- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
The Harmony Trial (NCT02851407), a prospective randomized trial conducted among adults and children, reported that defibrotide was not effective for the prevention of veno-occlusive
disease/sinusoidal obstruction syndrome (VOD/SOS) [1]. Importantly no safety concerns were raised [2], but consecutively, in May 2022, the European Medicines Agency (EMA) recommended not to
use defibrotide for prophylaxis of VOD/SOS (https://www.ema.europa.eu/en/medicines/dhpc/defitelio-defibrotide-do-not-use-prophylaxis-veno-occlusive-disease-vod-after-post-hematopoietic). A
decade ago, another prospective randomized trial, the Pediatric Prevention Trial (NCT00272948), clearly demonstrated efficacy in the prevention of VOD/SOS [3]. Prospective randomized trials
are considered the so-called “gold standard” of clinical medicine. What happened that such two prospective randomized trials yielded apparently contradictory results? The Pediatric
Prevention Trial was conceptualized between 2001 and 2005 and recruited 360 pediatric patients considered at high risk for VOD/SOS between 2006 and 2009, being informed by prior and ongoing
treatment studies at the time in which defibrotide had shown consistent efficacy in the setting of established disease [4]. The sample size calculation generated back in 2005 was based on
the presumed incidence in high-risk pediatric patients of up to 30%. In order to reduce this incidence by half, to 15%, the sample size calculation resulted in accrual of 270 patients for
both arms. In 2008, the interim analysis revealed that the actual incidence of VOD/SOS in this high-risk population based on investigator assessment was only 20% according to the modified
Seattle criteria. Consequently, the sample size was adjusted as part of a planned interim analysis to 360 patients. Eventually the trial demonstrated efficacy in the competing risk analysis
of the intent-to-treat population reducing the incidence from 20 to 12% (_p_ = 0.049; per protocol population: _p_ = 0.022) [3]. Whereas the Pediatric Prevention Trial focused exclusively on
the highest risk pediatric population, the Harmony Trial enrolled pediatric and adult patients and enrolled patients where high risk among other criteria was defined as a myeloablative
conditioning (MAC) with more than two (not further defined, see below) alkylators or TBI plus one or more alkylators. Current high-risk populations in adult disease—such as those exposed to
sirolimus—were excluded, due to regulatory considerations on the part of the sponsor at the time. Almost half of the patients enrolled were adults and half of the diagnoses were acute
leukemias. Only 15% and 7% of the enrolled patients, respectively, were very high-risk pediatric patients with neuroblastoma and osteopetrosis. In 2010, a meta-analysis including 25,000
pediatric and adult patients revealed an average VOD/SOS incidence of 13.7% [5]. The reported incidence in children was around 20% [6, 7] which was later confirmed in the Pediatric
Prevention Trial with an overall incidence of 22.2% and an even higher incidence of 29.3% reported in infants [3]. In 2018, a study including 13,000 patients defined the incidence in the
highest risk population as ~18% with the most significant non-linear relationship existing between age and risk of VOD/SOS [8], and a contemporaneous meta-analysis including more than 27,000
patients confirmed a VOD/SOS incidence of 15% [9, 10]. In summary, an average VOD/SOS incidence of ~15% can be considered realistic with a persistent and consistently higher incidence of
VOD/SOS in children, particularly in infants as compared to adults. The Harmony Trial had an adaptive design with a calculated sample size of 400 patients based on a presumed incidence of
28% in the best supportive care (BSC) group. This incidence in the control arm was not even reached in the 10 years older trial in high-risk pediatric patients as described above. With a
decade between the two trials with scientific advancements that improved the safety of hematopoietic stem cell transplantation (HSCT) significantly, the presumed incidence was not realistic.
One example, treosulfan, albeit an alkylator such as busulfan, demonstrates a reduced endothelial toxicity profile. This is most evident in infantile malignant osteopetrosis (IMO) with a
historical incidence of VOD/SOS as high as 60% [3]. Shadur et al. [11] reported no VOD/SOS on 31 IMO patients using a treosulfan, fludarabine and thiotepa-based MAC regimen with an overall
survival of 100%. In retrospect, these observations suggest that a power calculation based on an overestimated incidence of VOD/SOS of 28%, in an adult/pediatric study, resulting in an
estimated sample size of 400, was markedly insufficient to detect a significant reduction in a non-composite endpoint, i.e., the incidence of VOD/SOS. Moreover, this was not the primary
endpoint ultimately chosen by the sponsor, as based on guidance from regulatory authorities. The primary endpoint of the Harmony Trial was required to be VOD/SOS-free survival by day 30
post-HSCT. There proved to be even greater challenges with this composite endpoint than simply an overestimate of the incidence. VOD/SOS is graded according to severity and only the
severe/very severe grades are associated with a mortality of up to 80% [5] whereas the mortality of mild/moderate cases is by comparison very low. VOD/SOS with multiorgan failure (MOF;
sometimes referred to as multiorgan dysfunction) occurs in an estimated 30–40% of patients with VOD/SOS after HSCT [12]. Therefore, considering a population of 40% out of 15% that are at
risk of treatment-related mortality due to VOD/SOS, the trial population at risk for this composite endpoint was further reduced to less than 7%. Szmit et al. analyzed the impact of
preemptive treatment with defibrotide by comparing in a single institution Seattle criteria with the pediatric EBMT criteria [13]. They not only found a significantly improved survival but
also a shortened median hospitalization by 12 days correlating with a reduced admission to the pediatric intensive care unit (PICU) (3.9% vs. 14.6%) (personal communication), clearly
reflecting morbidity from VOD as a significant endpoint for treatment efficacy. In conclusion, survival is a crude primary endpoint in defining the benefit of an intervention in VOD/SOS by
ignoring the substantial morbidity related to this disease. In addition, and importantly, a statistically meaningful difference in outcome for the endpoint of survival was diluted in both
trials by the required and necessary equipoise of allowing defibrotide use for the treatment of any emergent VOD/SOS in the BSC group. In designing the study, there were also concerns about
correctly and reproducibly diagnosing VOD/SOS. To address this, the Harmony Trial implemented an independent Endpoint Adjudication Committee (EPAC) to provide centralized assessment of the
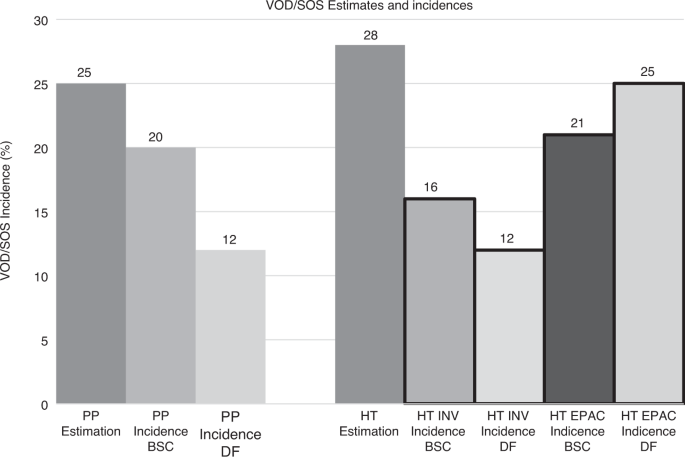
primary endpoint (VOD/SOS-free survival by day 30 post-HSCT) which comprised 4 independent HSCT specialists. Unexpectedly, the EPAC assessment of VOD/SOS resulted in detection of
significantly more cases of VOD/SOS (25% for prophylaxis vs. 21% for BSC) than that recorded by the investigators at the bedside (12% for prophylaxis vs. 16% for BSC) (Fig. 1). Disagreements
between EPAC and investigators proved asymmetric: there were many more cases of disagreement between EPAC and investigator in the direction of the EPAC calling VOD/SOS when the investigator
did not, compared to the opposite scenario. The primary endpoint was based on EPAC assessment alone. This significant discrepancy was not factored into the decision by the data and safety
monitoring committee (DSMC) to stop the trial for futility at the time of interim analysis, which was based upon an apparent lack of efficacy in a limited sample size, a decision worsened by
its timing during the pandemic and legitimate concerns regarding the pace of future enrollment. It is not possible to know if a trial with a more realistic sample size; recruited over a
longer period; using real world bedside assessment of VOD/SOS, and designed to assess the impact of defibrotide prophylaxis based upon VOD/SOS incidence, might have reached a different
conclusion, and above all, if it was focused on current higher risk patients, a conclusion that might then have matched the outcome of the previous Pediatric Prevention Trial [3]. Given the
totality of evidence, and in particular when faced with pediatric patients at very high risk of VOD/SOS, we would point to the results of the Pediatric Prevention Trial as providing
justification for use of prophylactic defibrotide in this smaller and highly selected group of patients for which there is exquisite unmet medical need. Given the substantive methodological
concerns raised with the Harmony Trial, we still regard this as an open question, and would not use this latter result over others to recommend against the use of defibrotide as prophylaxis
for patients at high risk. Therefore, before drawing any final conclusions, we advocate the consideration of the entire body of clinical benefit seen to date, especially when there is such a
discrepancy in outcome in two prospective randomized trials and would recommend further carefully designed studies in high-risk groups. The consequences of not recommending any prevention
of VOD/SOS are already devastating for a substantial number of transplant patients, and especially for high-risk infants, being amongst the most vulnerable populations undergoing HSCT, as
well as others. REFERENCES * Grupp SA, Corbacioglu S, Kang HJ, Teshima T, Khaw SL, Locatelli F, et al. Defibrotide plus best standard of care compared with best standard of care alone for
the prevention of sinusoidal obstruction syndrome (HARMONY): a randomised, multicentre, phase 3 trial. Lancet Haematol. 2023. https://doi.org/10.1016/s2352-3026(23)00011-x. * Grupp S,
Corbacioglu S, Jin Kang H, Teshima T, Zanette M, Lopez P, et al. A phase 3, randomized, adaptive study of defibrotide versus best supportive care (BSC) for the prevention of hepatic
veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) in patients undergoing hematopoietic cell transplantation (HCT): preliminary results. In: ASH. Atlanta, USA, 2021. *
Corbacioglu S, Cesaro S, Faraci M, Valteau-Couanet D, Gruhn B, Rovelli A, et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell
transplantation: an open-label, phase 3, randomised controlled trial. Lancet. 2012;379:1301–9. https://doi.org/10.1016/S0140-6736(11)61938-7. Article CAS PubMed Google Scholar *
Richardson P, Aggarwal S, Topaloglu O, Villa KF, Corbacioglu S. Systematic review of defibrotide studies in the treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS).
Bone Marrow Transplant. 2019;54:1951–62. https://doi.org/10.1038/s41409-019-0474-8. Article CAS PubMed PubMed Central Google Scholar * Coppell JA, Richardson PG, Soiffer R, Martin PL,
Kernan NA, Chen A, et al. Hepatic veno-occlusive disease following stem cell transplantation: incidence, clinical course, and outcome. Biol Blood Marrow Transplant. 2010;16:157–68.
https://doi.org/10.1016/j.bbmt.2009.08.024. Article PubMed Google Scholar * Barker CC, Butzner JD, Anderson RA, Brant R, Sauve RS. Incidence, survival and risk factors for the development
of veno-occlusive disease in pediatric hematopoietic stem cell transplant recipients. Bone Marrow Transplant. 2003;32:79–87. Article CAS PubMed Google Scholar * Cesaro S, Pillon M,
Talenti E, Toffolutti T, Calore E, Tridello G, et al. A prospective survey on incidence, risk factors and therapy of hepatic veno-occlusive disease in children after hematopoietic stem cell
transplantation. Haematologica. 2005;90:1396–404. PubMed Google Scholar * Strouse C, Zhang Y, Zhang MJ, DiGilio A, Pasquini M, Horowitz MM, et al. Risk score for the development of
veno-occlusive disease after allogeneic hematopoietic cell transplant. Biol Blood Marrow Transplant. 2018;24:2072–80. https://doi.org/10.1016/j.bbmt.2018.06.013. Article PubMed PubMed
Central Google Scholar * Yoon JH, Min GJ, Park SS, Park S, Lee SE, Cho BS, et al. Incidence and risk factors of hepatic veno-occlusive disease/sinusoidal obstruction syndrome after
allogeneic hematopoietic cell transplantation in adults with prophylactic ursodiol and intravenous heparin or prostaglandin E1. Bone Marrow Transplant. 2021;56:1603–13.
https://doi.org/10.1038/s41409-021-01215-y. Article CAS PubMed Google Scholar * Xia Y, Qin H, Yang J. Hepatic veno-occlusive disease development in the hematopoietic stem cell
transplantation patients: incidence and associated risk factors, a meta-analysis. Eur J Gastroenterol Hepatol. 2021;33:872–84. https://doi.org/10.1097/meg.0000000000001802. Article CAS
PubMed Google Scholar * Shadur B, Zaidman I, NaserEddin A, Lokshin E, Hussein F, Oron HC, et al. Successful hematopoietic stem cell transplantation for osteopetrosis using reduced
intensity conditioning. Pediatr Blood Cancer. 2018;65:e27010. https://doi.org/10.1002/pbc.27010. Article CAS PubMed Google Scholar * Carreras E, Diaz-Beya M, Rosinol L, Martinez C,
Fernandez-Aviles F, Rovira M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last
decade. Biol Blood Marrow Transplant. 2011;17:1713–20. https://doi.org/10.1016/j.bbmt.2011.06.006. Article PubMed Google Scholar * Szmit Z, Gorczynska E, Król A, Ussowicz M,
Mielcarek-Siedziuk M, Olejnik I, et al. Introduction of new pediatric EBMT criteria for VOD diagnosis: is it time-saving or money-wasting?: prospective evaluation of pediatric EBMT criteria
for VOD. Bone Marrow Transplant. 2020. https://doi.org/10.1038/s41409-020-0918-1. Download references FUNDING Open Access funding enabled and organized by Projekt DEAL. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * University of Regensburg, Regensburg, Germany Selim Corbacioglu * The Children’s Hospital of Philadelphia, Philadelphia, PA, USA Stephan A. Grupp * Jerome Lipper
Multiple Myeloma Center, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA Paul Gerard Richardson * Department of Hematology, Hospital Universitario Puerta de Hierro
Majadahonda, Madrid, Spain Rafael Duarte * King’s College Hospital and Anthony Nolan Research Institute, London, UK Antonio Pagliuca * Comprehensive Cancer Center, Helsinki University
Hospital, Helsinki, Finland Tapani Ruutu * Duke University Children’s Hospital, Durham, NC, USA Kris Mahadeo * Josep Carreras Foundation & Leukemia Research Institute, (Hospital
Clínic/Barcelona University Campus), Barcelona, Spain Enric Carreras Authors * Selim Corbacioglu View author publications You can also search for this author inPubMed Google Scholar *
Stephan A. Grupp View author publications You can also search for this author inPubMed Google Scholar * Paul Gerard Richardson View author publications You can also search for this author
inPubMed Google Scholar * Rafael Duarte View author publications You can also search for this author inPubMed Google Scholar * Antonio Pagliuca View author publications You can also search
for this author inPubMed Google Scholar * Tapani Ruutu View author publications You can also search for this author inPubMed Google Scholar * Kris Mahadeo View author publications You can
also search for this author inPubMed Google Scholar * Enric Carreras View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS SC designed and
drafted the manuscript. SAG, PGR, RD, AP, TR, KM and EC contributed to the writing of the manuscript. CORRESPONDING AUTHOR Correspondence to Selim Corbacioglu. ETHICS DECLARATIONS COMPETING
INTERESTS All authors have received honoraria and/or research support from JAZZ Pharmaceuticals whose product is discussed in this manuscript. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a
Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit
to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are
included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and
your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this
license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Corbacioglu, S., Grupp, S.A., Richardson, P.G. _et al._ Prevention
of veno-occlusive disease/sinusoidal obstruction syndrome: a never-ending story and no easy answer. _Bone Marrow Transplant_ 58, 839–841 (2023). https://doi.org/10.1038/s41409-023-02007-2
Download citation * Received: 29 March 2023 * Revised: 12 April 2023 * Accepted: 04 May 2023 * Published: 25 May 2023 * Issue Date: August 2023 * DOI:
https://doi.org/10.1038/s41409-023-02007-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative









