- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Hyperbilirubinaemia is a prevalent condition during the neonatal period, and if not promptly and effectively managed, it can lead to severe bilirubin-induced neurotoxicity.
Sunflower seeds are a nutrient-rich food source, particularly abundant in linoleic acid. Here, we provide compelling evidence that lactating maternal mice fed a sunflower seed diet
experience enhanced neurological outcomes and increased survival rates in hyperbilirubinemic offspring. We assessed histomorphological indices, including cerebellar Nissl staining, and
Calbindin staining, and hippocampal hematoxylin and eosin staining. Furthermore, we observed the transmission of linoleic acid, enriched in sunflower seeds, to offspring through lactation.
The oral administration of linoleic acid-rich sunflower seed oil by lactating mothers significantly prolonged the survival time of hyperbilirubinemic offspring mice. Mechanistically,
linoleic acid counteracts the bilirubin-induced accumulation of ubiquitinated proteins and neuronal cell death by activating autophagy. Collectively, these findings elucidate the novel role
of a maternal linoleic acid-supplemented diet in promoting child health. SIMILAR CONTENT BEING VIEWED BY OTHERS AN INTESTINAL SPHINGOLIPID CONFERS INTERGENERATIONAL NEUROPROTECTION Article
Open access 03 August 2023 CHEMOPREVENTION OF BILIRUBIN ENCEPHALOPATHY WITH A NANOCEUTICAL AGENT Article 06 July 2022 CHOLINE SUPPLEMENTATION PREVENTS THE EFFECTS OF BILIRUBIN ON
CEREBELLAR-MEDIATED BEHAVIOR IN CHOLINE-RESTRICTED GUNN RAT PUPS Article 07 October 2020 INTRODUCTION Bilirubin is the final metabolite of heme in mammals, primarily derived from the
degradation of senescent erythrocytes [1]. The initial step in heme catabolism involves heme oxygenase (HO), which generates biliverdin, carbon monoxide (CO), and iron. Biliverdin reductase,
expressed ubiquitously, converts water-soluble biliverdin into poorly water-soluble bilirubin. In the liver, bilirubin metabolism is facilitated by UDP glucuronosyltransferase family 1
member A1 (UGT1A1). This enzyme conjugates unconjugated bilirubin, increasing its water solubility and enabling excretion with bile into the intestine. The mutation in UGT1A1 results in
elevated serum bilirubin, resulting in conditions like Gilbert syndrome and Crigler–Najjar syndrome [2, 3]. Uncontrolled unconjugated hyperbilirubinemia in infants can progress to acute
bilirubin encephalopathy (kernicterus) and even result in fatality [4]. Recent evidence has increasingly highlighted the crucial role of bilirubin, at physiological concentrations, in
modulating various biological functions, acting as a yellow hormone within the human body [5]. Bilirubin is known for its antioxidant capacity that protects the gut or other human tissues
from oxidative stress [6]. However, excessive bilirubin entering the brain can cause irreversible neurological damage. The mechanisms underlying bilirubin neurotoxicity are not yet fully
understood, but they may involve neuronal excitotoxicity, mitochondrial energy depletion, calcium overload, and oxidative stress [7, 8]. Our previous study revealed that the neurotoxicity of
bilirubin is associated with proteasome inhibition, which is the main system for protein degradation in eukaryotic cells [9]. Exogenous bilirubin inhibits both the 19 S
proteasome-associated deubiquitinases (ubiquitin-specific protease 14 [USP14] and ubiquitin carboxy-terminal hydrolase L5 [UCHL5]) and the chymotrypsin-like (CT-like) peptidase activity of
20 S proteasomes, leading to the accumulation of ubiquitinated proteins and cytotoxicity. However, effective methods to alleviate the cytotoxicity resulting from bilirubin-induced
neurotoxicity through proteasome inhibition are still lacking. It is well-known that hyperbilirubinemic _Ugt1_−_/−_ mice experiences fatal outcomes within 8 days after birth [10]. In this
study, we made an incidental observation during the breeding of _Ugt1__+/_− female mice, which revealed a prolonged survival of hyperbilirubinemic _Ugt1_−_/−_ offspring when their maternal
diet was supplemented with sunflower seeds. Our investigation not only explored the effects of adding linoleic acid-rich sunflower seeds or sunflower oil to the maternal diet on
bilirubin-induced neurotoxicity in mice but also delved into the potential molecular mechanisms underlying the impact of dietary linoleic acid on bilirubin-induced neurotoxicity. This study
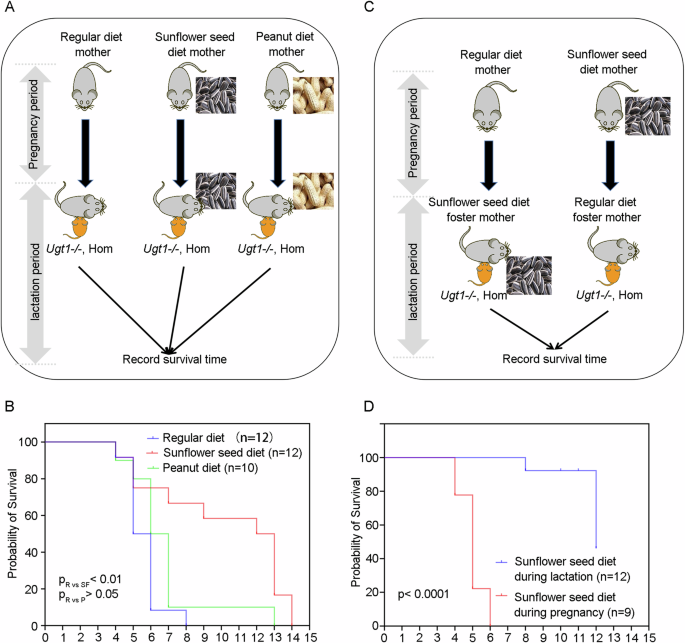
could offer new insights into the prevention or treatment of bilirubin neurotoxicity. RESULTS MATERNAL DIETARY SUPPLEMENTATION WITH SUNFLOWER SEEDS PROLONGS SURVIVAL OF UGT1− _/−_ OFFSPRING
As previously described in Refs. [10, 11], _Ugt1_−_/−_ mice experience fatal outcomes within 8 days after birth due to severe hyperbilirubinemia-induced bilirubin encephalopathy, with 50%
mortality at postnatal day 6 (P6). Since sunflower seeds are rich in nutrients, such as vitamin E and fatty acids, they were commonly included in the diet for breeding mice. To improve the
overall breeding success of _Ugt1_−_/−_ mice, we added sunflower seeds to the cages of maternal mice [12]. Interestingly, when sunflower seeds were added to the diet of _Ugt1__+/_− maternal
mice, we observed a prolonged survival of hyperbilirubinemic _Ugt1_−_/−_ offspring, with a median survival of postnatal 12.5 days (P12.5) (Fig. 1A, B). To investigate whether the maternal
nut diet could extend the survival of hyperbilirubinemic offspring, we also added peanuts to the cages of maternal mice. However, there were no significant difference between the regular
diet group and the peanut diet group (Fig. 1A, B). Since the sunflower seed diet was administered to the mothers in the above experiments in both the pregnancy and lactation periods, the
effect of the sunflower seed diet may be exerted during embryonic development, after birth, or from embryonic development to birth. To determine the specific period when the sunflower seed
diet exerts its effects, we conducted a cross-foster experiment. In this study, the offspring were divided into two groups: the pregnancy sunflower seed diet group and the lactation
sunflower seed diet group. In the pregnancy sunflower seed diet group, the offspring in the sunflower seed diet group were raised by mothers with a regular diet. In the lactation sunflower
seed diet group, the offspring in the regular diet group were raised by mothers with the sunflower seed diet (Fig. 1C). Results showed that hyperbilirubinemic offspring in the lactation
sunflower seed diet group (with a median survival of P12) exhibited a longer survival time compared to the pregnancy sunflower seed diet group (with a median survival of P5) (Fig. 1D). Thus,
maternal sunflower seed diet attenuates lethal phenotype of hyperbilirubinemic offspring mice in the postnatal period, and this effect is likely transmitted to the offspring through
mother’s milk. MATERNAL DIETARY SUPPLEMENTATION WITH SUNFLOWER SEEDS ATTENUATES NEUROLOGICAL DAMAGE IN UGT1− _/−_ OFFSPRING Neonatal mice with hyperbilirubinemia often exhibit
region-selective neurological injury, primarily affecting cerebellum, hippocampus, vestibule, and oculomotor nucleus [13, 14]. To further verify the beneficial effect of maternal sunflower
seed diet on hyperbilirubinemic offspring mice, histomorphological examinations were performed at 5 days of age. As shown in Fig. 2A, hyperbilirubinemic homozygote _Ugt1_−_/−_ offspring
(Hom) from maternal mice supplemented with regular diet demonstrated significantly fewer cerebellar Nissl vesicles, lighter staining, and slightly smaller cerebellar size, compared with wild
type/heterozygote _Ugt1__+/_− mice (WT/Het), which was significantly improved by the maternal sunflower seed diet. Measurement of cerebellar lobules IV and IX [11] revealed a statistically
significant improvement in the reduction of cerebellar external granule layer (EGL) thickness in hyperbilirubinemic offspring mice following the maternal sunflower seed diet (Fig. 2B). In
addition, the cerebellum of hyperbilirubinemic offspring mice in the maternal sunflower seed diet group exhibited an increased number of Purkinje cells compared to the regular diet group
(Fig. 2C). As expected, the HE staining results indicated no significant pathological changes in organs such as the heart, liver, spleen, lung, and kidney in hyperbilirubinemic offspring
mice when compared with WT/Het offspring mice, irrespective of the presence or absence of the maternal sunflower seed diet (Fig. 2D). Together, these results suggest that a maternal
sunflower seed diet is beneficial for mitigating neurological damage in hyperbilirubinemic offspring mice. MATERNAL DIETARY SUPPLEMENTATION WITH SUNFLOWER SEEDS DOES NOT AFFECT THE LEVEL OF
SERUM AND BRAIN BILIRUBIN Next, we investigated whether maternal dietary supplementation with sunflower seeds could decrease bilirubin levels in offspring mice. We initially examined the
appearance of the offspring mice and observed noticeable yellow staining in the abdomen, skin of the extremities, and brain tissue of both the regular diet group and the sunflower seed diet
group (Fig. 3A). In addition, there was no statistical difference (_p_ > 0.05) in serum unconjugated or conjugated bilirubin, as well as brain total or unconjugated bilirubin levels,
between the two groups of hyperbilirubinemic offspring mice (Fig. 3B–D). These findings suggest that the maternal sunflower seed diet may not exert its effects by reducing serum and brain
bilirubin levels in hyperbilirubinemic offspring mice. MATERNAL ORAL ADMINISTRATION OF LINOLEIC ACID-RICH SUNFLOWER SEED OIL PROLONGS SURVIVAL OF UGT1− _/−_ OFFSPRING Sunflower seeds contain
a large amount of fat, mainly composed of unsaturated fatty acids, with linoleic acid comprising up to 70% of their content [15]. Furthermore, the fatty acid composition of milk can be
influenced by maternal dietary changes among various nutrients [16]. Therefore, it is presumed that linoleic acid may play a pivotal role in the neuroprotective effects of sunflower seeds.
As expected, gas chromatography-mass spectrometry analysis revealed higher levels of linoleic acid in the gastric contents of offspring mice from the sunflower seed diet group compared to
the regular diet group (Fig. 4A). To further clarify the types of effector molecules present in sunflower seeds, lactating female mice were orally administered normal saline and linoleic
acid-rich sunflower oil at doses of 10 μl/g and 20 μl/g (Fig. 4B). As shown in Fig. 4C, the survival time of mice in the 20 μl/g sunflower oil group was significantly prolonged compared with
the saline group, although the survival time of the 10 μl/g sunflower oil group did not exhibit a significant extension compared with the saline group. These results suggest that the
predominant effector molecules in sunflower seeds are primarily present in sunflower oil, further indicating that linoleic acid may serve as the key effector molecule in sunflower seeds.
LINOLEIC ACID ANTAGONIZES BILIRUBIN-INDUCED CELL DEATH IN VITRO To examine the effect of linoleic acid on bilirubin-induced neurotoxicity, we conducted in vitro experiments using HT22 cells,
a mouse hippocampal neuronal cell line. As shown in Fig. 5A, bilirubin inhibited the cell viability of HT22, whereas linoleic acid dose-dependently ameliorated the cytotoxicity caused by
bilirubin. Furthermore, bilirubin triggered the cleavage of caspase 3 and PARP, a major caspase 3 substrate, which was significantly reversed upon the addition of linoleic acid. This
suggests that linoleic acid counteracts bilirubin-induced apoptosis in neuronal cells (Fig. 5B, C). Accordingly, PI staining was performed to verify that linoleic acid effectively inhibited
bilirubin-induced cell death in HT22 cells (Fig. 5D and Supplementary Fig. 1A). Previous studies have demonstrated that bilirubin induces neuronal cell death by inhibiting the
ubiquitin-proteasome system and activating endoplasmic reticulum (ER) stress [9, 17, 18]. Therefore, we examined the effects of linoleic acid on bilirubin-induced ubiquitinated protein
accumulation and endoplasmic reticulum stress. As illustrated in Fig. 5E and Supplementary Fig. 1B, bilirubin promoted the accumulation of ubiquitinated proteins and induced the expression
of endoplasmic reticulum (ER) stress-related markers (including C/EBP homologous protein [CHOP], activating transcription factor 4 [ATF4], phosphorylation of eukaryotic translation
initiation factor 2 A [p-eIF2α], and heat shock protein family A (Hsp70) member 5 [HSPA5/Bip]). These findings suggest that bilirubin inhibits ubiquitin proteasome system and induces ER
stress. Consistently, linoleic acid reversed the inhibitory effects of bilirubin on the ubiquitin-proteasome system and ER stress (Fig. 5E and Supplementary Fig. 1B). Furthermore, maternal
oral administration of linoleic acid-rich sunflower seed also inhibited bilirubin-induced the accumulation of ubiquitinated proteins, CHOP, and p-eIF2α in the brain tissue of _Ugt1_−_/−_
mice (Fig. 5F and Supplementary Fig. 1C). However, neither bilirubin nor linoleic acid appeared to affect ATF4 and Bip in the brain tissue of _Ugt1_−_/−_ mice (Fig. 5F and Supplementary Fig.
1C). Collectively, these results demonstrate that linoleic acid can inhibit bilirubin-induced cell death in neuronal cells, thereby contributing to the neuroprotective effect of a sunflower
seed diet against bilirubin-induced neurotoxicity. LINOLEIC ACID AMELIORATES BILIRUBIN-INDUCED NEUROTOXICITY BY ACTIVATING AUTOPHAGY One potential explanation is that linoleic acid might
directly bind to bilirubin, thereby neutralizing its toxic effects. To investigate this possibility, we examined the binding modes of bilirubin and linoleic acid in aqueous water. Our
findings revealed two likely binding modes, Mode A and Mode B (Fig. 6A). However, the binding energies of both modes A and B were low (−5.5 and −2.7 kcal/mol, respectively), indicating a
weak interaction between bilirubin and linoleic acid in aqueous solution. Thus, linoleic acid may not work through direct interaction with bilirubin. UnaG is a bilirubin-dependent
fluorescent protein and the binding of bilirubin to UnaG results in the emission of a fluorescence signal [19]. Interestingly, we observed that bilirubin treatment induced comparable levels
of fluorescence in HT22 cells expressing UnaG protein, regardless of the presence or absence of linoleic acid (Fig. 6B and Supplementary Fig. 2A). These findings suggest that a sufficient
amount of bilirubin still permeates into the cells even following linoleic acid treatment. Furthermore, hexanal, a metabolite of linoleic acid failed to attenuate bilirubin-induced cell
death (Fig. 6C). We also tested the effects of other derivatives of linoleic acid, such as arachidonic acid and conjugated linoleic acid, on bilirubin-induced neurotoxicity. Interestingly,
arachidonic acid exhibited a mitigating effect on bilirubin-induced cell death, whereas conjugated linoleic acid had a less pronounced effect and exhibited cytotoxicity in HT22 cells
(Supplementary Fig. 2B). Linoleic acid regulates various cell biological processes by modulating mutiple pathways or targets, including free fatty acid receptors (such as GPR40 or GPR120),
PPARs, PI3K/Akt, EGFR, or autophagy [20,21,22,23,24,25]. To explore whether linoleic acid acts through these pathways or targets, we employed agents that interfere with these pathways, such
as GPR40 antagonist (DC260126), GPR120 antagonist (AH-7614), PPARγ antagonist (GW9662), PPARβ/δ antagonist (GSK0660), autophagy inhibitors (chloroquine [CQ] and wortmannin), Akt inhibitor
(MK-2206), EGFR inhibitor (AG 1478), and EFGR and PI3K kinase inhibitor (MTX-211). Remarkably, the protective effect of linoleic acid was substantially blocked by autophagy inhibitors (CQ
and wortmannin) (Fig. 6D). Thus, we hypothesized that autophagy plays a crucial role in the neuroprotective effect of linoleic acid. Consistently, PI staining results demonstrated that
linoleic acid effectively inhibited bilirubin-induced neuronal cell death, which was reversed by the addition of autophagy inhibitors (CQ and wortmannin) (Fig. 6E and Supplementary Fig. 3A).
It was reported that the activation of autophagy protects neurons and astrocytes from bilirubin-induced cytotoxicity [26]. Therefore, we hypothesized that linoleic acid antagonized the
neurotoxicity of bilirubin by activating autophagy. To assess this, we examined the expression levels of LC3 and p62, which are proteins involved in the autophagy process. Our results showed
that linoleic acid increased LC3-II expression but had no significant effect on p62 expression, indicating the activation of cellular autophagy by linoleic acid (Fig. 6F). Furthermore,
linoleic acid attenuated bilirubin-induced the accumulation of ubiquitinated proteins and the cleavage of PARP and caspase 3. However, the protective effects of linoleic acid were blocked by
the addition of autophagy inhibitors (CQ and wortmannin) (Fig. 6G and Supplementary Figure 3B), while autophagy inhibitors treatment had no effect on bilirubin-induced cytotoxicity (Fig. 6H
and Supplementary Fig. 3C). Taken together, these data suggest that linoleic acid may ameliorate bilirubin neurotoxicity by activating cellular autophagy. DISCUSSION Excessive bilirubin
entering the brain can cause neurotoxicity. Currently, there are some measures currently available for the prevention and treatment of bilirubin-induced neurotoxicity. Clinical methods, such
as blue light therapy and exchange transfusion therapy, are commonly used to alleviate neurotoxicity associated with hyperbilirubinemia in patients with conditions like neonatal jaundice.
However, both methods have certain limitations. Blue light therapy may not be effective in severe cases and can lead to adverse effects [27], while exchange transfusion requires specialized
equipment and may involve procedure-related complications [28]. Thus, the development of safe, effective, and cost-efficient approaches for preventing and treating bilirubin-induced
neurotoxicity is crucial. In the present study, we investigated the effects of a maternal sunflower seed diet on the survival and neurological damage phenotype of hyperbilirubinemic
_Ugt1_−_/−_ offspring mice. Our findings revealed that the maternal sunflower seed diet significantly prolonged the survival and improved the neurological damage in hyperbilirubinemic
offspring mice. Additionally, we demonstrated that the effects of the linoleic acid-rich sunflower seed diet were mainly transmitted to the offspring through lactation, as shown by the
cross-foster experiment. Mechanistically, linoleic acid induced the activation of autophagy, which ameliorated bilirubin-induced accumulation of ubiquitinated proteins and cell death. As
sunflower seeds are a readily available and cost-effective source of linoleic acid, they hold promise for patients suffering from hyperbilirubinemia, particularly in resource-limited
regions. It has been established that unsaturated fatty acids, including linoleic acid, can readily cross the blood-brain barrier [29]. Our study further demonstrated that linoleic acid, an
omega-6 polyunsaturated fatty acid, may provide neuroprotection against hyperbilirubinemia-induced neurological damage in mice. Other studies have suggested that docosahexaenoic acid (DHA),
an omega-3 polyunsaturated fatty acid, exerts neuroprotective effects against bilirubin-induced neurotoxicity by enhancing the activity of superoxide dismutase (SOD) and catalase (CAT) [30,
31]. It is worth noting that under normal physiological conditions, unsaturated fatty acids may compete with bilirubin for binding sites on albumin in the bloodstream, potentially leading to
increased levels of free bilirubin [32]. Moreovers, high doses of unsaturated fatty acids (including linoleic acid) can act as inhibitors of UGT1A1 in vitro, potentially hindering the
conversion of unconjugated bilirubin into conjugated bilirubin and resulting in elevated levels of unconjugated bilirubin [33]. These properties of unsaturated fatty acids suggest a complex
role in the management of hyperbilirubinemia. Our previous study demonstrated that bilirubin can induce neurotoxicity by inhibiting the proteasome, leading to the accumulation of
ubiquitinated proteins [9]. In the present study, we discovered that linoleic acid, abundant in sunflower seeds, significantly mitigates bilirubin-induced ubiquitinated protein accumulation
both in vitro and in vivo. Apart from its neurotoxic effects, bilirubin is a potent antioxidant that plays crucial physiological roles in the human body. Therefore, instead of simply
reducing serum bilirubin levels, linoleic acid may preserve other properties of bilirubin, such as its antioxidant activity, while counteracting its neurotoxicity. Additionally, these
effects of linoleic acid appear to be attributed to the activation of autophagy. Hence, we propose that linoleic acid may counteract bilirubin-induced neurotoxicity by inducing autophagy. It
has been reported that linoleic acid activates autophagy through both mTOR-dependent and independent pathways [20]. Future studies should delve into the detailed mechanisms of linoleic
acid-induced autophagy activation. However, the present study has certain limitations. Firstly, sunflower seeds were not the exclusive dietary source for the maternal mice in the sunflower
seed group during the experiment, potentially introducing bias due to maternal food preferences. Secondly, although we compared whole-brain bilirubin levels, cerebellar bilirubin levels were
not assessed. Furthermore, it is essential to calculate the free bilirubin as a crucial parameter to delve into the detailed mechanism of fatty acid antagonism against bilirubin-induced
cytotoxicity. Thirdly, while the combination of phototherapy and sunflower seed diets may hold promise as a method for preventing and treating hyperbilirubinemia, there is presently
insufficient experimental evidence to firmly support this claim. In conclusion, our study provides novel insights into the protective effect of a maternal sunflower seed diet on
hyperbilirubinemic offspring mice, achieved through breastfeeding during lactation. Furthermore, we propose that this protective effect of sunflower seeds can be attributed to linoleic acid,
which alleviates bilirubin-induced accumulation of ubiquitinated proteins and neuronal cell death by activating autophagy (Fig. 7). These findings highlight the potential of dietary
interventions involving linoleic acid-rich sources, such as sunflower seeds, as a promising strategy for preventing and treating bilirubin-induced neurotoxicity. MATERIALS AND METHODS
REAGENTS Bilirubin (#B4126) was purchased from Merck. Linoleic acid (#90150) was purchased from Cayman. DC260126 (#HY-101906), AH-7614 (#HY-19996), GW9662 (#HY-16578), GSK0660 (#HY-12377),
chloroquine (#HY-17589A), Wortmannin (#HY-10197), MK2206 (#HY-10358), AG-1478 (#HY-13524), and MTX-211 (#HY-107364) were purchased from MedChemExpress. PARP (#9532), cleaved caspase 3
(#9661), CHOP (#2895), ATF4 (#11815), p-eIF2α (#3398), Bip (#3177), and LC3A/B (#4108) antibodies were purchased from Cell Signaling Technology. Ubiquitin antibody (#sc-8017, 1:500) was
purchased from Santa Cruz biotechnology. P62 (#ab56416) was purchased from Abcam. GAPDH (#AP0063) was purchased from Bioworld. With the exception of the specified antibodies, all other
antibodies utilized for western blot experiments were diluted at a ratio of 1:1000. CELL LINES HT22 mouse hippocampal neuronal cells were obtained from Wuhan Procell Life Science &
Technology Company (CL-0697) and cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum. The cells were recently authenticated, tested for mycoplasma
contamination, and maintained at 37 °C in a humidified atmosphere with 5% CO2. ANIMALS The study was conducted according to the guidelines of the Declaration of Helsinki. All animal handling
procedures were performed in compliance with the People’s Republic of China legislation for the care and use of laboratory animals. The experimental protocols involving animals were
approved by the Institutional Animal Care and Use Committee of Guangzhou Medical University. Heterozygous Ugt1 knockout adult male mice (Het; _Ugt1__+/_−) in the C57BL/6 background were
generously provided by Professor Zhongqiu Liu from Guangzhou University of Chinese Medicine [10, 34]. Homozygous Ugt1 knockout mice (Hom; _Ugt1_−_/−_) were generated by breeding _Ugt1__+/_−
mice. The parental mice were housed in the Animal Center of Guangzhou Medical University, following a 12 h light/12 h dark circadian rhythm, with free access to water. The mice were randomly
allocated to either control or treatment group. Both control and treatment groups of parental mice were fed with regular chow diet, while the treatment group received additional autoclaved
shelled sunflower seeds or peanuts in their cages alongside the regular chow diets. The regular chow diets for the parental mice were provided by Guangdong Medical Laboratory Animal Center.
For the assessment of orally administered sunflower oil, autoclaved pure sunflower oil (without being dissolved in other solvents) was administered to lactating female mice via gavage at the
indicated dose (10 ul/g or 20 ul/g once daily), commencing on the first day of pup’s life. Throughout the gavage period, females had ad libitum access to water and a regular diet. The assay
continued until all hyperbilirubinemic offspring mice succumbed. CEREBELLAR HISTOLOGICAL DETECTION The brains were carefully dissected from the skulls and fixed in 4% paraformaldehyde for
24 h. After fixation, the brains were dehydrated by immersion in a 20% sucrose solution, followed by transfer to a 30% sucrose solution until they settled. Excess sucrose solution was
removed by gently blotting with filter paper. Prior to sectioning, the cryostat and sample base were pre-cooled. The brains were then bisected along the median sagittal plane using a blade.
To ensure optimal orientation, the brain sections were meticulously embedded layer by layer with OCT embedding agent, ensuring that the largest section was parallel to the base and
positioned facing upwards. The resulting sections had a thickness of 20 μm. For the assessment of cerebellar development in neonatal offspring, Nissl staining and Calbindin staining were
performed on neonatal mice at 5 days of age (_n_ = 5–6 animals/group; 1–2 sections/animal). For Nissl staining, we utilized the corresponding reagents provided by Servicebio Biotechnology
and applied it to paraformaldehyde-fixed sections in accordance with the manufacturer’s instructions. For Calbindin staining, after blocking with 3% BSA for 30 minutes, the primary antibody
(abcam#ab108404, 1:150) was incubated overnight at 4 °C. Following three washes with PBS, a fluorescent secondary antibody incubation was performed and subsequently, the nuclei were
re-stained using DAPI. To capture images of the stained sections, an Aperio digital pathology slide scanner was employed. MEASUREMENT OF SERUM BILIRUBIN After administering anesthesia to the
offspring mice, a gentle inversion of the mice was performed to facilitate blood flow towards the head and face. The skin in the submandibular region was carefully disinfected using
alcohol-soaked cotton balls. A small incision was then made in the submandibular vein using a blade to collect blood samples. Microcapillary blood collection tubes were used to collect the
blood, which was transferred into eppendorf tubes (EPs). The collected blood samples were incubated at room temperature in the dark for 30 min. After incubation, the tubes were subjected to
centrifugation at 3000 rpm for 10 min at room temperature. This centrifugation step allowed for the separation of serum from the cellular components of the blood. The resulting serum was
carefully transferred to another EP tube and stored appropriately for further analysis. To determine the concentrations of direct and total bilirubin in the serum, a biochemical analyzer was
utilized (Hitachi Ltd. 3100 Serial, Tokyo, Japan). This analyzer employs specific assays or reagents to measure the bilirubin levels accurately. The serum was diluted 2-fold prior to being
tested on the biochemical analyzer. Approximately 10 μl of diluted serum is consumed per test. Additionally, the concentration of indirect bilirubin was calculated based on the total (Total
BilE-HA, Wako, Japan) and direct (Direct Bil E-HA, Wako, Japan) bilirubin measurements. MEASUREMENT OF BRAIN BILIRUBIN In order to detect brain bilirubin, we have referenced a research paper
and made enhancements based on it [35]. Preparation of samples for measurement of total bilirubin: The brains were extracted from the skull. After homogenizing the brain tissue, transfer 20
mg into a homogenization tube. Add exactly 200 μl of 10% oxalic acid and 200 μl of water-saturated dichloromethane. Seal the tube tightly and wrap it in tin foil to protect it from light.
Shake the mixture overnight at a temperature of 4 °C using a 360 rotary shaker. Afterward, centrifuge the sample at 7000 rpm for 5 min at a temperature of 4 °C. Carefully collect the lower
layer containing water-saturated dichloromethane, filter it, and use it for analysis under the corresponding chromatographic conditions. Preparation of samples for measurement of indirect
bilirubin: Follow a similar method to that used for total bilirubin, but replace the 10% oxalic acid and water-saturated dichloromethane with dichloromethane. The chromatographic analysis
was performed using an ODS-2 column (250 mm × 4.6 mm, 5 μm) under the following conditions: isocratic elution with a mobile phase consisting of acetonitrile - 1% glacial acetic acid aqueous
solution (95:5), at a volume flow rate of 1 ml/min. The detection wavelength was set at 450 nm, and the column temperature was maintained at 30 °C. An injection volume of 4 μl was used.
FATTY ACID DETERMINATION OF GASTRIC CONTENTS Gastric contents were collected on ice. Firstly, the entire gastric capsule was excised from cardia to pylorus, followed by careful dissection of
the gastric wall with scissors. Subsequently, the gastric contents were meticulously stripped into 1.5 ml centrifuge tubes and stored at −80 °C. For fatty acid determination of gastric
contents, 2 mL of petroleum ether-ether mixture and 1 ml of potassium hydroxide-methanol solution as methylation reagent were added to 500 mg (to 0.1 mg) of homogeneous gastric in a
stoppered tube with a capacity of 10 ml. The mixture was vortexed and shaken, and incubated at 65 °C for 1 h. Then, the mixture was vortexed and shaken again, and 2 ml of deionized water was
added, allowing the reaction to separate for 30 min. Finally, the mixture was centrifuged at a speed of 4500 rpm for 2 min before and the supernatant were analyzed by gas
chromatography-mass spectrometry (Agilent Technologies). CELL VIABILITY ASSAY HT22 cells were seeded in 96-well plates. After allowing the cells to adhere, they were treated with
experimental compounds for 24 h. The reagents were dissolved in RPMI-1640 medium with 10% fetal bovine serum. After the treatment period, 10 µl of Cell Counting Kit-8 reagent (DOJINDO, CK04)
was added to each well, followed by incubation for 2 h at 37 °C. The absorbance at a wavelength of 450 nm was then measured using a microplate reader (Thermo Scientific). PROPIDIUM IODIDE
STAINING HT22 cells were seeded in 24-well plates. After treatment with the indicated reagents in RPMI-1640 medium with 10% fetal bovine serum for 24 hours, the cells were subjected to
propidium iodide (PI) staining for 30 min using the PI staining kit (KeyGEN BioTECH#KGA108). The PI-positive cells were then imaged using a fluorescence microscope (ZEISS). UNAG ASSAY The
HT22 cells were cultured in glass-bottomed dishes, and after they adhered to the surface, the UnaG plasmid was transfected using Lipofectamine 3000 reagent according to the manufacturer’s
instructions. After 24 hours, HT22 cells expressing UnaG were treated with 20 μM bilirubin in the presence or absence of linoleic acid. Subsequently, fluorescence microscopy was employed to
observe cellular fluorescence after a 6-hour incubation period for each experimental group. WESTERN BLOTTING Western blotting was performed following a previously described protocol [36].
Briefly, equal amounts of protein extracts were separated by SDS-PAGE and electrotransferred to a polyvinylidene difluoride (PVDF) membrane (Millipore). Subsequently, the membranes were
blocked with 5% non-fat milk for 1 h at room temperature. Overnight incubation with primary antibodies was conducted at 4 °C. The following day, horseradish peroxidase-labeled secondary
antibodies were applied and incubated for 1 h at room temperature. Finally, the membranes were treated with an ECL reagent and visualized using x-ray film. STATISTICAL ANALYSIS The data were
analyzed using Graphpad Prism 9.0 software. Survival analysis was conducted by Log-rank test. The statistical analyses for group comparisons were conducted using t-tests and ANOVA,
respectively, in accordance with the experimental design. The results are presented as mean ± standard deviation. A _p_ value less than 0.05 indicates statistical significance. DATA
AVAILABILITY The data supporting the present findings are available from the corresponding author upon reasonable request. REFERENCES * Shibahara S, Kitamuro T, Takahashi K. Heme degradation
and human disease: diversity is the soul of life. Antioxid Redox Signal. 2002;4:593–602. Article CAS PubMed Google Scholar * Kadakol A, Ghosh SS, Sappal BS, Sharma G, Chowdhury JR,
Chowdhury NR. Genetic lesions of bilirubin uridine-diphosphoglucuronate glucuronosyltransferase (UGT1A1) causing Crigler-Najjar and Gilbert syndromes: correlation of genotype to phenotype.
Hum Mutat. 2000;16:297–306. Article CAS PubMed Google Scholar * Schwertner HA, Vítek L. Gilbert syndrome, UGT1A1*28 allele, and cardiovascular disease risk: possible protective effects
and therapeutic applications of bilirubin. Atherosclerosis. 2008;198:1–11. Article CAS PubMed Google Scholar * Dennery PA, Seidman DS, Stevenson DK. Neonatal hyperbilirubinemia. N. Engl
J Med. 2001;344:581–90. Article CAS PubMed Google Scholar * Vítek L, Tiribelli C. Bilirubin: The yellow hormone? J Hepatol. 2021;75:1485–90. Article PubMed Google Scholar * Stocker R,
Yamamoto Y, McDonagh AF, Glazer AN, Ames BN. Bilirubin is an antioxidant of possible physiological importance. Science. 1987;235:1043–6. Article CAS PubMed Google Scholar * Watchko JF.
Kernicterus and the molecular mechanisms of bilirubin-induced CNS injury in newborns. Neuromol Med. 2006;8:513–29. Article CAS Google Scholar * Ye H-B, Wang J, Zhang W-T, Shi H-B, Yin
S-K. Taurine attenuates bilirubin-induced neurotoxicity in the auditory system in neonatal guinea pigs. Int J Pediatr Otorhinolaryngol. 2013;77:647–54. Article PubMed Google Scholar *
Huang H, Guo M, Liu N, Zhao C, Chen H, Wang X, et al. Bilirubin neurotoxicity is associated with proteasome inhibition. Cell Death Dis. 2017;8:e2877. Article CAS PubMed PubMed Central
Google Scholar * Li Y, Liu H, Chen K, Wu X, Wu J, Yang Z, et al. Pathological significance and prognostic roles of indirect bilirubin/albumin ratio in hepatic encephalopathy. Front Med
(Lausanne). 2021;8:706407. Article PubMed Google Scholar * Bortolussi G, Baj G, Vodret S, Viviani G, Bittolo T, Muro AF. Age-dependent pattern of cerebellar susceptibility to bilirubin
neurotoxicity in vivo in mice. Dis Model Mech. 2014;7:1057–68. PubMed PubMed Central Google Scholar * Jugloff DG, Logan R, Eubanks JH. Breeding and maintenance of an Mecp2-deficient mouse
model of Rett syndrome. J Neurosci Methods. 2006;154:89–95. Article CAS PubMed Google Scholar * Vaz AR, Silva SL, Barateiro A, Falcão AS, Fernandes A, Brito MA, et al. Selective
vulnerability of rat brain regions to unconjugated bilirubin. Mol Cell Neurosci. 2011;48:82–93. Article CAS PubMed Google Scholar * Rose J, Vassar R. Movement disorders due to bilirubin
toxicity. Semin Fetal Neonatal Med. 2015;20:20–5. Article PubMed Google Scholar * Muhammad Anjum F, Nadeem M, Issa Khan M, Hussain S. Nutritional and therapeutic potential of sunflower
seeds: a review. Br Food J. 2012;114:544–52. Article Google Scholar * Innis SM. Impact of maternal diet on human milk composition and neurological development of infants. Am J Clin Nutr.
2014;99:734s–41s. Article CAS PubMed Google Scholar * Qaisiya M, Brischetto C, Jašprová J, Vitek L, Tiribelli C, Bellarosa C. Bilirubin-induced ER stress contributes to the inflammatory
response and apoptosis in neuronal cells. Arch Toxicol. 2017;91:1847–58. Article CAS PubMed Google Scholar * Ostrow JD, Pascolo L, Brites D, Tiribelli C. Molecular basis of
bilirubin-induced neurotoxicity. Trends Mol Med. 2004;10:65–70. Article CAS PubMed Google Scholar * Kumagai A, Ando R, Miyatake H, Greimel P, Kobayashi T, Hirabayashi Y, et al. A
bilirubin-inducible fluorescent protein from eel muscle. Cell. 2013;153:1602–11. Article CAS PubMed Google Scholar * Yang B, Zhou Y, Wu M, Li X, Mai K, Ai Q. ω-6 Polyunsaturated fatty
acids (linoleic acid) activate both autophagy and antioxidation in a synergistic feedback loop via TOR-dependent and TOR-independent signaling pathways. Cell Death Dis. 2020;11:607. Article
CAS PubMed PubMed Central Google Scholar * Serna-Marquez N, Diaz-Aragon R, Reyes-Uribe E, Cortes-Reynosa P, Salazar EP. Linoleic acid induces migration and invasion through FFAR4- and
PI3K-/Akt-dependent pathway in MDA-MB-231 breast cancer cells. Med Oncol. 2017;34:111. Article PubMed Google Scholar * Sum CS, Tikhonova IG, Neumann S, Engel S, Raaka BM, Costanzi S, et
al. Identification of residues important for agonist recognition and activation in GPR40. J Biol Chem. 2007;282:29248–55. Article CAS PubMed Google Scholar * Cartoni C, Yasumatsu K,
Ohkuri T, Shigemura N, Yoshida R, Godinot N, et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J Neurosci. 2010;30:8376–82. Article CAS PubMed PubMed Central
Google Scholar * Zuo X, Wu Y, Morris JS, Stimmel JB, Leesnitzer LM, Fischer SM, et al. Oxidative metabolism of linoleic acid modulates PPAR-beta/delta suppression of PPAR-gamma activity.
Oncogene. 2006;25:1225–41. Article CAS PubMed PubMed Central Google Scholar * O’Rourke EJ, Kuballa P, Xavier R, Ruvkun G. ω-6 polyunsaturated fatty acids extend life span through the
activation of autophagy. Genes Dev. 2013;27:429–40. Article PubMed PubMed Central Google Scholar * Qaisiya M, Mardešić P, Pastore B, Tiribelli C, Bellarosa C. The activation of autophagy
protects neurons and astrocytes against bilirubin-induced cytotoxicity. Neurosci Lett. 2017;661:96–103. Article CAS PubMed Google Scholar * Faulhaber FRS, Procianoy RS, Silveira RC.
Side effects of phototherapy on neonates. Am J Perinatol. 2019;36:252–7. Article PubMed Google Scholar * Behjati S, Sagheb S, Aryasepehr S, Yaghmai B. Adverse events associated with
neonatal exchange transfusion for hyperbilirubinemia. Indian J Pediatrics. 2009;76:83–5. Article Google Scholar * Avellini L, Terracina L, Gaiti A. Linoleic acid passage through the
blood-brain barrier and a possible effect of age. Neurochem Res. 1994;19:129–33. Article CAS PubMed Google Scholar * Hao W, Song J, Li G. Neuroprotective effect of ω-3 polyunsaturated
fatty acids on bilirubin encephalopathy in vitro and in vivo. Med Sci Monit. 2018;24:2631–8. Article CAS PubMed PubMed Central Google Scholar * Becerir C, Kılıç İ, Sahin Ö, Özdemir Ö,
Tokgün O, Özdemir B, et al. The protective effect of docosahexaenoic acid on the bilirubin neurotoxicity. J Enzym Inhib Med Chem. 2013;28:801–7. Article CAS Google Scholar * Amin SB.
Effect of free fatty acids on bilirubin-albumin binding affinity and unbound bilirubin in premature infants. J Parenter Enter Nutr. 2010;34:414–20. Article CAS Google Scholar * Lv X, Xia
Y, Finel M, Wu J, Ge G, Yang L. Recent progress and challenges in screening and characterization of UGT1A1 inhibitors. Acta Pharm Sin B. 2019;9:258–78. Article PubMed Google Scholar *
Nguyen N, Bonzo JA, Chen S, Chouinard S, Kelner MJ, Hardiman G, et al. Disruption of the ugt1 locus in mice resembles human Crigler-Najjar type I disease. J Biol Chem. 2008;283:7901–11.
Article CAS PubMed Google Scholar * Wang X, Li J, Di L, Li W, Wang H. HPLC method for determination of free and total bilirubin in liushen pills. Res Pract Chin Med. 2020;34:60–4. CAS
Google Scholar * Yan D, Li X, Yang Q, Huang Q, Yao L, Zhang P, et al. Regulation of Bax-dependent apoptosis by mitochondrial deubiquitinase USP30. Cell Death Discov. 2021;7:211. Article
CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Natural Science Foundation of China (82370067, 81972399 and
82272660), the Natural Science Foundation Research Team of Guangdong Province (2018B030312001), the Plan on Enhancing Scientific Research in GMU (02-410-2302289XM), the Basic and Applied
Basic Research Project of the Guangzhou Basic Research Program (202201011411), Guangdong Basic and Applied Basic Research Foundation (2021A1515011382), and the open research funds from the
Sixth Affiliated Hospital of Guangzhou Medical University, Qingyuan People’s Hospital (202011–106). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Guangzhou Municipal and Guangdong Provincial
Key Laboratory of Protein Modification and Degradation, State Key Laboratory of Respiratory Disease, School of Basic Medical Sciences, The Sixth Affiliated Hospital of Guangzhou Medical
University, Qingyuan People’s Hospital, Qingyuan, 511518, China Ding Yan, XinTian Wu, Xi Chen, Jiangtuan Wang, Feifei Ge, Meixuan Wu, Jiawen Wu, Na Zhang, Min Xiao, Xueheng Wu, Qian Xue,
Xiaofen Li, Xin Wang, Xin Chen & Jinbao Liu * Central Laboratory, the Second Affiliated Hospital of Guangzhou Medical University, Guangzhou, Guangdong, 510260, China Jinghong Chen *
Department of Neonatology, Guangzhou Women and Children’s Medical Center, Guangzhou Medical University, Guangzhou, Guangdong, 511436, China Ping Wang * Department of Surgery, UT Southwestern
Medical Center, Dallas, TX, 75390, USA Daolin Tang Authors * Ding Yan View author publications You can also search for this author inPubMed Google Scholar * XinTian Wu View author
publications You can also search for this author inPubMed Google Scholar * Xi Chen View author publications You can also search for this author inPubMed Google Scholar * Jiangtuan Wang View
author publications You can also search for this author inPubMed Google Scholar * Feifei Ge View author publications You can also search for this author inPubMed Google Scholar * Meixuan Wu
View author publications You can also search for this author inPubMed Google Scholar * Jiawen Wu View author publications You can also search for this author inPubMed Google Scholar * Na
Zhang View author publications You can also search for this author inPubMed Google Scholar * Min Xiao View author publications You can also search for this author inPubMed Google Scholar *
Xueheng Wu View author publications You can also search for this author inPubMed Google Scholar * Qian Xue View author publications You can also search for this author inPubMed Google
Scholar * Xiaofen Li View author publications You can also search for this author inPubMed Google Scholar * Jinghong Chen View author publications You can also search for this author
inPubMed Google Scholar * Ping Wang View author publications You can also search for this author inPubMed Google Scholar * Daolin Tang View author publications You can also search for this
author inPubMed Google Scholar * Xin Wang View author publications You can also search for this author inPubMed Google Scholar * Xin Chen View author publications You can also search for
this author inPubMed Google Scholar * Jinbao Liu View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS J.L., Xin Wang, and Xin Chen. designed and
supervised this study. D.Y., XinTian Wu, Xi C., J.W., F.G., M.W., J.W., N.Z., M.X., Xueheng Wu, Q.X., J.C., and X.L. performed experiments, analyzed the data, and performed the statistical
analyses. D.Y. interpreted the results and wrote the manuscript. P.W. interpreted the results. Xin C. and D.T. edited the manuscript. All authors had full access to all of the data in the
study and accept responsibility for the decision to submit for publication. CORRESPONDING AUTHORS Correspondence to Xin Wang, Xin Chen or Jinbao Liu. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. ETHICS APPROVAL AND CONSENT TO PARTICIPATE All animal experiments have been approved by the Animal Ethics Committee of Guangzhou Medical
University (Approval number: GY2022-019). The research does not involve human subjects, human material, or human data. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral
with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTAL FIGURES AND FIGURE LEGENDS FULL AND UNCROPPED WESTERN BLOTS
RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes
were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If
material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain
permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Yan, D., Wu, X., Chen, X. _et al._ Maternal linoleic acid-rich diet ameliorates bilirubin neurotoxicity in offspring mice. _Cell Death Discov._ 10, 329 (2024).
https://doi.org/10.1038/s41420-024-02099-9 Download citation * Received: 20 August 2023 * Revised: 02 July 2024 * Accepted: 11 July 2024 * Published: 19 July 2024 * DOI:
https://doi.org/10.1038/s41420-024-02099-9 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative






)
