- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT In the phase 3 POLLUX study, daratumumab plus lenalidomide and dexamethasone (DRd) significantly reduced the risk of progression/death and induced deeper responses vs. lenalidomide
and dexamethasone alone (Rd) in patients with relapsed/refractory multiple myeloma (RRMM). We report a subgroup analysis of East Asian (Japanese, Korean, and Taiwanese) patients from POLLUX
based on a longer follow-up of 24.7 months. Median progression-free survival was not reached (NR) for DRd vs. 13.8 months for Rd (hazard ratio [HR], 0.42; 95% confidence interval [CI],
0.23–0.76), and overall response rates were higher for DRd vs. Rd (90.2 vs. 72.1%). DRd extended the median duration of response vs. Rd (NR vs. 20.2 months), and minimal residual
disease–negative rates at the 10–5 sensitivity threshold were 21.2 vs. 9.1% for DRd vs. Rd. No new safety signals were observed. Similar efficacy and safety were observed in the smaller
subgroup of Japanese patients treated with DRd vs. Rd. These results demonstrate favorable efficacy and safety of DRd vs. Rd in East Asian patients and also in the Japanese-only patient
subgroup that are consistent with findings in the overall patient population of POLLUX. SIMILAR CONTENT BEING VIEWED BY OTHERS DARATUMUMAB PLUS LENALIDOMIDE/DEXAMETHASONE IN UNTREATED
MULTIPLE MYELOMA: ANALYSIS OF KEY SUBGROUPS OF THE MAIA STUDY Article 15 January 2025 DARATUMUMAB/LENALIDOMIDE/DEXAMETHASONE IN TRANSPLANT-INELIGIBLE NEWLY DIAGNOSED MYELOMA: MAIA LONG-TERM
OUTCOMES Article Open access 27 February 2025 SECOND- AND THIRD-LINE TREATMENT STRATEGIES IN MULTIPLE MYELOMA: A REFERRAL-CENTER EXPERIENCE Article Open access 06 December 2022 INTRODUCTION
Daratumumab, a human IgG1κ monoclonal antibody that targets the cell surface protein CD38, demonstrates on-tumor and immunomodulatory mechanisms of action in multiple myeloma
(MM)1,2,3,4,5,6. Daratumumab exerts its antimyeloma activity via multiple mechanisms, including direct apoptosis induction, complement-dependent cytotoxicity, antibody-dependent
cell-mediated cytotoxicity, antibody-dependent cellular phagocytosis, and modulation of the enzymatic activities of CD381,2,3,4,6. Daratumumab also binds CD38 on immunosuppressive regulatory
cells, triggering the expansion and activation of cytotoxic T-cells and elevation in T-cell clonality, which may provide additional antimyeloma effects5. Based on the results of
single-agent and combination therapy studies, daratumumab was approved as a monotherapy and in combination with standard of care regimens across many countries in patients with relapsed
and/or refractory multiple myeloma (RRMM)7,8,9,10,11,12. Recently, daratumumab in combination with lenalidomide and dexamethasone (Rd) or bortezomib and dexamethasone was approved for
treatment of adults with RRMM in Japan13. The POLLUX study compared the efficacy and safety of daratumumab in combination with Rd (DRd) vs. Rd alone in patients with RRMM who received at
least one prior line of therapy9. The addition of daratumumab to Rd significantly prolonged progression-free survival (PFS; median, not reached (NR) vs. 18.4 months; hazard ratio [HR], 0.37;
95% confidence interval [CI], 0.27–0.52; _P_ < 0.001) and increased the overall response rate (ORR; 92.9 vs. 76.4%; _P_ < 0.001). A novel aspect of the POLLUX study was a prospective
analysis of minimal residual disease (MRD) in RRMM patients. At a sensitivity threshold of one tumor cell per 100,000 white cells (10–5), 22.4% of patients treated with DRd were below this
threshold compared with 4.6% in the control group, highlighting the very deep responses induced by daratumumab-based treatment9. The adverse event profile was clinically manageable and was
consistent with those of daratumumab and Rd alone9. The efficacy and safety profiles of targeted anticancer therapies in East Asian patients may differ from those of the overall study
populations in clinical trials14,15. To understand the impact of DRd vs. Rd in the East Asian patient population, we performed subanalyses of POLLUX data to evaluate the efficacy and safety
of DRd vs. Rd in East Asian (Japanese, Korean, and Taiwanese) patients as well as in only Japanese patients. PATIENTS AND METHODS PATIENTS A total of 96 East Asian patients from the phase 3
POLLUX clinical trial (ClinicalTrials.gov identifier: NCT02076009) were included in this analysis. A separate subanalysis of the 36 Japanese patients alone was also conducted. Study design,
patient eligibility, treatment schedule, and statistical analyses were previously published9. In brief, eligible patients had documented MM, measurable disease at screening, and progressive
disease during or after receiving their last regimen, and had received and responded to one or more previous lines of therapy. Measurable disease was defined according to serum or urine
M-protein levels or serum-free light chain levels and abnormal serum immunoglobulin-free light chain ratios (kappa:lambda light chains). Progressive disease was defined according to
International Myeloma Working Group (IMWG) criteria16. Patients with lenalidomide-refractory disease or who had discontinued previous lenalidomide treatment due to adverse events were
excluded from the study. DOSING Patients were randomized (1:1) to receive 28-day cycles of DRd or Rd alone until disease progression, unacceptable toxicity, withdrawal of consent, or death.
Daratumumab was administered at 16 mg/kg intravenously once weekly during Cycles 1 and 2, every 2 weeks during Cycles 3–6, and every 4 weeks thereafter. Patients with creatinine clearance
>60 ml/min received 25 mg lenalidomide orally on Days 1–21 of each cycle; patients with creatinine clearance of 30–60 ml/min received 10 mg lenalidomide daily. Dexamethasone was
administered at 40 mg weekly. Patients in the DRd group received a split dose of dexamethasone on weeks when daratumumab was administered: 20 mg of dexamethasone before the daratumumab
infusion and 20 mg the day after the daratumumab infusion. Patients aged >75 years or with a body mass index <18.5 kg/m2 received a reduced dose of dexamethasone (20 mg) weekly at the
physician’s discretion. EVALUATION AND STATISTICAL ANALYSES Responses were evaluated based on IMWG criteria16,17. The Kaplan–Meier method was used to evaluate PFS, overall survival (OS), and
duration of response. A Cox regression model was used to estimate 95% CIs. MRD status was assessed by next-generation sequencing (NGS) using bone marrow obtained from patients who had a
suspected complete response (CR) and was measured at three sensitivity thresholds: 10–4, 10–5, and 10–6, corresponding to one tumor cell per 104, 105, and 106 white blood cells,
respectively. The MRD-negative rate was defined as the proportion of subjects who achieved MRD negativity at any time after their first dose of daratumumab. In our MRD analysis, patients in
the intent-to-treat population who did not undergo MRD assessment were considered to be MRD-positive. Cytogenetic risk status was assessed by fluorescence in situ hybridization or karyotype
testing. Patients were considered high risk if they had at least one of the following cytogenetic abnormalities: del(17p), t(4;14), or t(14;16); patients lacking all three of these
abnormalities were considered standard risk. RESULTS PATIENTS AND TREATMENT Patients in POLLUX were randomized between June 2014 and July 2015, and the clinical cutoff date for this analysis
was 7 March 2017. East Asian patients comprised 16.9% (96/569) of the overall population of patients in POLLUX, with Japanese patients constituting 6.3% (36/569) of the POLLUX study
population. Fifty-two East Asian (21 Japanese) patients were randomized to the DRd group, and 44 East Asian (15 Japanese) patients were randomized to the Rd group (Table 1). The median
(range) age was 64 (34–85) years for East Asian patients and 68 (45–81) years for Japanese patients. The median (range) time since diagnosis was 3.3 (0.8–27.0) years for East Asian patients
and 3.0 (0.9–27.0) years for Japanese patients. Among East Asian patients, 22 (43.1%) patients in the DRd group and 32 (72.7%) patients in the Rd group discontinued treatment. The most
common reasons for discontinuation for DRd vs. Rd were progressive disease (27.5 vs. 63.6%) and adverse events (9.8 vs. 2.3%). Among Japanese patients, 7 (35.0%) patients in the DRd group
and 11 (73.3%) patients in the Rd group discontinued treatment. The most common reasons for discontinuation for DRd vs. Rd were progressive disease (15.0 vs. 66.7%) and adverse events (10.0
vs. 0.0%). The median (range) number of prior lines of therapy was 2 (1–6) for East Asian patients and 1 (1–6) for Japanese patients; 7.3% of East Asian patients and 11.1% of Japanese
patients had received >3 prior lines of therapy. Prior therapies included proteasome inhibitors (PIs; in 79.2% of East Asian patients and 83.3% of Japanese patients) and immunomodulatory
drugs (in 59.4% of East Asian patients and 44.4% of Japanese patients), including lenalidomide (in 5.2% of East Asian patients and 13.9% of Japanese patients). A total of 62.5% of East Asian
patients and 50.0% of Japanese patients had undergone autologous stem cell transplantation, and 44.8% of East Asian patients and 44.4% of Japanese patients were refractory to their last
line of therapy. The median (range) number of treatment cycles received in East Asian patients was 23 (2–33) for DRd and 14.5 (1–32) for Rd. Among Japanese patients, the median (range)
number of treatment cycles received was 23 (3–27) for DRd and 11 (3–25) for Rd. The median (range) cumulative lenalidomide dose received for East Asian patients was 5,190.0 (150–15,750) mg
for DRd and 5,170.0 (70–15,750) mg for Rd; the median (range) cumulative lenalidomide dose received for Japanese patients was 4,927.5 (150–11,615) mg and 4,670.0 (340–12,950) mg,
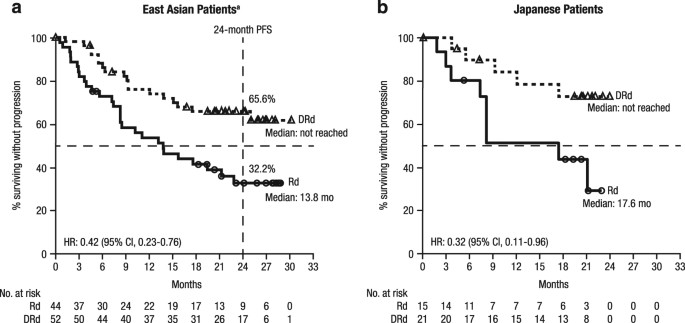
respectively. EFFICACY The median (range) duration of follow-up was 24.7 (0.7–30.5) months in East Asian patients and 21.4 (4.4–24.1) months in Japanese patients. The median PFS for DRd vs.
Rd was NR vs. 13.8 months in East Asian patients (HR, 0.42; 95% CI, 0.23–0.76; Fig. 1a) and NR vs. 17.6 months in Japanese patients (HR, 0.32; 95% CI, 0.11–0.96; Fig. 1b). The 24-month PFS
rate for DRd vs. Rd was 65.6% (95% CI, 50.5–77.0) vs. 32.2% (95% CI, 18.3–46.9) in East Asian patients; in Japanese patients, the 24-month PFS rate was not estimable (NE) in either treatment
group. In patients with a treatment-free interval of >12 months between receipt of last therapy and randomization, the median PFS for DRd vs. Rd was NR vs. 22.8 months (HR, 0.31; 95% CI,
0.09–1.03) in East Asian patients (Fig. 2a). Among these patients, the 24-month PFS rate for DRd vs. Rd was 79.7% (95% CI, 54.5–91.9) vs. 44.9% (95% CI, 18.8–68.1). In East Asian patients
with a treatment-free interval of ≤12 months, the median PFS for DRd vs. Rd was 25.0 months vs. 8.5 months (HR, 0.47; 95% CI, 0.24–0.94; Fig. 2b); the 24-month PFS rate for DRd vs. Rd was
55.8% (95% CI, 36.2–71.5) vs. 24.7% (95% CI, 10.1–42.6). The ORR for DRd vs. Rd was 90.2 vs. 72.1% in East Asian patients and 90.0 vs. 60.0% in Japanese patients (Table 2). Responses to DRd
vs. Rd in East Asian patients included 17 (33.3%) vs. 5 (11.6%) stringent complete responses (sCRs), 10 (19.6%) vs. 4 (9.3%) CRs, 11 (21.6%) vs. 8 (18.6%) very good partial responses
(VGPRs), and 8 (15.7%) vs. 14 (32.6%) partial responses (PRs). Consistent responses were observed across various subgroups, including those defined by International Staging Sysem staging,
cytogenetic risk, number of prior lines of therapy, prior PI exposure, and refractoriness to PIs. Responses to DRd vs. Rd in Japanese patients included 9 (45.0%) vs. 1 (6.7%) sCR(s), 1
(5.0%) vs. 0 (0.0%) CRs, 5 (25.0%) vs. 4 (26.7%) VGPRs, and 3 (15.0%) vs. 4 (26.7%) PRs. The median duration of response for DRd vs. Rd was NE (95% CI, 24.0-NE) vs. 20.2 (95% CI, 12.9-NE)
months in East Asian patients and NE (95% CI, NE-NE) vs. 20.2 (95% CI, 2.1-NE) months in Japanese patients. Among responders in the response-evaluable analysis set, the median (range) time
to first response for DRd vs. Rd was 1.0 (0.9–13.0) month vs. 1.1 (0.9–8.4) months in East Asian patients and 1.0 (1.0–2.0) months vs. 1.1 (1.0–2.9) months in Japanese patients. Among East
Asian patients, MRD-negative rates were higher for DRd vs. Rd (32.7 vs. 13.6% at 10–4, 21.2 vs. 9.1% at 10–5, and 11.5 vs. 4.5% at 10–6; Fig. 3a). Among Japanese patients, MRD-negative rates
were also higher for DRd vs. Rd (33.3 vs. 6.7% at 10–4, 23.8 vs. 6.7% at 10–5, and 14.3 vs. 6.7% at 10–6; Fig. 3b). SAFETY The most common treatment-emergent adverse events (TEAEs; >20%
of patients in any group) are listed in Table 3. Consistent with the overall patient population9, higher rates of neutropenia, diarrhea, nasopharyngitis, and pyrexia were noted in East Asian
patients in the DRd group (35 [68.6%] patients, 21 [41.2%] patients, 20 [39.2%] patients, and 13 [25.5%] patients, respectively) compared with those in the Rd group (21 [47.7%] patients, 8
[18.2%] patients, 12 [27.3%] patients, and 3 [6.8%] patients, respectively). Similar findings were observed in Japanese patients treated with DRd vs. Rd (Table 3). The most common grade 3 or
4 TEAEs (>5% of patients in any group) are summarized in Table 4. As expected, the rate of grade 3 or 4 neutropenia was higher in the DRd group compared with the Rd group (34 [66.7%] vs.
19 [43.2%] East Asian patients; 12 [60.0%] vs. 5 [33.3%] Japanese patients). The rate of grade 3 or 4 thrombocytopenia was lower for DRd vs. Rd in both East Asian patients (7 [13.7%]
patients vs. 10 [22.7%] patients) and Japanese patients (1 [5.0%] patient vs. 2 [13.3%] patients); these rates were comparable to those in the overall study population (36 [12.7%] patients
vs. 38 [13.5%] patients)9. The rate of grade 3 or 4 infections for the DRd group vs. the Rd group was 14 (27.5%) vs. 12 (27.3%) East Asian patients and 6 (30.0%) vs. 2 (13.3%) Japanese
patients. The most common grade 3 or 4 infection was pneumonia (for DRd vs. Rd, 7 [13.7%] vs. 4 [9.1%] East Asian patients and 2 [10.0%] vs. 2 [13.3%] Japanese patients). Among East Asian
patients, serious TEAEs were observed in 26 (51.0%) patients in the DRd group vs. 19 (43.2%) patients in the Rd group; among Japanese patients, they were observed in 10 (50.0%) vs. 4
(26.7%), respectively. The most common serious TEAE in the DRd group was pneumonia, which occurred in 7 (13.7%) East Asian patients treated with DRd and 5 (11.4%) treated with Rd, and in 2
(10.0%) Japanese patients treated with DRd and 1 (6.7%) treated with Rd. TEAEs led to discontinuation of study treatment in 8 (15.7%) East Asian patients and 3 (15.0%) Japanese patients in
the DRd group, and in 2 (4.5%) East Asian patients in the Rd group. No Japanese patients in the Rd group discontinued study treatment due to TEAEs. Among patients in the DRd group who
discontinued study treatment due to TEAEs, four East Asian patients (including one Japanese patient) discontinued due to TEAEs possibly or probably related to daratumumab; these TEAEs were
grade 3 Epstein-Barr virus-associated lymphoproliferative disorder, grade 3 diarrhea, grade 3 pneumonia, and grade 5 multiple organ dysfunction syndrome. Pneumonia was the most common TEAE
leading to discontinuation of study treatment and was observed in 3 (5.9%) East Asian patients and 1 (5.0%) Japanese patient in the DRd group and in 1 (2.3%) East Asian patient and none of
the Japanese patients in the Rd group. In East Asian patients, the median (range) duration of infusion was 7.1 (6.0–14.5) h for the first infusion, 4.4 (3.0–9.4) h for the second infusion,
and 3.5 (2.2–6.2) h for all subsequent infusions. In Japanese patients, the median (range) duration of infusion was 7.1 (6.1–14.0) h for the first infusion, 4.4 (4.0–7.5) h for the second
infusion, and 3.5 (2.5–4.7) h for all subsequent infusions. Infusion-related reactions (IRRs) among daratumumab-treated patients occurred in 25 (49.0%) East Asian patients and 7 (35.0%)
Japanese patients. Most occurred during the first infusion and were grade 1 or 2 in severity. Grade 3 IRRs occurred in 6 (11.8%) East Asian patients and 1 (5.0%) Japanese patient, and no
grade 4 IRRs were observed. The most common IRR was dyspnea, which occurred in 5 (9.8%) East Asian patients and 2 (10.0%) Japanese patients. No patients discontinued treatment due to IRRs.
In the overall POLLUX population, the rates of second primary malignancies were low and were balanced between the two treatment groups (6% in both treatment groups)18. Among patients in the
East Asian and Japanese subgroups, second primary malignancies were reported in 3 patients in the DRd group: 1 Korean patient (right flank skin site metastatic adenocarcinoma) and 2 Japanese
patients (worsening of Bowen’s disease in 1 patient and EBV-positive lymphoproliferative disorder in another). No second primary malignancies were reported in patients in the Rd group.
Among East Asian patients, 10 (19.6%) patients in the DRd group received a total of 49 blood transfusions (8 [15.7%] patients received a total of 26 packed red blood cell transfusions and 5
[9.8%] patients received a total of 23 platelet transfusions), and 12 (27.3%) patients in the Rd group received a total of 57 blood transfusions (12 [27.3%] patients received a total of 48
packed red blood cell transfusions, 1 [2.3%] patient received 2 fresh frozen plasma transfusions, and 5 [11.4%] patients received a total of 7 platelet transfusions). Among Japanese
patients, 2 (10.0%) patients in the DRd group received a total of 10 blood transfusions (all were packed red blood cell transfusions), and 2 (13.3%) patients in the Rd group received a total
of 8 blood transfusions (2 [13.3%] patients received a total of 6 packed red blood cell transfusions and 1 [6.7%] patient received 2 platelet transfusions). DISCUSSION Consistent with the
primary results of the POLLUX study, the addition of daratumumab to Rd significantly reduced the risk of progression/death and increased the rate of deeper responses while demonstrating a
favorable safety profile in East Asian patients based on longer follow-up9. The hazard ratio for disease progression or death in the daratumumab group vs. control group in these patients was
comparable to that of the international population in POLLUX9. In the East Asian subgroup described here, median PFS was NR in the DRd group. These data compare favorably with a subanalysis
of Japanese patients in the phase 3 ELOQUENT-2 study of elotuzumab plus Rd (ERd) in patients with RRMM19. In the subanalysis of the ELOQUENT-2 study, median PFS was 22.2 months in the ERd
arm compared with 18.5 months in the Rd arm19. At a milestone of 24 months, the PFS rate of 66% for DRd in East Asian patients in POLLUX compares favorably with the rate of 48% at the same
time point in Japanese patients treated with ERd in ELOQUENT-2, although a direct comparison between these two studies should be interpreted with caution due to differences in study design
and eligibility criteria19. While the ≥CR rates for DRd among East Asian and Japanese patients were similar to that of the overall POLLUX population, the sCR rates were numerically higher
(33.3 and 45.0% in East Asian and Japanese patients, respectively, compared with 26% in the overall POLLUX population)18. For the first time in a study of East Asian and Japanese patients
with RRMM, a prospective analysis of MRD was performed. At the sensitivity threshold of 10–5 for MRD recommended by the IMWG for NGS-based assays20, MRD-negative rates in both subgroups were
similar to the rates observed for the DRd and Rd treatment groups in the overall POLLUX population9. No new safety signals for the combination of daratumumab and Rd were observed9.
Neutropenia, a known lenalidomide-associated toxicity, was also the most frequently observed adverse event associated with DRd in these subgroups of patients, consistent with the findings in
the overall POLLUX population9. Although patients in the DRd group received more treatment cycles compared with those in the Rd group in both subpopulations (East Asian: median of 23.0 vs.
14.5; Japanese: median of 23.0 vs. 11.0), the cumulative doses of lenalidomide received were similar between the treatment groups. Whether the increased neutropenia rate in the DRd treatment
group is due to the longer exposure to lenalidomide or due to the introduction of daratumumab remains unclear, and analyses are ongoing based on longer follow-up. These findings further
support the results of other studies examining the efficacy and safety profile of daratumumab in Japanese patients, including the phase 1 MMY1002 study of daratumumab monotherapy in Japanese
patients with RRMM21 and the phase 1 MMY1005 study of daratumumab plus bortezomib and dexamethasone in Japanese patients with RRMM22. Taken together, these studies confirm that Japanese
patients derive a similar magnitude of clinical benefit with daratumumab-based regimens compared with patient populations across many other regions and that no new safety signals related to
daratumumab can be identified in Japanese or East Asian patients19. There are several limitations to the current study. First, the number of patients in each subgroup, especially in the
Japanese subgroup, was low. Second, this was a post hoc rather than a prespecified analysis, so no statistical testing was performed. Third, the impact of this regimen on OS has not yet been
determined, as these data were immature at the time of this analysis; follow-up is ongoing. In summary, the addition of daratumumab to Rd led to prolonged PFS with increased and deeper
responses compared with Rd alone in both East Asian patients and Japanese patients from POLLUX, consistent with findings in the international POLLUX population. Given the recent approval of
daratumumab in combination with lenalidomide and dexamethasone as a new treatment option in Japan13, these findings suggest that this regimen is a new standard of care for Japanese patients
with RRMM. REFERENCES * de Weers, M. et al. Daratumumab, a novel therapeutic human CD38 monoclonal antibody, induces killing of multiple myeloma and other hematological tumors. _J. Immunol._
186, 1840–1848 (2011). Article PubMed Google Scholar * Lammerts van Bueren, J., et al. Direct in vitro comparison of daratumumab with surrogate analogs of CD38 antibodies MOR03087,
SAR650984 and Ab79. _Blood_ 124, Abstract 3474 (2014). * Overdijk, M. B. et al. Antibody-mediated phagocytosis contributes to the anti-tumor activity of the therapeutic antibody daratumumab
in lymphoma and multiple myeloma. _mAbs_ 7, 311–321 (2015). Article CAS PubMed PubMed Central Google Scholar * van de Donk, N. W. C. J. et al. Monoclonal antibodies targeting CD38 in
hematological malignancies and beyond. _Immunol. Rev._ 270, 95–112 (2016). Article PubMed PubMed Central Google Scholar * Krejcik, J. et al. Daratumumab depletes CD38+ immune-regulatory
cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. _Blood_ 128, 384–394 (2016). Article CAS PubMed PubMed Central Google Scholar * Overdijk, M. B. et al.
The therapeutic CD38 monoclonal antibody daratumumab induces programmed cell death via Fcgamma receptor-mediated cross-linking. _J. Immunol._ 197, 807–813 (2016). Article CAS PubMed
Google Scholar * Lokhorst, H. M. et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. _N. Engl. J. Med._ 373, 1207–1219 (2015). Article CAS PubMed Google Scholar *
Lonial, S. et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. _Lancet_ 387, 1551–1560 (2016). Article
CAS PubMed Google Scholar * Dimopoulos, M. A. et al. Daratumumab, lenalidomide, and dexamethasone for multiple myeloma. _N. Engl. J. Med._ 375, 1319–1331 (2016). Article CAS PubMed
Google Scholar * Palumbo, A. et al. Daratumumab, bortezomib, and dexamethasone for multiple myeloma. _N. Engl. J. Med._ 375, 754–766 (2016). Article CAS PubMed Google Scholar * Chari,
A. et al. Daratumumab plus pomalidomide and dexamethasone in relapsed and/or refractory multiple myeloma. _Blood_ 130, 974–981 (2017). Article CAS PubMed PubMed Central Google Scholar *
_DARZALEX_® _(Daratumumab) Injection, for Intravenous Use [Package Insert]_. Janssen Biotech, Inc., Horsham, PA, 2017. * Genmab announces approval of DARZALEX® (daratumumab) for relapsed or
refractory multiple myeloma in Japan [news release]. Copenhagen, Denmark: Genmab A/S. September 27, 2017. http://ir.genmab.com/releasedetail.cfm?releaseid=1041758. Accessed September 29,
2017. * Ohtsu, A. et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. _J.
Clin. Oncol._ 29, 3968–3976 (2011). Article CAS PubMed Google Scholar * Kiyota, N. et al. Subgroup analysis of Japanese patients in a phase 3 study of lenvatinib in
radioiodine-refractory differentiated thyroid cancer. _Cancer Sci._ 106, 1714–1721 (2015). Article CAS PubMed PubMed Central Google Scholar * Rajkumar, S. V. et al. Consensus
recommendations for the uniform reporting of clinical trials: report of the International Myeloma Workshop Consensus Panel 1. _Blood_ 117, 4691–4695 (2011). Article CAS PubMed PubMed
Central Google Scholar * Durie, B. G. M. et al. International uniform response criteria for multiple myeloma. _Leukemia_ 20, 1467–1473 (2006). Article CAS PubMed Google Scholar *
Bahlis, N., et al. Daratumumab, lenalidomide, and dexamethasone (DRd) vs. lenalidomide and dexamethasone (Rd) in relapsed or refractory multiple myeloma (RRMM): efficacy and safety update
(POLLUX). _J. Clin. Oncol._ 35, Abstract 8025 (2017). * Suzuki, K. et al. Randomized phase 3 study of elotuzumab for relapsed or refractory multiple myeloma: ELOQUENT-2 Japanese patient
subanalysis. _Blood Cancer J._ 7, e540 (2017). Article CAS PubMed PubMed Central Google Scholar * Kumar, S. et al. International Myeloma Working Group consensus criteria for response
and minimal residual disease assessment in multiple myeloma. _Lancet Oncol._ 17, e328–e346 (2016). Article PubMed Google Scholar * Iida, S. et al. Safety and efficacy of daratumumab in
Japanese patients with relapsed or refractory multiple myeloma: a multicenter, phase 1, dose-escalation study. _Int. J. Hematol._ 106, 541–551 (2017). Article CAS PubMed Google Scholar *
Ichinohe, T., et al. Daratumumab with bortezomib+dexamethasone in Japanese pts with relapsed/refractory multiple myeloma. Presented at the 79th Annual Meeting of the Japanese Society of
Hematology (JSH); Abstract OS3-12D-3 (Tokyo, 20–22 October 2017). Download references ACKNOWLEDGEMENTS This study was sponsored by Janssen Global Services, LLC. Medical writing support was
provided by Kimberly Carmony, Ph.D., of MedErgy, and was funded by Janssen Global Services, LLC. AUTHORS’ CONTRIBUTIONS K.S., M.A.D., N.T., S.O., A.S., M.M., H.K., S.-S.Y., S.-Y.H., and S.I.
contributed to the accrual and treatment of patients. X.Q. and M.Q. collected and analyzed the data. All authors contributed to data interpretation. All authors drafted and reviewed the
manuscript, approved the final version, and decided to publish this report. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Hematology, Japanese Red Cross Medical Center, Tokyo,
Japan Kenshi Suzuki * National and Kapodistrian University of Athens, Athens, Greece Meletios A. Dimopoulos * Department of Hematology, National Hospital Organization Disaster Medical Center
of Japan, Tachikawa, Japan Naoki Takezako * Keio University Hospital, Tokyo, Japan Shinichiro Okamoto * Hitachi General Hospital, Hitachi, Japan Atsushi Shinagawa * Department of
Hematology, National Hospital Organization Shibukawa Medical Center, Shibukawa, Japan Morio Matsumoto * Department of Hematology, Ogaki Municipal Hospital, Ogaki, Japan Hiroshi Kosugi *
Seoul National University Hospital, Seoul, Korea Sung-Soo Yoon * National Taiwan University Hospital, Taipei, Taiwan Shang-Yi Huang * Janssen Research & Development, LLC, Spring House,
PA, USA Xiang Qin & Ming Qi * Nagoya City University Graduate School of Medical Sciences, Nagoya, Japan Shinsuke Iida Authors * Kenshi Suzuki View author publications You can also search
for this author inPubMed Google Scholar * Meletios A. Dimopoulos View author publications You can also search for this author inPubMed Google Scholar * Naoki Takezako View author
publications You can also search for this author inPubMed Google Scholar * Shinichiro Okamoto View author publications You can also search for this author inPubMed Google Scholar * Atsushi
Shinagawa View author publications You can also search for this author inPubMed Google Scholar * Morio Matsumoto View author publications You can also search for this author inPubMed Google
Scholar * Hiroshi Kosugi View author publications You can also search for this author inPubMed Google Scholar * Sung-Soo Yoon View author publications You can also search for this author
inPubMed Google Scholar * Shang-Yi Huang View author publications You can also search for this author inPubMed Google Scholar * Xiang Qin View author publications You can also search for
this author inPubMed Google Scholar * Ming Qi View author publications You can also search for this author inPubMed Google Scholar * Shinsuke Iida View author publications You can also
search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Shinsuke Iida. ETHICS DECLARATIONS CONFLICT OF INTEREST K.S. received honoraria from Celgene, Takeda,
and Janssen and consulted for Takeda. M.A.D. consulted for Amgen, Janssen, Takeda, and Celgene; received honoraria from Amgen, Novartis, Celgene, Takeda, Genesis Pharmaceuticals, Janssen,
and Bristol-Myers Squibb; received research funding from Janssen and Amgen; and received personal fees from Janssen, Genesis Pharmaceuticals, and Amgen. S.O. received honoraria from Celgene,
Takeda, Bristol-Myers Squibb, Ono, Sanofi, Novartis, and Janssen and received research funding from Novartis, Ono, Sanofi, and Takeda. A.S. received honoraria from Celgene, Takeda, Janssen,
Novartis, and Bristol-Myers Squibb. M.M. received honoraria from Celgene, Takeda, Bristol-Myers Squibb, Ono, and Janssen. S.-S.Y. received honoraria from Celgene, Takeda, and Janssen and
consulted for Celgene, Takeda, and Janssen. X.Q. and M.Q. are employees of Janssen. S.I. received honoraria from Janssen, Celgene, Ono, Takeda, Novartis, and Bristol-Myers Squibb and
received research funding from Celgene, Janssen, Ono, Takeda, Novartis, Bristol-Myers Squibb, Chugai, Sanofi, MSD, Kyowa Hakko Kirin, Astellas, Teijin Pharma, Toyama Chemical, Daiichi
Sankyo, and Bayer. N.T., H.K., and S.-Y.H. declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to
jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International
License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source,
provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons
license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Suzuki, K., Dimopoulos, M.A., Takezako, N. _et al._ Daratumumab, lenalidomide, and
dexamethasone in East Asian patients with relapsed or refractory multiple myeloma: subgroup analyses of the phase 3 POLLUX study. _Blood Cancer Journal_ 8, 41 (2018).
https://doi.org/10.1038/s41408-018-0071-x Download citation * Received: 18 January 2018 * Revised: 08 February 2018 * Accepted: 08 February 2018 * Published: 01 May 2018 * DOI:
https://doi.org/10.1038/s41408-018-0071-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative