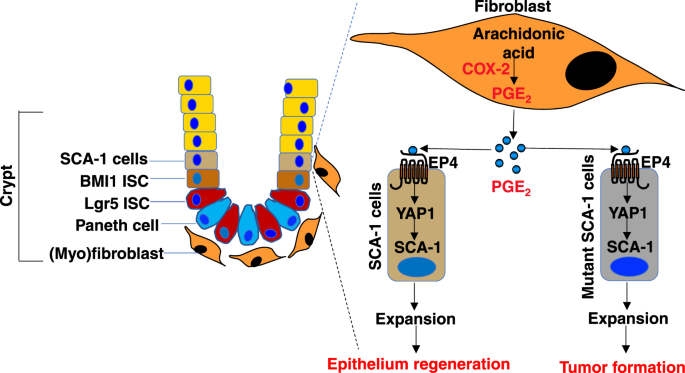
SCA-1+ reserve intestinal stem-like cells via EP4 receptor signaling in a 3D organotypic co-culture model of primary intestinal fibroblasts and fresh crypts (Fig. 1).3 However, further
studies are necessary to confirm whether these SCA-1+ cells are reserve ISCs. PGE2 has been shown to promote colonic epithelial regeneration via Yap1 in a mouse model of colitis.6 Similarly,
PGE2 secreted from mesenchymal stem cells (MSCs) attenuated acute liver failure by induction of hepatocyte proliferation and survival via YAP in a mouse model of acute liver failure.7 Given
that ISCs are responsible for injury-induced intestinal regeneration, it is conceivable that PGE2 promotes regeneration of intestinal epithelium via induction of ISC expansion following
injury. Indeed, Roulis et al. showed that PGE2 induced expansion of SCA-1+ reserve intestinal stem-like cells via an EP4-Yap1 pathway in their 3D organotypic co-culture model (Fig. 1).3 It
would be very interesting to determine whether PGE2 secreted from fibroblasts promotes intestinal epithelial regeneration by induction of reserve ISC expansion via an EP4-Yap1 pathway in
mouse models of inflammatory bowel disease. Cancer stem cells (CSCs) or tumor-initiating cells (mutant stem cells) are thought to be responsible for tumor initiation, growth, metastatic
spread, relapse, and recurrence. How CSCs are formed, maintained, and expanded are not fully understood. Similar to ISCs, stromal cells also contribute to CSC formation and expansion via
production of certain factors. For example, PGE2 secreted from MSCs induced colonic CSC formation in vitro.8 PGE2 secreted from myeloid-derived suppressive cells might also induce cervical
CSC formation and expansion in vitro.9 Moreover, PGE2 has been shown to induce colonic CSC expansion by activating NF-κB via EP4–PI3K and EP4–MAPK signaling and promote liver metastases in
an orthotopic mouse model of metastatic CRC.10 In CRC cell lines, PGE2 induced YAP1 expression and transcriptional activity, which resulted in induction of both _COX-2_ and _EP4_ gene
transcription.6 This positive signaling loop of PGE2-YAP1 induced cell proliferation as well.6 Roulis et al. presented data showing that epithelial-specific deletion of _Yap1_ or _Ep4_ in
_Apc__Min/+_ mice resulted in reduction of Yap1 target gene expression, Sca-1+ cell expansion, and tumor formation when compared to control mice. Since Sca-1 is one of the CSC markers and
also one of Yap-1 target genes, their results suggest that PGE2 promotes intestinal adenoma formation by induction of mutant ISC expansion via the EP4-Yap1 pathway in a spontaneous murine
model of CRC (Fig. 1). Further investigation is needed to provide direct evidence demonstrating that PGE2 secreted from fibroblasts accelerates intestinal adenoma formation by induction of
mutant ISC expansion via the EP4-Yap1 pathway. Collectively, these findings not only reveal a novel mechanism underlying contribution of fibroblast-derived PGE2 to expansion of SCA-1+
reserve intestinal stem-like cells and intestinal adenoma formation (Fig. 1), but also support the rationale for developing EP4 antagonists as new anti-tumor agents in CRC treatment.
Frankly, targeting colonic CSCs may present a novel therapeutic approach for CRC patients. REFERENCES * Wang, D. & DuBois, R. N. _J. Clin. Invest._ 128, 2732–2742 (2018). Article Google
Scholar * Hull, M. A. et al. _Sci. Rep._ 7, 6074 (2017). Article Google Scholar * Roulis, M. et al. _Nature_ 580, 524–529 (2020). Article CAS Google Scholar * Santos, A. J. M., Lo, Y.
H., Mah, A. T. & Kuo, C. J. _Trends Cell Biol._ 28, 1062–1078 (2018). Article CAS Google Scholar * Greicius, G. et al. _Proc. Natl. Acad. Sci. USA_ 115, E3173–E3181 (2018). Article
CAS Google Scholar * Kim, H. B. et al. _Gastroenterology_ 152, 616–630 (2017). Article CAS Google Scholar * Liu, Y. et al. _FASEB J._ 33, 2514–2525 (2019). Article CAS Google Scholar
* Li, H. J., Reinhardt, F., Herschman, H. R. & Weinberg, R. A. _Cancer Discov._ 2, 840–855 (2012). Article CAS Google Scholar * Kuroda, H. et al. _Oncotarget_ 9, 36317–36330 (2018).
Article Google Scholar * Wang, D., Fu, L., Sun, H., Guo, L. & DuBois, R. N. _Gastroenterology_ 149, 1884–1895 (2015). Article CAS Google Scholar Download references AUTHOR
INFORMATION AUTHORS AND AFFILIATIONS * Department of Biochemistry and Molecular Biology, Medical University of South Carolina, 601 Clinical Science Building, 96 Jonathan Lucas Street, Suite
601, Charleston, SC, 29425, USA Dingzhi Wang & Raymond N. DuBois Authors * Dingzhi Wang View author publications You can also search for this author inPubMed Google Scholar * Raymond N.
DuBois View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Raymond N. DuBois. RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wang, D., DuBois, R.N. Fibroblasts fuel intestinal tumorigenesis. _Cell Res_ 30, 635–636 (2020). https://doi.org/10.1038/s41422-020-0340-7
Download citation * Published: 03 June 2020 * Issue Date: August 2020 * DOI: https://doi.org/10.1038/s41422-020-0340-7 SHARE THIS ARTICLE Anyone you share the following link with will be
able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative