- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Prostate cancer (PCA) is one of the most common male genitourinary tumors. However, the molecular mechanisms involved in the occurrence and progression of PCA have not been fully
clarified. The present study aimed to investigate the biological function and molecular mechanism of the nuclear receptor peroxisome proliferator-activated receptor gamma 2 (_PPARG2_) in
PCA. Our results revealed that _PPARG2_ was downregulated in PCA, and overexpression of PPARG2 inhibited cell migration, colony formation, invasion and induced cell cycle arrest of PCA cells
in vitro. In addition, _PPARG2_ overexpression modulated the activation of the Akt signaling pathway, as well as inhibited tumor growth in vivo. Moreover, mechanistic analysis revealed that
_PPARG2_ overexpression induced increased expression level of miR-200b-3p, which targeted 3′ UTR of the downstream targets DNMT3A/3B, and facilitated interaction with demethylated _AKAP12_
gene promoter and suppressed cell proliferation in PCA. Our findings provided the first evidence for a novel PPARG2-AKAP12 axis mediated epigenetic regulatory network. The study identified a
molecular mechanism involving an epigenetic modification that could be possibly targeted as an antitumoral strategy against prostate cancer. SIMILAR CONTENT BEING VIEWED BY OTHERS
DNMT1-MEDIATED EPIGENETIC SILENCING OF TRAF6 PROMOTES PROSTATE CANCER TUMORIGENESIS AND METASTASIS BY ENHANCING EZH2 STABILITY Article 08 July 2022 NRP1 PROMOTES PROSTATE CANCER PROGRESSION
VIA MODULATING EGFR-DEPENDENT AKT PATHWAY ACTIVATION Article Open access 25 February 2023 YTHDF1/RNF7/P27 AXIS PROMOTES PROSTATE CANCER PROGRESSION Article Open access 18 April 2025
INTRODUCTION Prostate cancer (PCA) is one of the most common male genitourinary tumors with its high fatality rate in the western world1. Reports revealed that the United States is expected
to spend more than US$8 billion annually on the screening and treatment of PCA2. Incidence of PCA in China is lower than in the United States; however, in the past two decades, numbers of
PCA patients have increased significantly due to environmental pollution, westernized change in diet, and aging of the population3. The prostate-specific antigen (PSA) is currently
recognized as a useful tool for early screening of PCA4. However, screening based on serum PSA is still largely debated5, and up to 22% of newly diagnosed patients with PCA are advanced or
metastatic ones6. Once diagnosed, it is usually treated by active surveillance, prostatectomy, radiation therapy, hormone therapy, or chemotherapy7. So far, the complex molecular mechanisms
involved in the occurrence and progression of PCA have not been fully clarified. We believe it is necessary to explore the pathological mechanism and look for new molecular therapeutic
targets of PCA. The nuclear receptor peroxisome proliferator-activated receptor-γ (PPARG) is a ligand‑dependent transcription factor (TF) that plays a vital role in regulating the
differentiation of adipocytes and the transcription of multiple genes8,9,10,11. The human PPARG gene was found to be located on the short arm of chromosome 3 (3p25) in 1995. PPARG exists in
two protein isoforms, PPARG1 and PPARG211. Compared to PPARG1, PPARG2 contains 30 additional amino acids at the N terminus and the ligand-independent activation activity is 5–10 times than
that of PPARG112. It has been reported that PPARG plays a role in a variety of chronic diseases including tumors3,13, diabetes14, inflammation15, atherosclerosis16, and so on. As far as
PPARG2 is concerned, its role in PCA has not been clarified. DNA methylation is a common type of epigenetic modification. The existence of CpG islands in human genome is always closely
related to the methylation status and it is also related to a majority of the coding genes in the human genome17. DNA methylation plays an important role in the of gene expression, cell
proliferation, differentiation, and development, and is also closely related to human development and tumors18,19,20. When one gene promoter region is methylated, its transcription is often
inactivated, whereas demethylation is usually manifested as a transcriptional activation. In the present study, we showed that the downregulation of _PPARG2_ expression in PCA acted as a
tumor suppressor in suppressing malignancy of PCA cells in vitro and in vivo. Moreover, mechanistic analysis revealed that upregulated _PPARG2_ facilitated interaction with demethylated
A-Kinase anchoring protein 12 (_AKAP12_) gene promoter and suppressed cell proliferation in PCA. Our present results provide the first evidence for a novel PPARG2-AKAP12 axis-mediated
epigenetic regulatory network. The study identified a molecular mechanism involving an epigenetic modification that could be possibly targeted as an antitumoral strategy against PCA.
MATERIALS AND METHODS CELL LINES AND CULTURE The human PCA cell lines (LNCap, PC3, and DU145) were purchased from the Chinese Academy of Sciences (Shanghai, China) and were cultured with
Roswell Park Memorial Institute 1640 (10-040-CV, Corning, USA) containing 10% fetal bovine serum (FBS, Gibco, Billings, MT). The non-malignant immortalized human prostate epithelial cell
line named NHPrE1 has been described previously21,22. The cell line was routinely passaged in 50/50 Dulbecco’s modified Eagle’s medium (DMEM)/F12 (Gibco) culture medium containing 5% FBS, 1%
insulin-transferrin-selenium-X (Gibco), 0.4% bovine pituitary extract (Hammond Cell Tech, Windsor, CA), and 10 ng/ml epidermal growth factor (Sigma, Woodstock, VA) with 1% AB/AM (Gibco).
The miR-200b-3p mimic and inhibitor were purchased from GenePharma Co., Ltd (Shanghai, China). HEK 293T cell line was cultured with DMEM containing 10% FBS. Cells were cultured on different
sizes of cell culture dishes in a humidified atmosphere containing 5% CO2 at 37 °C. All cells were authenticated by short tandem repeat profiling. TISSUE SAMPLES Eight human PCA samples and
eight benign prostatic hyperplasia tissues were obtained from the Urology Department of Affiliated Hospital of Nantong University, between 2017 and 2018. The samples were frozen quickly in
liquid nitrogen and were stored at −80 °C. The study was in accordance with the International Ethical Guidelines for Biomedical Research Involving Human Subjects. The protocol was approved
by the Ethics Committee of the Affiliated Hospital of Nantong University. All subjects obtained informed consent to participate in this study. CONSTRUCTION OF LENTIVIRUS VECTORS The PPARG2
overexpression lentivirus vector-GV358 containing the human PPARG2 wild-type full-length sequence (PPARG2) for gain-of-function and the lentivirus empty vector (EV) as a control were
constructed by GeneChem Co., Ltd (Shanghai, China). In brief, the successful construction of the plasmid was first verified by restriction enzyme digestion, PCR identification, and
sequencing. Then, the constructed plasmid and lentivirus packaging plasmids phelper 1.0 and phelper 2.0 were co-transfected into the cultured HEK 293T cells. Lentiviral particles were
obtained by collecting supernatant using the kit for ultracentrifugation concentration and purification of lentiviral particles, and combined with fluorescent titer assay and enzyme-linked
immunosorbent assay. Virus titer was determined as 1 × 108 transducing U ml−1. GENE EXPRESSION PROFILING AND MIRNA-SEQ ANALYSIS The gene expression profiles of PC3-PPARG2 cDNA (PPARG2) and
PC3-EV were compared using Agilent SurePrint G3 Human Gene Expression 8 × 60K Microarray (Agilent Technologies, Santa Clara, CA) (Gene Expression Omnibus database accession number
GSE108309). High-throughput miRNA sequencing (miRNA-seq) between PPARG2 and EV group was conducted using the single-ended 50 bp sequencing mode of the Illumina Hiseq3000 sequencing platform
(Genergy Bio-technology, Shanghai, China). The Sequence Read Archive (SRA) accession number was PRJNA719139. Differential expression genes (DEGs) between PPARG2 and EV were screened, based
on a _t_-test of linear models for microarray analysis package in R (Version 3.3, http://www.bioconductor.org)23. DEGs fold change of gene expression was calculated with a threshold of fold
change > 1 and _P_-value < 0.05 for DEG selection. GO AND KEGG PATHWAY ENRICHMENT The Database for Annotation, Visualization, and Integrated Discovery (DAVID, Version 6.8,
http://david.abcc.ncifcrf.gov/) can provide a comprehensive set of functional annotation tools for investigators to understand biological meaning behind a large list of genes24. Gene
Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis were performed using DAVID online tool, to analyze the DEGs at the functional level. _P_ <
0.05 was considered statistically significant. REAL-TIME QUANTITATIVE PCR Real-time quantitative PCR (qPCR) was used to detect the expression levels of mRNAs and miRNAs that were involved in
this study. Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. The primer sequences (_AKAP12_, _PPARG2_, miR-200b-3p, U6, and _GAPDH_) were
listed in Supplementary Table S1. The conditions of qPCR amplification were as follows: the holding stage keeps 95 °C for 5 min (1 cycle), the cycling stage holds 95 °C for 15 s and 60 °C
for 45 s (40 cycles), the melt curve stage keeps 95 °C for 15 s, 60 °C for 1 min, and 95 °C for 15 s (1 cycle). The whole amplifications were on Step OnePlus Real-Time PCR Systems (ABI, Life
Technologies Corporation, USA). The 2−ΔΔCt method was applied to calculate the relative quantities of the target RNAs25. WESTERN BLOTTING Total proteins of the tissues and cells were
extracted using a protein extraction kit (Beyotime Biotechnology, Shanghai, China) according to the manufacturer’s instructions. The extracted protein concentrations were detected using a
bicinchoninic acid kit (Sigma-Aldrich, St. Louis, USA). The protein samples (each 40 μg) were separated using polyacrylamide gel electrophoresis (10% concentration) and transferred to a
polyvinylidene fluoride membrane. The membrane was then blocked with 5% fat-free milk at room temperature for 1 h and incubated with primary antibody at 4 °C overnight. The next day, the
membrane was washed with Tris-buffered Saline Tween 20 (TBST) for four times (3 min/time) and then incubated with the corresponding secondary antibody (1 : 2000; catalog number 7056 or 7054,
Cell Signaling Technology, Inc.) for 1 h at room temperature. Again, the membrane was washed with TBST for four times. The target bands were scanned and visualized using chemiluminescence
method with Bio-Rad Gel Doc EZ imager (Life Science Research, CA, USA). Image J software (National Institutes of Health, MD) was applied to analyze the intensity of the target bands. The
primary antibodies used were as follows: PPARG2 (1 : 800; catalog number sc-166731, Santa Cruz, CA), cyclinD1 (1 : 1000; catalog number sc-166731, Santa Cruz, CA), cyclinB1 (1 : 1000;
catalog number 12231, Cell Signaling Technology, Inc.), p21Cip1 (1 : 1000; catalog number 2947, Cell Signaling Technology, Inc.), p27Kip1 (1 : 1000; catalog number 3686, Cell Signaling
Technology, Inc.), Bcl-2 (1 : 1000; catalog number 4223, Cell Signaling Technology, Inc.), AKT (1 : 1000; catalog number SAB4500797, Sigma-Aldrich, Saint Louis, MO, USA), p-AKT (1 : 1000;
catalog number 05–802 R, Sigma-Aldrich, Saint Louis, MO, USA), DNA methyltransferase 3A (DNMT3A (1 : 1000; catalog number 32578, Cell Signaling Technology, Inc.), and DNMT3B (1 : 1000;
catalog number 72335, Cell Signaling Technology, Inc.). β-Actin (1 : 1000; catalog number 3700, Cell Signaling Technology, Inc.) was used as an internal reference. CELL PROLIFERATION, COLONY
FORMATION, MIGRATION, AND INVASION For cell proliferation, cells (3 × 103 per well) were seeded to a 96-well plate and cultured for 0, 24, 48, 72, and 96 h. Then, 100 μl of medium
containing 10 μl Cell Counting Kit-8 (CCK-8) reagent (Beyotime Biotechnology, Shanghai, China) was added to each well for incubation of another 2 h at 37 °C. The absorbance was then measured
at 450 nm according to the manufacturer’s instruction. For 5-ethynyl-2′-deoxyuridine (EdU) incorporation assay, cells (5 × 104 per well) were cultured in 96-well plates at 37 °C for 48 h.
Then, 100 μl of medium containing 50 μM EDU (catalog number C10310-1, RiboBio Biotechnology, Guangzhou, China) was added to each well for another 2 h at 37 °C and fixed with 4%
paraformaldehyde for 30 min. Then, the fixed cells were permeabilized with 0.5% Triton X-100 for 10 min. Finally, the cells were stained with Apollo® 567 and Hoechst33342, respectively. For
colony formation, cells were seeded to a six-well plate and cultured for 2 weeks. Then the cells were fixed with 4% paraformaldehyde for half an hour and stained with crystal violet
(Sigma-C3886) for 10 min, and then the colonies comprising over 50 cells were counted. For migration of wound-healing assay, in brief, the cell monolayers of each well in a six-well plate
were scratched using a 100 µl pipette tip and photographed at 0 and 24 h, respectively. For cell invasion assay, the diluted Matrigel (catalog number 356234; BD Biosciences) was added to
each Transwell upper chamber. Then, cells (1 × 105) that cultured in serum-free medium were added to the upper chambers and the complete medium was added to the lower chambers. After 36 h,
the cells were fixed with 4% paraformaldehyde for half an hour and stained with 0.5% crystal violet for 10 min at room temperature. Cells were counted under a microscope (Leica DM2500, Leica
Microsystems, Inc.) at ×200 magnification. FLOW CYTOMETRY Cells (1 × 106 per well) were seeded to a six-well plate. After 24 h, cells were trypsinized and washed with precooled
phosphate-buffered saline (PBS) and fixed with 70% ethyl alcohol at 4 °C overnight. Then, the cell suspension was incubated with propidium iodide (0.5 mg/ml) (Beyotime Biotech, Shanghai,
China) for 15 min. DNA content was analyzed using a flow cytometer (BD Biosciences, San Jose, CA). TUMOR XENOGRAFT MODELS The experiments were approved by the Research Ethics Committee of
Nantong University according to Council on Animal Care Guidelines of Nantong University. A total of 12 BALB/c 5-week-old male nude mice were randomly divided into EV and PPARG2 groups (6 per
group). EV or PPARG2-transfected PC3 cells were injected subcutaneously into the flanks of the mice (1 × 106 cells/100 μL per flank). Tumor growth of mice was observed every 7 days, using a
caliper for the tumor volumes. Thirty-five days later, all mice were killed and the tumors were weighted and photographed. Then, tumor tissues were used for hematoxylin and eosin (H&E)
staining and immunohistochemical analysis of Ki67 protein expression. The tumor volumes were measured and calculated using the following formula: volume (_V_) = width (_W_)2 × length
(_L_)/2. METHYLATION-SPECIFIC AND BISULFITE-SEQUENCING PCR For methylation-specific PCR (MSP), the methylation status of CpG islands in _AKAP12_ gene promoter region were screened using MSP
method initially in PCA cells. In brief, DNA samples that modified with bisulfite were extracted according to instructions of the manufacturer (Zymo Research, Orange, CA) first. Then, a
total of 40 ng of bisulfite-modified DNA was used for PCR amplification. After that, 10 µl of the amplificated product was taken to analyze using Agarose gel electrophoresis. For
interpretation of the MSP results, the methylated and unmethylated state is represented by the methylation (M) and unmethylation (U) band, respectively. Occasionally, if the site is
methylated partially, the two bands may appear. Bisulfite-sequencing PCR (BSP) is a sequencing method to detect the methylation status of CpG islands. Briefly, DNA samples were treated with
bisulfite and amplified by PCR. Then, the PCR products were purified using a TIANgel Midi Purification Kit (Tiangen Biotech, Beijing, China). After that, the purified products were cloned
into a pGEM-T easy vector (Promega, Madison, WI, USA). Nine colonies were randomly chosen for plasmid DNA extraction using a Promega Spin Mini kit (Promega) and then sequenced by an ABI 3130
Genetic Analyzer (Applied Biosystems, Foster City, CA, USA). DUAL LUCIFERASE REPORTER In brief, the 3′-untranslated region (3′-UTR) of DNMT3A/3B (wild type and mutant) were amplified first
and cloned into the pmiR-RB-ReportTM vector (Ribobio, Guangzhou, China), respectively. Then HEK 293T cells were co-transfected with pmiR-RB-ReportTM-WT- DNMT3A/3B or
pmiR-RB-ReportTM-MUT-DNMT3A/3B and miR-200b-3p mimic/mimic control. After 48 h, the Dual Luciferase Reporter Detection System (Promega, Madison, WI, USA) was used to detect luciferase
activity. Firefly luciferase (_hLuc_+) was the reporter gene and _Renilla_ luciferase (_hRluc_) was the internal reference gene. The relative activity changes of _hLuc_+/_hRluc_ were
detected to determine whether miRNAs could target 3′-UTR of the corresponding gene. For _AKAP12_ gene promoter analysis, pGL3-Basic vector was selected for construction. Relative luciferase
activity was detected by the Dual Luciferase Assay system (Promega). The phRL-TK vectors (Promega) were used as the internal reference. CHROMATIN IMMUNOPRECIPITATION Chromatin
immunoprecipitation (ChIP) assay was performed using ChIP Enzymatic Chromatin IP Kit (Magnetic beads, Cell Signaling, Danvers, MA) according to the manufacturer’s instructions. Briefly, the
cells were crosslinked with formaldehyde of 1% final concentration first. Then, they were washed with pre-cold PBS and collected, followed by sonication crush. The solution complexes were
immunoprecipitated using the anti-PPARG2 antibody (1 : 100; catalog number sc-166731, Santa Cruz, CA) or rabbit immunoglobulin G (IgG, negative control). After that, the immunoprecipitated
complexes were collected using protein G-agarose beads. The precipitates were eluted from the beads and the DNA–protein complexes were de-crosslinked at last. The DNA samples were
recollected and used for PCR analysis. The PCR conditions were as follows: the holding stage keeps 95 °C for 5 min (1 cycle), the cycling stage holds 95 °C for 30 s, 55 °C for 30 s, and 72
°C for 30 s (35 cycles), and 72 °C for 10 min (1 cycle). ChIP primers for detailed sequences were shown in Supplementary Table S1. DATA ANALYSIS Statistical analysis was performed using the
SPSS 17.0 statistical package (Chicago, IL, USA). Data were expressed as the mean ± SD. The Student’s _t_-test was used for comparison of two groups. Differences of multiple groups were
compared using one-way analysis of variance (ANOVA). When ANOVA detects significant differences, the data were then compared using a Tukey’s test as post hoc test. Correlation coefficient
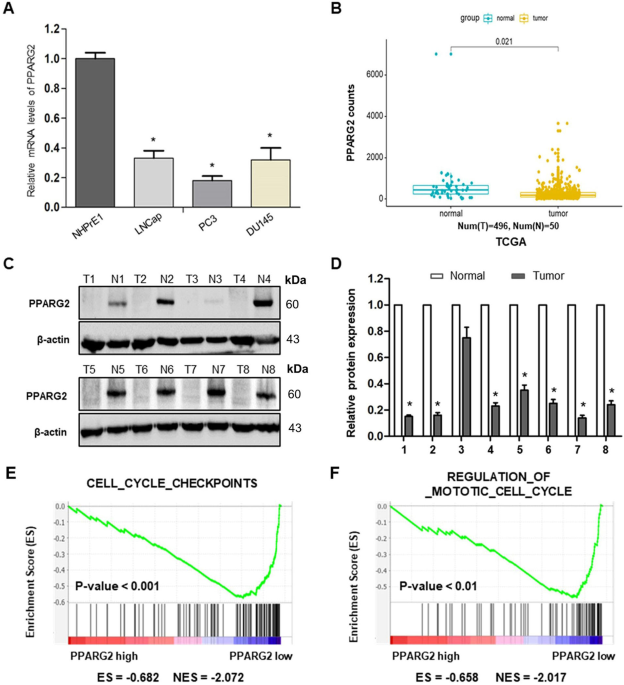
(_r_) and _P_-values were calculated by Pearson’s correlation analysis. _P_ < 0.05 was considered statistically significant. RESULTS _PPARG2_ IS DOWNREGULATED IN PCA CELL LINES AND
TISSUES To determine the expression levels of _PPARG2_ in PCA cell lines and tissues, we first detected the relative mRNA expression levels in different PCA cell lines (LNCap, PC3, and
DU145) and a normal prostate epithelial progenitor cell line NHPrE1 by qRT-PCR. We found it was significantly downregulated in the three PCA cell lines compared with the normal cell line
(Fig. 1A). Then, the data extracted from The Cancer Genome Atlas (TCGA) showed that PPARG2 was downregulated in PCA tissue samples (496 cases) compared with the normal tissues (50 cases)
(Fig. 1B). To examine the protein expression level of _PPARG2_ in clinical PCA specimens, eight PCA tissues (T) and eight prostate hyperplasia tissues (N) were collected and analyzed. The
results revealed lower PPARG2 protein expression was in the Tumor group than that in the Normal group (Fig. 1C, D). In addition, to obtain further insight into the function of _PPARG2_, the
gene set enrichment analysis26 of TCGA profiles based on _PPARG2_ single gene expression was performed. The results indicated that PPARG2 expression levels were correlated negatively with
cell proliferation by affecting genes in cell cycle regulation (Fig. 1E, F). Taken together, the above results reveal obviously that _PPARG2_ is downregulated in PCA. _PPARG2_ SUPPRESSES
CELL MIGRATION, COLONY FORMATION, INVASION, AND INDUCES CELL CYCLE ARREST OF PCA CELLS IN VITRO To study the effects of _PPARG2_ on the biological behaviors, PC3 and LNCaP cell lines were
selected as research represents of PCA cells in the following studies. The _PPARG2_-overexpressing lentivirus vector-GV358 containing the human _PPARG2_ wild-type full-length sequence
(_PPARG2_) for gain-of-function and the lentivirus EV as a control were transfected respectively into the PCA cells. Results from wound-healing assays indicated that overexpression of
_PPARG2_ suppressed cell migration significantly in PC3 and LNCaP cell lines (Fig. 2A, B). Overexpression of _PPARG2_ also significantly inhibited the colony numbers of PPARG2 group in the
colony formation assay compared to those of the EV group (Fig. 2C, D). At the same time, Transwell cell invasion tests showed that the cells’ abilities of the _PPARG2_ group to migrate and
penetrate Matrigel was significantly reduced compared with those of the EV group (Fig. 2E, F). These results indicate that _PPARG2_ may inhibit the proliferation and tumorigenicity of PCA
cells. To further evaluate the potential suppressing effects of _PPARG2_ on cell proliferation, CCK-8 assay was performed in 1, 2, 3, and 4 days after Lv-PPARG2 and Lv-EV transfection.
Compared with the EV group, a significant decrease of cell viability was detected in PC3 and LNCaP cells in the PPARG2 group (Fig. 3A). Then, EdU retention assays were performed to assess
the inhibiting effect of PPARG2 on DNA replication. Following transfection with Lv-PPARG2, the percentage of EdU-positive cells was decreased significantly in PC3 and LNCaP cells compared to
EV group (Fig. 3B, C). Moreover, to identify the mechanism through which PPARG2 overexpression inhibits the proliferation of PCA cells, we checked the cell cycle distribution in PC3 and
LNCaP cells transfected with Lv-PPARG2 or Lv-EV using flow cytometry (Fig. 3D, E). The results showed cell cycle of G1 arrest that the cell populations in the G1 phase of the cell cycle
increased significantly in the PPARG2 group compared with EV-transfected controls of PC3 and LNCaP cells. However, the cell populations were reduced in the S stage of the cell cycle compared
to the EV controls in the two cell lines. The results indicated that progression of G1-S cell cycle was inhibited by PPARG2 overexpression in PC3 and LNCaP cells. In addition, the protein
expression levels of cyclinD1, cyclinB1, p21Cip1 and p27Kip1, Bcl-2, p-AKT, and AKT were also analyzed between the two groups in PC3 and LNCaP cells (Fig. 3F–H). Western blotting analysis
revealed that cyclinD1, Bcl-2, and p-AKT were decreased significantly in PPARG2-transfected cells. Conversely, cell cycle inhibitors p21Cip1 and p27Kip1 were upregulated in
PPARG2-transfected cells. Moreover, altering expression of PPARG2 in the two groups had no effect on the protein expression of AKT and cyclinB1. _PPARG2_ INHIBITS TUMOR GROWTH IN VIVO To
further study the anticancer effects of _PPARG2_ on PCA progression in vivo, xenograft models were established via injection subcutaneously of PC3 cells treated with _PPARG2_ or EV into
BALB/c nude mice. Compared with the EV group, the xenograft tumor volumes and weights in the PPARG2 group were all markedly decreased (Fig. 4A–C). Moreover, H&E and proliferating
cell-associated antigen Ki67 staining were performed to study the proliferation level of the subcutaneous tumors. H&E-staining results showed the nuclei in both groups were large and
deeply stained, whereas Ki67-staining results in tumor xenografts indicated that the Ki67-positive rate was decreased markedly in the PPARG2 group, suggesting that PPARG2 can inhibit
tumorigenicity of PCA cells in vivo (Fig. 4D, E). _PPARG2_-MEDIATED INDUCTION OF _AKAP12_ MRNA UPREGULATION IN VITRO To further explore the potential mechanisms of PPARG2-suppressing cell
proliferation of PCA, gene expression microarray was performed using PC3 cells between overexpressing PPARG2 cDNA (PPARG2) and EV. The clustering heat map of differentially expressed genes
between sample groups was shown in Supplementary Fig. S1A. Then, differentially expressed genes were screened to meet fold change > 1 and _P_-value < 0.05 between the two groups, of
which 716 genes were upregulated and 822 genes were downregulated (Supplementary Fig. S1B). From the upregulated gene set, we screened the _AKAP12_ gene (Fig. 5A) and the expression levels
were significantly lower in PCA tissues than those in normal ones from the TCGA database (Fig. 5B). Moreover, correlation analysis from Gene Expression Profiling Interactive Analysis (GEPIA)
(Fig. 5C) and TCGA (Fig. 5D) database indicated that the expressions between the two genes was positively correlated significantly. In addition, _PPARG2_ also showed a positive correlation
in mRNA expression with _AKAP12_ in most cancer and normal tissues or cell lines (Fig. 5E, F). Then, functional analysis of GO enrichment and KEGG pathway were performed to investigate the
functions and processes of the target gene set using the online software DAVID. The results revealed that GO enrichment involved in (i) biological process (BP) (Supplementary Fig. S2A), such
as positive regulation of transcription, positive regulation of apoptotic process, and DNA methylation; (ii) involved in molecular function (Supplementary Fig. S2B), such as protein
binding, TF binding, and poly(A) RNA binding; and (iii) involved in cellular component (Supplementary Fig. S2C), such as nucleoplasm, nucleus, and cytoplasm. The KEGG pathway analysis
suggested that the differentially expressed genes of PC3-PPARG2 cells were involved in cell cycle, PI3K-Akt signaling pathway, and transcriptional misregulation in cancer, etc.
(Supplementary Fig. S2D). UPREGULATED _PPARG2_ INDUCES DEMETHYLATION OF THE _AKAP12_ PROMOTER REGION IN VITRO From the above analysis results of BP, which is under GO classification, we
found the target gene set were involved in DNA methylation. Then, expression levels of the three DNA methyltransferase (_DNMT1_, _DNMT3A_, and _DNMT3B_) were extracted from the microarray
data. The results indicated that the expression levels of _DNMT3A_ and _DNMT3B_ were all downregulated markedly in the PPARG2 group compared with those of the EV group (Supplementary Fig.
S3B, C), whereas _DNMT1_ expression level showed a nonsignificant upregulation in the PPARG2-treated group (Supplementary Fig. S3A). To further investigate the intrinsic mechanism of
_PPARG2_-induced upregulation of _AKAP12_ and downregulation of _DNMT3A/3B_, we then conducted the following experiments to clarify it from the perspective of epigenetics. We first scanned
the _AKAP12_ promoter for potential regions of DNA methylation and found obvious CpG islands existed in the promoter region near the transcription start site (TSS) (Fig. 6A). Then, the CpG
island demethylation statuses were detected between the PPARG2 and EV group by using MSP. The demethylation levels of the _AKAP12_ promoter region were significantly increased in the PPARG2
group (Fig. 6B), which could be the result of downregulated DNMT3A/3B expression in the PPARG2 group. Moreover, to obtain further demethylation status details of specific CpG sites near the
promoter region of _AKAP12_ between the PPARG2 and EV group, a 294 bp length of PCR product (−315 to −22 bp) was analyzed following sodium bisulfite treatment using BSP method (Fig. 6C). The
results revealed lower methylation frequencies in the PPARG2 group than those in the EV group (Fig. 6D, E). In addition, to determine which CpG sites were responsible for the
demethylation-related activation of the _AKAP12_ gene under the condition of _PPARG2_ upregulation in PCA cells, two _AKAP12_ gene promoter regions (PGL3-180 and PGL3-315) were constructed
and treated with _SssI_ methylase in vitro before being transfected into the PC3 cell line (Fig. 6F). In comparison with the untreated promoter construct, the _SssI_ methylase-treated
construct showed a significant suppression of promoter activity. Although there were no significant differences in the promoter activity between the region of PGL3-180 and PGL3-315 with or
without _SssI_ methylase treatment, it indicated that the promoter region at −180 bp may play an important role in regulating _AKAP12_ gene transcription. Finally, relative _AKAP12_ mRNA
expression levels were detected after PC3 cells were treated with different concentrations (0, 1, 2, and 3 μM) of DNMT inhibitor 5-Aza-2′-deoxycytidine (5-Aza-dc). The detection results
showed a 5-Aza-dc dose-dependent significant upregulation of _AKAP12_ mRNA expression levels (Fig. 6G), which indicated that methylation statuses of the related region in _AKAP12_ promoter
were involved in regulation of its expression. UPREGULATION OF MIR-200B-3P REGULATES EXPRESSION OF THE DOWNSTREAM TARGET GENES _DNMT3A/3B_ IN PPARG2-OVEREXPRESSED PCA CELLS To further
explore the mechanism of upregulated _PPARG2_ causing downregulation of _DNMT3A/3B_, one of the possibilities we thought was that miRNAs may be involved in the regulatory function of the
genes. Then, miRNA-seq was performed between PPARG2 and EV group of the PC3 cell line. The clustering heat map of differentially expressed miRNAs between sample groups were shown in
Supplementary Fig. S4A. Differentially expressed miRNAs were screened, and 18 miRNAs were upregulated and 44 miRNAs were downregulated (Supplementary Fig. S4B). From the upregulated 18
miRNAs, we identified miR-200b-3p as the study target, which was upregulated significantly in the PPARG2 group compared with the EV group extracted from miRNA-seq results (Fig. 7A), and it
has been then confirmed by experimental verification (Fig. 7B). The next step, the regulatory relationship between miR-200b-3p and _DNMT3A/3B_, was confirmed via experimental verification.
Bioinformatics revealed that _DNMT3A/3B_ 3′-region contained one conserved target site of miR-200b-3p, respectively (Fig. 7C). To evaluate this prediction, the wild-type 3′-UTR sequence of
_DNMT3A/3B_ (wild type) or its mutant sequence (Mut) (Fig. 7D, E) was subcloned into the pmiR luciferase reporter and then co-transfected with miR-CON or miR-200b-3p mimic into 293T cells.
The results indicated that the relative luciferase activity of the pmiR wild type was decreased significantly by 45.0% (Fig. 7D) and 51.3% (Fig. 7E), respectively, when miR-200b-3p mimic was
co-transfected into the cells. However, no differences of relative luciferase activity of pmiR-Mut showed when co-transfected with miR-CON or miR-200b-3p mimic into PC3 cells. Moreover, the
qPCR-detected results verified that relative miR-200b-3p expression levels showed significant upregulation or downregulation in miR-200b-3p mimic or miR-200b-3p inhibitor-treated PC3 cells
(Fig. 7F). Finally, we examined DNMT3A/3B expression in PC3 cells by western blotting. As expected, miR-200b-3p mimic triggered a significant suppressing effect on DNMT3A/3B protein
expression. However, the protein expression levels of DNMT3A/3B were regained in miR-200b-3p inhibitor-treated PC3 cells (Fig. 7G–J). The results further confirmed the regulatory effect of
miR-200b-3p on DNMT3A/3B mRNA. ENHANCED BINDING OF _PPARG2_ TO _AKAP12_ PROMOTER REGION IN PPARG2-OVEREXPRESSED PC3 CELLS To further explore the mechanism by which _PPARG2_ induces the
upregulation of _AKAP12_, we thought of _PPARG2_, which, as a kind of TF, may be involved in binding to the promoter region of _AKAP12_. We predicted the potential TF-binding site near the
_AKAP12_ promoter region using the online software JASPAR (http://jaspar.genereg.net/) and found two potential _PPARG2_-binding sites (site 1, −1540 to −1521 bp and site 2, −160 to −141 bp)
(Fig. 8A). Then ChIP assay was performed to verify the prediction. The results revealed that _PPARG2_ could bind to the two binding sites. Meanwhile, enhanced binding of _PPARG2_ can be
found in _PPARG2_-overexpressed PC3 cells (Fig. 8B). Moreover, to further verify the efficiency of the binding sites from ChIP assay results, PGL3 plasmids containing serially truncated and
mutated _AKAP12_ gene promoter were constructed and co-transfected with siControl or siPPARG2 into 293T cells, to determine the most effective functional binging site that caused
_PPARG2_-regulated _AKAP12_ gene promoter activation (Fig. 8C). Luciferase reporter activity detection results showed a significant reduction of promoter activity in the cells that
co-transfected with siPPARG2 of both PGL3-WT and PGL3-MT groups. At the same time, the reduced promoter activity caused by sequence truncation was not very obvious. From the luciferase
results of serially mutated _AKAP12_ gene promoters (pGL3-MT1, pGL3-MT2,and pGL3-MT3), a more significant reduction in AKAP12 gene promoter activity was found in pGL3-MT2 (site 1, −160 to
−141 bp) or pGL3-MT3 (two sites mutation simultaneously). The results suggested that the _PPARG2_-binding site 2 may play a more important role in _AKAP12_ transcriptional activation.
DISCUSSION Peroxisome proliferator-activated receptors (PPARs) are involved in many diseases such as cancer27,28, diabetes29, and inflammation30. Studies have revealed that PPARG acts as a
tumor suppressor and plays an important role in tumorigenesis31,32. Additional studies showed PPARG as an oncogene in the development of tumors33,34. Regarding the dual role of PPARG gene
played in the occurrence and development of tumors, we believe it may be related to multiple factors involved in tumor types and tumor progression in a specific environment. In this study,
our research target PPARG2, one of the PPARG protein isoforms, was found to be downregulated in PCA and inhibited cell migration, colony formation, invasion, and induced cell cycle arrest of
PCA cells in vitro. Moreover, _PPARG2_ overexpression modulates the activation of the Akt signaling pathway, as well as inhibit tumor growth in vivo. This is our first report to elaborate
the functional significance of _PPARG2_ expression in human PCA and experiment results indicated that _PPARG2_ functioned as a tumor suppressor and inhibits PCA malignant progression. Thus,
_PPARG2_ holds great prospects as a new therapeutic target for PCA. As TFs, most of members of PPARs can bind to the specific sites of the target gene promoter region to regulate their
expression35. Therefore, for the next step for mechanism exploration, we screened differentially expressed genes via gene expression microarray from the PPARG2 and EV groups in vitro first.
We found that _AKAP12_ mRNA was upregulated, mediated by PPARG2 overexpression. Reports have revealed that _AKAP12_ is a protein kinase C substrate and a potential tumor suppressor in many
types of cancers including hepatocellular carcinoma (HCC)36, colorectal cancer37, and breast cancers38. Moreover, interestingly, GO enrichment results indicated _PPARG2_ overexpression
involved in DNA methylation of BP. Accordingly, we then associated _AKAP12_ gene with DNA methylation. We found that obvious CpG islands existed in its promoter region near the TSS and
confirmed that upregulated _PPARG2_ induced demethylation of the _AKAP12_ promoter region via MSP and BSP experiments. Moreover, relative AKAP12 mRNA expression levels increased in a
5-Aza-dc dose-dependent concentration, which indicated that methylation statuses of _AKAP12_ promoter were involved in regulation of its expression. The above results indicated PPARG2-AKAP12
axis-mediated epigenetic regulatory network may play a role in PCA. Enzymes that catalyze CpG methylation in DNA sequence include DNA methyltransferase 1 (DNMT1), DNMT3A, and DNMT3B. These
DNA methyltransferases are essential for mammalian tissue development and homeostasis, and also related to human developmental disorders and cancer, which supports that DNA methylation plays
a key role in the specification and maintenance of cell fate39,40. In this study, we found that expression levels of _DNMT3A_ and _DNMT3B_ were all downregulated markedly in the PPARG2
group compared with those in EV the group. This data coincided with the upregulation of _AKAP12_ due to its promoter demethylation. The next step, we explored the potential mechanism of
_DNMT3A/3B_ downregulation induced by _PPARG2_ overexpression in PCA. It may be two ways: a direct regulation or an indirect effect through a mediator. Regarding the possible indirect
influence mechanism, miRNAs could be suitable mediators, which belong to noncoding RNAs family. They mainly cause mRNA translational repression or degradation by targeting to the 3′-UTR of
mRNAs41. Thus, we screened out miR-200b-3p using miRNA-seq and the _DNMT3A/3B_ 3′-UTR region contains conserved target sites of miR-200b-3p. Studies have shown that miR-200b-3p was
downregulated in androgen-independent PCA42, HCC43, and glioma44. Our experiments confirmed the regulatory relationship between miR-200b-3p and _DNMT3A/3B_. The data indicated that
miR-200b-3p participated indirectly in PPARG2-AKAP12 axis-mediated epigenetic regulatory network in PCA. In addition, the ChIP results demonstrated the enhanced binding of PPARG2 to _AKAP12_
gene promoter in _PPARG2_-overexpressed PC3 cells and the sequential deletion and mutation analyses revealed that binding site 2 (−160 to −141 bp) may play a more important role in the
_AKAP12_ gene promoter activity. Coincidentally, the binding site 2 is exactly where CG island is located. It is obvious that increased affinity of _PPARG2_ to the specific binding site is
due to the CG island demethylation. The data revealed that DNA demethylation enhanced the binding of _PPARG2_ to the _AKAP12_ gene promoter and participated in regulation of the _AKAP12_
gene expression. In summary, the present work elucidates a PPARG2-AKAP12 axis-mediated epigenetic regulatory network in PCA (Fig. 8D). _PPARG2_ acts as a tumor suppressor in suppressing
malignancy of PCA cells in vitro and in vivo. As a transcriptional factor, _PPARG2_ overexpression induces the increased expression level of miR-200b-3p, which targeted 3′-UTR of the
downstream targets _DNMT3A/3B_, facilitates interaction with demethylated _AKAP12_ gene promoter and suppresses cell proliferation in PCA via AKT signaling pathway. Of course, we recognize
that upregulated _PPARG2_ potentially regulate a cluster of miRNAs, while one miRNA can target lots of genes, and involves in cross-talk pathways in the complex and elaborate epigenetic
regulatory network in PCA. Therefore, we need to make more efforts to better explore the sophisticated mechanisms of _PPARG2_ regulation in the progression of PCA. In brief, our findings
provided the first evidence for a novel PPARG2-AKAP12 axis-mediated epigenetic regulatory network. The study identified a molecular mechanism involving an epigenetic modification that could
be possibly targeted as an antitumoral strategy against prostate PCA. REFERENCES * Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018. _CA Cancer J. Clin._ 68, 7–30 (2018).
Article PubMed Google Scholar * Roth, J. A., Gulati, R., Gore, J. L., Cooperberg, M. R. & Etzioni, R. Economic analysis of specific antigen screening and selective treatment
strategies. _JAMA Oncol._ 2, 890–898 (2016). Article PubMed PubMed Central Google Scholar * Xu, Y. et al. Family history is significantly associated with prostate cancer and its early
onset in Chinese population. _Prostate_ 79, 1762–1766 (2019). Article CAS PubMed Google Scholar * Fenton, J. J. et al. Prostate-specific antigen-based screening for prostate cancer:
evidence report and systematic review for the US preventive services task force. _JAMA_ 319, 1914–1931 (2018). Article PubMed Google Scholar * Eldred-Evans, D. et al. Rethinking prostate
cancer screening: could MRI be an alternative screening test? _Nat. Rev. Urol._ 17, 526–539 (2020). Article PubMed Google Scholar * Thurairaja, R., McFarlane, J., Traill, Z. & Persad,
R. State-of-the-art approaches to detecting early bone metastasis in prostate cancer. _Bju. Int._ 94, 268–271 (2004). Article PubMed Google Scholar * Litwin, M. S. & Tan, H. J. The
diagnosis and treatment of prostate cancer: a review. _JAMA_ 317, 2532–2542 (2017). Article PubMed Google Scholar * Dubois, V., Eeckhoute, J., Lefebvre, P. & Staels, B. Distinct but
complementary contributions of PPAR isotypes to energy homeostasis. _J. Clin. Invest._ 127, 1202–1214 (2017). Article PubMed PubMed Central Google Scholar * Jiang, M., Shappell, S. B.
& Hayward, S. W. Approaches to understanding the importance and clinical implications of peroxisome proliferator-activated receptor gamma (PPARgamma) signaling in prostate cancer. _J.
Cell. Biochem._ 91, 513–527 (2004). Article CAS PubMed Google Scholar * Jiang, M., Strand, D. W., Franco, O. E., Clark, P. E. & Hayward, S. W. PPARgamma: a molecular link between
systemic metabolic disease and benign prostate hyperplasia. _Differentiation_ 82, 220–236 (2011). Article CAS PubMed PubMed Central Google Scholar * Rosen, E. D. & Spiegelman, B. M.
PPARgamma: a nuclear regulator of metabolism, differentiation, and cell growth. _J. Biol. Chem._ 276, 37731–37734 (2001). Article CAS PubMed Google Scholar * Werman, A. et al.
Ligand-independent activation domain in the N terminus of peroxisome proliferator-activated receptor gamma (PPARgamma). Differential activity of PPARgamma1 and -2 isoforms and influence of
insulin. _J. Biol. Chem._ 272, 20230–20235 (1997). Article CAS PubMed Google Scholar * Jiang, M., Jerome, W. G. & Hayward, S. W. Autophagy in nuclear receptor PPARgamma-deficient
mouse prostatic carcinogenesis. _Autophagy_ 6, 175–176 (2010). Article PubMed Google Scholar * Li T., et al. PPARG polymorphisms are associated with unexplained mild vision loss in
patients with type 2 diabetes mellitus. _J. Ophthalmol_. 2019, 5284867 (2019). * Liu, C. et al. Pparg promotes differentiation and regulates mitochondrial gene expression in bladder
epithelial cells. _Nat. Commun._ 10, 4589 (2019). Article PubMed PubMed Central CAS Google Scholar * Merchan B. B., Tinahones F. J. & Macias-Gonzalez M. Commonalities in the
Association between PPARG and Vitamin D Related with Obesity and Carcinogenesis. _PPAR Res_. 2016, 2308249 (2016). * Yamada, Y. et al. A comprehensive analysis of allelic methylation status
of CpG islands on human chromosome 21q. _Genome Res._ 14, 247–266 (2004). Article CAS PubMed PubMed Central Google Scholar * Shen, L. et al. Genome-wide profiling of DNA methylation
reveals a class of normally methylated CpG island promoters. _PLoS Genet._ 3, 2023–2036 (2007). Article CAS PubMed Google Scholar * Feinberg, A. P. Phenotypic plasticity and the
epigenetics of human disease. _Nature_ 447, 433–440 (2007). Article CAS PubMed Google Scholar * Skvortsova, K., Stirzaker, C. & Taberlay, P. The DNA methylation landscape in cancer.
_Essays Biochem._ 63, 797–811 (2019). Article CAS PubMed PubMed Central Google Scholar * Jiang, M. et al. Functional remodeling of benign human prostatic tissues in vivo by
spontaneously immortalized progenitor and intermediate cells. _Stem Cells_ 28, 344–356 (2010). CAS PubMed PubMed Central Google Scholar * Bhatia, B. et al. Critical and distinct roles of
p16 and telomerase in regulating the proliferative life span of normal human prostate epithelial progenitor cells. _J. Biol. Chem._ 283, 27957–27972 (2008). Article CAS PubMed PubMed
Central Google Scholar * Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. _Genome Biol._ 15, 550 (2014). Article
PubMed PubMed Central CAS Google Scholar * Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics
resources. _Nat. Protoc._ 4, 44–57 (2009). Article CAS PubMed Google Scholar * Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative
PCR and the 2(-Delta Delta C(T)) Method. _Methods_ 25, 402–408 (2001). Article CAS PubMed Google Scholar * Subramanian, A. et al. Gene set enrichment analysis: a knowledge-based
approach for interpreting genome-wide expression profiles. _Proc. Natl Acad. Sci. USA_ 102, 15545–15550 (2005). Article CAS PubMed PubMed Central Google Scholar * Brunetti, L.,
Loiodice, F., Piemontese, L., Tortorella, P. & Laghezza, A. New approaches to cancer therapy: combining fatty acid amide hydrolase (FAAH) inhibition with peroxisome
proliferator-activated receptors (PPARs) activation. _J. Med. Chem._ 62, 10995–11003 (2019). Article CAS PubMed Google Scholar * Youssef, J. & Badr, M. Peroxisome
proliferator-activated receptors and cancer: challenges and opportunities. _Br. J. Pharmacol._ 164, 68–82 (2011). Article CAS PubMed PubMed Central Google Scholar * Ngala, R. A. et al.
A new, highly selective murine peroxisome proliferator-activated receptor delta agonist increases responsiveness to thermogenic stimuli and glucose uptake in skeletal muscle in obese mice.
_Diabetes Obes. Metab._ 13, 455–464 (2011). Article CAS PubMed Google Scholar * Korbecki, J., Bobinski, R. & Dutka, M. Self-regulation of the inflammatory response by peroxisome
proliferator-activated receptors. _Inflamm. Res._ 68, 443–458 (2019). Article CAS PubMed PubMed Central Google Scholar * Cesario, R. M., Stone, J., Yen, W. C., Bissonnette, R. P. &
Lamph, W. W. Differentiation and growth inhibition mediated via the RXR:PPARgamma heterodimer in colon cancer. _Cancer Lett._ 240, 225–233 (2006). Article CAS PubMed Google Scholar * Yu,
J. et al. Inhibitory role of peroxisome proliferator-activated receptor gamma in hepatocarcinogenesis in mice and in vitro. _Hepatology_ 51, 2008–2019 (2010). Article CAS PubMed Google
Scholar * Ahmad, I. et al. Sleeping Beauty screen reveals Pparg activation in metastatic prostate cancer. _Proc. Natl Acad. Sci. USA_ 113, 8290–8295 (2016). Article CAS PubMed PubMed
Central Google Scholar * Rogenhofer, S. et al. Enhanced expression of peroxisome proliferate-activated receptor gamma (PPAR-gamma) in advanced prostate cancer. _Anticancer Res._ 32,
3479–3483 (2012). CAS PubMed Google Scholar * Mirza, A. Z., Althagafi, I. I. & Shamshad, H. Role of PPAR receptor in different diseases and their ligands: Physiological importance and
clinical implications. _Eur. J. Med. Chem._ 166, 502–513 (2019). Article CAS PubMed Google Scholar * Han, S. et al. MicroRNA-1251-5p promotes tumor growth and metastasis of
hepatocellular carcinoma by targeting AKAP12. _Biomed. Pharmacother._ 122, 109754 (2020). Article PubMed CAS Google Scholar * He, P. et al. Upregulation of AKAP12 with HDAC3 depletion
suppresses the progression and migration of colorectal cancer. _Int. J. Oncol._ 52, 1305–1316 (2018). CAS PubMed Google Scholar * Soh, R. Y. Z., Lim, J. P., Samy, R. P., Chua, P. J. &
Bay, B. H. A-kinase anchor protein 12 (AKAP12) inhibits cell migration in breast cancer. _Exp. Mol. Pathol._ 105, 364–370 (2018). Article CAS PubMed Google Scholar * Aluru, N. et al.
Developmental exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin alters DNA methyltransferase (dnmt) expression in zebrafish (_Danio rerio_). _Toxicol. Appl. Pharmacol._ 284, 142–151 (2015).
Article CAS PubMed PubMed Central Google Scholar * Ceccarelli, V. et al. Molecular mechanisms underlying eicosapentaenoic acid inhibition of HDAC1 and DNMT expression and activity in
carcinoma cells. _Biochim. Biophys. Acta Gene. Regul. Mech._ 1863, 194481 (2020). Article CAS PubMed Google Scholar * Pu, M. et al. Regulatory network of miRNA on its target:
coordination between transcriptional and post-transcriptional regulation of gene expression. _Cell. Mol. Life. Sci._ 76, 441–451 (2019). Article CAS PubMed Google Scholar * He, M. et al.
Down-regulation of miR-200b-3p by low p73 contributes to the androgen-independence of prostate cancer cells. _Prostate_ 73, 1048–1056 (2013). Article CAS PubMed Google Scholar *
Moh-Moh-Aung, A. et al. Decreased miR-200b-3p in cancer cells leads to angiogenesis in HCC by enhancing endothelial ERG expression. _Sci. Rep._ 10, 10418 (2020). Article CAS PubMed PubMed
Central Google Scholar * Wu, J., Cui, H., Zhu, Z. & Wang, L. MicroRNA-200b-3p suppresses epithelial-mesenchymal transition and inhibits tumor growth of glioma through down-regulation
of ERK5. _Biochem. Biophys. Res. Commun._ 478, 1158–1164 (2016). Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Dr. Changlai Zhu for flow cytometer
operation. FUNDING This study was supported by the National Natural Science Foundation of China (Grant number 81874171), Postgraduate Research & Practice Innovation Program of Jiangsu
Province (Grant number KYCX20_2797), Nantong Science and Technology Project (numbers JC2020027, MS12018066, and MSZ19216). AUTHOR INFORMATION Author notes * These authors contributed
equally: Feng Li, Tingting Lu AUTHORS AND AFFILIATIONS * Department of Pathology, Key Laboratory of Microenvironment and Translational Cancer Research, Medical School of Nantong University,
Nantong, 226001, Jiangsu, China Feng Li, Tingting Lu, Dongmei Liu & Fulu Dong * Department of Laboratory Medicine, Affiliated Hospital of Nantong University, Nantong, 226001, Jiangsu,
China Feng Li * Basic Medical Research Centre in Medical College of Nantong University, Nantong, 226001, Jiangsu, China Feng Li, Tingting Lu, Dongmei Liu, Chong Zhang, Yonghui Zhang &
Fulu Dong Authors * Feng Li View author publications You can also search for this author inPubMed Google Scholar * Tingting Lu View author publications You can also search for this author
inPubMed Google Scholar * Dongmei Liu View author publications You can also search for this author inPubMed Google Scholar * Chong Zhang View author publications You can also search for this
author inPubMed Google Scholar * Yonghui Zhang View author publications You can also search for this author inPubMed Google Scholar * Fulu Dong View author publications You can also search
for this author inPubMed Google Scholar CONTRIBUTIONS F.D. and F.L. concepted and designed the study. F.L., T.L., and D.L. performed experiments and collated the data. T.L. and Y.Z.
contributed to conducting in vivo experiments. F.L. and C.Z. contributed to data analysis and manuscript drafting. All authors have read and approved the final submitted manuscript.
CORRESPONDING AUTHOR Correspondence to Fulu Dong. ETHICS DECLARATIONS ETHICS STATEMENT The protocol was approved by the Ethics Committee of the Affiliated Hospital of Nantong University. All
patients obtained informed consent to participate in this study. CONSENT FOR PUBLICATION All the authors agree to publish this paper. CONFLICT OF INTEREST The authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Edited by F.
Pentimalli SUPPLEMENTARY INFORMATION SUPPLEMENTAL FIGURE AND TABLE LEGENDS FIG. S1. CLUSTERING AND SCREENING OF DIFFERENTIALLY EXPRESSED GENES BETWEEN EV AND PPARG2 GROUPS. FIG. S2.
FUNCTIONAL ANALYSIS OF GO ENRICHMENT AND KEGG PATHWAY OF TARGET GENE SET. FIG. S3. EXPRESSION LEVELS OF DNA METHYLTRANSFERASE EXTRACTED FROM THE MICROARRAY DATA. FIG. S4. CLUSTERING AND
SCREENING OF DIFFERENTIALLY EXPRESSED MIRNAS BETWEEN EV AND PPARG2 GROUPS. SUPPLEMENTARY TABLE S1: PRIMERS SEQUENCES USED IN THIS STUDY RIGHTS AND PERMISSIONS OPEN ACCESS This article is
licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give
appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in
this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative
Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a
copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Li, F., Lu, T., Liu, D. _et al._ Upregulated _PPARG2_
facilitates interaction with demethylated _AKAP12_ gene promoter and suppresses proliferation in prostate cancer. _Cell Death Dis_ 12, 528 (2021). https://doi.org/10.1038/s41419-021-03820-7
Download citation * Received: 18 December 2020 * Revised: 11 May 2021 * Accepted: 11 May 2021 * Published: 22 May 2021 * DOI: https://doi.org/10.1038/s41419-021-03820-7 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative




