- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
The 2016 revision of the World Health Organization classification of lymphoid neoplasms introduces the umbrella category “nodal T-cell lymphomas with T-follicular helper (TFH) phenotype”,
which includes angioimmunoblastic T-cell lymphoma (AITL), follicular T-cell lymphoma and nodal peripheral T-cell lymphoma (PTCL) with a TFH phenotype1. One of the genetic features clustering
TFH cell-derived lymphomas is a recurrent _RHOA G17V_ mutation, which is present in approximately 60% of investigated cases2,3. _RHOA_ is a member of the Rho family of GTPases which
function as molecular regulators of diverse cellular functions4. Mutant RHOA acts as a dominant-negative signaling protein sequestering guanine nucleotide exchange factors (GEFs) thereby
inhibiting wildtype RHOA and potentially other GEF-dependent proteins3. In vivo, mutant _RHOA_ has recently been shown to skew CD4+ T-cell differentiation towards the TFH lineage and promote
AITL lymphomagenesis5. Thus, the _RHOA_ _G17V_ mutation can be viewed as pivotal genetic aberration in AITL and potentially other TFH cell-derived lymphomas. Mutations contributing to
lymphomagenesis in wildtype _RHOA_ AITL cases remain largely unknown. This mutational heterogeneity points towards the existence of distinct AITL lymphomagenic pathways. In this report, we
explore the mutational landscape of AITL by assessing the data from large sequencing studies focusing on the association between _RHOA_ mutational status and recurrent mutations in other
genes to provide evidence for the existence of distinct lymphomagenic pathways in AITL. Sequencing studies of AITL and/or PTCL published between 01-01-2014 and 28-02-2017 using an English
language restriction were identified with PubMed. In total, 117 abstracts were screened. Only 34 articles were eligible for full text review. Studies were included in our analysis if they
contained ten or more AITL cases and used targeted deep sequencing of _RHOA_, _TET2_, _DNMT3A_, _IDH2_, _CD28_ and multiple other genes or whole genome/exome/transcriptome approaches. Also,
the original dataset had to be available to the authors. Five of the 34 articles met the prespecified inclusion criteria and were included in our analysis6,7,8,9,10. The article selection
process was performed by two authors. In total, these studies analyzed 239 AITL cases using various sequencing techniques. Of interest, in 13.8% (33/239) of investigated AITL cases no
detectable mutations were reported. _RHOA_ was mutated in 61.1% (146/239) of the investigated AITL cases. The remaining 25.1% (60/239) of cases were wildtype, but carried mutations in other
genes (Table 1a). We focused on the data extract of all wildtype _RHOA_ AITL cases to identify potentially recurrent mutations contributing to AITL lymphomagenesis other than _RHOA_. Only
mutations occurring in more than 5% of targeted cases and identified in two or more studies were classified as recurrent. _TET2_, _CD28_, _DNTM3A_, _PLCG1_, _IDH2_, _VAV1_, _FYN_ and _STAT3_
were mutated in 60.7% (34/56), 18.6% (8/43), 17.9% (10/56), 14.0% (6/43), 13.8% (8/58), 11.6% (5/43), 7.8% (4/51) and 7.0% (3/43) of targeted wildtype _RHOA_ AITL cases, respectively
(Supplementary Data set 1). As these mutations also frequently occur in mutant _RHOA_ AITL cases, we performed Mantel–Haenszel statistics to assess the association between these mutations
and _RHOA_ mutational status across different studies (SPSS v21 IBM Corp., Armonk, NY, USA). A _p_-value ≤0.05 was considered significant. Statistical analysis showed that mutations in
_TET2_ and _IDH2_ were associated with mutant _RHOA_ status (_p_ < 0.001 for both genes). Mutations in _DNMT3A_ and _CD28_, including _CTLA4_–_CD28_ fusion, also tend to show this
association (_p_ = 0.076 and 0.093). Interestingly, despite being mutated in a low number of cases, mutations in _VAV1_ tend to associate with wildtype _RHOA_ status (_p_ = 0.268). Mutations
in _FYN_, _PLCG1_ and _STAT3_ showed no significant association with _RHOA_ mutational status (_p_ = 0.972, 0.960 and 0.979) (Table 1b). This study reports on the association between _RHOA_
mutational status and other recurrent mutations in AITL. We found an association between mutant _RHOA_ and mutations in _TET2_ and _IDH2_. Despite being mutually exclusive in acute myeloid
leukemia, mutations in _IDH2_ and _TET2_ tend to co-occur in _AITL_11. Gene expression profiling and promoter methylation analysis of double mutant AITL cases showed upregulation of genes
associated with TFH phenotype and downregulation of genes associated with TH1 phenotype11. Mutant _IDH2_ and _TET2_ potentially cooperate with mutant _RHOA_ to induce a potent TFH phenotype
in vivo. This mechanism would explain the association found between these mutations in the present study. We also identified a strong tendency towards association between mutant _RHOA_ and
mutations in _DNMT3A_. The exact mechanism by which mutations in epigenetic modifiers contribute to lymphomagenesis remain to be elucidated, but alterations in hematopoietic stem cell
differentiation is an attractive theory. The present study also identified a strong tendency towards association between mutant _RHOA_ and mutations in _CD28_, including _CTLA4_–_CD28_ gene
fusion. _CD28_ mutations in AITL are confined to hotspot residues D124 and T195 and render CD28 constitutively active10,12. The _CTLA4_–_CD28_ fusion gene has only been reported in an Asian
cohort10. Therefore, validation of this fusion gene in other cohorts is essential to confirm the association between mutant _RHOA_ and mutations in _CD28_. Altogether, these findings point
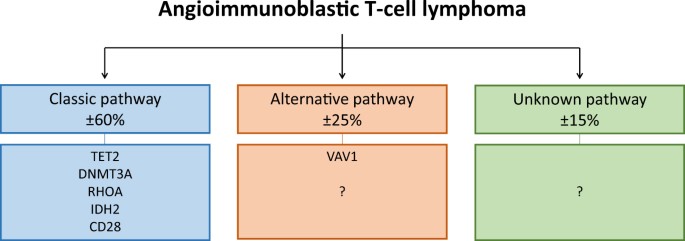
towards a classic AITL lymphomagenic pathway (Fig. 1). Several therapeutic approaches targeting epigenetic modifiers, IDH2 or CD28 are currently in clinical trials or have already been
approved for other diseases13,14. The tendency of these mutations to cluster will potentially help to develop novel combinatorial therapeutic regimens. Despite being mutated in rather a low
number of AITL cases, this study identified the tendency of mutations in _VAV1_ to associate with wildtype _RHOA_. _VAV1_ encodes a Rho GTPase family-specific GEF which is primarily
expressed in the hematopoietic system15. The studies that targeted _VAV1_ identified three missense mutations (_E524D_, _E556D_ and _D797G_), two frameshift deletions (_151_158del_ and
_778_783del_), one fusion gene (_VAV1_–_S100A7_) and one in-frame deletion (_778_786del_)6,9,10. Abate et al. found the _778_786_ in-frame deletion and _VAV1_–_S100A7_ fusion gene to be
locked in a constitutively active conformation, indicated by high levels of Tyr174 phosphorylation6. Both genetic aberrations resulted in increased VAV1 catalytic-dependent functions
downstream of RAC1, another member of the Rho family of GTPases6. These findings are in accordance with previous experiments showing that constitutively active VAV1 predominantly increases
nucleotide exchange of RAC1 and to a lesser extend of RHOA16. Interestingly, the RAC1 pathway is upregulated in mutant _RHOA_ compared to wildtype _RHOA_ AITL cases, providing evidence that
both mutations have similar effects on VAV1 catalytic-dependent pathways17. Additionally, Abate et al. found that the _VAV1_–_S100A7_ fusion gene resulted in increased NFAT activity, a
functional readout of VAV1 non-catalytic activity, whereas both the _778_786_ in-frame deletion and _VAV1_–_S100A7_ fusion gene increased expression of NFAT target genes6. A recently
published study, not yet indexed by PubMed at the time of our search, identified activating _VAV1_ mutations in 8.2% (7/85) of wildtype _RHOA_ AITL cases, compared to 0% (0/41) in mutant
_RHOA_ AITL cases, respectively18. They also showed that mutant RHOA enhances the non-catalytic functions of VAV1 through increased Tyr174 phosphorylation, thereby increasing NFAT activity
and expression of NFAT target genes. Together, these data not only strengthen the association between mutant _VAV1_ and wildtype _RHOA_, but also provide evidence that mutant _RHOA_ and
mutant _VAV1_ have similar effects on catalytic and non-catalytic signaling pathways downstream of VAV1. Therefore, we deduce from these data that mutant _RHOA_ and mutant _VAV1_ contribute
to AITL lymphomagenesis in a similar manner. This would mean that _VAV1_ is part of an alternative AITL lymphomagenic pathway (Fig. 1). Previous clinicopathological studies have shown that
mutant _RHOA_ AITL cases have worse performance status, more frequent B-symptoms and splenomegaly and a more potent TFH immunophenotype compared to wildtype _RHOA_ AITL cases19,20. These
data provide additional justification for separating AITL subgroups. According to our analysis, no mutations were detectable in approximately 15% (range 3–25%) of AITL cases (Fig. 1).
Exploring the mutational landscape of AITL using targeted deep sequencing panels enriched with members of the Rho family of GTPases and their regulatory proteins might identify driver
mutations in this subgroup. It is also possible that other lymphomagenic mechanisms contribute to some AITL cases, for example mutations in signaling pathways directly regulating TFH
differentiation. We are aware that there are some limitations to our study. Our findings are entirely based on retrospective data from a relatively small sample size. Furthermore, there is
significant technical heterogeneity between the sequencing studies from which the data is derived. The individual studies use different sequencing techniques, bioinformatics pipelines for
data processing and mutation calling methods. Despite these limitations, this study remains noteworthy as it provides a unique perspective on associations and possible collaborations between
the most common genetic aberrations in AITL as well as providing a rationale for future research. In short, using data from large sequencing studies this study reports on varying
associations between _RHOA_ mutational status and other recurrent mutations in AITL. These findings enable us to identify three potentially distinct AITL lymphomagenic pathways. First, the
classic pathway with the _RHOA_ _G17V_ mutation which is associated with mutations in _TET2_, _DNMT3A_, _IDH2_ and _CD28_. Secondly, the alternative pathway with mutations in _VAV1_ or
potentially yet unidentified mutations in members of the Rho family of GTPases or their regulatory proteins. Third, AITL cases with unknown mutations which might arise from direct mutations
in pathways regulating TFH differentiation. To what extend these different lymhpomagenic pathways result in different clinical behavior of AITL is largely unknown. Additional evidence on the
mutational landscape of AITL, especially wildtype _RHOA_ AITL cases, is needed to either confirm or refute our findings. Furthermore, prospective data is needed to identify potential
clinical differences between the distinct lymphomagenic pathways of AITL proposed in this manuscript. REFERENCES * Swerdlow, S. H. et al. The 2016 revision of the World Health Organization
classification of lymphoid neoplasms. _Blood_ 127, 2375–2390 (2016). Article CAS PubMed PubMed Central Google Scholar * Dobay, M. P. et al. Integrative clinicopathological and molecular
analyses of angioimmunoblastic T-cell lymphoma and other nodal lymphomas of follicular helper T-cell origin. _Haematologica_ 102, e148–e151 (2017). Article PubMed PubMed Central Google
Scholar * Cools, J. RHOA mutations in peripheral T cell lymphoma. _Nat. Genet._ 46, 320–321 (2014). Article CAS PubMed Google Scholar * Hodge, R. G. & Ridley, A. J. Regulating Rho
GTPases and their regulators. _Nat. Rev. Mol. Cell. Biol._ 17, 496–510 (2016). Article CAS PubMed Google Scholar * Cortes, J. R. et al. Role and Mechanisms of Rhoa G17V in the
Pathogenesis of AITL. _Blood_ 128, 621 (2016). (Abstract 608). Google Scholar * Abate, F. et al. Activating mutations and translocations in the guanine exchange factor VAV1 in peripheral
T-cell lymphomas. _Proc. Natl. Acad. Sci. USA_ 114, 764–769 (2017). Article CAS PubMed PubMed Central Google Scholar * Nguyen, T. et al. Identification of cell-type-specific mutations
in nodal T-cell lymphomas. _Blood Cancer J._ 7, e516 (2017). Article CAS PubMed PubMed Central Google Scholar * Palomero, T. et al. Recurrent mutations in epigenetic regulators, RHOA
and FYN kinase in peripheral T cell lymphomas. _Nat. Genet._ 46, 166–170 (2014). Article CAS PubMed PubMed Central Google Scholar * Vallois, D. et al. Activating mutations in genes
related to TCR signaling in angioimmunoblastic and other follicular helper T-cell–derived lymphomas. _Blood_ 128, 1490–1502 (2016). Article CAS PubMed Google Scholar * Yoo, H. Y. et al.
Frequent CTLA4-CD28 gene fusion in diverse types of T cell lymphoma. _Haematologica_ 101, 757–763 (2016). Article CAS PubMed PubMed Central Google Scholar * Wang, C. et al. IDH2R172
mutations define a unique subgroup of patients with angioimmunoblastic T-cell lymphoma. _Blood_ 126, 1741–1752 (2015). Article CAS PubMed PubMed Central Google Scholar * Rohr, J. et al.
Recurrent activating mutations of CD28 in peripheral T-cell lymphomas. _Leukemia_ 30, 1062–1070 (2016). Article CAS PubMed Google Scholar * Willemsen, M. & Schouten, H. C.
Inappropriate costimulation and aberrant DNA methylation as therapeutic targets in angioimmunoblastic T-cell lymphoma. _Biomark. Res._ 5, 6 (2017). Article PubMed PubMed Central Google
Scholar * Schmitz, N. & Leval, L. How I manage peripheral T‐cell lymphoma, not otherwise specified and angioimmunoblastic T‐cell lymphoma: current practice and a glimpse into the
future. _Br. J. Haematol._ 176, 851–866 (2017). Article CAS PubMed Google Scholar * Bustelo, X. R. Vav family exchange factors: an integrated regulatory and functional view. _Small
GTPases_ 5, e973757 (2014). Article PubMed Central Google Scholar * Crespo, P., Schuebel, K. E., Ostrom, A. A., Gutkind, J. S. & Bustelo, X. R. Phosphotyrosine-dependent activation of
Rac-1 GDP/GTP exchange by the vav proto-oncogene product. _Nature_ 385, 169–172 (1997). Article CAS PubMed Google Scholar * Manso, R. et al. The RHOA G17V gene mutation occurs
frequently in peripheral T-cell lymphoma and is associated with a characteristic molecular signature. _Blood_ 123, 2893–2894 (2014). Article CAS PubMed Google Scholar * Fujisawa M. et
al. Activation of RHOA-VAV1 signaling in angioimmunoblastic T-cell lymphoma. _Leukemia._ https://doi.org/10.1038/leu.2017.273 (2017). * Nagao, R. et al. Clinicopathologic analysis of
angioimmunoblastic T-cell lymphoma with or without RHOA G17V mutation using formalin-fixed paraffin-embedded sections. _Am. J. Surg. Pathol._ 40, 1041–1050 (2016). Article PubMed Google
Scholar * Ondrejka, S. L. et al. Angioimmunoblastic T-cell lymphomas with the RHOA p. Gly17Val mutation have classic clinical and pathologic features. _Am. J. Surg. Pathol._ 40, 335–341
(2016). Article PubMed Google Scholar Download references AUTHORS’ CONTRIBUTIONS M.W. developed the initial hypothesis that lead to this work and wrote the manuscript. M.A.H. and A.Z.H.
were involved in critical reviewing and revising the manuscript. B.W. supervised the statistical analysis. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pathology, GROW-School
for Oncology & Developmental Biology, Maastricht University Medical Centre, Maastricht, The Netherlands Mathijs Willemsen, Myrurgia Abdul Hamid & Axel zur Hausen * Department of
Methodology and Statistics, CAPHRI-Care and Public Health Research Institute, Maastricht University, Maastricht, The Netherlands Bjorn Winkens Authors * Mathijs Willemsen View author
publications You can also search for this author inPubMed Google Scholar * Myrurgia Abdul Hamid View author publications You can also search for this author inPubMed Google Scholar * Bjorn
Winkens View author publications You can also search for this author inPubMed Google Scholar * Axel zur Hausen View author publications You can also search for this author inPubMed Google
Scholar CORRESPONDING AUTHOR Correspondence to Mathijs Willemsen. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION
PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY
DATASET 1 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and
reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if
changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the
material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to
obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Willemsen, M., Abdul Hamid, M., Winkens, B. _et al._ Mutational heterogeneity of angioimmunoblastic T-cell lymphoma indicates distinct lymphomagenic pathways. _Blood Cancer Journal_
8, 6 (2018). https://doi.org/10.1038/s41408-017-0047-2 Download citation * Received: 19 November 2017 * Revised: 19 November 2017 * Accepted: 29 November 2017 * Published: 17 January 2018 *
DOI: https://doi.org/10.1038/s41408-017-0047-2 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is
not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative



