- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BAX and BAK are pro-apoptotic members of the BCL2 family that are required to permeabilize the mitochondrial outer membrane. The proteins can adopt a non-activated monomeric
conformation, or an activated conformation in which the exposed BH3 domain facilitates binding either to a prosurvival protein or to another activated BAK or BAX protein to promote pore
formation. Certain cancer cells are proposed to have high levels of activated BAK sequestered by MCL1 or BCLXL, thus priming these cells to undergo apoptosis in response to BH3 mimetic
compounds that target MCL1 or BCLXL. Here we report the first antibody, 14G6, that is specific for the non-activated BAK conformer. A crystal structure of 14G6 Fab bound to BAK revealed a
binding site encompassing both the α1 helix and α5-α6 hinge regions of BAK, two sites involved in the unfolding of BAK during its activation. In mitochondrial experiments, 14G6 inhibited BAK
unfolding triggered by three diverse BAK activators, supporting crucial roles for both α1 dissociation and separation of the core (α2-α5) and latch (α6-α9) regions in BAK activation. 14G6
bound the majority of BAK in several leukaemia cell lines, and binding decreased following treatment with BH3 mimetics, indicating only minor levels of constitutively activated BAK in those
cells. In summary, 14G6 provides a new means of assessing BAK status in response to anti-cancer treatments. SIMILAR CONTENT BEING VIEWED BY OTHERS STRUCTURE OF THE BAK-ACTIVATING ANTIBODY
7D10 BOUND TO BAK REVEALS AN UNEXPECTED ROLE FOR THE Α1-Α2 LOOP IN BAK ACTIVATION Article 12 March 2022 DECIPHERING MOLECULAR SPECIFICITY IN MCL-1/BAK INTERACTION AND ITS IMPLICATIONS FOR
DESIGNING POTENT MCL-1 INHIBITORS Article 03 February 2025 CHEMICAL MODULATION OF CYTOSOLIC BAX HOMODIMER POTENTIATES BAX ACTIVATION AND APOPTOSIS Article Open access 16 December 2023
INTRODUCTION The mitochondrial pathway of apoptosis is regulated by the BCL2 family of proteins that includes prosurvival proteins (e.g. BCL2 and MCL1) and two sets of pro-apoptotic
proteins, the BH3-only proteins (e.g. BIM, BID) and the pore-forming proteins (BAX, BAK and BOK) [1, 2]. Apoptosis is initiated by upregulation of the BH3-only proteins which can bind to
prosurvival members, but can also trigger BAX and BAK to unfold and convert into an activated conformation. Activated BAX and BAK can be sequestered by prosurvival proteins or can pair up as
homodimers capable of pore formation (Fig. 1a). Thus, for BCL2 signalling to achieve mitochondrial pore formation and subsequent cell death depends on protein levels and protein-protein
interactions, as well as on the BAX and BAK activation status. From a structural point of view, several features of BAX and BAK unfolding into their activated conformations to form
heterodimers and homodimers have been identified. Activator BH3-only proteins (e.g. BIM) can bind to a hydrophobic surface groove (α3–α5) on the BAX and BAK proteins [3,4,5,6] to trigger
major BAX and BAK unfolding. This activation process includes dissociation of α1 [7,8,9], and separation of α2–α5 core from the α6-α9 latch [4, 5]. The resulting exposure of the BH3 domain
(in α2) allows reciprocal BH3:groove interactions with a second activated BAX or BAK molecule to generate stable symmetric homodimers (Fig. 1a) [10,11,12]. Homodimers cluster on the
mitochondrial outer membrane to trigger membrane permeabilization and release apoptogenic factors such as cytochrome _c_ [13,14,15,16]. Binding of BAX and BAK by prosurvival proteins is less
well characterized, but involves capture of the exposed BH3 domain of activated BAX or BAK in the hydrophobic groove of a prosurvival protein (Fig. 1a) [17, 18]. During apoptosis in cells,
BAX and BAK activation status can be assessed via a range of techniques including epitope exposure, limited proteolysis, co-immunoprecipitation, Blue-Native PAGE, site-directed spin
labelling and size exclusion chromatography [19,20,21,22,23]. Antibodies to N-terminal epitopes recognize all forms of activated BAX or BAK (i.e. homodimers and heterodimers both display a
solvent-exposed N-terminus) [7,8,9]. What has been lacking to date are antibodies that can distinguish between heterodimers and homodimers, or that specifically bind the non-activated BAK
conformer. Consideration of BAX and BAK activation in the context of BCL2 signalling in cells has resulted in several models. Here, those models will be referred to as “BAX/BAK cell death
models” to differentiate them from BAX and BAK activation defined by structural unfolding. These models fall into two broad classes: the direct and the indirect cell death models. In the
“direct” BAX/BAK cell death model, BAX and BAK are in the non-activated conformation in healthy cells, and become activated by BH3-only proteins [24]. In the “indirect” model, BAK (and BAX)
are constitutively activated and sequestered by prosurvival proteins, and then competed off by upregulated BH3-only proteins [25]. Elements of both direct and indirect models may occur
according to the unified, embedded and interconnected cell death models [26,27,28,29]. In the recent “membrane-mediated spontaneous” model, BAX and BAK can convert to the activated
conformations upon translocation to the mitochondrial outer membrane [30]. BAX and BAK status is of particular interest to cancer treatment as most anti-cancer therapies aim to achieve
mitochondrial permeabilization (by BAX or BAK) that efficiently induces apoptosis. For example, BAX mutations can cause resistance to BH3 mimetic based therapies in leukaemia patients [31,
32]. Assessment of BAK activation status in leukaemia and ovarian cancer cell lines found that up to 80% of BAK was constitutively activated and bound to prosurvival proteins as heterodimers
[33, 34], an example of the indirect BAX/BAK cell death model. Moreover, those heterodimers predicted responses to BH3 mimetics and other anti-cancer agents. Finally, insufficient BAX/BAK
function can result in sublethal cytochrome _c_ release and DNA damage as well as cells with a persister phenotype [35,36,37]. Thus, improved means of measuring BAX and BAK status, before
and after anti-cancer treatments, may help improve patient responses. In addition to BAX and BAK regulation by BCL2 signalling, antibodies that bind directly to BAX and BAK can regulate
their function. Antibodies that activate BAK (or the mitochondria-targeted BAX variant S184L) bind to the N-terminal α1-α2 loop region to trigger activation-associated conformational
rearrangements [38,39,40]. Antibodies that inhibit BAX prevent its translocation [39] or obstruct the N-terminal activation site [41]. Here we identify and characterize the first antibody
(clone 14G6) specific for the non-activated conformation of BAK. The antibody binds with nanomolar affinity and prevents BAK activation triggered by three distinct activators: cBID, a
BAK-activating antibody, and heat. A crystal structure revealed that 14G6 embraces a complex non-linear epitope of both the α5–α6 hinge region and the α1 helix in non-activated BAK,
supporting previous evidence that activation requires separation of the latch and the α1 helix from the α2-α5 core. In several untreated leukaemia cell lines 14G6 antibody recognized the
majority of BAK, indicating minimal constitutively activated BAK before apoptotic signalling. Thus, this antibody provides a new tool for assessing BAK activation status both before and
after anti-cancer treatments. RESULTS THE 14G6 ANTIBODY SPECIFICALLY BINDS TO NON-ACTIVATED BAK To understand the major conformational changes involved in BAX and BAK activation and
oligomerization, we and others have generated several antibodies to different regions in the two proteins [10, 38, 40]. To generate additional antibodies for interrogating BAK-mediated
apoptosis, mice were immunized with human BAK lacking endogenous cysteine residues, the flexible N terminus and the C terminal transmembrane anchor (BAKΔN22ΔC25Δcys, herein referred to as
BAKΔTM). Using mouse B cell cloning, B cells that bind to BAKΔTM were selected and the recovered antibody variable regions expressed as chimeras containing human constant regions. When
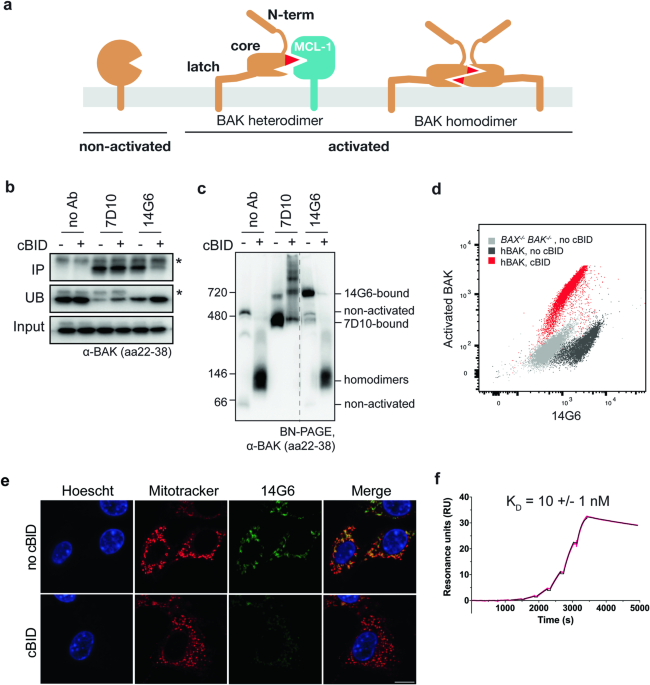
tested by ELISA, several antibodies were found to bind BAKΔTM. When tested for binding to full-length BAK in mitochondria, the 14G6 clone showed specificity for non-activated BAK (Fig.
1b–e). These experiments were performed using _bak__−/−__bax__−/−_ mouse embryonic fibroblasts (MEFs) stably expressing human BAK, and the cell membrane permeabilized with digitonin to allow
access of cBID and antibodies. Immunoprecipitation showed that clone 14G6 could bind BAK before but not after incubation with cBID (Fig. 1b). In contrast, clone 7D10, that binds all forms
of BAK [38, 40] could bind BAK both before and after BAK activation by cBID (Fig. 1b). The same pattern of antibody binding was shown by blue-native PAGE (BN-PAGE), with 7D10 binding
indicated by its ability to shift BAK to a higher molecular weight native complex (gel-shift) both before and after cBID treatment, while 14G6 gel-shifted BAK only prior to cBID (Fig. 1c).
When tested on flow cytometry and immunocytochemistry, 14G6 also showed high staining before but not after BAK activation by cBID (Figs. 1d, e and S1a). Surface Plasmon Resonance (SPR)
analysis of 14G6 with BAKΔTM showed tight binding (_K_D of 10 ± 1 nM) with a slow dissociation rate (Fig. 1d; Table 1). These data, together with the failure of 14G6 to bind to any BAK
peptide on peptide array (Fig. S1b), indicate that 14G6 binds to a non-linear epitope, and is specific for the non-activated conformer of BAK. 14G6 PREVENTS BAK ACTIVATION We next asked if
14G6 might activate BAK as does the 7D10 antibody [38], or if it might prevent BAK activation. Permeabilized cells were incubated with 14G6, with or without subsequent incubation with cBID
or 7D10 (Fig. 2a, b). 14G6 was able to block cytochrome _c_ release initiated by cBID or 7D10 (Fig. 2a) and to block BAK conformation change assessed by the ability of proteinase K to cleave
in the α1–α2 loop (Fig. 2b). When assessed by flow cytometry, 14G6 was again found to block cytochrome _c_ release and BAK activation after treatment with cBID or 7D10 as well as heat (Fig.
2c). In these experiments, 7D10 could still bind BAK that was bound by 14G6, as shown by gel-shift on BN-PAGE (Fig. S2). STRUCTURAL CHARACTERIZATION OF 14G6 FAB BINDING TO BAK REVEALS
NON-LINEAR EPITOPE To identify the 14G6 epitope we determined the crystal structure of the 14G6 Fab bound to BAKΔTM. The 14G6 Fab was generated by papain cleavage of the full-length antibody
and purified by cation exchange. The 14G6 Fab: BAKΔTM complex runs as a monodisperse complex on size exclusion chromatography, with a molecular weight of _ca_ 65 kDa (Fig. 3a). The
asymmetric unit of the crystal contains two copies of the 14G6 Fab: BAK complex (Table 2). The shape complementarity metric Sc is 0.617 (over the trimmed surface area of 534 Å2), which is
typical of an antibody-antigen interface [42, 43]. 14G6 binds BAK by cradling the α5-α6 hinge region and making contacts with BAK α1 (Fig. 3b). Binding at the α5-α6 hinge is mediated by all
three heavy chain CDR loops and CDR-L2 and L3 (Fig. 3c). The backbone of CDR-H1 L33 and CDR-H3 P99 interacts with BAK Y143, while the side-chain carboxamide of CDR-H2 N52 interacts with the
backbone carbonyl of BAK Q144. The side chains of CDR-L3 S93 and R95 interact with BAK T148 hydroxyl and L147 backbone carbonyl, respectively, while CDR-L2 W50 makes a hydrophobic contact
with the end of α6 (Fig. 3d). The BAK α1-α2 loop has been displaced from its usual position and the residues are mostly unresolved in the electron density, suggesting some flexibility (Fig.
3e). This loop movement allows the antibody to interact directly with α1 through its two heavy chain CDR loops (Fig. 3d). CDR-H1 N31 and Y32 both form hydrogen bonds with BAK Q47, with
additional hydrophobic contacts extending along the C-terminal half of α1. CDR-H3 L101 has inserted itself into the pocket usually occupied by the linchpin residue M60 [40], making
hydrophobic contacts with V39 and H43 (Fig. 3d). Thus CDR-H1 and CDR-H3 may act to stabilize BAK α1 in the closed conformation. BAK is in the non-activated conformation as shown in previous
structures, with an RMSD of 0.72 Å (chain C) −0.76 Å (chain D) when aligned with 2IMS [44]. There are a few noticeable differences between the structures of 2IMS and 14G6-bound BAK (Fig.
S3a, b). In the 14G6-bound structure, α3 is extended at both the N- and C-terminal ends, with the α3-α4 loop displaying a different conformation compared with 2IMS. This results in a more
closed BH3 binding groove (Fig. S3a, b). However, the altered conformation of α3 may be influenced by crystal contacts, since BAK H99 (at the α3 C-terminus) in chain D makes a hydrogen bond
with the antibody light chain from the other copy of the complex (Fig. S3c). MECHANISM OF INHIBITION – MUTATIONS IN BAK N-TERMINUS SUGGEST A ROLE FOR BINDING TO THE Α1 REGION The structure
of 14G6-bound to BAK suggests three possible mechanisms of inhibition. Firstly, the closed groove may prevent BH3-only proteins from binding (Fig. S3a, b). Second, contact with α1 may
prevent dissociation of this helix early on in BAK activation. Lastly, contact with the α5–α6 hinge may prevent unlatching of the α2–α5 core from α6–α8. To test whether 14G6-bound BAK could
still bind BH3-only proteins, we used a mutant of BAKΔTM with a cysteine in the groove (K113C), along with a cysteine-mutant of BID (R84C) and observed the BAK band shift on non-reducing
SDS-PAGE. The covalent linkage between BAK and BID forms even in the absence of oxidizing agent (Fig. S3d). This link can still form even when BAK is pre-incubated with excess 14G6,
suggesting that 14G6 binding does not prevent BID from binding to BAK and that the groove can adopt both open and closed conformations when bound to 14G6. Thus, 14G6 binding appears not to
limit binding of either cBID (Fig. S3d) or the activating antibody 7D10 (see Fig. S2). We next tested mutation of the BAK M60 residue, to assess if 14G6 binding requires displacement of the
α1–α2 loop (Fig. 3e). Our recent study showed that mutagenesis of the α1–α2 loop (M60G) could not only “open” the α1-α2 loop, but could also promote dissociation of α1 (and BAK activation)
[40]. Accordingly, SPR analysis of BAK M60G binding to 14G6 resulted in a stronger binding affinity (0.6 nM), primarily due to an increase in the association rate by 18-fold (Table 1, Fig.
S4d). We had also found that mutation of M60 to a tryptophan appeared to “close” the loop and increased the thermal stability of BAK and reduced spontaneous activation [40]. Surprisingly,
binding of 14G6 to BAK M60W showed no significant difference in kinetics or affinity compared with the WT (Table 2, Fig. S4e). Although lower spontaneous activity in the M60W mutant does
suggest that the loop exists more often in the closed orientation or that the threshold for releasing the loop is increased, perhaps neither of these are significant enough to alter the
binding kinetics of 14G6. Another potential explanation is that 14G6 binds to the α5–α6 hinge and then induces the movement of the α1–α2 loop, and that the threshold for this induced
movement is similar in the M60W mutant compared with wild-type. 14G6 BINDS MOUSE BAK WITH LOWER AFFINITY Mouse and human BAK share >90% sequence identity and have a high degree of
structural similarity (RMSD 0.617 Å between 2IMS and 6MCY) (Fig. S5a, b). As such, while 14G6 was raised against human BAK, we wondered whether it might also bind mouse BAK. We found that
14G6 readily bound and inhibited human BAK when pre-incubated with membrane fractions on ice (Figs. 1–4). In contrast, pre-incubation at 30 °C was required for efficient 14G6 binding (Fig.
S5c) and inhibition of mouse BAK (Fig. S5d). To rationalize this difference, we determined the kinetic and affinity parameters by SPR and found that 14G6-bound mouse BAK with a _K_D of 1.14
µM, which is 100-fold lower than human BAK (Table 1, Fig. S4f). This is due to changes in both the association (0.81 × 103 1/Ms, tenfold slower) and dissociation (9.2 × 10−4 1/s, tenfold
faster) rates. Despite the 100-fold difference in affinity, the epitope is essentially conserved within both human and mouse BAK. The single amino acid difference in the region that
interacts with 14G6 is hBAK E50 which makes a charge-assisted hydrogen bond to CDRH1-N31 on 14G6, whereas in mBAK this residue is a glutamine. This subtle change may contribute to the faster
dissociation rate observed for 14G6-mBAK interactions. Outside of the direct interaction region, the mBAK sequence differences may contribute to the lower affinity. For example, in mBAK
there is a hydrogen bond between mD59 (α1–α2 loop) and mQ45 (α1) that is not present in hBAK and this may stabilize the α1–α2 loop in the closed conformation compared to hBAK, which cannot
form this hydrogen bond (V61 and Q47, respectively) (Fig. S5b). This may account for the slower on-rate observed for 14G6-mBAK interactions. There are also differences in amino acids in α3,
α4, α5 and α6 (Fig. S5a, b) that despite not directly contacting 14G6, may have (difficult to predict) allosteric effects or effects on flexibility that promote the lower binding affinity
observed for mBAK. 14G6 AS A NEW TOOL TO ASSESS BAK STATUS IN CANCER CELLS Due to the specificity of 14G6 for non-activated BAK, this antibody provides a new means of assessing BAK status in
each cell context. In MEFs, the majority of BAK is not constitutively activated based on 14G6 binding by immunoprecipitation and gel-shift (Fig. 1b, c), and on the lack of cleavage by
proteinase K (lane 2, Fig. 2b). Although less quantitative, single cell analysis also indicated the presence of significant non-activated BAK in untreated MEFs, as 14G6 binding decreased
after cBID treatment (Fig. 1d, e, S1a). In contrast, up to 80% of BAK was found to be constitutively activated (i.e. forming heterodimers with prosurvival proteins) in certain cell lines,
including MV4;11 and HL-60 acute myeloid leukaemia cells [33, 34]. To examine if constitutively activated BAK is common in cancer cell lines, a series of blood cancer cell lines established
from patients with acute lymphoblastic leukaemia (ALL) or acute myeloid leukaemia (AML) were examined for BAK activation status using limited proteolysis (Fig. 4a) or 14G6 gel-shift (Fig.
4b). If a significant portion of BAK was constitutively activated, BN-PAGE would show minimal gel-shift by 14G6 both before and after apoptotic signalling. However, in each case, only
minimal BAK was constitutively activated as most BAK was cleaved only to the ~23 kD band (lane 2; Fig. 4a), and could be gel-shifted by 14G6 (lane 3 vs lane 1; Fig. 4b). After treatment with
cBID or heat, BAK became activated as shown by cleavage to smaller fragments (lanes 3 and 4; Fig. 4a) and less gel-shift by 14G6 (lane 4 vs lane 3; Fig. 4b). Thus, minimal constitutively
activated BAK is present in these cells. To test BAK activation in response to BH3 mimetics, four cell lines were incubated with inhibitors specific for BCL2, MCL1 or BCLxL, alone or in
combination. A combination of inhibitors was able to activate the majority of BAK in each cell line, as 14G6 was no longer able to gel-shift BAK (lane 10 vs lane 6; Fig. 4c). Moreover, BAK
(and BAX) became susceptible to limited proteolysis (lane 6 vs lane 2; Fig. S6). The MCL1 inhibitor alone could trigger BAK and BAX activation in three cell lines (Figs. 4c and S6). Thus, in
these cells, BH3 mimetics are not acting by displacing constitutively activated BAK from prosurvival proteins. Rather, BH3 mimetics displace BH3-only proteins which then activate BAK (Fig.
4d). DISCUSSION Since BAX and BAK were shown to be essential for apoptotic cell death [45], these proteins have been widely studied [1]. In their non-activated forms, BAK and BAX form a
globular bundle of 8 or 9 helices. Upon activation, the proteins partially unfold and form complexes with like molecules to form homodimers, or with prosurvival proteins to form heterodimers
(Fig. 1a). Each cell may contain a mixture of the three conformers dependent on the balance of BCL2 family members present in each cell and the cell stresses encountered. Determining the
proportion of each conformer in individual cells is not yet possible, but is required to better understand responses to anti-cancer therapies such as the BH3 mimetics. We report the
generation and characterization of 14G6, the first antibody specific for the non-activated form of BAK. Specificity is explained by 14G6 being a conformational antibody with a binding site
encompassing two regions, α1 and the α5–α6 hinge, both of which undergo major changes upon BAK activation. Previous studies have shown that disulfide tethering of α5-α6 was able to block the
unlatching leading to MOMP [5], with evidence that cBID could still bind and trigger partial α1 dissociation [7]. The requirement for α1 dissociation for BAK activation is consistent with a
role for the BH4 domain in stabilizing the non-activated protein [7, 46]. By binding to both the α5-α6 hinge and α1, 14G6 appears able to prevent both unlatching and α1 dissociation,
maintaining BAK in a fully non-activated state. Accordingly, 14G6 binding inhibited BAK activation, as addition of 14G6 to mitochondria-enriched membranes inhibited BAK-mediated cytochrome
_c_ release initiated by three distinct BAK activators, the BH3-only protein cBID, the 7D10 antibody, and mitochondrial incubation at 43 °C. While many inhibitory antibodies act by
preventing activators from binding to their activation sites, 14G6 did not prevent binding of either cBID or 7D10, but bound to BAK at two sites critical for its unfolding. No other known
antibodies to BAK involve an epitope spanning two distinct binding sites [7]. There is, however, such an antibody (3G11) to BAX whose epitope is similar to that of 14G6 [41]. Although
high-resolution structural information is not available for the BAX-3G11 complex, hydrogen-deuterium exchange mass-spectrometry (HDX-MS) identified the epitope as involving residues along
the length of α1 and α6, along with some residues at the C-terminal end of α5, suggesting binding at the hinge region of α5–α6, similar to 14G6. A key difference, however, is that 3G11
appears to bind directly to the BAX α1–α2 loop, possibly locking it in place [41], while 14G6 displaces the α1–α2 loop and stabilizes BAK in its loop-open inactive conformation. Displacement
of the BAK α1–α2 loop allowed direct binding of 14G6 to α1. Similar displacement was evident in the structure of BAK bound by the 7D10 activating antibody, whose epitope was in the α1-α2
loop itself [40]. 7D10 binding removes M60 (within the α1–α2 loop) from its pocket (involving α1) to promote α1 dissociation and BAK activation [40]. In the 14G6 crystal structure, 14G6
appears to substitute for M60 by inserting Leu101 from CDR-H3 into the M60 pocket to bind directly to α1 and hold it in place to prevent BAK activation. Thus, structures of the 14G6
inhibiting antibody and the 7D10 activating antibody together reveal molecular events involved in BAK N-terminus exposure during apoptosis. Inhibition by 14G6 shares a common theme to two
other reported BAK inhibitors, despite binding at distinct sites. The BH3 peptide inhibitor of BAK, Bim-H3Pc-RT, binds at the canonical groove binding site, where a pentyl-carboxylate side
chain inserts into a pocket to make a key charge-charge interaction with R42 on α1, thus stabilizing the helix and preventing α1 dissociation during activation [47]. This interaction also
contributes to specificity for human BAK, as the corresponding residue in mouse BAK is L40. A small-molecule inhibitor of BAK activation that is specific for mouse BAK has also been
described [48]. It binds the BAK-VDAC2 complex, enhancing VDAC2’s ability to restrain BAK in a non-activated state. Chimera experiments identified residues in α1 and α9 as being important
for activity, specifically mouse BAK L40 (human R42) [48,49,50,51]. This small-molecule inhibitor prevents α1 exposure. While each of the inhibitors binds at a distinct site, either directly
or indirectly influencing BAK, they all function by blocking major structural rearrangements, namely, α1 dissociation and α5-α6 hinge unlatching. As the first antibody specific for
non-activated BAK, 14G6 provides a new means of characterizing BCL2 family signalling in cells, complementing current BAK activation assays [19,20,21,22,23]. 14G6 specificity is evident in
both bulk and single cell assays, and in the presence or absence of 1% digitonin. The antibody binds mouse as well as human BAK, although with lower affinity. The use of 14G6 in single cell
analyses can address questions that bulk assays cannot. For example, in flow cytometry 14G6 can be multiplexed with other antibodies (Figs. 1d and S1a) with the potential to identify
heterogeneity in patient samples and their response to therapy. In single cell imaging, 14G6 may identify heterogeneity of BAK activation within a cell. Here we used 14G6 binding in bulk
assays to quantitate the proportion of BAK in the non-activated conformation prior to apoptotic signalling. 14G6 gel-shift showed that the majority of BAK is in the non-activated
conformation in six cancer cell lines as well as in SV40-immortalized MEFs (Fig. 4). This contrasts with reports of up to 80% constitutively activated BAK in certain leukaemia and ovarian
cancer cell lines [33, 34]. In those studies, spurious BAK activation by detergents appeared not to be involved, as size exclusion identified constitutively activated BAK oligomers after
extraction with either CHAPS or digitonin [33], detergents generally considered unable to unfold BAK. Our findings also provide no evidence for membrane-mediated activation of BAK [30, 52]
as non-activated BAK is present in the mitochondria-enriched membrane fraction in all cells examined (Fig. 4a,b and S6), and in MEFs was shown to be membrane-integrated via a C-terminal
transmembrane domain [53]. In the cells examined here, BAK binding to prosurvival proteins must occur after apoptotic signalling (including BH3 mimetic treatments), and contribute to
resistance (Fig. 4d) rather than to sensitization [33, 34]. Hence, BH3 mimetics principally act upstream to compete off BAK-activating BH3-only proteins, as well as prevent sequestration of
activated BAK (Fig. 4d). Whether BH3 mimetics can also compete off BAK from MCL1 or BCLXL is less clear, with evidence that such heterodimers are relatively insensitive to BH3 mimetics [29,
54]. These findings suggest that measuring interactions between BCL2 family members in individual cells may help clarify mechanisms of resistance to BH3 mimetics and other cancer treatments.
METHODS AND MATERIALS CELL LINES Mouse embryonic fibroblasts (MEFs) derived from _bax__−/−_ or _bak__−/_−_bax_−_/−_ C57BL/6 mice were transformed with SV40 as previously described [10, 55].
For cells expressing human BAK, full-length BAK was stably expressed in SV40-immortalized _bak__−/−__bax__−/−_ mouse embryonic fibroblasts (MEFs) [38]. MEFs were maintained in Dulbecco’s
modified Eagle’s medium (DMEM) supplemented with 10% foetal bovine serum, 0.1 mM L-asparagine and 55 μM 2-mercaptoethanol at 37 °C in a humidified 10% CO2 incubator, and regularly tested
negative for mycoplasma (Lonza MycoAlert). The human ALL cell line RS4;11 [56] was originally imported to WEHI from ATCC (American Type Culture Collection;VI USA). Human AML cell lines
MOLM-13 [57], KG-1 [58], HL-60 [59] and OCI-AML3 [60] were originally imported to WEHI from DSMZ (Deutsche Sammlung von Mikroorganismen unde Zellkulturen, Leibniz, Germany), and MV4;11 [61]
and THP-1 [62] from ATCC (American Type Culture Collection;VI USA). RS4;11, MOLM-13, KG-1 and HL-60 cell lines were cultured in RPMI-1640 medium (Gibco #31800089) supplemented with 10%
heat-inactivated FCS, 100 U/mL penicillin and 100 µg/mL streptomycin (Gibco #15140122). MV4;11, and THP-1 cell lines were cultured in RPMI-1640 medium supplemented with 15% heat-inactivated
FCS, 100 U/mL penicillin and 100 µg/mL streptomycin. The OCI-AML3 cell line was cultured in MEM alpha medium with GlutaMAX™ supplement (Gibco #32561037) supplemented with 10%
heat-inactivated FCS (Sigma #F9423). All human leukaemia cell lines were maintained at 37 °C with 5% CO2 for <2 months, verified by STR profiling at the Australian Genomics Research
Facility (AGRF) and regularly tested negative for mycoplasma. PREPARATION OF MITOCHONDRIA-ENRICHED MEMBRANE FRACTIONS FROM MEFS AND CANCER CELL LINES To generate mitochondria-enriched
membrane fractions from MEFs and the human cancer cell lines, cells were spun and resuspended (at 1 × 107 ml−1 for MEFs and THP-1 cells and at 3 × 107 ml−1 for the smaller RS4;11, MOLM-13,
OCI-AML3, MV4;11, KG-1 cells) in MELB buffer (93.5 mM sucrose, 20 mM HEPES, pH 7.4, 2.5 mM MgCl2 and 100 mM KCl) supplemented with Complete Protease Inhibitors (Roche, Castle Hill, NSW,
Australia). Cell membranes were permeabilized by addition of 0.025% w/v digitonin and incubation on ice for 10 min, then the pellet (mitochondria-enriched membrane) fractions separated from
the supernatant (cytosolic) fraction by centrifugation at 13,000 × _g_ for 5 min. Membrane fractions were resuspended in MELB buffer supplemented with Complete Protease Inhibitors (Roche)
and 8 μg/ml pepstatin A (Sigma; #26305-03-3). MITOCHONDRIA INCUBATION AND CYTOCHROME _C_ RELEASE ASSAYS To trigger activation (unfolding) of BAK, mitochondria-enriched membrane fractions (50
μl) were incubated with 100 nM caspase-8-cleaved human BID (cBID) or human BIDM97A (cBIDM97A) or with 7D10 antibody (0.1 μg/μl) at 30 °C for 30 min. To test if 14G6 blocked BAK activation,
membranes fractions were pre-incubated with 14G6 (0.1 μg/μl) on ice for 30 min. To monitor cytochrome _c_ release from mitochondria, reactions were spun at 13,000 x _g_ and the supernatant
and pellet fractions immunoblotted for cytochrome _c_. BH3 MIMETIC TREATMENT For BAK activation by BH3 mimetics cells were supplemented with the broad spectrum caspase inhibitor Q-VD-OPh
(MedChem Express, # HY-12305; 50 µM) for 30 min before addition of iBCL2 (Venetoclax/ABT-199, Active Biochem #A-1231), iMCL1 (S63845, Active Biochem #6044) and iBCLXL (A-1331852, AbbVie,
provided by G. Lessene) in dimethyl sulfoxide (DMSO), and incubated for a further 3 h. Either membrane fractions or whole cell lysates were assessed for BAK activation by proteinase K
cleavage or 14G6 gel-shift on BN-PAGE as described. BAK ACTIVATION ASSESSED BY LIMITED PROTEOLYSIS BAK conformation change was assessed by limited proteolysis [19]. Briefly, 50 μl of
membrane fractions resuspended in MELB with 8 μg/ml pepstatin A were pre-chilled to 0 °C then incubated with 30 μg/ml proteinase K (Sigma) on ice for 20 min. Reactions were stopped by
addition of 1 mM phenylmethylsulfonyl fluoride, and samples immunoblotted with antibody to the BAK BH3 domain (clone 4B5) [19]. IMMUNOPRECIPITATION Membrane fractions (at least 2.5 × 106
cells per treatment) with or without cBID treatment were solubilized with 1% w/v digitonin in lysis buffer (20 mM Tris, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10% glycerol, pH 7.4) on ice for
1 h. Lysates were centrifuged at 13,000 × _g_ for 5 min and supernatants added to Protein G Sepharose and allowed to pre-clear at 4 °C for 1 h. To immunoprecipitate BAK, pre-cleared samples
were added to fresh Protein G sepharose supplemented with 4 μg 7D10 rat monoclonal antibody or 14G6 mouse monoclonal antibody and incubated at 4 °C for 1–2 h. Unbound fractions were
collected and the resin washed with lysis buffer containing 0.1% w/v digitonin, and bound protein eluted by boiling in sample buffer. Unbound and total lysates were immunoblotted for BAK.
BLUE-NATIVE PAGE OF MEMBRANE FRACTIONS BN-PAGE of extracted proteins was performed as previously described [63]. Cells or membrane fractions were solubilized in 20 mM Bis-Tris (pH 7.4), 50
mM NaCl, 10% glycerol, 1% (w/v) digitonin with 10 mM DTT before centrifugation at 13,000 × _g_ to pellet insoluble debris. WESTERN BLOTTING Immunoprecipitated samples and samples from
mitochondrial assays were resolved by SDS-PAGE using Tris-Glycine gels (Bio-Rad) or by BN-PAGE using Bis-Tris gels (Invitrogen), and transferred to 0.22 μm nitrocellulose or PVDF membranes.
Molecular weights were estimated using Precision Plus Protein Kaleidoscope Prestained Protein Standard (Bio-Rad #1610375). Primary antibodies included rabbit polyclonal anti-BAK aa23-38
(1:5000, Sigma #B5897, RRID:AB_258581), rat monoclonal anti-BAK (clone 4B5, in-house), anti-cytochrome _c_ (1:2000, BD Pharmingen #556433, RRID: AB_396417). These two anti-BAK antibodies
recognize all BAK after its denaturation on SDS-PAGE or transfer in SDS after BN-PAGE, as they both bind to linear epitopes (aa23-38 and BH3 domain, respectively). Detection was achieved
using horseradish peroxidase (HRP)-conjugated anti-rabbit (1:5000, Southern Biotech #4010-05, RRID: AB_2632593), anti-rat (1:5000, Southern Biotech #3010-05, RRID: AB_2795801) and anti-mouse
(1:2000, Southern Biotech #1010-05, RRID: AB_2728714) secondary antibodies. To avoid signals from antibody light chains in immunoprecipitation samples, heavy chain-specific HRP-conjugated
goat anti-rabbit IgG (1:5000, Southern Biotech #4041-05, RRID: AB_2795946) and goat anti-rat IgG (1:5000, Southern Biotech #3030-05, AB_2716837) were also used. Proteins were visualized by
Luminata Forte Western HRP substrate (Millipore #WBLUF0500) on a ChemiDoc TM Touch Imaging System (Bio-Rad Cat#1708370), and images processed with ImageLab Software (Bio-Rad v6.1). Uncropped
blots are shown in Supplementary Material File. RECOMBINANT PROTEIN EXPRESSION AND PURIFICATION hBAKΔN22ΔC25Δcys (BAKΔTM) wild-type, BAK mutants (M60G, M60W), avi-tagged BAKΔTM and
mBAKΔN20ΔC25Δcys were produced as previously described [40, 47]. Here and elsewhere Δcys means replacement of wild-type cysteine residues with serine. BAK constructs were cloned into the
pGEX 6P3 vector and the corresponding plasmids were transformed into BL21 (DE3) _Escherichia coli_ cells and grown in Super Broth (induction at OD (600 nm) = 1 with 1 mM IPTG for 3 h at 37
°C). Cells were lyzed in 50 mM Tris pH 8.0, 150 mM NaCl, 1 mM EDTA and 1.5 µg/mL DNase I (Roche, Mannheim, Germany). Supernatants were passed over glutathione resin (GenScript, Piscataway,
NJ, USA) and the GST-tag removed by proteolysis using HRV-3C protease (1 ml at 0.2 mg/ml) overnight at 4 °C. BAK proteins were eluted as the flowthrough using lysis buffer without the DNase.
All proteins were further purified by size exclusion chromatography (Superdex S75 10/300) in TBS (20 mM Tris pH 8.0, 150 mM NaCl) at 4 °C and snap frozen in liquid nitrogen and stored at
−80 °C. PREPARATION OF 14G6 FAB-BAK COMPLEX FOR CRYSTALLIZATION Papain (P3125-100 mg, Sigma) was activated by diluting to 1 mg/ml in PBS containing 10 mM cysteine and 20 mM EDTA and
incubated on ice for 10 min. Activated papain (final concentration 0.05 mg/ml in PBS) was added to 14G6 (1 mg/ml) and cleaved for 2 h at 37 °C. The reaction was stopped by addition of
iodoacetamide to a concentration of 30 mM and incubation on ice for 30 min. The cleaved mixture was dialyzed into 10 mM sodium acetate, pH 4.5 and purified by cation exchange on a Mono S
5/50 column at 2 ml/min 0–100% Buffer B (10 mM sodium acetate, 500 mM NaCl) over 20 column volumes. 1 M Tris pH 8 was added to fractions containing Fab. Purified 14G6 Fab was mixed with a
1.5-fold excess of BAKΔTM and purified on a Superdex 200 Increase 10/300 column (Cytiva) in TBS buffer. The complex was concentrated to 5.4 mg/ml, flash frozen and stored at −80 °C until
use. CRYSTALLIZATION AND STRUCTURE DETERMINATION Crystals were obtained by sitting drop vapour diffusion with a reservoir containing 0.09 M Bis-tris chloride pH 5.5, 22.5% PEG 3350, 4%
acetonitrile. X-ray diffraction data were collected at the Australian Synchrotron MX2 Beamline using an Eiger 16 M detector [64]. Data were processed using XDS [65]. The structure was
determined by molecular replacement using MolRep [66] with 2YV6 [67] (BAK) and 5W5Z [39] (Fab) as the search models. The structure was refined in Refmac5 [68] and Phenix [69], with iterative
model building in COOT [70]. SURFACE PLASMON RESONANCE Surface Plasmon Resonance (SPR) data were collected on a Biacore S200 (Cytiva). 14G6 was diluted to 2 µg/mL in running buffer and
approximately 150 Resonance Units (RU) captured on a Protein A chip (Cytiva). Kinetics for each BAK construct were determined using a single-cycle kinetic method, with injection time 300 sec
and a final dissociation time of 2400 sec. All curves were reference and blank subtracted and analyzed with Biacore S200 Evaluation software using 1:1 binding kinetic model. All experiments
were carried out at 25 °C using DPBS + 0.005% Tween-20 as running buffer. Kinetic and affinity parameters are quoted as the average ± standard deviation of three independent experiments.
BID BINDING TO BAK BID R84C BH3 peptide (Biotin-DSESQEDIICNIARHLAQVGDSMDRSIPPGLVNGL-NH2) was synthesized by Mimotopes and stored as a 2 mM stock in DMSO. 9 µM BAKΔcysΔN22ΔC25 CT His with
K113C mutation was pre-incubated with 1.5× excess of 14G6 antibody before addition of 4× excess of BID R84C BH3 peptide and treatment with a final concentration of 2 mM copper (II)
1,10-phenanthroline (CuPhe). The reaction was incubated for 1 h at room temperature and stopped by addition of EDTA to 20 mM. Samples were then run on non-reducing SDS-PAGE (Bolt Bis-Tris
Plus 4–12% (Invitrogen, NW04120BOX) using MES running buffer). DATA AVAILABILITY The coordinates for BAK-14G6 have been deposited in the PDB with accession code 8UKY. All other data are
available from the corresponding authors upon request. REFERENCES * Moldoveanu T, Czabotar PE. BAX, BAK, and BOK: a coming of age for the BCL-2 family effector proteins. Cold Spring Harb
Perspect Biol. 2019;12:a036319. Article Google Scholar * Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta.
2011;1813:521–31. Article CAS PubMed Google Scholar * Dai H, Smith A, Meng XW, Schneider PA, Pang YP, Kaufmann SH. Transient binding of an activator BH3 domain to the Bak BH3-binding
groove initiates Bak oligomerization. J Cell Biol. 2011;194:39–48. Article CAS PubMed PubMed Central Google Scholar * Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, et
al. Bax crystal structures reveal How BH3 domains activate bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–31. Article CAS PubMed Google Scholar * Brouwer
JM, Westphal D, Dewson G, Robin AY, Uren RT, Bartolo R, et al. Bak core and latch domains separate during activation, and freed core domains form symmetric homodimers. Mol Cell.
2014;55:938–46. Article CAS PubMed Google Scholar * Moldoveanu T, Grace CR, Llambi F, Nourse A, Fitzgerald P, Gehring K, et al. BID-induced structural changes in BAK promote apoptosis.
Nat Struct Mol Biol. 2013;20:589–97. Article CAS PubMed PubMed Central Google Scholar * Alsop AE, Fennell SC, Bartolo RC, Tan IKL, Dewson G, Kluck RM. Dissociation of Bak α 1 helix from
the core and latch domains is required for apoptosis. Nat Commun. 2015;6:6841. Article CAS PubMed Google Scholar * Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM,
et al. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–14. Article CAS PubMed PubMed Central
Google Scholar * Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–34. Article CAS PubMed Google Scholar *
Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, et al. To trigger apoptosis, Bak exposes Its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell.
2008;30:369–80. Article CAS PubMed Google Scholar * Dewson G, Ma S, Frederick P, Hockings C, Tan I, Kratina T, et al. Bax dimerizes via a symmetric BH3:groove interface during apoptosis.
Cell Death Differ. 2012;19:661–70. Article CAS PubMed Google Scholar * Subburaj Y, Cosentino K, Axmann M, Pedrueza-Villalmanzo E, Hermann E, Bleicken S, et al. Bax monomers form dimer
units in the membrane that further self-assemble into multiple oligomeric species. Nat Commun. 2015;6:8042. Article CAS PubMed Google Scholar * Westphal D, Dewson G, Menard M, Frederick
P, Iyer S, Bartolo R, et al. Apoptotic pore formation is associated with in-plane insertion of Bak or Bax central helices into the mitochondrial outer membrane. Proc Natl Acad Sci USA.
2014;111:E4076–85. Article CAS PubMed PubMed Central Google Scholar * Uren RT, O’Hely M, Iyer S, Bartolo R, Shi MX, Brouwer JM, et al. Disordered clusters of bak dimers rupture
mitochondria during apoptosis. eLife. 2017;6:e19944. Article PubMed PubMed Central Google Scholar * Grosse L, Wurm CA, Bruser C, Neumann D, Jans DC, Jakobs S. Bax assembles into large
ring-like structures remodeling the mitochondrial outer membrane in apoptosis. EMBO J. 2016;35:402–13. Article CAS PubMed PubMed Central Google Scholar * Salvador-Gallego R, Mund M,
Cosentino K, Schneider J, Unsay J, Schraermeyer U, et al. Bax assembly into rings and arcs in apoptotic mitochondria is linked to membrane pores. EMBO J. 2016;35:389–1. Article CAS PubMed
PubMed Central Google Scholar * Czabotar PE, Lee EF, Thompson GV, Wardak AZ, Fairlie WD, Colman PM. Mutations to Bax beyond the BH3 domain disrupts interactions with pro-survival
proteins and promotes apoptosis. J Biol Chem. 2011;286:7123–31. Article CAS PubMed PubMed Central Google Scholar * Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M,
et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–6. Article CAS PubMed Google Scholar * Iyer S, Uren RT, Kluck RM.
Probing BAK and BAX activation and pore assembly with cytochrome c release, limited proteolysis, and oxidant-induced linkage. Methods Mol Biol. 2019;1877:201–16. Article CAS PubMed Google
Scholar * Mandal T, Hustedt EJ, Song L, Oh KJ. CW EPR and DEER methods to determine BCL-2 family protein structure and interactions: application of site-directed spin labeling to BAK
apoptotic pores. Methods Mol Biol. 2019;1877:257–3. Article CAS PubMed Google Scholar * Murad F, Garcia-Saez AJ. Quantification of the interactions between BCL-2 proteins by fluorescence
correlation spectroscopy. Methods Mol Biol. 2019;1877:337–50. Article CAS PubMed Google Scholar * Osterlund EJ, Hirmiz N, Tardif C, Andrews DW. Rapid imaging of BCL-2 family
interactions in live cells using FLIM-FRET. Methods Mol Biol. 2019;1877:305–35. Article CAS PubMed Google Scholar * Singh G, Moldoveanu T. Methods to probe conformational activation and
mitochondrial activity of proapoptotic BAK. Methods Mol Biol. 2019;1877:185–200. Article CAS PubMed Google Scholar * Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer
SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–92. Article CAS PubMed Google Scholar *
Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak.[see comment]. Science.
2007;315:856–9. Article CAS PubMed Google Scholar * Chen HC, Kanai M, Inoue-Yamauchi A, Tu HC, Huang Y, Ren D, et al. An interconnected hierarchical model of cell death regulation by the
BCL-2 family. Nat Cell Biol. 2015;17:1270–81. Article CAS PubMed PubMed Central Google Scholar * Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3
domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–35. Article CAS PubMed Google
Scholar * Leber B, Lin J, Andrews DW. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene. 2010;29:5221–30. * Llambi F, Moldoveanu T, Tait SW,
Bouchier-Hayes L, Temirov J, McCormick LL, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–31. Article CAS PubMed PubMed
Central Google Scholar * Luo X, O’Neill KL, Huang K. The third model of Bax/Bak activation: a Bcl-2 family feud finally resolved? F1000Res. 2020;9:F1000. Article PubMed PubMed Central
Google Scholar * Blombery P, Lew TE, Dengler MA, Thompson ER, Lin VS, Chen X, et al. Clonal hematopoiesis, myeloid disorders and BAX-mutated myelopoiesis in patients receiving venetoclax
for CLL. Blood. 2022;139:1198–207. Article CAS PubMed Google Scholar * Moujalled DM, Brown FC, Chua CC, Dengler MA, Pomilio G, Anstee NS, et al. Acquired mutations in BAX confer
resistance to BH3-mimetic therapy in acute myeloid leukemia. Blood. 2023;141:634–44. Article CAS PubMed Google Scholar * Dai H, Ding H, Meng XW, Peterson KL, Schneider PA, Karp JE, et
al. Constitutive BAK activation as a determinant of drug sensitivity in malignant lymphohematopoietic cells. Genes Dev. 2015;29:2140–52. Article CAS PubMed PubMed Central Google Scholar
* Liu D, Hou X, Wu W, Zanfagnin V, Li Y, Correia C, et al. Constitutive BAK/MCL1 complexes predict paclitaxel and S63845 sensitivity of ovarian cancer. Cell Death Dis. 2021;12:789. Article
CAS PubMed PubMed Central Google Scholar * Ichim G, Lopez J, Ahmed SU, Muthalagu N, Giampazolias E, Delgado ME, et al. Limited mitochondrial permeabilization causes DNA damage and
genomic instability in the absence of cell death. Mol Cell. 2015;57:860–72. Article CAS PubMed PubMed Central Google Scholar * Kalkavan H, Chen MJ, Crawford JC, Quarato G, Fitzgerald P,
Tait SWG, et al. Sublethal cytochrome c release generates drug-tolerant persister cells. Cell. 2022;185:3356–74 e3322. Article CAS PubMed PubMed Central Google Scholar * Shen S, Vagner
S, Robert C. Persistent cancer cells: the deadly survivors. Cell. 2020;183:860–74. Article CAS PubMed Google Scholar * Iyer S, Anwari K, Alsop AE, Yuen WS, Huang DC, Carroll J, et al.
Identification of an activation site in Bak and mitochondrial Bax triggered by antibodies. Nat Commun. 2016;7:11734. Article CAS PubMed PubMed Central Google Scholar * Robin AY, Iyer S,
Birkinshaw RW, Sandow J, Wardak A, Luo CS, et al. Ensemble Properties of Bax Determine Its Function. Structure. 2018;26:1346–1359.e1345. Article CAS PubMed Google Scholar * Robin AY,
Miller MS, Iyer S, Shi MX, Wardak AZ, Lio D, et al. Structure of the BAK-activating antibody 7D10 bound to BAK reveals an unexpected role for the alpha1-alpha2 loop in BAK activation. Cell
Death Differ. 2022;29:1757–68. Article CAS PubMed PubMed Central Google Scholar * Uchime O, Dai Z, Biris N, Lee D, Sidhu SS, Li S, et al. Synthetic antibodies inhibit Bcl-2-associated X
protein (BAX) through blockade of the N-terminal activation site. J Biol Chem. 2016;291:89–102. Article CAS PubMed Google Scholar * Lawrence MC, Colman PM. Shape complementarity at
protein/protein interfaces. J Mol Biol. 1993;234:946–50. Article CAS PubMed Google Scholar * Epa VC, Colman PM. Shape and electrostatic complementarity at viral antigen-antibody
complexes. Curr Top Microbiol Immunol. 2001;260:45–53. CAS PubMed Google Scholar * Moldoveanu T, Liu Q, Tocilj A, Watson MH, Shore G, Gehring K. The x-ray structure of a BAK homodimer
reveals an inhibitory zinc binding site. Mol Cell. 2006;24:677–88. Article CAS PubMed Google Scholar * Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA, et al. The combined
functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–99. Article CAS PubMed PubMed Central Google
Scholar * Kvansakul M, Yang H, Fairlie WD, Czabotar PE, Fischer SF, Perugini MA, et al. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly
selective subset of BH3-containing death ligands. Cell Death Differ. 2008;15:1564–71. Article CAS PubMed Google Scholar * Brouwer JM, Lan P, Cowan AD, Bernardini JP, Birkinshaw RW, van
Delft MF, et al. Conversion of Bim-BH3 from activator to inhibitor of Bak through structure-based design. Mol Cell. 2017;68:659–672.e659. Article CAS PubMed Google Scholar * van Delft
MF, Chappaz S, Khakham Y, Bui CT, Debrincat MA, Lowes KN, et al. A small molecule interacts with VDAC2 to block mouse BAK-driven apoptosis. Nat Chem Biol. 2019;15:1057–66. Article PubMed
Google Scholar * Chin HS, Li MX, Tan IKL, Ninnis RL, Reljic B, Scicluna K, et al. VDAC2 enables BAX to mediate apoptosis and limit tumor development. Nat Commun. 2018;9:4976. Article
PubMed PubMed Central Google Scholar * Lazarou M, Stojanovski D, Frazier AE, Kotevski A, Dewson G, Craigen WJ, et al. Inhibition of Bak activation by VDAC2 is dependent on the Bak
transmembrane anchor. J Biol Chem. 2010;285:36876–83. Article CAS PubMed PubMed Central Google Scholar * Naghdi S, Varnai P, Hajnoczky G. Motifs of VDAC2 required for mitochondrial Bak
import and tBid-induced apoptosis. Proc Natl Acad Sci USA. 2015;112:E5590–9. Article CAS PubMed PubMed Central Google Scholar * O’Neill KL, Huang K, Zhang J, Chen Y, Luo X. Inactivation
of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane. Genes Dev. 2016;30:973–88. Article PubMed PubMed Central Google Scholar * Iyer S, Bell F,
Westphal D, Anwari K, Gulbis J, Smith BJ, et al. Bak apoptotic pores involve a flexible C-terminal region and juxtaposition of the C-terminal transmembrane domains. Cell Death Differ.
2015;22:1665–75. Article CAS PubMed PubMed Central Google Scholar * Hockings C, Alsop AE, Fennell SC, Lee EF, Fairlie WD, Dewson G, et al. Mcl-1 and Bcl-x(L) sequestration of Bak
confers differential resistance to BH3-only proteins. Cell Death Differ. 2018;25:721–34. Article CAS PubMed Google Scholar * Ma SB, Nguyen TN, Tan I, Ninnis R, Iyer S, Stroud DA, et al.
Bax targets mitochondria by distinct mechanisms before or during apoptotic cell death: a requirement for VDAC2 or Bak for efficient Bax apoptotic function. Cell Death Differ.
2014;21:1925–35. * Stong RC, Korsmeyer SJ, Parkin JL, Arthur DC, Kersey JH. Human acute leukemia cell line with the t(4;11) chromosomal rearrangement exhibits B lineage and monocytic
characteristics. Blood. 1985;65:21–31. Article CAS PubMed Google Scholar * Matsuo Y, MacLeod RA, Uphoff CC, Drexler HG, Nishizaki C, Katayama Y, et al. Two acute monocytic leukemia
(AML-M5a) cell lines (MOLM-13 and MOLM-14) with interclonal phenotypic heterogeneity showing MLL-AF9 fusion resulting from an occult chromosome insertion, ins(11;9)(q23;p22p23). Leukemia.
1997;11:1469–77. Article CAS PubMed Google Scholar * Koeffler HP, Golde DW. Acute myelogenous leukemia: a human cell line responsive to colony-stimulating activity. Science.
1978;200:1153–4. Article CAS PubMed Google Scholar * Gallagher R, Collins S, Trujillo J, McCredie K, Ahearn M, Tsai S, et al. Characterization of the continuous, differentiating myeloid
cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood. 1979;54:713–33. Article CAS PubMed Google Scholar * Quentmeier H, Martelli MP, Dirks WG, Bolli N, Liso A,
Macleod RA, et al. Cell line OCI/AML3 bears exon-12 NPM gene mutation-A and cytoplasmic expression of nucleophosmin. Leukemia. 2005;19:1760–7. Article CAS PubMed Google Scholar * Lange
B, Valtieri M, Santoli D, Caracciolo D, Mavilio F, Gemperlein I, et al. Growth factor requirements of childhood acute leukemia: establishment of GM-CSF-dependent cell lines. Blood.
1987;70:192–9. Article CAS PubMed Google Scholar * Tsuchiya S, Yamabe M, Yamaguchi Y, Kobayashi Y, Konno T, Tada K. Establishment and characterization of a human acute monocytic leukemia
cell line (THP-1). Int J Cancer. 1980;26:171–6. Article CAS PubMed Google Scholar * Ma S, Hockings C, Anwari K, Kratina T, Fennell S, Lazarou M, et al. Assembly of the Bak apoptotic
pore: a critical role for the Bak protein alpha6 helix in the multimerization of homodimers during apoptosis. J Biol Chem. 2013;288:26027–38. Article CAS PubMed PubMed Central Google
Scholar * Aragao D, Aishima J, Cherukuvada H, Clarken R, Clift M, Cowieson NP, et al. MX2: a high-flux undulator microfocus beamline serving both the chemical and macromolecular
crystallography communities at the Australian Synchrotron. J Synchrotron Radiat. 2018;25:885–91. Article CAS PubMed PubMed Central Google Scholar * Kabsch W. Integration, scaling,
space-group assignment and post-refinement. Acta Crystallogr D Biol Crystallogr. 2010;66:133–44. Article CAS PubMed PubMed Central Google Scholar * Vagin A, Teplyakov A. Molecular
replacement with MOLREP. Acta Crystallogr D Biol Crystallogr. 2010;66:22–25. Article CAS PubMed Google Scholar * Wang H, Takemoto C, Akasaka R, Uchikubo-Kamo T, Kishishita S, Murayama K,
et al. Novel dimerization mode of the human Bcl-2 family protein Bak, a mitochondrial apoptosis regulator. J Struct Biol. 2009;166:32–37. Article CAS PubMed Google Scholar * Vagin AA,
Steiner RA, Lebedev AA, Potterton L, McNicholas S, Long F, et al. REFMAC5 dictionary: organization of prior chemical knowledge and guidelines for its use. Acta Crystallogr D Biol
Crystallogr. 2004;60:2184–95. Article PubMed Google Scholar * Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, et al. PHENIX: a comprehensive Python-based system for
macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–21. Article CAS PubMed PubMed Central Google Scholar * Emsley P, Lohkamp B, Scott WG, Cowtan K.
Features and development of Coot. Acta Crystallogr D Biol Crystallogr. 2010;66:486–1. Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We thank Paul
Masendycz, Kaye Wycherley and the WEHI Antibody Facility for mouse immunizations and collection of sera and splenocytes, the WEHI Biologics Initiative for assistance with mouse B cell
cloning, and the WEHI Protein Production Facility for 14G6 expression and purification. We acknowledge the support of the CSIRO Collaborative Crystallization Centre (C3). This research was
undertaken in part using the MX beamlines at the Australian Synchrotron, part of ANSTO, and made use of the Australian Cancer Research Foundation (ACRF) detector. FUNDING This work was
supported by a Program Grant (GNT1113133) to PEC and RMK, and Research Fellowship (GNT2009062) and Ideas Grant (2001406) to PEC from the Australian NHMRC, the Leukemia & Lymphoma Society
of America (Specialized Centre of Research [SCOR] grant no. 7015-18 to RK), the Lady Tata Memorial Trust (S.I.) and The Jack Brockhoff Foundation (SI). Work in the laboratories of the
authors was made possible through Victorian State Government Operational Infrastructure Support (OIS) and Australian Government NHMRC Independent Research Institute Infrastructure Support
(IRIIS) Scheme. Open Access funding enabled and organized by CAUL and its Member Institutions. AUTHOR INFORMATION Author notes * Hema Preethi Subas Satish Present address: Vanderbilt Vaccine
Center, Vanderbilt University Medical Center, Nashville, TN, 37232, USA * These authors contributed equally: Hema Preethi Subas Satish, Sweta Iyer. * These authors jointly supervised this
work: Michelle S. Miller, Ruth M. Kluck. AUTHORS AND AFFILIATIONS * Walter and Eliza Hall Institute of Medical Research, 1G Royal Parade, Parkville, VIC, 3052, Australia Hema Preethi Subas
Satish, Sweta Iyer, Melissa X. Shi, Agnes W. Wong, Karla C. Fischer, Ahmad Z. Wardak, Daisy Lio, Jason M. Brouwer, Rachel T. Uren, Peter E. Czabotar, Michelle S. Miller & Ruth M. Kluck *
Department of Medical Biology, The University of Melbourne, Parkville, VIC, 3010, Australia Hema Preethi Subas Satish, Sweta Iyer, Melissa X. Shi, Agnes W. Wong, Karla C. Fischer, Ahmad Z.
Wardak, Daisy Lio, Jason M. Brouwer, Rachel T. Uren, Peter E. Czabotar, Michelle S. Miller & Ruth M. Kluck Authors * Hema Preethi Subas Satish View author publications You can also
search for this author inPubMed Google Scholar * Sweta Iyer View author publications You can also search for this author inPubMed Google Scholar * Melissa X. Shi View author publications You
can also search for this author inPubMed Google Scholar * Agnes W. Wong View author publications You can also search for this author inPubMed Google Scholar * Karla C. Fischer View author
publications You can also search for this author inPubMed Google Scholar * Ahmad Z. Wardak View author publications You can also search for this author inPubMed Google Scholar * Daisy Lio
View author publications You can also search for this author inPubMed Google Scholar * Jason M. Brouwer View author publications You can also search for this author inPubMed Google Scholar *
Rachel T. Uren View author publications You can also search for this author inPubMed Google Scholar * Peter E. Czabotar View author publications You can also search for this author inPubMed
Google Scholar * Michelle S. Miller View author publications You can also search for this author inPubMed Google Scholar * Ruth M. Kluck View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS HPSS and SI designed, supervised, and performed research, analyzed data, prepared figures, and wrote the manuscript. MSM and RMK conceived,
designed, supervised research, and wrote the manuscript. MSM performed research and analyzed data. MXS, AWW, KCF, AZW, DL, RTU designed and performed some of the research that led to this
work and analyzed data. DL and JMB provided important reagents. RTU, KCF and PEC contributed to discussions of the data. All authors reviewed the manuscript. CORRESPONDING AUTHORS
Correspondence to Michelle S. Miller or Ruth M. Kluck. ETHICS DECLARATIONS COMPETING INTERESTS HPSS, SI, MXS, AWW, KCF, AZW, DL, JMB, RTU, PEC, MSM and RMK are or were employees of WEHI
which receives royalties from AbbVie and Genentech from the sale of Venetoclax. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims
in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY DATA SUPPLEMENTARY ORIGINAL BLOTS RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under
a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate
credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article
are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Subas Satish, H.P., Iyer, S., Shi, M.X. _et al._ A novel inhibitory
BAK antibody enables assessment of non-activated BAK in cancer cells. _Cell Death Differ_ 31, 711–721 (2024). https://doi.org/10.1038/s41418-024-01289-3 Download citation * Received: 13
November 2023 * Revised: 21 March 2024 * Accepted: 22 March 2024 * Published: 06 April 2024 * Issue Date: June 2024 * DOI: https://doi.org/10.1038/s41418-024-01289-3 SHARE THIS ARTICLE
Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided
by the Springer Nature SharedIt content-sharing initiative