- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
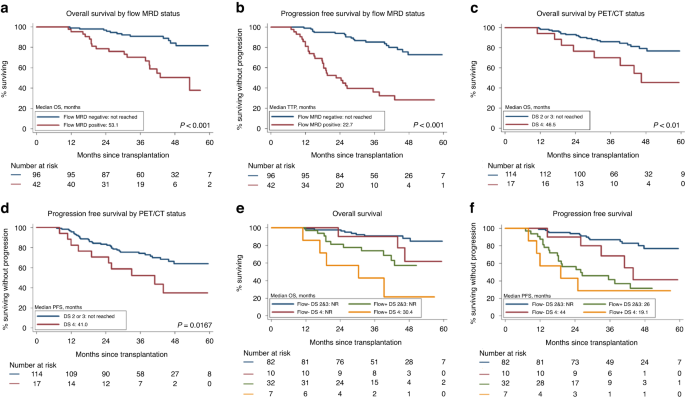
Access through your institution Buy or subscribe Measurable residual disease (MRD) is a surrogate endpoint for progression-free survival (PFS) in Multiple myeloma (MM) and meta analysis have
confirmed that bone marrow (BM) MRD negativity is associated with superior PFS both in transplant and non transplant setting [1]. BM next generation multiparametric flow cytometry (MFC) and
next generation sequencing (NGS) are currently being employed to assess MRD and have been included in the International Myeloma Working Group (IMWG) criteria for defining MRD negative state
[2]. 18F-FDG PET/CT scan with its ability to distinguish between metabolically active and inactive disease has helped to evaluate the extramedullary disease component which is present in up
to 20% of patients [3, 4]. We hypothesized that a combined assessment of MRD with BM flow and PET/CT scan may be more predictive and prospectively evaluated both on day + 100 post ASCT. In
addition, we also assessed the clinical and laboratory predictors of of flow MRD status and the risk factors influencing time to progression after transplant. Between April, 2016 and
November, 2019, 160 consecutive MM patients underwent first ASCT post novel agent based induction. Responses to induction and transplant were assessed using the IMWG consensus criteria. [2].
Post ASCT patients received maintenance therapy with lenalidomide 10 mg daily for 21 days every month for ≥2 years. The BM flow MRD was performed on 138 patients using previously described
protocol (supplementary File: Protocol) with an overall sensitivity of 10−6. Whole body 18F-FDG PET/CT after ASCT was done in 131 patients and the FDG uptake in the target lesions was
quantified according to the 5-point Deauville scale [5]. Four patients were PET negative at diagnosis as well as on day + 100 evaluation. The median interval from diagnosis to transplant was
12.1 months. The median follow up of the cohort is 39 months (range: 11 to 63.9 months). This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through
your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant
access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions *
Read our FAQs * Contact customer support REFERENCES * Munshi N, Avet-Loiseau H, Rawstron AC, Owen RG, Child JA, Thakurta A, et al. Association of minimal residual disease with superior
survival outocmes in patiens with Multiple myeloma: a meta-analysis. JAMA Oncol. 2017;3:28–35. Article PubMed PubMed Central Google Scholar * Kumar S, Paiva B, Anderson KC, Durie B,
Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17:e328–46.
Article PubMed Google Scholar * Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S, et al. Role of 18F-FDG PET/CT in the diagnosis and management of multiple myeloma and other
plasma cell disorders: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18:e206–e217. https://doi.org/10.1016/S1470-2045(17)30189-4. Article PubMed
Google Scholar * Kumar L, Gogi R, Patel AK, Mookerjee A, Sahoo RK, Malik PS, et al. Multiple myeloma with extramedullary disease: impact of autologous stem cell transplantation on outcome.
Bone Marrow Transpl. 2017;52:1473–5. https://doi.org/10.1038/bmt.2017.165. Article CAS Google Scholar * Boellaard R, Delgado-Bollon R, Oyen WJG, Giammarile F, Tatsch K, Eschner W, et al.
FDG PET/CT EANM procedure guidelines for tumor imaging 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. Article CAS PubMed Google Scholar * Paiva B, San-Miguel JF, Avet-Loiseau H. MRD in
multiple myeloma: does CR really matter? Blood. 2022:2022016170. https://doi.org/10.1182/blood.2022016170. * Attal M, Lauwers-Cances V, Hulin C, Caillot D, Escoffre M, Arnulf B, et al. IFM
2009 Study. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20. Article CAS PubMed PubMed Central Google Scholar * Rasche L,
Alapat D, Kumar M, Gershner G, McDonald J, Wardell CP, et al. Combination of flow cytometry and functional imaging for monitoring of residual disease in myeloma. Leukemia. 2019;33:1713–22.
https://doi.org/10.1038/s41375-018-0329-0. Article CAS PubMed Google Scholar * Meignan M, Gallamini A, Haioun C. Report on the First International Workshop on interim-PET scan in
lymphoma. Leuk Lymphoma. 2009;50:1257–60. Article PubMed Google Scholar * Zamagni E, Nanni C, Dozza L, Carlier T, Bailly C, Tacchetti P, et al. Standardization of (18)F-FDG-PET/CT
according to Deauville criteria for metabolic complete response definition in newly diagnosed multiple myeloma. J Clin Oncol. 2021;39:116–25. Article CAS PubMed Google Scholar * Diamond
BT, Rustad E, Maclachlan K, Thoren K, Ho C, Roshal M, et al. Defining the undetectable: the current landscape of minimal residual disease assessment in multiple myeloma and goals for future
clarity. Blood Rev. 2021;46:100732. https://doi.org/10.1016/j.blre.2020.100732. Article CAS PubMed Google Scholar Download references ACKNOWLEDGEMENTS We wish to thank all the patients
and their families for having participated in the study. We are also grateful to the resident doctors, laboratory and nursing staff, scientists and faculty of the departments of Medical
Oncology, Laboratory Oncology Unit and Nuclear Medicine. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Medical Oncology, All India Institute of Medical Sciences, New Delhi,
110029, India Anjali Mookerjee, Atul Sharma & Lalit Kumar * Lab Oncology, All India Institute of Medical Sciences, New Delhi, 110029, India Ritu Gupta * Nuclear Medicine, All India
Institute of Medical Sciences, New Delhi, 110029, India Rakesh Kumar * Bio-Statistics, All India Institute of Medical Sciences, New Delhi, 110029, India Ravindra Mohan Pandey Authors *
Anjali Mookerjee View author publications You can also search for this author inPubMed Google Scholar * Ritu Gupta View author publications You can also search for this author inPubMed
Google Scholar * Rakesh Kumar View author publications You can also search for this author inPubMed Google Scholar * Atul Sharma View author publications You can also search for this author
inPubMed Google Scholar * Ravindra Mohan Pandey View author publications You can also search for this author inPubMed Google Scholar * Lalit Kumar View author publications You can also
search for this author inPubMed Google Scholar CONTRIBUTIONS AM: designing and performed the experiments, collating data, manuscript writing. RG: review of flow cytometry experiments/data,
discussion. RK: review of PET scan data. AS: patients clinical management RMP Statistical analysis. LK: conceptualization, experiments design, treatment and monitoring, manuscript writing
and editing. CORRESPONDING AUTHOR Correspondence to Lalit Kumar. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY MATERIAL RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Mookerjee, A., Gupta, R., Kumar, R. _et al._ Dual assessment with multiparameter flow cytometry and 18F-FDG PET/CT scan
provides enhanced prediction of measurable residual disease after autologous haemopoietic stem cell transplant in myeloma—a prospective study. _Bone Marrow Transplant_ 58, 1045–1047 (2023).
https://doi.org/10.1038/s41409-023-02017-0 Download citation * Received: 20 January 2023 * Revised: 14 May 2023 * Accepted: 07 June 2023 * Published: 16 June 2023 * Issue Date: September
2023 * DOI: https://doi.org/10.1038/s41409-023-02017-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








