- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
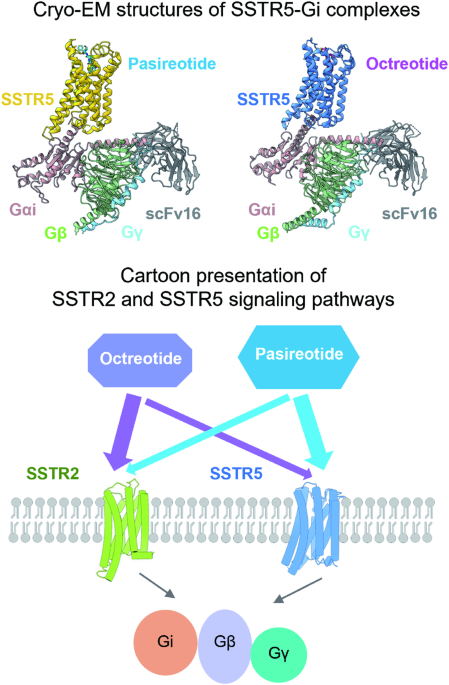
ABSTRACT Somatostatin receptor 5 (SSTR5) is highly expressed in ACTH-secreting pituitary adenomas and is an important drug target for the treatment of Cushing’s disease. Two cyclic SST
analog peptides (pasireotide and octreotide) both can activate SSTR5 and SSTR2. Pasireotide is preferential binding to SSTR5 than octreotide, while octreotide is biased to SSTR2 than SSTR5.
The lack of selectivity of both pasireotide and octreotide causes side effects, such as hyperglycemia, gastrointestinal disturbance, and abnormal glucose homeostasis. However, little is
known about the binding and selectivity mechanisms of pasireotide and octreotide with SSTR5, limiting the development of subtype-selective SST analog drugs specifically targeting SSTR5.
Here, we report two cryo-electron microscopy (cryo-EM) structures of SSTR5-Gi complexes activated by pasireotide and octreoitde at resolutions of 3.09 Å and 3.24 Å, respectively. In
combination with structural analysis and functional experiments, our results reveal the molecular mechanisms of ligand recognition and receptor activation. We also demonstrate that
pasireotide preferentially binds to SSTR5 through the interactions between Tyr(Bzl)/DTrp of pasireotide and SSTR5. Moreover, we find that the Q2.63, N6.55, F7.35 and ECL2 of SSTR2 play a
crucial role in octreotide biased binding of SSTR2. Our results will provide structural insights and offer new opportunities for the drug discovery of better selective pharmaceuticals
targeting specific SSTR subtypes. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read
our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS STRUCTURAL INSIGHTS INTO THE ACTIVATION OF SOMATOSTATIN RECEPTOR 2 BY CYCLIC SST ANALOGUES Article Open access 20
May 2022 STRUCTURAL INSIGHTS INTO LIGAND RECOGNITION AND SELECTIVITY OF SOMATOSTATIN RECEPTORS Article Open access 23 June 2022 PLASTICITY IN LIGAND RECOGNITION AT SOMATOSTATIN RECEPTORS
Article 24 February 2022 DATA AVAILABILITY The cryo-EM density maps and corresponding atomic coordinates of SSTR5–Gi complexes bound with pasireotide and octreotide have been deposited in
the Electron Microscopy Data Bank and the Protein Data Bank under the accession codes of EMD-39931, EMD-39901 and 8ZCJ, 8ZBE, respectively. REFERENCES * Theodoropoulou M, Stalla GK.
Somatostatin receptors: from signaling to clinical practice. Front Neuroendocrinol. 2013;34:228–52. PubMed CAS Google Scholar * Gu YZ, Schonbrunn A. Coupling specificity between
somatostatin receptor sst2A and G proteins: isolation of the receptor-G protein complex with a receptor antibody. Mol Endocrinol. 1997;11:527–37. PubMed CAS Google Scholar * Günther T,
Tulipano G, Dournaud P, Bousquet C, Csaba Z, Kreienkamp HJ, et al. International union of basic and clinical pharmacology. CV. Somatostatin receptors: structure, function, ligands, and new
nomenclature. Pharmacol Rev. 2018;70:763–835. PubMed PubMed Central Google Scholar * Patel YC. Somatostatin and its receptor family. Front Neuroendocrinol. 1999;20:157–98. PubMed CAS
Google Scholar * Barnett P. Somatostatin and somatostatin receptor physiology. Endocrine. 2003;20:255–64. PubMed CAS Google Scholar * Eychenne R, Bouvry C, Bourgeois M, Loyer P, Benoist
E, Lepareur N. Overview of radiolabeled somatostatin analogs for cancer imaging and therapy. Molecules. 2020;25:4012. PubMed PubMed Central CAS Google Scholar * Shimon I, Taylor JE, Dong
JZ, Bitonte RA, Kim S, Morgan B, et al. Somatostatin receptor subtype specificity in human fetal pituitary cultures. Differential role of SSTR2 and SSTR5 for growth hormone,
thyroid-stimulating hormone, and prolactin regulation. J Clin Invest. 1997;99:789–98. PubMed PubMed Central CAS Google Scholar * Melmed S. Medical progress: acromegaly. N Engl J Med.
2006;355:2558–73. PubMed CAS Google Scholar * Schmid HA. Pasireotide (SOM230): development, mechanism of action and potential applications. Mol Cell Endocrinol. 2008;286:69–74. PubMed
CAS Google Scholar * Shimon I. Somatostatin receptors in pituitary and development of somatostatin receptor subtype-selective analogs. Endocrine. 2003;20:265–9. PubMed CAS Google Scholar
* Tirosh A, Stemmer SM, Solomonov E, Elnekave E, Saeger W, Ravkin Y, et al. Pasireotide for malignant insulinoma. Hormones. 2016;15:271–6. PubMed Google Scholar * Reubi JC, Waser B,
Schaer JC, Laissue JA. Somatostatin receptor sst1-sst5 expression in normal and neoplastic human tissues using receptor autoradiography with subtype-selective ligands. Eur J Nucl Med.
2001;28:836–46. PubMed CAS Google Scholar * Sun L, Coy DH. Somatostatin and its analogs. Curr Drug Targets. 2016;17:529–37. PubMed CAS Google Scholar * Sawicka-Gutaj N, Owecki M,
Ruchala M. Pasireotide - mechanism of action and clinical applications. Curr Drug Metab. 2018;19:876–82. PubMed CAS Google Scholar * Colao A, Petersenn S, Newell-Price J, Findling JW, Gu
F, Maldonado M, et al. A 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med. 2012;366:914–24. PubMed CAS Google Scholar * Feelders RA, Yasothan U, Kirkpatrick P.
Pasireotide. Nat Rev Drug Discov. 2012;11:597–8. PubMed CAS Google Scholar * Gueorguiev M, Grossman AB. Pituitary tumors in 2010: a new therapeutic era for pituitary tumors. Nat Rev
Endocrinol. 2011;7:71–3. PubMed CAS Google Scholar * Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G. SOM230: a novel somatostatin peptidomimetic with broad somatotropin release
inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol. 2002;146:707–16. PubMed CAS Google Scholar * Bolanowski M, Kałużny M, Witek P,
Jawiarczyk-Przybyłowska A. Pasireotide-a novel somatostatin receptor ligand after 20 years of use. Rev Endocr Metab Disord. 2022;23:601–20. PubMed PubMed Central CAS Google Scholar *
Silverstein JM. Hyperglycemia induced by pasireotide in patients with Cushing’s disease or acromegaly. Pituitary. 2016;19:536–43. PubMed PubMed Central CAS Google Scholar * Husni H, Khan
SA, Alghaieb B, Abusamaan MS, Donner TW, Hamrahian AH. Pasireotide use for the treatment of endogenous hyperinsulinemic hypoglycemia refractory to conventional medical therapy: a case
report and review of the literature. Clin Case Rep. 2022;10:e05650. PubMed PubMed Central Google Scholar * Gadelha MR, Wildemberg LE, Bronstein MD, Gatto F, Ferone D. Somatostatin
receptor ligands in the treatment of acromegaly. Pituitary. 2017;20:100–8. PubMed CAS Google Scholar * Melmed S. Acromegaly pathogenesis and treatment. J Clin Invest. 2009;119:3189–202.
PubMed PubMed Central CAS Google Scholar * Gomes-Porras M, Cárdenas-Salas J, Álvarez-Escolá C. Somatostatin analogs in clinical practice: a review. Int J Mol Sci. 2020;21:1682. PubMed
PubMed Central CAS Google Scholar * Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat
Rev Drug Discov. 2003;2:999–1017. PubMed CAS Google Scholar * Gadelha MR, Bronstein MD, Brue T, Coculescu M, Fleseriu M, Guitelman M, et al. Pasireotide versus continued treatment with
octreotide or lanreotide in patients with inadequately controlled acromegaly (PAOLA): a randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2014;2:875–84. PubMed CAS Google Scholar *
Öberg K, Lamberts SW. Somatostatin analogues in acromegaly and gastroenteropancreatic neuroendocrine tumours: past, present and future. Endocr Relat Cancer. 2016;23:R551–r66. PubMed Google
Scholar * Lamberts SW, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med. 1996;334:246–54. PubMed CAS Google Scholar * Chan MM, Chan MM, Mengshol JA, Fish DN, Chan ED.
Octreotide: a drug often used in the critical care setting but not well understood. Chest. 2013;144:1937–45. PubMed CAS Google Scholar * Lamberts SWJ, Hofland LJ. Anniversary review:
octreotide, 40 years later. Eur J Endocrinol. 2019;181:R173–r83. PubMed CAS Google Scholar * Xing C, Zhuang Y, Xu TH, Feng Z, Zhou XE, Chen M, et al. Cryo-EM structure of the human
cannabinoid receptor CB2-G(i) signaling complex. Cell. 2020;180:645–54.e13. PubMed PubMed Central CAS Google Scholar * Duan J, Shen DD, Zhou XE, Bi P, Liu QF, Tan YX, et al. Cryo-EM
structure of an activated VIP1 receptor-G protein complex revealed by a NanoBiT tethering strategy. Nat Commun. 2020;11:4121. PubMed PubMed Central CAS Google Scholar * Zheng SQ,
Palovcak E, Armache JP, Verba KA, Cheng Y, Agard DA. MotionCor2: anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat Methods. 2017;14:331–2. PubMed
PubMed Central CAS Google Scholar * Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat Methods.
2017;14:290–6. PubMed CAS Google Scholar * Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–32. PubMed Google
Scholar * Afonine PV, Poon BK, Read RJ, Sobolev OV, Terwilliger TC, Urzhumtsev A, et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr D Struct Biol.
2018;74:531–44. PubMed PubMed Central CAS Google Scholar * Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera-a visualization system for
exploratory research and analysis. J Comput Chem. 2004;25:1605–12. PubMed CAS Google Scholar * Pettersen EF, Goddard TD, Huang CC, Meng EC, Couch GS, Croll TI, et al. UCSF ChimeraX:
structure visualization for researchers, educators, and developers. Protein Sci. 2021;30:70–82. PubMed CAS Google Scholar * Maeda S, Koehl A, Matile H, Hu H, Hilger D, Schertler GFX, et
al. Development of an antibody fragment that stabilizes GPCR/G-protein complexes. Nat Commun. 2018;9:3712. PubMed PubMed Central Google Scholar * Heo Y, Yoon E, Jeon YE, Yun JH, Ishimoto
N, Woo H, et al. Cryo-EM structure of the human somatostatin receptor 2 complex with its agonist somatostatin delineates the ligand-binding specificity. Elife. 2022;11:e76823. PubMed PubMed
Central CAS Google Scholar * Chen LN, Wang WW, Dong YJ, Shen DD, Guo J, Yu X, et al. Structures of the endogenous peptide- and selective non-peptide agonist-bound SSTR2 signaling
complexes. Cell Res. 2022;32:785–8. PubMed PubMed Central CAS Google Scholar * Cuevas-Ramos D, Fleseriu M. Pasireotide: a novel treatment for patients with acromegaly. Drug Des Devel
Ther. 2016;10:227–39. PubMed PubMed Central CAS Google Scholar * Dalm VA, Hofland LJ, Lamberts SW. Future clinical prospects in somatostatin/cortistatin/somatostatin receptor field. Mol
Cell Endocrinol. 2008;286:262–77. PubMed CAS Google Scholar * Zhao W, Han S, Qiu N, Feng W, Lu M, Zhang W, et al. Structural insights into ligand recognition and selectivity of
somatostatin receptors. Cell Res. 2022;32:761–72. PubMed PubMed Central CAS Google Scholar * Zhao J, Fu H, Yu J, Hong W, Tian X, Qi J, et al. Prospect of acromegaly therapy: molecular
mechanism of clinical drugs octreotide and paltusotine. Nat Commun. 2023;14:962. PubMed PubMed Central CAS Google Scholar * Robertson MJ, Meyerowitz JG, Panova O, Borrelli K, Skiniotis
G. Plasticity in ligand recognition at somatostatin receptors. Nat Struct Mol Biol. 2022;29:210–7. PubMed PubMed Central CAS Google Scholar * Robertson MJ, Papasergi-Scott MM, He F,
Seven AB, Meyerowitz JG, Panova O, et al. Structure determination of inactive-state GPCRs with a universal nanobody. Nat Struct Mol Biol. 2022;29:1188–95. PubMed CAS Google Scholar * Yuan
Y, Jia G, Wu C, Wang W, Cheng L, Li Q, et al. Structures of signaling complexes of lipid receptors S1PR1 and S1PR5 reveal mechanisms of activation and drug recognition. Cell Res.
2021;31:1263–74. PubMed PubMed Central CAS Google Scholar * Liapakis G, Fitzpatrick D, Hoeger C, Rivier J, Vandlen R, Reisine T. Identification of ligand binding determinants in the
somatostatin receptor subtypes 1 and 2. J Biol Chem. 1996;271:20331–9. PubMed CAS Google Scholar * Bo Q, Yang F, Li Y, Meng X, Zhang H, Zhou Y, et al. Structural insights into the
activation of somatostatin receptor 2 by cyclic SST analogues. Cell Discov. 2022;8:47. PubMed PubMed Central CAS Google Scholar Download references ACKNOWLEDGEMENTS The cryo-EM data were
collected at the Center for Integrative Imaging of University of Science and Technology of China (Hefei). This project was supported by “USTC Research Funds of the Double First-Class
Initiative” (YD9990002027 to FY, YD9100002021 to PS), the National Natural Science Foundation of China (22207100 to FY, 22277114, 31971152 to PS, 21825703 to CLT, 22307113 to SLL), the
National Key Research & Development Project, Ministry of Science and Technology the China (2022YFC3400500 to CLT), Postdoctoral Science Foundation (2022M713054 to FY), the Strategic
Priority Research Program of Chinese Academy of Sciences (XDB37000000 to CLT), Anhui Provincial Natural Science Foundation (2108085J16 to PS). And the National Key R&D Program of China
(2021YFA1200104 to SLL), supported by “the Fundamental Research Funds for the Central Universities”. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * The First Affiliated Hospital of USTC,
School of Life Sciences, Division of Life Sciences and Medicine, Joint Center for Biological Analytical Chemistry, Anhui Engineering Laboratory of Peptide Drug, Anhui Laboratory of Advanced
Photonic Science and Technology, University of Science and Technology of China, Hefei, 230026, China Ying-ge Li, Xian-yu Meng, Xiru Yang, Sheng-long Ling, Pan Shi, Chang-lin Tian & Fan
Yang * The Anhui Provincial Key Laboratory of High Magnetic Resonance Image, High Magnetic Field Laboratory, Chinese Academy of Sciences, Hefei, 230031, China Chang-lin Tian Authors *
Ying-ge Li View author publications You can also search for this author inPubMed Google Scholar * Xian-yu Meng View author publications You can also search for this author inPubMed Google
Scholar * Xiru Yang View author publications You can also search for this author inPubMed Google Scholar * Sheng-long Ling View author publications You can also search for this author
inPubMed Google Scholar * Pan Shi View author publications You can also search for this author inPubMed Google Scholar * Chang-lin Tian View author publications You can also search for this
author inPubMed Google Scholar * Fan Yang View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS FY, CLT and PS conceived and supervised the whole
project; YGL designed the expression constructs, YGL and XRY expressed, optimized and purified the receptor; XYM and YGL prepared the cryo-EM grids, collected the cryo-EM data, performed
cryo-EM map calculation, model building; YGL designed the constructs for functional assays and performed the functional experiments; FY, YGL, XYM and SLL analyzed the structures, FY and YGL
prepared the figures and participated manuscript writing; FY, CLT and PS wrote the manuscript with inputs from the authors. CORRESPONDING AUTHORS Correspondence to Pan Shi, Chang-lin Tian or
Fan Yang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS Springer Nature or
its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the
accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Li,
Yg., Meng, Xy., Yang, X. _et al._ Structural insights into somatostatin receptor 5 bound with cyclic peptides. _Acta Pharmacol Sin_ 45, 2432–2440 (2024).
https://doi.org/10.1038/s41401-024-01314-8 Download citation * Received: 31 January 2024 * Accepted: 15 May 2024 * Published: 26 June 2024 * Issue Date: November 2024 * DOI:
https://doi.org/10.1038/s41401-024-01314-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * G protein-coupled receptors * Somatostatin receptor 5 *
pasireotide * octreotide * cryo-EM









