- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Natural killer/T cell lymphoma (NKTCL) exhibits highly aggressive clinical behavior, and the outcomes for relapsed/refractory patients are still poor. Recently, the mechanism
underlying the effect of Epstein-Barr virus (EBV) infection, which has not been fully defined in NKTCL, has attracted great attention. We explored how LMP1 promoted aerobic glycolysis via
metabolic sequencing combined with mRNA sequencing and immunoprecipitation coupled to mass spectrometry. Experimental assays were used to determine the effects of LMP1 and its downstream
pathway on the function and glucose metabolism of NKTCL cells. The correlations between LMP1 expression in patients and their clinical features, treatment response, and prognosis were
analyzed. Results show that LMP1 enhances NKTCL cell proliferation in vitro and in vivo, inhibits apoptosis, and decreases gemcitabine sensitivity. In addition, LMP1 also enhances aerobic
glycolysis in NKTCL cells, as indicated by increases in glucose uptake, lactate production, and extracellular acidification rate. Clinically, LMP1 expression is correlated with risk
stratification, treatment response, and prognosis, and higher LMP1 expression indicates greater SUVmax for NKTCL patients. Mechanistically, LMP1 competitively binds to TRAF3 to promote cell
proliferation and aerobic glycolysis by regulating the noncanonical NF-κB pathway. The application of an NF-κB pathway inhibitor or reactivation of the NF-κB pathway affects aerobic
glycolysis and the biological function of NKTCL cells. In summary, this study is the first to describe and define in detail how LMP1 affects glucose metabolism in NKTCL and might provide a
novel perspective for further treatment. SIMILAR CONTENT BEING VIEWED BY OTHERS DEPENDENCE ON MITOCHONDRIAL RESPIRATION OF MALIGNANT T CELLS REVEALS A NEW THERAPEUTIC TARGET FOR
ANGIOIMMUNOBLASTIC T-CELL LYMPHOMA Article Open access 19 June 2024 MULTI-OMICS ANALYSES REVEAL THAT HIV-1 ALTERS CD4+ T CELL IMMUNOMETABOLISM TO FUEL VIRUS REPLICATION Article 25 March 2021
A FUNCTIONAL SINGLE-CELL METABOLIC SURVEY IDENTIFIES _ELOVL1_ AS A TARGET TO ENHANCE CD8+ T CELL FITNESS IN SOLID TUMOURS Article Open access 10 March 2025 INTRODUCTION Natural killer/T
cell lymphoma (NKTCL), a subtype of T cell and NK-cell lymphoid proliferations and lymphomas classified by the latest World Health Organization (WHO) [1], presents highly aggressive clinical
behavior and is particularly prevalent in Asian and South American populations [2]. In recent decades, therapeutic regimens containing L-asparaginase, including DDGP [3], modified SMILE
[4], and P-GemOx [5], have been recommended by the National Comprehensive Cancer Network guidelines based on improved clinical prognosis [6]. However, the outcomes for relapsed and
refractory patients are still suboptimal [7]. Considering the close association of NKTCL with Epstein-Barr virus (EBV) infection [8], targeting EBV provides a promising strategy for future
treatment. Latent membrane protein 1 (LMP1), encoded by EBV, promotes oncogenesis to facilitate malignant transformation in EBV-associated malignancies [9], and has been reported to play
fundamental roles in nasopharyngeal carcinoma (NPC) [10, 11]. Thus, studies on the therapeutic potential of LMP1 for the treatment of NKTCL are urgently needed. The reprogramming of
metabolic pathways, regarded as a crucial hallmark of tumors, is associated with both tumorigenesis and disease progression [12]. Among them, the metabolism of glucose for aerobic glycolysis
rather than oxidative phosphorylation, known as the Warburg effect, is an important metabolic feature of malignant lymphomas [13, 14], and can support infinite replication, self-sufficiency
in growth signals, resistance to antigrowth signals, tumor invasion and metastasis, angiogenesis, and disturbance of the microenvironment [15]. It has been widely reported that viral
infection is related to tumor pathogenesis and development, and the mechanism underlying the effect of viral infection on tumor metabolism has attracted great attention in recent years.
Studies have shown that EBV and its encoded LMP1 can potentially alter glucose metabolism in NPC, through increasing the expression of vascular endothelial growth factor to stimulate
angiogenesis [16], inhibiting glucose deprivation and metabolism to restore sensitivity to apoptosis induction [17], and decreasing monocyte migration and T cell activation to promote immune
escape [18]. Hence, targeting LMP1 may represent a promising approach for treating EBV-positive malignancies by interfering with aerobic glycolysis. However, the effect of LMP1 on glucose
metabolism in EBV-associated NKTCL and the detailed mechanism have not been fully defined. In this study, we explored how LMP1 promoted aerobic glycolysis via metabolic sequencing combined
with mRNA sequencing and immunoprecipitation (IP) coupled to mass spectrometry. Experimental assays determined the effects of LMP1 and its downstream pathway on the function and glucose
metabolism of NKTCL cells. Clinically, the correlations between LMP1 expression in NKTCL patients and their clinical features, treatment response, prognosis, and the baseline maximum
standardized uptake value (SUVmax) before treatment were analyzed. In summary, this study is the first to describe and define in detail the effect of LMP1 on glucose metabolism in NKTCL and
might provide a novel perspective for further treatment. MATERIALS AND METHODS PATIENTS AND CLINICAL DATA A total of 58 formalin-fixed, paraffin-embedded tumor tissues from NKTCL patients
were obtained from the First Affiliated Hospital of Zhengzhou University. All patients were reviewed and interpreted independently by three experienced pathologists, and diagnoses were made
according to the latest WHO classification criteria. After diagnosis, enrolled patients received DDGP regimen, including gemcitabine, dexamethasone, cisplatin, and pegaspargase, as the
first-line treatment. Immunohistochemistry (IHC) of LMP1 was performed according to standard procedures. The LMP1 antibody was listed in Additional File 1: Table S1. Staining was assessed
according to the staining intensity and the positively stained area by a pathologist and verified by two other pathologists without prior knowledge of the patients’ information, and the
specific evaluation criteria and definition of cut-off value were described in Additional File 2. The overall response rate (ORR) was defined as the proportion of patients who achieved a
complete response (CR) or partial response (PR). Efficacy evaluation was conducted every 2 treatment cycles. Overall survival (OS) was defined as the interval from the date of first
treatment to the date of death for any reason. Progression-free survival (PFS) was defined as the interval from the date of first treatment to the date of disease progression or death for
any reason. The SUVmax was collected from the reports on pretreatment 18F-FDG PET-CT examinations. The clinical features of the patients and the correlations of these features with LMP1
expression were summarized in Table 1. CELL LINES AND CULTURE YT and NKYS cell lines were obtained from Dr. Wing C. Chan (City of Hope Medical Center), the SNT16 cell line was a gift from
Guangzhou Bairui Biomedical Technology Company, Ltd. (China), and the SNK6 cell line was kindly provided by Dr. Norio Shimizu and Yu Zhang of Chiba University. The culture conditions were
mentioned in Additional File 2. CONSTRUCTION OF STABLE CELL LINES Sequences for over-expression (OE)-LMP1 and its vector (OE-vector) were designed by Guangzhou Bairui Biomedical Technology
Company, Ltd. (China). Sequences for short hairpin RNA (shRNA) of LMP1 (shLMP1) and negative control (shNC), and OE-RelB and its vector (OE-vector) were designed by Shanghai Genechem
Company, Ltd. (China). Brief procedures were mentioned in Additional File 2. CELL PROLIFERATION ASSAY Cell proliferation assay was conducted with Cell Counting Kit-8 (CCK-8) reagent
(UElandy, China). Brief procedures were mentioned in Additional File 2. Independent experiments were repeated at least three times. CELL APOPTOSIS ASSAY Cell apoptosis assay was conducted
with APC-Annexin V/PI Apoptosis Detection Kit (UElandy, China). Brief procedures were mentioned in Additional File 2. Independent experiments were repeated at least three times. GEMCITABINE
SENSITIVITY ASSAY Gemcitabine sensitivity assay was conducted with CCK-8 reagent (UElandy, China). Brief procedures were mentioned in Additional File 2. Independent experiments were repeated
at least three times. MRNA SEQUENCING AND METABOLIC SEQUENCING ANALYSIS Total RNA extraction, mRNA library construction and sequencing, sample preparation, LC–MS detection, and data
analysis were performed by Suzhou PANOMIX Biomedical Tech Co., LTD. (China). Brief procedures were mentioned in Additional File 2. DROPLET DIGITAL PCR (DDPCR) RNA was extracted using
RNAsimple Total RNA Kit (TIANGEN, China). cDNA was synthesized by UEIris RT mix with DNase (UElandy, China). The primers were synthesized by Hangzhou Shangyasai Biotechnology Co., Ltd.
(China) and Sangon Biotech Co., Ltd. (China), with sequences listed in Additional File 1: Table S2. Brief procedures were mentioned in Additional File 2. Independent experiments were
repeated at least three times. GLUCOSE UPTAKE AND LACTATE PRODUCTION DETECTION ASSAYS Glucose uptake detection assay was conducted with the Glucose Oxidase Method Kit (APPLYGEN, China), and
the lactate production detection assay was conducted with the Lactic Acid Assay kit (Nanjing Jiancheng Bioengineering Institute, China). Brief procedures were mentioned in Additional File 2.
Independent experiments were repeated at least three times. GLYCOLYSIS STRESS ASSAY Extracellular acidification rate (ECAR) of NKTCL cells was conducted with Seahorse XF96 Flux Analyzer
(Agilent, USA) and Glycolysis Stress Test Kit (Agilent, USA). Cell adhesion was described in Additional File 2. Independent experiments were repeated at least three times. MASS SPECTROMETRY
ANALYSIS Total sample preparation, mass spectrometry detection, and data analysis were performed as previously [19]. Brief procedures were mentioned in Additional File 2. The raw sequencing
data was mentioned in Additional File 3. IP, CO-IP, AND SILVER STAINING Silver staining was conducted with Fast Silver Stain Kit (Beyotime, China). Brief procedures were mentioned in
Additional File 2. The antibodies were listed in Additional File 1: Table S1. WESTERN BLOTTING Brief procedures were mentioned in Additional File 2. Primary and secondary antibodies were
listed in Additional File 1: Table S1. XENOGRAFT TUMOR ASSAY BALB/c‐Nu nude mice and NOD-Scid mice were purchased from the GemPharmatech Company (China). Brief procedures were mentioned in
Additional File 2. Animals were randomly selected and at least five animals in each group. The antibodies were listed in Additional File 1: Table S1. STATISTICAL ANALYSIS Statistical
analyses were performed using SPSS software version 25.0 (IBM Corp., USA) and GraphPad Prism version 8.0 (GraphPad Software, Inc., USA). Data was expressed as the mean ± standard deviation
for repeated measurements. Comparisons between groups were performed using Student’s _t_-test and analysis of variance. PFS and OS were analyzed using the Kaplan–Meier method and log-rank
test. The correlation between LMP1 expression with clinical features and treatment response was assessed using the χ2-test. A value of _P_ < 0.05 was considered statistically significant.
RESULTS LMP1 INFLUENCES THE AGGRESSIVE BIOLOGICAL BEHAVIORS OF NKTCL CELLS AND IS RELATED TO THE CLINICAL CHARACTERISTICS OF PATIENTS To determine the role of LMP1 in the tumorigenesis and
development of NKTCL, we examined LMP1 expression in 6 NKTCL cell lines (Fig. 1A). From this, we selected YT and SNT16 to generate OE-LMP1 cells (Additional File 4: Fig. S1A), and NKYS and
SNK6 to generate shLMP1 cells (Additional File 4: Fig. S1B). Subsequently, cell proliferation, apoptosis resistance, and gemcitabine sensitivity were assessed in vitro. As expected, LMP1
overexpressing NKTCL cells, including YTOE-LMP1, SNT16OE-LMP1 cells, NKYSshNC, and SNK6shNC cells, exhibited significantly enhanced cell proliferation (Fig. 1B), inhibition of the
starvation-induced apoptosis (Fig. 1C) and reduced gemcitabine sensitivity (Fig. 1D). The role of LMP1 in tumorigenesis and tumor growth in vivo was also assessed in NKTCL xenograft mouse
models (Additional File 4: Fig. S1C). We found that the mice injected with YTOE-LMP1 cells exhibited a greater tumor burden than the mice injected with YTOE-vector cells (Fig. 1E, F) and
that the mice injected with NKYSshLMP1 cells exhibited a lower tumor burden (Fig. 1G, H). In addition, the expression of Ki67 was related to the expression of the related protein LMP1 in
mouse tissues (Fig. 1I). Furthermore, LMP1 expression was analyzed and scored by IHC staining in samples from 58 patients with pathologically verified NKTCL from the First Affiliated
Hospital of Zhengzhou University (Fig. 1J). Interestingly, we found that LMP1 expression was significantly related to the Prognostic Index for Natural Killer cell lymphoma-Epstein-Barr virus
(PINK-E) score of NKTCL patients (Table 1). Moreover, the results demonstrated that patients with low LMP1 expression were more likely to achieve a CR or a PR after treatment (Table 2), and
patients with aberrantly high LMP1 expression had a worse prognosis, indicated by shorter OS and PFS (Fig. 1K). Taken together, these findings indicate that LMP1 supports NKTCL cell
biological functions both in vitro and in vivo and is associated with the risk stratification, treatment response, and prognosis of NKTCL patients. LMP1 INFLUENCES THE AEROBIC GLYCOLYSIS IN
NKTCL CELLS AND IS RELATED TO SUVMAX OF PATIENTS To explore the functional role of metabolism in LMP1-enhanced NKTCL cell proliferation, untargeted metabolomics sequencing was conducted, and
778 differentially expressed metabolites between NKYSshNC and NKYSshLMP1 cells were identified (Additional File 4: Fig. S2A–D). KEGG enrichment analysis revealed that the main
differentially expressed metabolic pathways were glucose-related metabolic pathways (Fig. 2A), and the function of LMP1 in aerobic glycolysis in NPC has been confirmed [20]. Therefore, we
assessed glucose uptake and lactate production in the 6 NKTCL cell lines. Interestingly, the LMP1-positive cell lines SNK6, KAI3, and NKYS took up more glucose and produced more lactate than
did the LMP1-negative cell lines KHYG1, SNT16 and YT (Fig. 2B). OE-LMP1 and shLMP1 had the same effects on YT and SNT16 cells (Fig. 2C), and on NKYS and SNK6 cells (Fig. 2D). Moreover, the
changes in the ECAR induced by LMP1 were measured using a glycolysis stress assay. As expected, the OE-LMP1 cells had an increased ECAR, while the shLMP1 cells had a significantly decreased
ECAR (Fig. 2E, F). In addition, the expression of glycolysis-related genes and proteins was also investigated and recapitulated (Fig. 2G, H). Subsequently, 18F-FDG uptake was analyzed in 42
NKTCL patients with LMP1 expression scored who underwent PET-CT examination before treatment. These findings suggested that aberrantly high LMP1 expression was accompanied by a greater
SUVmax than low LMP1 expression (Fig. 2I, J). In summary, these results demonstrate that LMP1 strengthens NKTCL cell biological function by contributing to increased aerobic glycolysis. LMP1
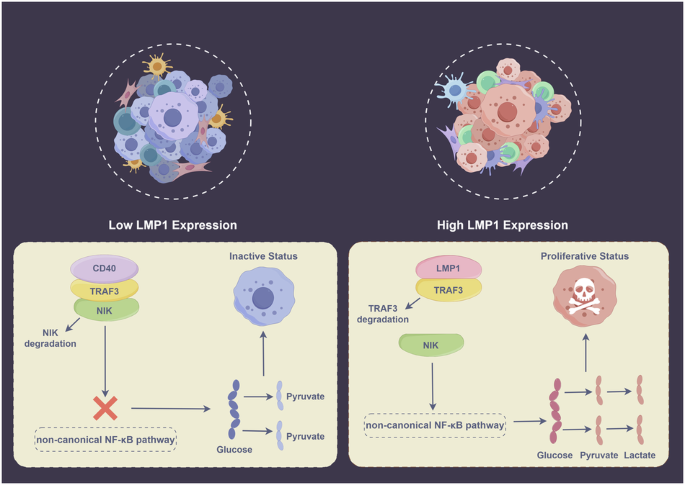
REGULATES AEROBIC GLYCOLYSIS VIA COMPETITIVE BINDING WITH TRAF3 TO ACTIVATE THE NONCANONICAL NF-ΚB PATHWAY The underlying mechanism by which LMP1 regulates aerobic glycolysis in NKTCL
remains unclear, and to address this problem, we performed RNA sequencing using RNA extracted from NKYSshNC and NKYSshLMP1 cells, and KEGG enrichment analysis indicated that the NF-κB
signaling pathway was significantly enriched in the shNC group (Fig. 3A). Furthermore, we identified one LMP1 binding partner, TRAF3, from the SNK6 cell line using IP coupled to mass
spectrometry according to Significance Analysis of the INTeractome (SAINT) score [21, 22] (Fig. 3B, Additional File 3). It has been previously reported that LMP1 sequesters and binds TRAF3
more effectively than CD40 to regulate the NF-κB pathway in B-cell lymphoma cells [23]. Accordingly, a CO-IP assay was implemented to verify the competitive binding of LMP1 and CD40 with
TRAF3 in NKTCL cells. The results showed that NKYSshNC and SNK6shNC cells bound more TRAF3, and NKYSshLMP1 and SNK6shLMP1 cells bound more CD40 (Fig. 3C). Depending on the dysregulated
function of TRAF3 in the noncanonical NF-κB pathway, degradation of TRAF3 after competitive binding to LMP1 with increased affinity also promoted the accumulation of NIK, which activated the
downstream noncanonical NF-κB signaling pathway, as confirmed by CO-IP assay (Fig. 3C). The expression of noncanonical NF-κB pathway-related genes and proteins was tested by ddPCR (Fig. 3D)
and western blotting in NKTCL cells (Fig. 3E), and by IHC staining in NKTCL xenograft tumor tissues (Fig. 3F), and these results collectively suggested that shLMP1 inhibited the activation
of the downstream noncanonical NF-κB pathway. In brief, LMP1 competes with CD40 for TRAF3 binding and subsequently activates the noncanonical NF-κB signaling pathway in NKTCL cells to
promote aerobic glycolysis (Fig. 4). ACTIVATION OF THE NONCANONICAL NF-ΚB PATHWAY AFFECTS AEROBIC GLYCOLYSIS IN NKTCL CELLS To confirm the effect of the noncanonical NF-κB pathway
reactivation on aerobic glycolysis in NKTCL, NKYSshLMP1 and SNK6shLMP1 cells with OE-RelB were generated (Additional File 4: Fig. S3). In addition, the NF-κB pathway inhibitor BAY 11-7082
was applied to the YT and SNT16 cells with OE-LMP1. BAY 11-7082 greatly reduced glucose uptake and lactate production compared with those in OE-LMP1 cells (Fig. 5A, B), and
NKYSshLMP1+OE-RelB and SNK6shLMP1+OE-RelB cells took up more glucose and produced more lactate than NKYSshLMP1+OE-vector and SNK6shLMP1+OE-vector cells (Fig. 5C, D). Moreover, the glycolysis
stress assay indicated that the ECAR was significantly decreased by the application of BAY 11-7082 in YT and SNT16 cells (Fig. 5E, F) and increased by OE-RelB in NKYS and SNK6 cells (Fig.
5G, H). Furthermore, the expression of glycolysis-related proteins was also confirmed by western blotting (Fig. 5I, J). In summary, the inhibition or reactivation of the NF-κB pathway
affects aerobic glycolysis in NKTCL cells. ACTIVATION OF THE NONCANONICAL NF-ΚB PATHWAY AND AEROBIC GLYCOLYSIS AFFECT THE AGGRESSIVE BIOLOGICAL FUNCTION OF NKTCL CELLS Whether the activation
of the noncanonical NF-κB pathway and aerobic glycolysis affect the tumorigenesis and development of NKTCL cells remains to be studied. Thus, cell proliferation, apoptosis resistance, and
gemcitabine sensitivity were assessed in vitro. As expected, inhibition of the NF-κB pathway significantly suppressed the proliferation of NKTCL cells (Fig. 6A), and interestingly, OE-RelB
attenuated the decrease in NKTCL cell proliferation caused by shLMP1, which was also inhibited by 2-DG, an aerobic glycolysis inhibitor (Fig. 6B). Consistently, in the apoptosis resistance
and gemcitabine sensitivity experiments, BAY 11-7082 increased starvation-induced cell apoptosis (Fig. 6C), and OE-RelB decreased this effect, and the effect of OE-RelB was inhibited by
treatment with 2-DG (Fig. 6D). Moreover, BAY 11-7082 reduced gemcitabine sensitivity in NKTCL cells (Fig. 6E) and OE-RelB promoted gemcitabine sensitivity, which was inhibited by 2-DG
treatment (Fig. 6F). In summary, the activation of the noncanonical NF-κB pathway and aerobic glycolysis affect the proliferation and development of NKTCL cells modulated by LMP1. DISCUSSION
Previous studies have comprehensively illuminated the phenotypic effects and mechanism of LMP1 on NPC. LMP1 plays an important role in the tumorigenesis and development of NPC through
activating multiple signaling pathways, including cell proliferation and survival, angiogenesis, and invasion pathways [11, 24, 25]. In addition, LMP1 can affect cell-cell interactions,
antigen presentation, cytokine and chemokine production, and modulation of the tumor microenvironment [10, 26, 27]. LMP1 is also reported to enhance cell proliferation in EBV-driven
malignancies and serves as a prognostic marker for NKTCL patients [28, 29]. Therefore, we detected LMP1 expression in 6 NKTCL cell lines and established stable OE-LMP1 and shLMP1 cells. Our
results showed that high LMP1 expression promoted NKTCL cell proliferation in vitro and in vivo, and was positively correlated with Ki67 expression in xenograft mouse tissues. In addition,
experimental assays demonstrated that LMP1 inhibited starvation-induced apoptosis and reduced the gemcitabine sensitivity of NKTCL cells. Clinically, LMP1 expression was scored in 58 NKTCL
patient tissues, and its correlations with clinical features, treatment response, and prognosis were analyzed. These findings suggest that LMP1 affects the tumorigenesis and development of
NKTCL cells and serves as an indicator of the risk stratification, treatment response, and prognosis of NKTCL patients. In recent years, studies on metabolic reprogramming, especially
glucose metabolism, have attracted increased attention. Based on the results of untargeted metabolic sequencing, we found that compared with LMP1-negative cells, LMP1-positive NKTCL cells
exhibited greater glucose uptake and lactate production. Consistent results were obtained for stable OE-LMP1 and shLMP1 cells. Moreover, the glycolysis stress assay suggested that the ECAR
was affected by LMP1 expression. The expression of glycolysis-related genes and proteins also recapitulated this finding. In addition, we analyzed the available 18F-FDG uptake of NKTCL
patients who underwent PET-CT examination before treatment, and the correlation between the SUVmax and LMP1 expression showed that aberrantly high LMP1 expression was accompanied by a
greater SUVmax in NKTCL patients. Mechanistically, some studies have indicated that LMP1 promotes aerobic glycolysis by regulating oncogenic signaling pathways [17, 30], involving in
epigenetic processes [31, 32], and activating glucose metabolic enzymes in NPC [20]. To date, few investigations have focused on the mechanism by which LMP1 regulates aerobic glycolysis in
NKTCL, which is the novel aspect of our study. To further explain the role of LMP1 in aerobic glycolysis in NKTCL, RNA sequencing was conducted and KEGG enrichment analysis revealed that the
NF-κB signaling pathway was significantly enriched in the high LMP1 expression group. Subsequently, via IP coupled to mass spectrometry, we identified one LMP1-interacting protein, TRAF3,
which plays a negative role in regulating the NF-κB signaling pathway [33]. Previous studies demonstrated that LMP1 led to TRAF3 sequestration in B-lymphoma cells, which inhibited the
negative regulation of pro-survival membrane, cytoplasmic, and nuclear signaling events by TRAF3 [23]. Therefore, the interactions between LMP1 and TRAF3 and between TRAF3 and CD40 upon LMP1
availability were examined in our research. The results showed that cells with high LMP1 expression exhibited greater binding of LMP1 and TRAF3, and shLMP1 cells exhibited greater binding
of LMP1 and CD40. Because of its competitive binding to LMP1 with greater affinity, the subsequent degradation of TRAF3 also protected NIK from self-degradation and subsequently caused its
accumulation, which activated the downstream noncanonical NF-κB signaling pathway. The expression of noncanonical NF-κB pathway-related genes and proteins also confirmed this phenomenon.
Several studies have shown that the NF-κB pathway governs glycolysis via direct engagement of the cellular networks, with profound implications on inflammation, metabolic diseases, and
tumorigenesis [34, 35]. A study on diffuse large B-cell lymphoma (DLBCL) showed that TP53 mutations cooperated with c-Rel to promote NF-κB functions and led to enhanced invasion and
metastasis in malignant cells [36]. Moreover, the activation of the NF-κB signaling pathway increased glucose uptake by inducing the plasma membrane localization of GLUT1, blocking
apoptosis, and promoting B-cell lymphoma growth [37]. However, this effect on NKTCL is not fully understood, and whether the activation of the noncanonical NF-κB signaling pathway affects
aerobic glycolysis and the biological function of NKTCL cells remains to be explored. Thus, in our study, an NF-κB pathway inhibitor was applied to YTOE-LMP1 and SNT16 OE-LMP1 cells, and
OE-RelB cells were established from NKYSshLMP1 and SNK6shLMP1 cells. The results showed that with the inhibition of the NF-κB pathway, NKTCL cells exhibited suppression of aerobic glycolysis
including decreased glucose uptake, lactate production, and ECAR, while OE-RelB cells showed promotion of aerobic glycolysis. In addition, glycolysis-related genes and proteins were also
examined. Further experiments confirmed that inhibition of the NF-κB pathway could suppress the aggressive behavior of NKTCL cells and that of OE-RelB cells could restore these behaviors.
Moreover, the addition of 2-DG, an inhibitor of glycolysis, significantly inhibited biological cellular functions. Recently, accumulating evidence has also revealed the immune-related
effects of LMP1 in NKTCL. Li et al. highlighted the crucial role of malignant NK cells with LMP1 expression in reshaping the cellular landscape and fostering an immunosuppressive
microenvironment [38], and two other studies suggested that LMP1 expression is positively correlated with PD-L1 expression in NKTCL [8, 39]. In addition, LMP1 also serves as a promising
target for novel adaptive T cell immunotherapy for the treatment of NKTCL [40]. Moreover, glycolysis has been implicated in the regulation of the tumor microenvironment and development by
inhibiting monocyte migration, suppressing T cell activation, and promoting the release of cytokines in DLBCL [41]. Thus, whether enhancement of aerobic glycolysis is involved in the
regulation of the NKTCL microenvironment and whether immunotherapy can attenuate abnormal glycolysis in NKTCL cells need to be elucidated in further studies. In summary, the present study is
the first to reveal the role and detailed mechanism of LMP1 in promoting aerobic glycolysis and aggressive biological functions in NKTCL. Our results elucidate the effects of viral
infection on abnormal metabolism in NKTCL patients, which expands the understanding of the pathogenesis and progression of this disease and might provide a promising perspective for the
treatment of NKTCL. DATA AVAILABILITY Raw untargeted metabolic sequencing data are available in the Metabolights under the accession number MTBLS9482. Raw mRNA sequencing data are deposited
in the NCBI BioProject under the accession number PRJNA1071043. Any other data is available from the authors upon reasonable request. REFERENCES * Alaggio R, Amador C, Anagnostopoulos I,
Attygalle AD, Araujo IBO, Berti E, et al. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720–48. Article
PubMed PubMed Central Google Scholar * Tse E, Zhao WL, Xiong J, Kwong YL. How we treat NK/T-cell lymphomas. J Hematol Oncol. 2022;15:74. Article CAS PubMed PubMed Central Google
Scholar * Wang X, Zhang L, Liu X, Li X, Li L, Fu X, et al. Efficacy and safety of a pegasparaginase-based chemotherapy regimen vs an L-asparaginase-based chemotherapy regimen for newly
diagnosed advanced extranodal natural killer/T-cell lymphoma: a randomized clinical trial. JAMA Oncol. 2022;8:1035–41. Article PubMed PubMed Central Google Scholar * Ghione P, Qi S,
Imber BS, Seshan V, Moskowitz A, Galasso N, et al. Modified SMILE (mSMILE) and intensity-modulated radiotherapy (IMRT) for extranodal NK-T lymphoma nasal type in a single-center population.
Leuk Lymphoma. 2020;61:3331–41. Article CAS PubMed PubMed Central Google Scholar * Zhang Y, Ma S, Cai J, Yang Y, Jing H, Shuang Y, et al. Sequential P-GEMOX and radiotherapy for
early-stage extranodal natural killer/T-cell lymphoma: a multicenter study. Am J Hematol. 2021;96:1481–90. Article CAS PubMed PubMed Central Google Scholar * Tian XP, Cao Y, Cai J,
Zhang YC, Zou QH, Wang JN, et al. Novel target and treatment agents for natural killer/T-cell lymphoma. J Hematol Oncol. 2023;16:78. Article CAS PubMed PubMed Central Google Scholar *
Lim SH, Hong JY, Lim ST, Hong H, Arnoud J, Zhao W, et al. Beyond first-line non-anthracycline-based chemotherapy for extranodal NK/T-cell lymphoma: clinical outcome and current perspectives
on salvage therapy for patients after first relapse and progression of disease. Ann Oncol. 2017;28:2199–205. Article CAS PubMed Google Scholar * Bi XW, Wang H, Zhang WW, Wang JH, Liu WJ,
Xia ZJ, et al. PD-L1 is upregulated by EBV-driven LMP1 through NF-κB pathway and correlates with poor prognosis in natural killer/T-cell lymphoma. J Hematol Oncol. 2016;9:109. Article
PubMed PubMed Central Google Scholar * Montes-Mojarro IA, Fend F, Quintanilla-Martinez L. EBV and the pathogenesis of NK/T cell lymphoma. Cancers. 2021;13:1414. Article CAS PubMed
PubMed Central Google Scholar * Lo AK, Dawson CW, Lung HL, Wong KL, Young LS. The role of EBV-encoded LMP1 in the NPC tumor microenvironment: from function to therapy. Front Oncol.
2021;11:640207. Article CAS PubMed Central Google Scholar * Tao Y, Shi Y, Jia J, Jiang Y, Yang L, Cao Y. Novel roles and therapeutic targets of Epstein-Barr virus-encoded latent membrane
protein 1-induced oncogenesis in nasopharyngeal carcinoma. Expert Rev Mol Med. 2015;17:e15. Article PubMed Google Scholar * Hanahan D, Weinberg RA. Hallmarks of cancer: the next
generation. Cell. 2011;144:646–74. Article CAS PubMed Google Scholar * Tian T, Li J, Shi D, Zeng Y, Yu B, Li X, et al. SMYD3 promotes aerobic glycolysis in diffuse large B-cell lymphoma
via H3K4me3-mediated PKM2 transcription. Cell Death Dis. 2022;13:763. Article CAS PubMed PubMed Central Google Scholar * Pang Y, Lu T, Xu-Monette ZY, Young KH. Metabolic reprogramming
and potential therapeutic targets in lymphoma. Int J Mol Sci. 2023;24:5493. Article CAS PubMed PubMed Central Google Scholar * Yang T, You C, Meng S, Lai Z, Ai W, Zhang J. EBV infection
and its regulated metabolic reprogramming in nasopharyngeal tumorigenesis. Front Cell Infect Microbiol. 2022;12:935205. Article CAS PubMed PubMed Central Google Scholar * Polet F,
Feron O. Endothelial cell metabolism and tumour angiogenesis: glucose and glutamine as essential fuels and lactate as the driving force. J Intern Med. 2013;273:156–65. Article CAS PubMed
Google Scholar * Zhang J, Jia L, Lin W, Yip YL, Lo KW, Lau VMY, et al. Epstein-Barr virus-encoded latent membrane protein 1 upregulates glucose transporter 1 transcription via the
mTORC1/NF-κB signaling pathways. J Virol. 2017;91:e02168–16. Article CAS PubMed PubMed Central Google Scholar * Marshall NA, Vickers MA, Barker RN. Regulatory T cells secreting IL-10
dominate the immune response to EBV latent membrane protein 1. J Immunol. 2003;170:6183–9. Article CAS PubMed Google Scholar * Li Z, Zhang X, Xue W, Zhang Y, Li C, Song Y, et al.
Recurrent GNAQ mutation encoding T96S in natural killer/T cell lymphoma. Nat Commun. 2019;10:4209. Article PubMed PubMed Central Google Scholar * Zhang J, Jia L, Liu T, Yip YL, Tang WC,
Lin W, et al. mTORC2-mediated PDHE1α nuclear translocation links EBV-LMP1 reprogrammed glucose metabolism to cancer metastasis in nasopharyngeal carcinoma. Oncogene. 2019;38:4669–84. Article
CAS PubMed PubMed Central Google Scholar * Teo G, Koh H, Fermin D, Lambert JP, Knight JD, Gingras AC, et al. SAINTq: Scoring protein-protein interactions in affinity purification -
mass spectrometry experiments with fragment or peptide intensity data. Proteomics. 2016;16:2238–45. Article CAS PubMed Google Scholar * Teo G, Liu G, Zhang J, Nesvizhskii AI, Gingras AC,
Choi H. SAINTexpress: improvements and additional features in Significance Analysis of INTeractome software. J Proteom. 2014;100:37–43. Article CAS Google Scholar * Bangalore-Prakash P,
Stunz LL, Mambetsariev N, Whillock AL, Hostager BS, Bishop GA. The oncogenic membrane protein LMP1 sequesters TRAF3 in B-cell lymphoma cells to produce functional TRAF3 deficiency. Blood
Adv. 2017;1:2712–23. Article CAS PubMed PubMed Central Google Scholar * Dawson CW, Port RJ, Young LS. The role of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the
pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol. 2012;22:144–53. Article CAS PubMed Google Scholar * Tsao SW, Tramoutanis G, Dawson CW, Lo AK, Huang DP. The
significance of LMP1 expression in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12:473–87. Article CAS PubMed Google Scholar * Yao L, Setsuda J, Sgadari C, Cherney B, Tosato G.
Interleukin-18 expression induced by Epstein-Barr virus-infected cells. J Leukoc Biol. 2001;69:779–84. Article CAS PubMed Google Scholar * Teichmann M, Meyer B, Beck A, Niedobitek G.
Expression of the interferon-inducible chemokine IP-10 (CXCL10), a chemokine with proposed anti-neoplastic functions, in Hodgkin lymphoma and nasopharyngeal carcinoma. J Pathol.
2005;206:68–75. Article CAS PubMed Google Scholar * Jiang M, Lu H, Lu C, Geng X, Jia Y, Wang P, et al. Specific soft-tissue invasion and LMP1 expression are potential indicators of
extranodal NK/T cell lymphoma, nasal type. Med Sci Monit. 2018;24:7603–13. Article CAS PubMed PubMed Central Google Scholar * Mao Y, Zhang DW, Zhu H, Lin H, Xiong L, Cao Q, et al. LMP1
and LMP2A are potential prognostic markers of extranodal NK/T-cell lymphoma, nasal type (ENKTL). Diagn Pathol. 2012;7:178. Article CAS PubMed PubMed Central Google Scholar * Lo AK,
Dawson CW, Young LS, Ko CW, Hau PM, Lo KW. Activation of the FGFR1 signalling pathway by the Epstein-Barr virus-encoded LMP1 promotes aerobic glycolysis and transformation of human
nasopharyngeal epithelial cells. J Pathol. 2015;237:238–48. Article CAS PubMed Google Scholar * Jiang Y, Yan B, Lai W, Shi Y, Xiao D, Jia J, et al. Repression of Hox genes by LMP1 in
nasopharyngeal carcinoma and modulation of glycolytic pathway genes by HoxC8. Oncogene. 2015;34:6079–91. Article CAS PubMed PubMed Central Google Scholar * Luo X, Hong L, Cheng C, Li N,
Zhao X, Shi F, et al. DNMT1 mediates metabolic reprogramming induced by Epstein-Barr virus latent membrane protein 1 and reversed by grifolin in nasopharyngeal carcinoma. Cell Death Dis.
2018;9:619. Article PubMed Central Google Scholar * Bishop GA, Stunz LL, Hostager BS. TRAF3 as a multifaceted regulator of B lymphocyte survival and activation. Front Immunol.
2018;9:2161. Article PubMed PubMed Central Google Scholar * Tornatore L, Thotakura AK, Bennett J, Moretti M, Franzoso G. The nuclear factor kappa B signaling pathway: integrating
metabolism with inflammation. Trends Cell Biol. 2012;22:557–66. Article CAS PubMed Google Scholar * Pi M, Kuang H, Yue C, Yang Q, Wu A, Li Y, et al. Targeting metabolism to overcome
cancer drug resistance: a promising therapeutic strategy for diffuse large B cell lymphoma. Drug Resist Updat. 2022;61:100822. Article CAS PubMed Google Scholar * Li L, Xu-Monette ZY, Ok
CY, Tzankov A, Manyam GC, Sun R, et al. Prognostic impact of c-Rel nuclear expression and REL amplification and crosstalk between c-Rel and the p53 pathway in diffuse large B-cell lymphoma.
Oncotarget. 2015;6:23157–80. Article PubMed PubMed Central Google Scholar * Sommermann TG, O’Neill K, Plas DR, Cahir-McFarland E. IKKβ and NF-κB transcription govern lymphoma cell
survival through AKT-induced plasma membrane trafficking of GLUT1. Cancer Res. 2011;71:7291–7300. Article CAS PubMed PubMed Central Google Scholar * Li YQ, Luo CL, Jiang JX, He S, Liu
Y, Yan WX, et al. Single-cell analysis reveals malignant cells reshape the cellular landscape and foster an immunosuppressive microenvironment of extranodal NK/T-cell lymphoma. Adv Sci.
2023;10:e2303913. Article Google Scholar * Kume A, Shinozaki-Ushiku A, Kunita A, Kondo A, Ushiku T. Enhanced PD-L1 expression in LMP1-positive cells of Epstein-Barr virus-associated
malignant lymphomas and lymphoproliferative disorders: a single-cell resolution analysis with multiplex fluorescence immunohistochemistry and in situ hybridization. Am J Surg Pathol.
2022;46:1386–96. Article PubMed Google Scholar * Li H, Song W, Wu J, Shi Z, Gao Y, Li J, et al. CAR-T cells targeting CD38 and LMP1 exhibit robust antitumour activity against NK/T cell
lymphoma. BMC Med. 2023;21:330. Article CAS PubMed PubMed Central Google Scholar * Cui Y, Leng C. A glycolysis-related gene signatures in diffuse large B-Cell lymphoma predicts
prognosis and tumor immune microenvironment. Front Cell Dev Biol. 2023;11:1070777. Article PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS YT and NKYS cell
lines were obtained from Dr. Wing C. Chan (City of Hope Medical Center), SNT16 cell line was a gift from Guangzhou Bairui Biomedical Technology Company, Ltd. (China), and SNK6 cell line was
kindly provided by Dr. Norio Shimizu and Yu Zhang of Chiba University. I would like to show great gratitude to them all. FUNDING This work was supported by the National Natural Science
Foundation of China (81970184; 82170183; 82070209; U1904139), Funding for Scientific Research and Innovation Team of The First Affiliated Hospital of Zhengzhou University (QNCXTD2023012),
Henan Province Youth Health Science and Technology Innovation Project (LJRC2023014), Joint Construction Project of Medical Science and Technology of Henan Province of China (LHGJ20220386),
and Henan Province Health Commission Co-Construction Project (SB201901044). The work was also supported by the Oncology Department and State Key Laboratory of Esophageal Cancer Prevention
& Treatment and Henan Key Laboratory for Esophageal Cancer Research of the First Affiliated Hospital of Zhengzhou University. AUTHOR INFORMATION Author notes * These authors contributed
equally: Wenting Song, Yuyang Gao, Jiazhuo Wu. AUTHORS AND AFFILIATIONS * Department of Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China Wenting Song,
Yuyang Gao, Jiazhuo Wu, Hongwen Li, Zhuangzhuang Shi, Chen Gong, Zihe Zhang, Zhaoming Li & Mingzhi Zhang * State Key Laboratory of Esophageal Cancer Prevention & Treatment and Henan
Key Laboratory for Esophageal Cancer Research, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China Wenting Song, Yuyang Gao & Jiazhuo Wu Authors * Wenting
Song View author publications You can also search for this author inPubMed Google Scholar * Yuyang Gao View author publications You can also search for this author inPubMed Google Scholar *
Jiazhuo Wu View author publications You can also search for this author inPubMed Google Scholar * Hongwen Li View author publications You can also search for this author inPubMed Google
Scholar * Zhuangzhuang Shi View author publications You can also search for this author inPubMed Google Scholar * Chen Gong View author publications You can also search for this author
inPubMed Google Scholar * Zihe Zhang View author publications You can also search for this author inPubMed Google Scholar * Zhaoming Li View author publications You can also search for this
author inPubMed Google Scholar * Mingzhi Zhang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Wenting Song: Investigation, Project
administration, Formal analysis, Data curation, Visualization, Writing-Original draft; Yuyang Gao: Investigation, Formal analysis, Validation; Jiazhuo Wu: Investigation, Formal analysis,
Validation; Hongwen Li: Formal analysis, Project administration; Zhuangzhuang Shi: Formal analysis; Chen Gong: Formal analysis; Zihe Zhang: Formal analysis; Zhaoming Li: Funding acquisition,
Resources, Supervision, Writing-review & editing; Mingzhi Zhang: Conceptualization, Funding acquisition, Resources, Supervision, Writing-review & editing. CORRESPONDING AUTHOR
Correspondence to Mingzhi Zhang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICS APPROVAL This study was conducted in accordance with the
declaration of Helsinki and approved by the Ethics Committee for Scientific Research and Clinical Trials of the First Affiliated Hospital of Zhengzhou University (approval number:
2023-KY-0258-002). ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by
Nirmal Robinson SUPPLEMENTARY INFORMATION ADDITIONAL FILE 1 ADDITIONAL FILE 2 ADDITIONAL FILE 3 ADDITIONAL FILE 4 CHECKLIST ORIGINAL DATA FILE RIGHTS AND PERMISSIONS OPEN ACCESS This article
is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you
give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material
in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Song, W., Gao, Y., Wu, J. _et al._ LMP1 enhances
aerobic glycolysis in natural killer/T cell lymphoma. _Cell Death Dis_ 15, 604 (2024). https://doi.org/10.1038/s41419-024-06999-7 Download citation * Received: 11 March 2024 * Revised: 09
August 2024 * Accepted: 13 August 2024 * Published: 20 August 2024 * DOI: https://doi.org/10.1038/s41419-024-06999-7 SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative









