- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Increased neutrophil extracellular traps (NETs) formation has been found to be associated with intestinal inflammation, and it has been reported that NETs may drive the progression
of gut dysregulation in sepsis. However, the biological function and regulation of NETs in sepsis-induced intestinal barrier dysfunction are not yet fully understood. First, we found that
both circulating biomarkers of NETs and local NETs infiltration in the intestine were significantly increased and had positive correlations with markers of enterocyte injury in abdominal
sepsis patients. Moreover, the levels of local citrullinated histone 3 (Cit H3) expression were associated with the levels of BIP expression. To further confirm the role of NETs in
sepsis-induced intestinal injury, we compared peptidylarginine deiminase 4 (PAD4)-deficient mice and wild-type (WT) mice in a lethal septic shock model. In WT mice, the Cit H3-DNA complex
was markedly increased, and elevated intestinal inflammation and endoplasmic reticulum (ER) stress activation were also found. Furthermore, PAD4 deficiency alleviated intestinal barrier
disruption and decreased ER stress activation. Notably, NETs treatment induced intestinal epithelial monolayer barrier disruption and ER stress activation in a dose-dependent manner in
vitro, and ER stress inhibition markedly attenuated intestinal apoptosis and tight junction injury. Finally, TLR9 antagonist administration significantly abrogated NETs-induced intestinal
epithelial cell death through ER stress inhibition. Our results indicated that NETs could contribute to sepsis-induced intestinal barrier dysfunction by promoting inflammation and apoptosis.
Suppression of the TLR9–ER stress signaling pathway can ameliorate NETs-induced intestinal epithelial cell death. SIMILAR CONTENT BEING VIEWED BY OTHERS TOLL-LIKE RECEPTOR 4-MEDIATED
ENDOPLASMIC RETICULUM STRESS INDUCES INTESTINAL PANETH CELL DAMAGE IN MICE FOLLOWING CLP-INDUCED SEPSIS Article Open access 10 September 2022 NEUTROPHIL EXTRACELLULAR TRAPS-TRIGGERED
IMPAIRED AUTOPHAGIC FLUX VIA METTL3 UNDERLIES SEPSIS-ASSOCIATED ACUTE LUNG INJURY Article Open access 27 August 2022 MACROPHAGE-DERIVED EXOSOMAL AMINOPEPTIDASE N AGGRAVATES SEPSIS-INDUCED
ACUTE LUNG INJURY BY REGULATING NECROPTOSIS OF LUNG EPITHELIAL CELL Article Open access 06 June 2022 INTRODUCTION Sepsis is defined as life-threatening organ dysfunction caused by the
dysregulation of the host response secondary to infection, which remains a leading cause of high mortality in the intensive care unit [1, 2]. The gastrointestinal tract is the most easily
and frequently involved organ in the process of sepsis and the gut has been deemed the motor of sepsis [3, 4]. The breakdown of the gut barrier can result in many bacteria and toxins
entering the internal environment, driving lethal sepsis and even multiple organ dysfunction syndrome (MODS) [5]. Hence, a more thorough understanding of the inflammatory mechanism involved
in intestinal barrier dysfunction is pivotal for developing more efficient treatments against sepsis and MODS. Since neutrophil extracellular traps (NETs) were firstly described by Brinkman
in 2004, excessive NETs formation has been shown to be involved in the pathophysiology of sepsis [6, 7]. NETs are web-like structures protruding from the membrane of activated neutrophils,
comprising decondensed DNA fibers accompanied by intracellular proteins, including histones, myeloperoxidase (MPO), and other antimicrobial proteins [6]. Histone citrullination induced by
peptidylarginine deiminase 4 (PAD4) plays a pivotal role in chromatin decondensation, which is one of the most crucial processes in NETs extrusion [8]. NETs can trap and kill a broad range
of pathogens, including bacteria and viruses [9]. However, uncontrolled NETs formation is generally considered a double-edged sword [10]. Excessive NETs formation has been indicated to have
a role in both infectious and noninfectious diseases, including but not limited to thrombosis, diabetes, vasculitis, and cancer [11, 12]. NETs are widely recognized as endogenous
damage-related molecular patterns (DAMPs) that can be recognized by TLR receptors [13]. Strategies targeting NETs formation have shown therapeutic and can improve survival in animal models
of sepsis [14]. Our preliminary research detected increased NETs infiltration in the intestines, and NETs disruption ameliorated intestinal injury in endotoxin mice [15]. However, the
biological function and downstream signaling pathway of NETs in sepsis-induced intestinal barrier dysfunction are not yet fully understood. The endoplasmic reticulum (ER) is the primary
cellular organelle in which protein synthesis, maturation, folding, modification, and degradation take place [16]. If the optimal balances of protein folding are interrupted in the ER, a
condition known as “ER stress” may occur [17]. Sustained ER stress can initiate inflammation through various mechanisms, including the production of reactive oxygen species (ROS) [18].
Quillard et al. reported that TLR2 stimulation followed by NETs participation may render smooth muscle cell-rich plaques susceptible to superficial erosion and thrombotic complications by
inducing ER stress and ROS production [19]. ER stress is implicated in the progression of intestinal barrier impairments in inflammatory bowel diseases [20]. Endoplasmic reticulum stress can
be regulated by TLR receptors and has a role in the process of sepsis-induced intestinal injury [21, 22]. We hypothesized that in sepsis, NETs can induce TLR receptor–ER stress–ROS
signaling and activation of the inflammatory response, increasing intestinal permeability and bacterial translocation. In the current study, to our knowledge, increased local NET
infiltration in the intestine in abdominal sepsis patients was firstly found, and the levels of local citrullinated histone 3 (Cit H3) were associated with the level of ER stress activation.
We further determined that, in vivo, NETs depletion by PAD4 deficiency alleviated intestinal inflammation and decreased ER stress activation in a lipopolysaccharide (LPS)-induced lethal
septic shock model and that, in vitro, NETs treatment induced ER stress activation and intestinal epithelial monolayer barrier disruption in a dose-dependent manner, which confirmed the
detrimental effect of NETs on intestinal barrier functions. Notably, we also observed that NETs-induced intestinal barrier dysfunction was mediated by ER stress, which is regulated by
Toll-like receptor 9 (TLR9). RESULTS INCREASED INTESTINAL NETS INFILTRATION AND ER STRESS ACTIVATION IN HUMAN ABDOMINAL SEPSIS To determine NETs performance and ER stress levels in sepsis
and their possible pathological impact on intestinal barrier dysfunction, we compared the relative expression in serum and relative protein levels in intestinal samples of healthy and
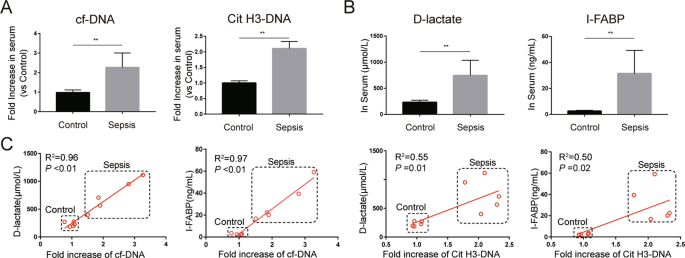
abdominal sepsis patients. We firstly investigated whether the expression of NETs is altered in the peripheral system of these patients. Serum cell-free DNA (cf-DNA), a rough biomarker of
NETs, and serum Cit H3-DNA complex, a specific biomarker of NETs, were significantly elevated in abdominal sepsis patients compared with healthy controls (Fig. 1A). In addition, the levels
of serum D-lactate and intestinal fatty-acid binding protein (I-FABP), biomarkers of intestinal damage, were markedly increased in abdominal sepsis patients (Fig. 1B). Moreover, there were
significant correlations between NETs markers and intestinal damage markers in serum (Fig. 1C). Increased intestinal injuries were further confirmed by hematoxylin and eosin (HE) staining of
intestines, evidenced by elevated Chiu’s scores in the abdominal sepsis group (Fig. 2A). Immunostaining analysis also demonstrated that NETs (MPO and Cit H3) infiltration was significantly
elevated in abdominal sepsis patients (Fig. 2B). In addition, activation of CHOP, a biomarker of ER stress, was induced in abdominal sepsis patients compared with controls (Fig. 2C). We then
investigated the correlation of protein levels between NETs, ER stress, and intestinal apoptosis biomarkers in intestinal specimens. Compared to those in healthy controls, the protein
levels of MPO, which is a biomarker of neutrophil activation and a main component of NETs, were significantly elevated in abdominal sepsis patients (Fig. 2D). In contrast to elevated levels
of Cit H3 in intestinal samples from abdominal sepsis patients, only low levels of Cit H3 were detected in intestines from healthy controls. Increased cleaved caspase 3, a biomarker of
apoptosis, and decreased Bcl-2, a biomarker of anti-apoptosis, were also found in human abdominal sepsis patients compared to healthy controls, and line regression analysis demonstrated a
positive correlation between Cit H3 and cleaved/full caspase 3 and a negative correlation between Cit H3 and Bcl-2 (Fig. 2F). In addition, elevated levels of BIP, a biomarker of ER stress
activation, were significantly higher in abdominal sepsis patients than in healthy controls (Fig. 2D, E). Simultaneously, there was a significant correlation between Cit H3 and BIP
expression (Fig. 2F). Collectively, these findings support a detrimental role of NETs in intestinal damage and a potential role of ER stress in this pathological process during human sepsis.
PAD4 DEFICIENCY DECREASES SYSTEMIC INFLAMMATION AND ORGAN DAMAGE IN A SEPTIC SHOCK MODEL To investigate the role of NETs in sepsis, a lethal dose of LPS was administered to induce septic
shock in PAD4-deficient mice and wild-type (WT) mice. We firstly evaluated the expression of NETs in WT and PAD4-deficient mice. The Cit H3-DNA complex was markedly increased in WT mice
after LPS challenge and it was abrogated in PAD4-deficient mice (Fig. 3A), which indicated that NETs formation was inhibited in PAD4-deficient mice in the LPS-induced septic shock model.
Survival analysis was then carried out to evaluate the effect of NETs on post-endotoxic shock survival rate (Fig. 3B). Thirty percent of PAD4-deficient mice survived beyond 100 h and this
result is in contrast to that obtained with WT mice, which all died within 80 h after LPS challenge. We next sought to investigate whether PAD4-deficient mice are more resistant to systemic
inflammation and organ damage in endotoxin shock. Serum proinflammatory cytokines, which have been shown to lead to sepsis, were detected and the concentrations of alanine transaminase (ALT)
and creatinine (Cr) were used to evaluate tissue injury. The levels of serum proinflammatory cytokines (including IL-6, IL-1β, and TNF-α) significantly increased after lethal LPS challenge
(Fig. 3C). In comparison, lower proinflammatory cytokines were found in PAD4-deficient mice. Moreover, WT mice also showed higher levels of serum ALT and Cr (Fig. 3D), accompanied by
increased lung edema (Fig. 3E), while these organ damage markers were significantly lower in PAD4-deficient mice. Collectively, these data indicate that NETs may play a pivotal role in
severe sepsis. PAD4 DEFICIENCY AMELIORATES SEPSIS-INDUCED INTESTINAL INFLAMMATION, ER STRESS ACTIVATION, AND INTESTINAL BARRIER DYSFUNCTION As a motor of sepsis, intestinal barrier functions
were further researched. Compared to that in WT mice, decreased intestinal water content was found in PAD4-deficient mice (Fig. 4A). As expected, LPS challenge resulted in increased
intestinal permeability, as evidenced by higher levels of plasma FD4 compared to the sham treatment (Fig. 4B). The level of FD4 was significantly reduced in the PAD4-deficient group. We then
investigated whether NETs participate in the process of sepsis-induced intestinal inflammation and apoptosis, which contribute to increased intestinal permeability. LPS challenge induced
increased local inflammatory cytokines (including IL-6, IL-1β, and TNF-α) in the intestinal mucosa of WT mice compared with sham mice (Fig. 4C). However, decreased production of these
inflammatory cytokines in the intestinal mucosa was found in PAD4-deficient mice. Moreover, histological evaluation by HE staining revealed increased histological inflammatory scores in WT
mice that underwent LPS challenge compared with sham mice, and there was a significant reduction in intestinal damage scores in PAD4-deficient mice compared to WT mice in LPS-induced septic
shock (Fig. 4D). Tight junction proteins play a critical role in regulating intestinal epithelial permeability and maintaining barrier functions. Therefore, the levels of tight junction
proteins in the intestinal mucosa were evaluated. In this research, in LPS-induced endotoxin shock, the levels of occludin and claudin 1 were substantially increased in the PAD4-deficient
group compared with the WT group (Fig. 4E). Consistently, compared to WT mice, PAD4-deficient mice exhibited decreased cleaved caspase 3 expression and increased Bcl-2 levels in the
intestine. These results indicate that NETs participate in sepsis-induced intestinal apoptosis. Collectively, these findings suggested a key role of NETs in sepsis-induced intestinal barrier
dysfunctions. We then investigated whether ER stress was involved in this pathological process and compared the relative expression of ER stress biomarkers between PAD4-deficient mice and
WT mice. The expression of BIP, an ER stress-related protein, in the intestine was significantly elevated in WT mice, whereas the expression in septic shock PAD4-deficient mice was lower
(Fig. 4E). Simultaneously, endotoxin shock increased the mRNA expression of XBP-1s, an ER stress biomarker, in the intestine of WT mice compared with control mice (Fig. 4G). Significantly
decreased mRNA levels of XBP-1s were found in PAD4-deficient mice in the LPS-induced septic shock model. Compared to control mice, WT mice showed a significant increase in ROS levels in the
intestine after septic shock, and PAD4 knockout significantly reduced ROS aggregation (Fig. 4H). These findings indicated an important role of ER stress in NETs-induced intestinal injuries
during sepsis. NETS IMPAIR THE INTEGRITY OF INTESTINAL EPITHELIAL CELL MONOLAYER BARRIERS IN VITRO To further confirm the detrimental role of NETs, we then separated NETs isolates from
healthy donors and cocultured them with Caco2 monolayers or Caco2 epithelial barriers. As listed in Fig. 5A, after coculture with NETs, the transepithelial electrical resistance (TEER)
values decreased in line with the NETs concentrations, indicating that NETs impaired the functions of Caco2 epithelial barriers in a dose-dependent manner. The TEER assay showed that NETs
induced intestinal epithelial barrier dysfunction in a time-dependent manner within 12 h after coculture. Thus, Caco2 cells were subjected to NETs isolates for 12 h in the following
experiments. As shown in Fig. 5B, NETs disrupted Caco2 monolayers in a dose-dependent manner. Moreover, a dose-dependent cytotoxicity was also revealed, as evidenced by decreased Caco2 cell
viability with increasing NETs treatment (Fig. 5C). Immunofluorescence analysis also showed that, after NETs treatment, ZO-1 localization at cellular junctions became discontinuous (Fig.
5D). Consistently, the protein levels of occludin and claudin 1 decreased and cleaved caspase 3 expression increased after NETs costimulation with Caco2 epithelial barriers (Fig. 5E). In
addition, the apoptotic cell rates increased after NETs treatment (Fig. 5G). Taken together, in vitro studies confirmed the detrimental effect of NETs on intestinal barrier functions. ER
STRESS REGULATES NETS-INDUCED DISRUPTIONS OF INTEGRITY IN THE INTESTINAL EPITHELIAL CELL MONOLAYER BARRIER After coculturing with NETs, the expression of BIP and phospho-eIF2α increased in a
dose-dependent manner in Caco2 epithelial barriers in vitro (Fig. 6A, B). In addition, in animal models, it was found that PAD4 deficiency inhibited endotoxin shock-induced ER stress
activation. Moreover, PAD4 deficiency decreased sepsis-induced intestinal ROS production, and ER stress activation has been suggested to be a cause of ROS generation [23]. Therefore, we
wondered whether ER stress and ROS production participate in NETs-induced epithelial injury. To further investigate the impact of ER stress and ROS in NETs-induced intestinal barrier
dysfunction, 4-phenylbutyrate (4-PBA), an inhibitor of ER stress, and N-acetylcysteine (NAC), a kind of ROS scavenger, were used. First, we found that NETs isolates treatment could induce
ROS production in Caco2 cells (Fig. 6C). ER stress inhibition by 4-PBA and NAC administration inhibited NETs-induced ROS generation. In addition, both 4-PBA treatment and NAC administration
significantly increased cell viability after NETs isolates treatment (Fig. 6D). Besides, TEER values were protected by 4-PBA treatment and NAC administration (Fig. 6E). Then, further
research was carried out to investigate the impact of ER stress inhibition on tight junctions and epithelial apoptosis. 4-PBA administration significantly decreased ER stress-related protein
levels of BIP and phospho-eIF2α (Fig. 6F). Moreover, decreased cleaved caspase 3 and increased occludin and claudin 1 levels were found after 4-PBA treatment. Collectively, the regulation
of ER stress and its downstream effector ROS can alleviate NETs-induced intestinal epithelial barrier dysfunction. TLR9 MEDIATES NETS-INDUCED ER STRESS ACTIVATION AND INTESTINAL BARRIER
DYSFUNCTION Histones and DNA are the major components of NETs, we next sought to determine which signaling pathway was the main intermediary between NETs and the disruption of intestinal
barriers. First, Caco2 monolayers were costimulated with NETs and TLR antagonists, such as C29 (TLR2 antagonist), TAK-242 (TLR4 antagonist), or ODN2088 (TLR9 antagonist). Surprisingly,
compared to administration of the TLR2 antagonist and TLR4 antagonist, administration of the TLR9 antagonist markedly alleviated NETs-induced cell morphological changes (Fig. 7A). Moreover,
these three different TLR antagonists significantly increased cell viability after costimulation with NETs (Fig. 7B). Compared to TLR2 antagonist and TLR4 antagonist, TLR9 antagonist
treatment more efficiently diminished NETs-induced cell injuries. Thus, we thought TLR9 may play a pivotal role in NETs-induced cell injuries, and further researches were carried out. TLR9
antagonist treatment resulted in a significant reduction in cleaved caspase 3 and an increase in occludin and claudin 1 levels (Fig. 7C). Moreover, the TLR9 antagonist efficiently reduced
BIP levels after NETs isolates treatment. Collectively, these observations suggested that the TLR9–ER stress–ROS signaling pathway may contribute to NETs-induced intestinal barrier
dysfunction. DISCUSSION In the present study, we firstly confirmed the increased infiltration of NETs and the correlation of NETs with intestinal injury and ER stress activation in
intestinal samples from abdominal sepsis patients. Moreover, NETs directly impaired the Caco2 intestinal epithelial cell monolayer barrier in vitro and NETs inhibition by PAD4 deficiency
ameliorated endotoxin shock-induced intestinal barrier dysfunction in vivo, which supported the detrimental effect of NETs in intestinal injury in sepsis. Another novel finding of the
current investigation is that NETs can activate ER stress in intestinal epithelial cells and that targeting ER stress attenuates NETs-induced intestinal damage. Notably, a TLR9 antagonist
reversed NETs-induced intestinal epithelial cell death and inhibited NETs-induced ER stress activation. Excessive NETs can result in vital organ damage and serve as proinflammatory mediators
in sepsis [24]. Our data revealed similar results that NETs depletion could improve the overall survival rate in LPS-induced lethal septic shock. Moreover, systemic inflammatory cytokines
and vital organ damage markers were significantly reduced after NETs inhibition. Therefore, NETs regulation will be crucial in targeting organ dysfunction and the proinflammatory response in
sepsis. Circulating NETs biomarkers have been reported to be associated with prognosis in sepsis patients [25, 26]. Similar results were also found in our research, in which circulating
NETs, both cf-DNA and the Cit H3-DNA complex, were significantly increased in abdominal sepsis patients. Moreover, circulating NETs had a positive correlation with biomarkers of intestinal
injury. However, the contribution of NETs to sepsis-induced intestinal inflammation and gut barrier dysfunction remains to be elucidated. Our preliminary research suggested that NETs may
promote intestinal injury in different animal models and observed increased formation of NETs in the intestines of critically ill surgical patients [15, 27,28,29]. Consistently, compared to
that in healthy controls, excessive NETs infiltration was also found in intestinal samples from abdominal sepsis patients. These results indicate a potential role of NETs in intestinal
epithelial injury. Lang et al. reported that NETs degradation could ameliorate intestinal inflammation in diabetic mice [30]. In addition, Dinallo et al. showed that increased NETs were
released in colonic mucosal tissues in ulcerative colitis patients and suggested a role for NETs in sustaining mucosal inflammation [31]. Li et al. demonstrated that NET regulation decreased
serum inflammatory cytokines and alleviated colon tissue damage in DSS-induced colitis [32]. In an LPS-induced lethal septic shock model, we found that NETs inhibition by PAD4 deficiency
decreased intestinal proinflammatory cytokines. Moreover, alleviated intestinal permeability and increased tight junction levels were also found in PAD4-deficient mice. In addition, to
directly investigate the role of NETs on intestinal barrier functions, NETs were separated from human neutrophils after phorbol myristate acetate (PMA) activation and costimulate with Caco2
epithelial barriers in vitro. We found that NETs isolates treatment impaired the integrity of Caco2 epithelial cell monolayer barrier in a time- and dose-dependent manner. Collectively,
these results confirmed the detrimental role of NETs in sepsis-induced intestinal barrier dysfunction. NETs were suggested to directly induce or aggravate endothelial cell death [19, 33].
Binet et al. also reported that NETs could dose-dependently induce apoptosis in human umbilical vein endothelial cells [34]. A similar result was observed in our research: in human
intestinal samples, the protein levels of Cit H3 had a significant correlation with cleaved caspase 3. With increasing NETs concentrations, the rate of apoptotic cells and the protein levels
of cleaved caspase 3 increased in vitro. However, it is unclear how NETs trigger intestinal injuries. Liu et al. reported that NETs sustained the inflammatory response by inducing NLRP3
activation [13]. Meegan et al. showed that Cit H3, which is a main component of NETs, causes endothelial barrier dysfunction by reorganizing the actin cytoskeleton, and this process is not
dependent on the Rho or MLCK signaling pathways [35]. In this study, increased ER stress activation was found in the intestines of abdominal sepsis patients and we also found that Cit H3 had
a significant correlation with ER stress in human intestinal samples. Moreover, ER stress has been reported to be detrimental to vital organ functions in sepsis, and ER stress was suggested
to promote intestinal inflammation [21, 36]. Therefore, we speculated that increased NETs infiltration activates ER stress and then promotes intestinal injury. We found that PAD4 deficiency
could decrease endotoxin shock-induced ER stress activation. To further confirm the role of ER stress in NETs-induced epithelial damage, Caco2 cells were preincubated with the ER stress
inhibitor, 4-PBA, before NETs isolates treatment. NETs can dose-dependently increase the level of ER stress-related proteins. ER stress inhibition alleviated NETs-induced intestinal barrier
disruption and ameliorated intestinal apoptosis. Therefore, NETs exert important roles in ER stress activation, which could subsequently cause intestinal injury and intestinal barrier
dysfunction in sepsis. Excessive ROS production activates the intestinal inflammatory response and intestinal epithelial cell death [37, 38]. It is therefore of great interest to explore
whether ROS mediate NETs-induced cell death. Our data showed that endotoxin shock induced intestinal oxidative stress and that PAD4 deficiency reduced ROS generation in vivo. Moreover, we
demonstrated that exposure to NETs isolates triggered Caco2 cells to produce ROS. Furthermore, diminishing ROS by NAC significantly reduced NETs-induced cell death. These results suggest
that ROS participate in NETs-induced intestinal injury. Excessive ROS generation can be induced by uncontrolled ER stress activation and then act downstream to trigger the inflammatory
response and tissue injury [39]. In our experiment, ER stress inhibition inhibited NETs-induced ROS production. Based on these data together, we could possibly conclude that ER stress was
activated by NETs and ROS was subsequently induced. Then, ROS initiated the inflammatory response and caused epithelial cell death. Extracellular histones and DNA, the main components of
NETs, are important endogenous DAMPs that induce proinflammatory signaling via TLR receptors [40]. Allam et al. demonstrated that histones were cytotoxic to renal endothelial cells and
induced TLR2- and TLR4-dependent inflammatory responses [41]. Saffarzadeh et al. demonstrated the predominant role of histones in NETs-induced cell death [33]. Interestingly, Meegan et al.
reported that Cit H3 could not induce cell death [35]. Moreover, DNA was previously reported as an antimicrobial component of NETs [42]. The DNA backstone contains unmethylated CpG motifs
that provoke inflammatory signaling via TLR9 [43]. Moreover, Afrazil et al. demonstrated that ER stress was induced by TLR4 within intestinal stem cells, resulting in crypt apoptosis in
necrotizing enterocolitis [44]. TLR2 ligands could induce endothelial ER stress in vitro and TLR9 antagonists attenuated the kainic acid-elicited ER stress response in neurons in vivo [19,
22]. In our current study, to determine which signaling pathway was mainly involved in NETs-induced disruption of intestinal barriers, antagonists of TLR2, TLR4, and TLR9 were administered.
Surprisingly, although the TLR2 antagonist and the TLR4 antagonist can reduce NETs-induced cell death and morphological changes in Caco2 monolayers, a great beneficial effect was found after
TLR9 antagonist costimulation with NETs isolates treatment. Moreover, the TLR9 antagonist can significantly decrease NETs-induced ER stress activation and alleviate intestinal barrier
disruptions. Thus, we hypothesize that in the process of NETs-induced intestinal barrier dysfunction, compared to histones, the DNA backbone may play the predominant role in NETs directly
inducing intestinal barrier dysfunction via TLR9 signaling. In conclusion, our study revealed that NETs were involved in sepsis-induced vital organ dysfunction and intestinal injury.
Increased NETs infiltration was associated with elevated intestinal inflammation and apoptosis in sepsis patients. NETs inhibition by PAD4 deficiency ameliorated sepsis-induced intestinal
barrier dysfunction, ER stress activation, and ROS production. Furthermore, inhibition of ER stress and ROS alleviated NETs-induced intestinal epithelial cell death. Moreover, TLR9
antagonist significantly inhibited NETs-induced intestinal injury and ER stress activation. These findings indicated that suppression of the NETs–TLR9–ER stress–ROS signaling pathway may be
protective in sepsis-induced organ injury and gut barrier dysfunction (Fig. 8). METHODS ETHIC STATEMENT This study was carried out according to the Recommendations of Guidelines for Clinical
Trials by the Ethics Committee of Jinling Hospital. The protocol in our study was carried out according to the principles of the Declaration of Helsinki and was approved by the Ethics
Committee of Jinling Hospital (2020NZKY-011-01). Written informed consent was obtained before any study-related procedure was performed. All experiments on mice were performed in accordance
with the guidelines approved by the Animal Review Committee at Jinling Hospital. PATIENTS Five adult abdominal trauma patients with confirmed intraperitoneal infection, caused by bowel
perforation, leading to life-threatening organ dysfunction (Sepsis 3 definition) were enrolled in this study. Detailed clinical characteristics of abdominal sepsis patients are shown in
Supplementary Table 1. Resection of the involved intestine and ostomy were performed at the surgeons’ discretion, and intestinal biopsies were obtained through stoma. Intestinal biopsies
were obtained from patients, undergoing definite intestinal anastomosis of ostomy and served as controls. Informed consent was obtained from all participants. ANIMALS PAD4-deficient
(C57BL/6) mice were purchased from Jackson Laboratory through the Model Animals Research Center of Nanjing University. WT (C57BL/6) mice were obtained from the Model Animals Research Center
of Nanjing University. Animals were maintained under specific conditions in a temperature-controlled room. Experiments were performed with randomly chosen male mice of 8–10 weeks of age (6
in each group). To induce lethal sepsis, adult mice were injected intraperitoneally with LPS (20 mg/kg, L2880, Sigma-Aldrich). Samples were obtained at 24 h after LPS injection.
ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA) Human D-lactate (K667-100, BioVision), human I-FABP (CSB-E08024h, CUSABIO, Wuhan, China), murine TNF-α (EK282/3-96, MultiSciences Biotech, Hangzhou,
China), murine IL-1β (EK201B/3-96, MultiSciences Biotech), murine IL-6 (EK206/3-96, MultiSciences Biotech), murine ALT, and Cr (Nanjing Jiancheng Bioengineering Institute) in serum or
intestinal tissue were detected by using commercially available ELISA kits according to the manufacturer’s protocols. QUANTIFICATION OF NETS To quantify NETs in mouse and human serum, cf-DNA
in serum was quantified according to the manufacturer’s instructions using the Quant-iT PicoGreen dsDNA Assay kit (P11496, Invitrogen). We also developed a capture ELISA based on
citrullinated histone H3 associated with DNA (Cit H3-DNA complex) according to previous research with minor modifications [45]. For the capture of antibody, 5-μg/ml anti-histone H3
(citrulline R2 + R8 + R17) antibody (ab5103, Abcam) was coated onto 96-well plates (dilution 1:500 in 50 μl) overnight at 4 °C. The plates were then blocked with 5% BSA for 2 h. After
washing three times (300 μl each), 50 μl of sample was added to the wells with 80 μl incubation buffer containing a peroxidase-labeled anti-DNA mAb (Cell Death ELISAPLUS, 11774425001,
Roche). The plate was incubated for 2 h, and shaken at 300 rpm at room temperature. After three washes (300 μl each), 100-μl peroxidase substrate (ABTS) was added. Absorbance at 405 nm was
measured after 30 min of incubation at room temperature in the dark. Values for soluble NET formation are expressed as percentage increase in absorbance above control. MEASUREMENT OF
INTESTINAL PERMEABILITY AND WATER CONTENT DETERMINATION In the intestinal permeability assay, mice were gavaged orally with FITC-dextran (FD4, MW 3000–5000, 400 mg/kg, 60842-46-8,
Sigma-Aldrich) 4 h before sacrifice. Food was withdrawn from mice 4 h prior to gavage. Mice were euthanized with CO2 inhalation followed by cervical dislocation. Blood was collected by
cardiac puncture into EDTA-K2 tubes and centrifuged at 2000 _g_ for 10 min. Plasma was recovered from the supernatant and processed for measuring fluorescence intensity on a SpectraMax M5
microplate reader (Molecular Device) at 485-nm excitation/525-nm emission. A standard curve was prepared by making serial dilutions of an FD4 standard within the linear range of the curve.
The concentration of FD4 in plasma was calculated using the standard curve as a reference. Gut and lung edema were estimated by comparing tissue water content. Briefly, an ∼5-cm long ileal
segment or lung tissue was harvested from each animal at 24 h after LPS administration. Tissues were dried in a 68 °C oven for 48 h. Organ water content was calculated as % H2O = (1 − dry
weight/wet weight) × 100%. HISTOLOGY AND IMMUNOFLUORESCENCE For histology, specimens of fresh small intestine were fixed in 10% formalin, cut into 4-μm sections, and processed on HE slides
for light microscopy. A gastrointestinal pathologist expert, blinded to the experiments assessed the severity of intestinal injury using the histological score of intestine injury based on
the method by Chiu et al. [46]. For immunofluorescence staining, slides were blocked with 5% bovine serum albumin in PBS at 37 °C for 1 h and the sections were incubated with 1:100 dilutions
of primary antibodies, including, anti-histone H3 (citrulline R2 + R8 + R17) (ab5103, Abcam), anti-MPO (66177-1-Ig, Proteintech, Wuhan, China), anti-CHOP (15204-1-AP, Proteintech), and ZO-1
(ab96587, Abcam). Nuclei were counterstained with DAPI according to the manufacturer’s instructions. ISOLATION OF HUMAN BLOOD POLYMORPHONUCLEAR NEUTROPHILS AND NETS ISOLATION Peripheral
blood from healthy donors was collected in EDTA-K2 blood collection tubes, and neutrophils were isolated from whole blood using PolymorphPrep (114683, Axis-Shield) according to the
manufacturer’s instructions with minor modifications. Namely, the lysis of red blood cells was performed using a hypotonic solution (NO.R1010, Solarbio, Beijing, China) according to the
manuscript. Isolated neutrophils were then resuspended in RPMI medium (11875-093, Gibco) supplemented with HEPES buffer (15630-080, Gibco). Freshly isolated neutrophils were seeded in
six-well culture plates (3 × 106 cells/well) and stimulated with PMA (100 nM, P1585, Sigma-Aldrich). After 4 h at 37 °C in the presence of 5% CO2, each well was carefully washed twice with
1-mL PBS and resuspended in DMEM. The supernatant of each well was collected and centrifuged for 5 min at 200 _g_ at 4 °C to remove whole cells and debris. NETs isolates were quantified by
evaluating the concentrations of DNA by the Quant-iT PicoGreen dsDNA Assay kit [47, 48]. CELL CULTURE AND ESTABLISHMENT OF INTESTINAL MONOLAYER BARRIERS Caco2 cells were purchased from the
Cell Bank of Chinese Academy of Sciences (Shanghai, China), and maintained in DMEM (10-013-CVR, Corning, China) supplemented with 10% fetal bovine serum (10099-141, Gibco), 100-U/mL
streptomycin, 100-U/mL benzylpenicillin, 1% glutamine, and 1% nonessential amino acids (Gibco) in a humidified, 5% CO2 atmosphere at 37 °C. Cells between passages 40 and 60 were selected for
experimentation to maintain relatively constant cellular phenotypes. To create monolayer barriers, Caco2 cells were seeded in 12-well transwell plates, on polyester membrane filters (pore
size 0.4 μm, surface area 1.12 cm2, 12-mm diameter; 3401, Corning Costar Corporation) at a density of 1 × 105 cells/cm2 with 0.5-mL culture medium on the apical side and 1.5-mL culture
medium on the basolateral side. The media were replaced every 2 days for the first 7 days and then daily thereafter. The cells grew for 21 days to build a confluent monolayer. The integrity
of the Caco2 monolayers was evaluated by the TEER. The TEER was measured by using an epithelial voltohmmeter (EVOM2, World Precision Instruments, USA). The TEER value was calculated using
the following equation: TEER value = [Ω cell monolayer – Ω filter (cell-free)] × filter area. The monolayer with a TEER value >400 Ω * cm2 was considered qualified for the following
tests. The TEER values were normalized to the initial values, and expressed as percentages of the initial resistance values. MEASUREMENT OF ROS PRODUCTION The fluorescent probe
2′,7′-dichlorofluorescein diacetate (DCFH-DA, E004-1-1, Nanjing Jiancheng Bioengineering Institute) was used to detect the ROS level according to the manufacturer’s instructions with minor
revisions. For detection of intestinal ROS expression, fresh ileum tissue (0.1 g of each sample) was cut into 1-mm pieces, homogenized in PBS, and centrifuged twice for 5 min each (1500
rpm), and the supernatant was subsequently discarded. The solution was digested using trypsin at room temperature for 5 min, and digestion was terminated by the addition of a small amount of
serum. Subsequently, the solution was filtered through a 100-μm filter, and 10-mL PBS was used to rinse the tissue. The filtrate was collected and centrifuged twice (5 min, 4 °C, 1500 rpm),
and the residual pellet was collected and resuspended in 10-µM DCFH-DA in serum-free DMEM. An hour later, the fluorescence was measured at excitation wavelengths of 485 nm and emission
wavelengths of 530 nm with a SpectraMax M5 microplate reader (Molecular Device). For detection of intracellular ROS, Caco2 cells (5 × 105 cells per well) were plated on six-well plates for
24 h. Caco2 cells were preincubated with different inhibitors for 1.5 h followed by treatment with NETs isolation for 12 h. Subsequently, intracellular ROS were detected by flow cytometry.
ANALYSIS OF APOPTOSIS AND CELL VIABILITY The FITC Annexin-V Apoptosis Detection Kit (556547, BD Biosciences) was used to detect apoptosis in Caco2 cells following the manufacturer’s
instructions. Briefly, after specific treatment with or without NETs isolation, cells were collected and washed three times with cold PBS, and then resuspended in 100-μl binding buffer, to
which 5-μl Annexin V and 5-μl PI were added. After incubation in dark for 15 min at room temperature, the cells were diluted with 200-μl binding buffer. Finally, the apoptotic cells were
analyzed on a FACS flow cytometer (BD Biosciences). Cell viability was determined using a commercially available Cell Counting Kit (CCK-8) (C0038, Beyotime, Shanghai, China) according to the
manufacturer’s instructions. Briefly, Caco2 cells were seeded in a 96-well plate at a density of 5 × 104/well and maintained in DMEM for 24 h at 37 °C. Caco2 cells were preincubated with or
without various inhibitors for 1.5 h, such as ER stress inhibitor (4-PBA, 5 µM, HY-A0281, MedChem Express), TLR2 inhibitor (C29, 50 µM, HY-100461, MedChem Express), TLR4 inhibitor (TAK-242,
1 µM, HY-11109, MedChem Express), TLR9 inhibitor (ODN2088, 5 µM, tlrl-2088, InvivoGen), or NAC (10 mM, A7250, Sigma-Aldrich). Subsequently, the cells were exposed to NETs isolates at
different concentrations. Following incubation for 24 h at 37 °C, 10 µl/well CCK-8 solution was added to the medium and cultured for 3 h at 37 °C. Subsequently, the absorbance of each well
at 450 nm was determined using SpectraMax M5 microplate reader (Molecular Device). Each sample was analyzed in triplicate, and the mean absorbance was calculated as the final result. WESTERN
BLOT ANALYSIS Total proteins in the intestinal tissues of mice with LPS-induced sepsis or cells were extracted using lysis buffer (SD001, Invent Biotechnologies). Proteins were separated by
SDS-PAGE and transferred to PVDF membranes. The membranes were blocked with 5% nonfat milk for 2 h and then incubated overnight at 4 °C with specific primary antibodies, including
anti-histone H3 (citrulline R2 + R8 + R17) (ab5103, Abcam), MPO (66177-1-Ig, Proteintech), Bcl-2 (26593-1-AP, Proteintech), occludin (ab216327, Abcam), claudin 1 (ab15098, Abcam), caspase 3
(9662S, Cell Signaling Technology), BIP (11587-1-AP, Proteintech), phospho-eIF2α (3398T, Cell Signaling Technology), and eIF2α (5324T, Cell Signaling Technology). The next day, membranes
were incubated with HRP-conjugated secondary antibody, and protein bands were visualized by using ECL. Protein quantification was measured in optical density units using Image J or Image Lab
software (Bio-Rad) and was normalized to the corresponding sample expression of GAPDH. QUANTITATIVE REAL-TIME PCR ANALYSIS Total RNA was extracted from intestinal tissue using TRIzol
reagent (15596026, Invitrogen) according to the manufacturer’s instructions, and its purity and concentration were determined by a Nanodrop instrument (Agilent Technologies). Single-strand
cDNA was generated by reverse transcription using HiScript II QRT SuperMix for qPCR (R223-01, Vazyme, Nanjing, China) according to the manufacturer’s instructions, and relatively
quantitative real-time PCR was performed using ChamQ SYBR Color qPCR Master Mix (Q431-02, Vazyme) and a Fast 7500 real-time PCR system (Applied Biosystems), all according to the
manufacturers’ manuals. Fold changes in the expression levels of targets were calculated by the 2−ΔΔCT method using GAPDH as an endogenous reference. The PCR was run in duplicate for each
sample. Primers used in qRT-PCR were synthesized by TSINGKE Biological Technology (Nanjing, China). A list of the primers used in these studies is provided in Supplementary Table 2.
STATISTICAL ANALYSIS The related results were shown as the mean ± standard deviation or median with interquartile range. At least three independent experiments were conducted to confirm the
results and differences between groups were tested by the Student’s _t_-test or one-way analysis of variance. Statistical analyses were conducted using GraphPad Prism software 7.0. _P_ <
0.05 was considered statistically significant (*_P_ < 0.05; **_P_ < 0.01). DATA AVAILABILITY The datasets used and/or analyzed during the current study are available from the
corresponding author on reasonable request. REFERENCES * Coopersmith CM, De Backer D, Deutschman CS, Ferrer R, Lat I, Machado FR, et al. Surviving Sepsis Campaign: research priorities for
sepsis and septic shock. Crit Care Med. 2018;46:1334–56. * Fleischmann C, Scherag A, Adhikari NK, Hartog CS, Tsaganos T, Schlattmann P, et al. Assessment of global incidence and mortality of
hospital-treated sepsis. current estimates and limitations. Am J Respir Crit Care Med. 2016;193:259–72. * Mittal R, Coopersmith CM. Redefining the gut as the motor of critical illness.
Trends Mol. Med. 2014;20:214–23. * Haseeb MA, Salwen MJ. Collateral damage: sepsis-induced gut injury. Crit Care Med. 2005;33:2439–40. * Catalioto RM, Maggi CA, Giuliani S. Intestinal
epithelial barrier dysfunction in disease and possible therapeutical interventions. Curr Med Chem. 2011;18:398–426. Article CAS Google Scholar * Brinkmann V, Reichard U, Goosmann C,
Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–35. * Shen XF, Cao K, Jiang JP, Guan WX, Du JF. Neutrophil dysregulation during
sepsis: an overview and update. J. Cell Mol Med. 2017;21:1687–97. * Wang Y, Li M, Stadler S, Correll S, Li P, Wang D, et al. Histone hypercitrullination mediates chromatin decondensation and
neutrophil extracellular trap formation. J Cell Biol. 2009;184:205–13. * Deng Q, Pan B, Alam HB, Liang Y, Wu Z, Liu B, et al. Citrullinated histone H3 as a therapeutic target for endotoxic
shock in mice. Front Immunol. 2019;10:2957. Article CAS Google Scholar * Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol.
2012;189:2689–95. * Chen L, Zhao Y, Lai D, Zhang P, Yang Y, Li Y, et al. Neutrophil extracellular traps promote macrophage pyroptosis in sepsis. Cell Death Dis. 2018;9:597. Article Google
Scholar * Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23:279–87. * Liu D, Yang P, Gao M, Yu T, Shi Y, Zhang M, et al.
NLRP3 activation induced by neutrophil extracellular traps sustains inflammatory response in the diabetic wound. Clin Sci. 2019;133:565–82. * Biron BM, Chung CS, O’Brien XM, Chen Y, Reichner
JS, Ayala A. Cl-amidine prevents histone 3 citrullination and neutrophil extracellular trap formation, and improves survival in a murine sepsis model. J Innate Immun. 2017;9:22–32. Article
CAS Google Scholar * Gao X, Hao S, Yan H, Ding W, Li K, Li J. Neutrophil extracellular traps contribute to the intestine damage in endotoxemic rats. J Surg Res. 2015;195:211–18. * Wang
R, Wang Z, Ma Y, Liu G, Shi H, Chen J, et al. Particle-induced osteolysis mediated by endoplasmic reticulum stress in prosthesis loosening. Biomaterials. 2013;34:2611–23. * Oakes SA, Papa
FR. The role of endoplasmic reticulum stress in human pathology. Annu Rev Pathol. 2015;10:173–94. * Zhang K, Kaufman RJ. From endoplasmic-reticulum stress to the inflammatory response.
Nature. 2008;454:455–62. * Quillard T, Araújo HA, Franck G, Shvartz E, Sukhova G, Libby P. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for
superficial erosion. Eur Heart J. 2015;36:1394–1404. * Ma X, Dai Z, Sun K, Zhang Y, Chen J, Yang Y, et al. Intestinal epithelial cell endoplasmic reticulum stress and inflammatory bowel
disease pathogenesis: an update review. Front Immunol. 2017;8:1271. Article Google Scholar * Liu L, Wu H, Zang J, Yang G, Zhu Y, Wu Y, et al. 4-Phenylbutyric acid reveals good beneficial
effects on vital organ function via anti-endoplasmic reticulum stress in septic rats. Crit Care Med. 2016;44:e689–701. Article CAS Google Scholar * Acioglu C, Mirabelli E, Baykal AT, Ni
L, Ratnayake A, Heary RF, et al. Toll like receptor 9 antagonism modulates spinal cord neuronal function and survival: direct versus astrocyte-mediated mechanisms. Brain Behav Immun.
2016;56:310–24. * Cullinan SB, Diehl JA. Coordination of ER and oxidative stress signaling: the PERK/Nrf2 signaling pathway. Int J Biochem Cell Biol. 2006;38:317–32. * Czaikoski PG, Mota JM,
Nascimento DC, Sônego F, Castanheira FV, Melo PH, et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS ONE. 2016;11:e0148142. Article
Google Scholar * Abrams ST, Morton B, Alhamdi Y, Alsabani M, Lane S, Welters ID, et al. A novel assay for neutrophil extracellular trap formation independently predicts disseminated
intravascular coagulation and mortality in critically Ill patients. Am J Respir Crit Care Med. 2019;200:869–80. * Maruchi Y, Tsuda M, Mori H, Takenaka N, Gocho T, Huq MA, et al. Plasma
myeloperoxidase-conjugated DNA level predicts outcomes and organ dysfunction in patients with septic shock. Crit Care. 2018;22:176. Article Google Scholar * Chu C, Yang C, Wang X, Xie T,
Sun S, Liu B, et al. Early intravenous administration of tranexamic acid ameliorates intestinal barrier injury induced by neutrophil extracellular traps in a rat model of trauma/hemorrhagic
shock. Surgery. 2020;167:340–51. * Wang S, Xie T, Sun S, Wang K, Liu B, Wu X, et al. DNase-1 treatment exerts protective effects in a rat model of intestinal ischemia-reperfusion injury. Sci
Rep. 2018;8:17788. Article CAS Google Scholar * Hu Q, Ren H, Hong Z, Wang C, Zheng T, Ren Y, et al. Early enteral nutrition preserves intestinal barrier function through reducing the
formation of neutrophil extracellular traps (NETs) in critically Ill surgical patients. Oxid Med Cell Longev. 2020;2020:8815655. PubMed PubMed Central Google Scholar * Lang J, Wang X, Liu
K, He D, Niu P, Cao R, et al. Oral delivery of staphylococcal nuclease by Lactococcus lactis prevents type 1 diabetes mellitus in NOD mice. Appl Microbiol Biotechnol. 2017;101:7653–62. *
Dinallo V, Marafini I, Di Fusco D, Laudisi F, Franzè E, Di Grazia A, et al. Neutrophil extracellular traps sustain inflammatory signals in ulcerative colitis. J Crohns Colitis.
2019;13:772–84. * Li T, Wang C, Liu Y, Li B, Zhang W, Wang L, et al. Neutrophil extracellular traps induce intestinal damage and thrombotic tendency in inflammatory bowel disease. J Crohns
Colitis. 2020;14:240–53. * Saffarzadeh M, Juenemann C, Queisser MA, Lochnit G, Barreto G, Galuska SP, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell
death: a predominant role of histones. PLoS ONE. 2012;7:e32366. Article CAS Google Scholar * Binet F, Cagnone G, Crespo-Garcia S, Hata M, Neault M, Dejda A, et al. Neutrophil
extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science. 2020;369:eaay5356. * Meegan JE, Yang X, Beard RS JR Jr., Jannaway M, Chatterjee V,
Taylor-Clark TE, et al. Citrullinated histone 3 causes endothelial barrier dysfunction. Biochem Biophys Res Commun. 2018;503:1498–1502. * Hosomi S, Grootjans J, Tschurtschenthaler M, Krupka
N, Matute JD, Flak MB, et al. Intestinal epithelial cell endoplasmic reticulum stress promotes MULT1 up-regulation and NKG2D-mediated inflammation. J Exp Med. 2017;214:2985–97. * Zhuang Y,
Wu H, Wang X, He J, He S, Yin Y. Resveratrol attenuates oxidative stress-induced intestinal barrier injury through PI3K/Akt-mediated Nrf2 signaling pathway. Oxid Med Cell Longev.
2019;2019:7591840. Article Google Scholar * Zou Z, Liu B, Zeng L, Yang X, Huang R, Wu C, et al. Cx43 inhibition attenuates sepsis-induced intestinal injury via downregulating ROS transfer
and the activation of the JNK1/Sirt1/FoxO3a signaling pathway. Mediators Inflamm. 2019;2019:7854389. Article Google Scholar * Wang Z, Yin F, Xu J, Zhang T, Wang G, Mao M, et al.
CYT997(Lexibulin) induces apoptosis and autophagy through the activation of mutually reinforced ER stress and ROS in osteosarcoma. J Exp Clin Cancer Res. 2019;38:44. Article CAS Google
Scholar * Marsman G, Zeerleder S, Luken BM. Extracellular histones, cell-free DNA, or nucleosomes: differences in immunostimulation. Cell Death Dis. 2016;7:e2518. Article CAS Google
Scholar * Allam R, Scherbaum CR, Darisipudi MN, Mulay SR, Hägele H, Lichtnekert J, et al. Histones from dying renal cells aggravate kidney injury via TLR2 and TLR4. J Am Soc Nephrol.
2012;23:1375–88. * Halverson TW, Wilton M, Poon KK, Petri B, Lewenza S. DNA is an antimicrobial component of neutrophil extracellular traps. PLoS Pathog. 2015;11:e1004593. Article Google
Scholar * Pisetsky DS. The origin and properties of extracellular DNA: from PAMP to DAMP. Clin Immunol. 2012;144:32–40. Article CAS Google Scholar * Afrazi A, Branca MF, Sodhi CP, Good
M, Yamaguchi Y, Egan CE, et al. Toll-like receptor 4-mediated endoplasmic reticulum stress in intestinal crypts induces necrotizing enterocolitis. J Biol Chem. 2014;289:9584–99. *
Caudrillier A, Kessenbrock K, Gilliss BM, Nguyen JX, Marques MB, Monestier M, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest.
2012;122:2661–71. * Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg.
1970;101:478–83. * Najmeh S, Cools-Lartigue J, Giannias B, Spicer J, Ferri LE. Simplified human neutrophil extracellular traps (NETs) isolation and handling. J Vis Exp. 2015;98:e52687. *
Barrientos L, Marin-Esteban V, de Chaisemartin L, Le-Moal VL, Sandré C, Bianchini E, et al. An improved strategy to recover large fragments of functional human neutrophil extracellular
traps. Front Immunol. 2013;4:166. Article Google Scholar Download references ACKNOWLEDGEMENTS We are grateful to Professor Mark Looney for directions in testing the Cit H3-DNA complex.
FUNDING This work was supported by the National Natural Science Foundation of China (81770532) and Jiangsu Province Medical Foundation for Youth Talents (QNRC2016901). AUTHOR INFORMATION
Author notes * These authors contributed equally: Shilong Sun, Zehua Duan, Xinyu Wang. AUTHORS AND AFFILIATIONS * Division of Trauma and Surgical Intensive Care Unit, Research Institute of
General Surgery, Affiliated Jinling Hospital, Medical School of Nanjing University, Nanjing, 210002, Jiangsu, P. R. China Shilong Sun, Zehua Duan, Xinyu Wang, Chengnan Chu, Chao Yang, Fang
Chen & Weiwei Ding * Key Laboratory of Intestinal Injury, Research Institute of General Surgery, Affiliated Jinling Hospital, Medical School of Nanjing University, Nanjing, 210002,
Jiangsu, P. R. China Shilong Sun, Chenyang Wang & Qiurong Li * School of Medicine, Southeast University, Nanjing, 210009, P. R. China Fang Chen * State Key Laboratory of Analytical
Chemistry for Life Science & Jiangsu Key Laboratory of Molecular Medicine, Medical School of Nanjing University, Nanjing, 210093, Jiangsu, P. R. China Daojuan Wang * The First School of
Clinical Medicine, Southern Medical University, Nanjing, 210002, Jiangsu, P. R. China Weiwei Ding Authors * Shilong Sun View author publications You can also search for this author inPubMed
Google Scholar * Zehua Duan View author publications You can also search for this author inPubMed Google Scholar * Xinyu Wang View author publications You can also search for this author
inPubMed Google Scholar * Chengnan Chu View author publications You can also search for this author inPubMed Google Scholar * Chao Yang View author publications You can also search for this
author inPubMed Google Scholar * Fang Chen View author publications You can also search for this author inPubMed Google Scholar * Daojuan Wang View author publications You can also search
for this author inPubMed Google Scholar * Chenyang Wang View author publications You can also search for this author inPubMed Google Scholar * Qiurong Li View author publications You can
also search for this author inPubMed Google Scholar * Weiwei Ding View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS WD and QL designed this
research; SS, ZD, and XW conducted the research; CC, CY, and FC collected clinical samples and related information; DW and CW performed the statistical analysis; and SS, ZD, XW, and WD wrote
and revised the paper. CORRESPONDING AUTHORS Correspondence to Qiurong Li or Weiwei Ding. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by S. He. SUPPLEMENTARY INFORMATION
SUPPLEMENTARY TABLE 1 SUPPLEMENTARY TABLE 2 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use,
sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative
Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds
the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Sun, S., Duan, Z., Wang, X. _et al._ Neutrophil extracellular traps impair intestinal barrier functions in sepsis by regulating TLR9-mediated
endoplasmic reticulum stress pathway. _Cell Death Dis_ 12, 606 (2021). https://doi.org/10.1038/s41419-021-03896-1 Download citation * Received: 28 January 2021 * Revised: 12 May 2021 *
Accepted: 13 May 2021 * Published: 11 June 2021 * DOI: https://doi.org/10.1038/s41419-021-03896-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




