- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Chronic myeloid leukemia (CML) is a clonal hematopoietic stem cell neoplasm characterized by an uncontrolled proliferation of moderately and well differentiated cells of the
granulocytic lineage. LW-213, a newly synthesized flavonoid compound, was found to exert antitumor effects against breast cancer through inducing G2/M phase arrest. We investigated whether
LW-213 exerted anti-CML effects and the underlying mechanisms. We showed that LW-213 inhibited the growth of human CML cell lines K562 and imatinid-resistant K562 (K562r) in dose- and
time-dependent manners with IC50 values at the low μmol/L levels. LW-213 (5, 10, 15 μM) caused G2/M phase arrest of K562 and K562r cells via reducing the activity of G2/M phase
transition-related proteins Cyclin B1/CDC2 complex. LW-213 treatment induced apoptosis of K562 and K562r cells via inhibiting the expression of CDK9 through lysosome degradation, thus
leading to the suppression of RNAPII phosphorylation, down-regulation of a short-lived anti-apoptic protein MCL-1. The lysosome inhibitor, NH4Cl, could reverse the anti-CML effects of LW-213
including CDK9 degradation and apoptosis. LW-213 treatment also degraded the downstream proteins of BCR-ABL1, such as oncoproteins AKT, STAT3/5 in CML cells, which was blocked by NH4Cl. In
primary CML cells and CD34+ stem cells, LW-213 maintained its pro-apoptotic activity. In a K562 cells-bearing mice model, administration of LW-213 (2.5, 5.0 mg/kg, ip, every other day for 4
weeks) dose-dependently prolonged the survival duration, and significantly suppressed huCD45+ cell infiltration and expression of MCL-1 in spleens. Taken together, our results demonstrate
that LW-213 may be an efficient agent for CML treatment. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS THE COMBINATION OF
VENETOCLAX AND QUERCETIN EXERTS A CYTOTOXIC EFFECT ON ACUTE MYELOID LEUKEMIA Article Open access 02 November 2024 PTEROSTILBENE DOWNREGULATES BCR/ABL AND INDUCES APOPTOSIS OF T315I-MUTATED
BCR/ABL-POSITIVE LEUKEMIC CELLS Article Open access 13 January 2022 BDA-366, A PUTATIVE BCL-2 BH4 DOMAIN ANTAGONIST, INDUCES APOPTOSIS INDEPENDENTLY OF BCL-2 IN A VARIETY OF CANCER CELL
MODELS Article Open access 17 September 2020 INTRODUCTION Chronic myeloid leukemia (CML) affects approximately one individual per 100,000 people per year with a slight male preponderance and
accounts for 15% of all new cases of leukemia [1]. The leukemic cells proliferate in an uncontrolled manner with the reciprocal translocation t(9;22)(q34;q11), which results in the
formation of the _bcr-abl_ oncogene [2]. Constitutive expression of BCR-ABL1 transforms hematopoietic stem cells (HSCs) into CML stem cells that self-renew, proliferate and differentiate to
give rise to myeloproliferative diseases [3]. BCR-ABL1 exhibits constitutive tyrosine kinase activity, and many proproliferation signaling molecules, such as RAS/RAF/MAP kinases,
phosphoinositide 3-kinase (PI3-kinase), and signal transducer and activator of transcription 5 (STAT5), can be activated by BCR-ABL1 [2]. BCR-ABL1 also activates STAT3 via the JAK and MEK
pathways [4]. The median survival of CML patients was 5–7 years before the tyrosine kinase inhibitors (TKIs) were used in the clinic [1]. Although TKIs achieved an excellent curative effect,
CML stem cells do not respond to TKIs and persist in all patients who undergo long-term therapy. The presence of these cells is associated with poor prognosis, acquisition of TKI
resistance, relapse, and disease progression [3]. LW-213, a newly synthesized flavonoid, is the derivative of wogonin. Previous studies suggested that wogonin and its structurally related
natural flavones, such as apigenin, chrysin and luteolin, are inhibitors of cyclin-dependent kinase 9 (CDK9) [5]. CDK9 is an important member of the CDK family and affects transcription.
CDK9 can phosphorylate S2 residues in the CTD (C-terminal domain) of RNAPII (RNA polymerase II), which is required for transcript elongation [6]. Inhibition of CDK9 activity prevents the
transcription of RNAPII, which leads to the downregulation of myeloid cell leukemia 1 (MCL-1), a short-lived antiapoptotic protein. Therefore, apoptosis can be induced [7]. MCL-1 is an
antiapoptotic member of the BCL-2 family. It differs from other members of the BCL-2 family by its short half-life due to the degradation through the proteasome pathway [8]. MCL-1 has been
considered the most relevant therapeutic target in multiple types of cancer and a relevant therapeutic target in acute and chronic lymphoid malignancies. Previous studies showed that
inhibition of MCL-1 expression with RNA interference is sufficient to promote mitochondrial membrane depolarization and apoptosis in leukemic cells [9]. In CML cells, BCR-ABL1 promotes the
expression of MCL-1, and MCL-1 is expressed in primary CML cells in a constitutive manner at the mRNA and protein levels [2]. LW-213 has been suggested to possess antitumor effects by
inducing G2/M arrest in breast cancer [10]. We also proved that LW-213 could inhibit the proliferation of CML cells by inducing G2/M phase arrest as well as noteworthy apoptosis effects.
LW-213 inhibited the activity of CDK9, decreased the expression of MCL-1 and interfered with the downstream proteins of BCR-ABL1 in both CML cell lines and primary cells. In this article, a
new mechanism by which LW-213 exerts its anti-CML effects was investigated. MATERIALS AND METHODS COMPOUNDS AND REAGENTS LW-213 (99% purity, MW = 445.52) was synthesized and provided by
Prof. Zhi-yu Li in our lab. For in vitro experiments, LW-213 was dissolved in dimethyl sulfoxide (DMSO, Sigma-Aldrich, St. Louis, MO, USA) as a stock solution at a concentration of 0.02 M.
The stock solution was stored at −20 °C and freshly diluted to an indicated concentration with RPMI-1640 medium (GIBCO, Carlsbad, CA, USA). For in vivo experiments, LW-213 was prepared for
intraperitoneal injection by Dr. Xue Ke from College of Pharmacy, China Pharmaceutical University. Primary CDK9 antibody was obtained from Cell Signaling Technology (Danvers, MA). Primary
antibodies for Cyclin B1, CDC2, p-CDC2 (Y15), β-tubulin, MCL-1, p-CDK9 (Thr186), AKT, p-AKT (Ser473), β-actin, BCL-2, STAT5, STAT3, p-STAT5 (Y694), p-RNAPII-S2, p-RNAPII-S5, ABL1, LC3,
P62/SQSTM1, caspase 3, and caspase 9 were obtained from ABclonal Technology (Wuhan, China). IRDyeTM 800-conjugated secondary antibodies were purchased from Rockland (Philadelphia, PA, USA).
MG-132 was purchased from KeyGENE BioTECH (Jiangsu, China). MG-132 powder was dissolved in DMSO and stored at −20 °C. 3-MA (Sigma-Aldrich, St. Louis, MO) was dissolved in ddH2O and stored at
−20 °C. CELL CULTURE The human CML cell line K562 and imatinib-resistant K562 cells (K562r) were purchased from the Cell Bank of Shanghai Institute of Biochemistry & Cell Biology.
Primary leukemia cells from newly diagnosed CML patients at the First Affiliated Hospital of Nanjing Medical University, Nanjing, China, who did not receive therapy were collected using
lymphocyte-monocyte separation medium (Jingmei, Nanjing, China). Signed informed consent was obtained from each patient. The cell lines and primary CML cells were cultured in RPMI-1640
medium supplemented with 10% fetal bovine serum (FBS) (GIBCO, Carlsbad, USA), 100 U/mL benzylpenicillin and 100 µg/mL streptomycin at 37 °C in a humidified environment with 5% CO2. ANIMAL
MODELS Female NOD/SCID nude mice (5–6 weeks old, weighing 18–22 g) (Slaccas Shanghai Laboratory Animal Co., Ltd, Shanghai, China) were sublethally irradiated (1.8 Gy) and engrafted with K562
cells (5 × 106) via tail vein injection 24 h following radiation treatment. After 7 days, animals were randomly assigned to the control group, the low dose LW-213 group, and the high dose
LW-213 group (_n_ = 10 per group). The animals were intraperitoneally (i.p.) injected with 2.5 mg/kg LW-213 in the low dose LW-213 group and 5 mg/kg in the high dose LW-213 group every other
day for 4 weeks. The animals in the control group received i.p. injection of physiological saline solution. Finally, the cells from spleen tissue were collected for immunofluorescence
detection after labeling with human CD45-PE (huCD45-PE, Miltenyi Biotec, Auburn, CA, USA) and MCL-1 antibody. The images were captured with a confocal microscope at ×1000 magnification
(FluoView FV1000, Olympus, Tokyo, Japan). The number of surviving mice was counted. The animal study was carried out according to the regulations of the China Food and Drug Administration
(CFDA) on Animal Care. Animals were maintained in an air-conditioned and pathogen-free environment (23 ± 2 °C, 55% ± 5% humidity) under controlled lighting (12 h light/day) and supplied with
standard laboratory food and water ad libitum throughout the experimental period. CELL PROLIFERATION ASSAYS Cell growth was assessed using the trypan blue exclusion method by manual cell
counting using a hemocytometer (Qiujing, Shanghai, China) [11]. The results are reported as the cell number. MTT assays were performed to evaluate the growth inhibitory effect of the drug
treatments [11]. The concentration that caused 50% inhibition of cell viability (IC50) values was calculated with GraphPad software. CELL CYCLE DETECTION The cell cycle distribution was
analyzed by propidium iodide (PI) staining and quantified using a BD FACSCalibur flow cytometer (Becton, Dickinson, San Jose, CA). The cells were collected and then fixed with 70% ethanol
for 2 h at 4 °C. After fixation, the cells were washed with PBS buffer and stained with PI for 30 min. The fluorescence was detected by flow cytometry. The percentage of cells in G0/G1, S,
and G2/M phases of the cell cycle was determined by the PI fluorescence signal peak versus the integral and quantitated with FlowJo software. APOPTOSIS DETECTION An Annexin V/PI staining
assay was used to detect apoptosis of cells after LW-213 treatment. The cells were collected and washed with PBS buffer. The cells were resuspended in 100 μL of binding buffer and stained
with 5 μL of Annexin V and 5 μL of PI for 10 min (The Annexin V/PI apoptosis kit was purchased from Vazyme Biotech, Nanjing, China). The fluorescence was detected by flow cytometry on a
Becton-Dickinson FACSCalibur, and the data analysis was performed with FlowJo software and BD C6 flow cytometry software. WESTERN BLOT ASSAYS After LW-213 treatment for the indicated time,
total protein was extracted from cells with RIPA Lysis and Extraction Buffer (Thermo Fisher Scientific, USA). The lysates were centrifuged at 12 000 × _g_ for 15 min at 4 °C. The
concentration of proteins was determined with a BCA Protein Assay Kit (Thermo Fisher Scientific, USA). Then, equal amounts of protein were separated with 8%–12% SDS-PAGE and transferred to
PVDF membranes (Millipore, Boston, MA, USA). After the membranes were blocked with 1% BSA for 2 h at room temperature, they were incubated with primary antibodies overnight at 4 °C. After
washes with PBST, the membranes were incubated with secondary antibodies for 1 h at room temperature. Protein band detection was performed with the Odyssey Infrared Imaging System (LI-COR
Inc., Lincoln, NE, USA). MAGNETIC-ACTIVATED CELL SORTING (MACS) Primary CML cells or the cells collected from umbilical cord blood were thoroughly washed, and the cell counts were set at 2 ×
107. The cells were resuspended in 250 μL of 1 × BD IMag™ buffer. The CD34 antibody (Miltenyi Biotec, USA) was added at 4 °C for 15 min, and the cells were washed twice with 1 × BD IMag™
buffer. Then, 50 μL of magnetic bead solution was added to 100 μL of the cell suspension and incubated for 30 min at room temperature. The columns were set on a magnet. The column was primed
by adding 500 μL of wash buffer and letting the solution drip through completely. The cell suspension was then added to the column in a magnetic field for 6 ~ 8 min and the supernatant was
removed. Next, buffer was added to wash the cells, and the column was placed on the magnet for 2 ~ 4 min. The washing process was repeated two times, and the bound cells were incubated in
medium or buffer for treatment or detection. CFSE PROLIFERATION ASSAY The CD34+ cells isolated by MACS were centrifuged at 1000 r/min for 5 min and resuspended in serum-free medium to 1 mL.
The CFSE stock solution was added to the cell suspension (1:1000). The cells were incubated for 20 min at 37 °C in the dark. The medium was replaced after incubation, and the cells were
treated with different concentrations of LW-213. Fluorescence was detected by flow cytometry on a Becton-Dickinson FACSCalibur. IMMUNOFLUORESCENCE The paraffin sections of spleens on cover
slips were fixed in ice-cold methanol for 15 min and permeabilized in 0.15% (v/v) Triton X-100 for 20 min. After blocking with 3% BSA for 1 h at room temperature, the cells were incubated
with primary anti-MCL-1 antibody overnight at 4 °C, followed by incubation with Alexa Fluor 488 goat anti-rabbit lgG antibody (1:500) for 1 h. The huCD45-PE antibody was incubated with the
cells for 50 min at 37 °C, and DAPI (Beyotime Biotechnology, Shanghai, China) was added to the cells at room temperature for 15 min before the sections were sealed. For the cell lines, K562
and K562r cells treated with LW-213 were seeded on the cover slips. After fixation and blocking, the cells were incubated with primary anti-β-tubulin antibody overnight at 4 °C, followed by
incubation with Alexa Fluor 488 goat anti-rabbit lgG antibody. The cells were then stained with PI for 30 min before the samples were sealed. The cells were observed on a confocal laser
scanning microscope (FluoView FV1000, Olympus, Tokyo, Japan). CELL TRANSFECTION WITH CDK9 PLASMID CDK9 plasmid (3 μg) was added to 200 μL of serum-free medium without antibiotic and
incubated at room temperature for 5 min. Next, 8 μL of Lipofectamine 2000 reagent (Life Technologies, Grand Island, NY, USA) was added to serum-free medium without antibiotic to final volume
of 200 µL and incubated at room temperature for 5 min. The two solutions above were mixed, incubated at room temperature for 20 min and then added to 2 mL of cell suspension. The cells were
incubated at 4 °C for 8 h, and the transfection medium was replaced with complete medium, which was incubated with the cells for 40 h. STATISTICAL ANALYSIS All data are expressed as the
mean ± S.E.M. from at least three independent experiments performed in a parallel manner. Statistical analysis of multiple group comparisons was performed by one-way analysis of variance
(ANOVA) followed by the Bonferroni post hoc test. Comparisons between two groups were analyzed using two-tailed Student’s _t_-tests. A _P_-value < 0.05 was considered statistically
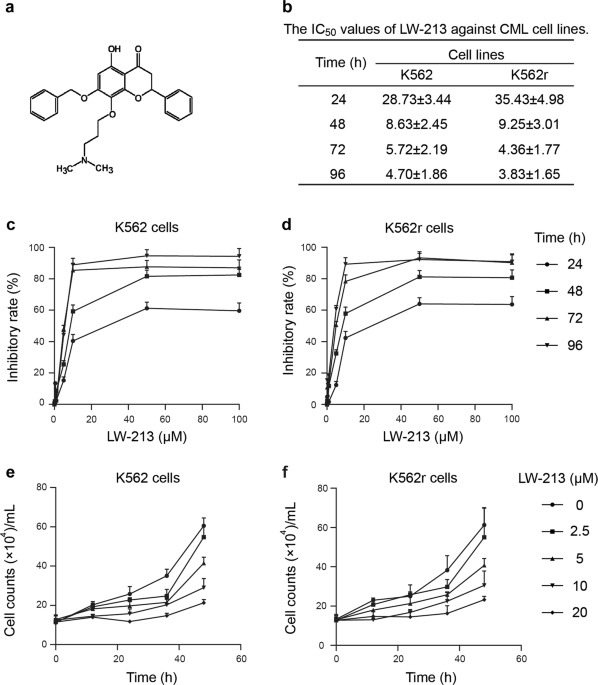
significant. RESULTS LW-213 DECREASES THE VIABILITY OF CML CELL LINES IN VITRO The chemical structure of LW-213 is shown in Fig. 1a. We first assessed the cytotoxic effects of LW-213 using
the MTT assay. The CML cell lines K562 and K562r were incubated with various concentrations of LW-213 for 24, 48, 72 and 96 h. Cell viability decreased in a concentration- and time-dependent
manner (Fig. 1b–d). We also determined the antiproliferative effects of LW-213 on K562 and K562r cells by the trypan blue dye exclusion assay (Fig. 1e, f). The results showed that LW-213
inhibited the growth of CML cell lines. Because inhibition of cell viability may result either from cell cycle arrest or cell death [12], we then investigated both in subsequent experiments.
LW-213 BLOCKS CML CELL CYCLE PROGRESSION AT G2/M PHASE The effects of LW-213 on cell cycle progression were evaluated by PI staining analysis. CML cell lines treated with 0, 5, 10, and 15
μM LW-213 showed an accumulation of cells in G2/M phase at 12 and 24 h (Fig. 2a–c). Comparison with the untreated cells revealed an apparent concentration- and time-dependent increase in the
percentage of G2/M cells. The immunofluorescence assays showed that the number of cells in mitosis increased by 5.03% and 3.91% in K562 and K562r cells, respectively, suggesting that G2
phase arrest was induced by LW-213 (Fig. 2d, e). To further investigate the pathways affected by LW-213, we examined the expression levels of G2/M phase-transition-related proteins under the
same conditions (Fig. 2f, g). Progression through G2/M requires activated Cyclin B1/CDC2 complexes [10]. After treatment with LW-213, a decrease in Cyclin B1 expression was observed. In
K562 cells (24 h) and K562r cells, LW-213 increased the level of p-CDC2, an inactivated form of CDC2 (Fig. 2f). This result indicated that the activity reduction of the Cyclin B1/CDC2
complex resulted in G2/M arrest. LW-213 INDUCES LYSOSOME-DEPENDENT APOPTOSIS VIA THE CDK9-RNAPII-MCL-1 AXIS AND PROMOTES PROTEIN DEGRADATION OF BCR-ABL1 DOWNSTREAM To evaluate whether the
antiproliferation effects of LW-213 were associated with apoptosis, K562 and K562r cells were treated with 0, 5, 10, and 15 μM of LW-213, and statistically profound increase in apoptotic
rates was detected by flow cytometric analysis after 12 and 24 h of treatment (Annexin V and PI double-positive, Fig. 3a–c). Apoptosis was further investigated by Western blot, and
concentration-dependent cleavage of caspase 3/9 was revealed (Fig. 4a). Furthermore, the apoptosis induced by LW-213 could be reversed by the lysosome inhibitor NH4Cl (10 mM) (Fig. 3d, e),
indicating that this type of apoptosis might be lysosome-dependent. Previous studies have demonstrated that wogonin and other structurally related natural flavones are inhibitors of CDK9
[5]. To investigate the molecular mechanisms by which LW-213 induces apoptosis in cancer cells, we first investigated the effects of LW-213 on CDK9, and the results revealed that LW-213
decreased the levels of both phosphorylated CDK9 and total CDK9 (Fig. 4b). Next, we analyzed the expression of BCL-2 family proteins. The cellular levels of MCL-1 protein decreased
significantly after treatment with LW-213 (Fig. 4a). MCL-1 is an antiapoptosis protein and has been considered to be the most relevant therapeutic target [13]. CDK9 plays a major role in the
regulation of transcription and promotes the phosphorylation of the carboxyl-terminal domain of RNA polymerase II (RNAPII) at Ser2 (S2), which is required for transcript elongation. The
activation of RNAPII contributes to the transcription of MCL-1 mRNA and its subsequent translation [5]. We then determined the phosphorylation of RNAPII at S2. Western blot analysis of CML
cell lines treated with different concentrations of LW-213 showed that S2 phosphorylation and S5 phosphorylation were diminished in a concentration-dependent manner after 12 h of treatment
(Fig. 4b). We also determined the effects of NH4Cl on the expression of CDK9 after treatment with LW-213. NH4Cl could significantly prevent LW-213-induced degradation of CDK9 (Fig. 4c–e).
This demonstrated that degradation of CDK9 proteins is involved in LW-213-induced apoptosis. Then, K562 cells were transfected with the CDK9 plasmid. The overexpression of CDK9 rescued the
reduction of MCL-1 expression induced by treatment with LW-213 (Fig. 4f, g), and the cells were rescued from LW-213-induced apoptosis (Fig. 4h, i). The ubiquitin-proteasome pathway is a
major intracellular system for protein degradation [14]. However, the ubiquitin-proteasome pathway might not be involved in LW-213-induced apoptosis because of the inability of MG-132 to
rescue cells from death (Fig. 3f, g). LW-213 INDUCES THE EXPRESSION REDUCTION OF PROTEINS DOWNSTREAM OF BCR-ABL1 We determined whether LW-213-induced lysosome degradation could affect
oncoproteins in CML cells. AKT, STAT3 and STAT5 protein expression was determined; decreases in the levels of these proteins were observed after LW-213 treatment, and the reduction could be
reversed by NH4Cl (Fig. 5a, b). Previous studies demonstrated that the activity of STAT5 contributes to the expression of MCL-1 [2]. PI3K/AKT and MEK/ERK/STAT3 also promote cell
proliferation [4, 15]. Deregulation of proteins in the cancer cell proliferation signal transduction pathway by LW-213 contributed to apoptosis and cell death. LW-213 treatment also resulted
in the degradation of these proteins in primary CML cells (Fig. 5c). LW-213 PROMOTES APOPTOSIS IN CD34+ CELLS AND PRIMARY CML CELLS Primary CML cells were isolated from CML patients.
Samples #1~#4 were treated with 0 ~ 15 μM LW-213, and an increase in the proportion of apoptotic cells was observed (Fig. 6a–d). LW-213 reduced the levels of p-CDK9, CDK9, pro-caspase 3 and
MCL-1 in primary CML cells from sample #1 (Fig. 6e). Although TKIs targeting BCR-ABL1 achieve an excellent therapeutic effect, strong evidence shows that CML stem cells are resistant to TKIs
[3]. The CML stem cells are not fully dependent on BCR-ABL1 for their survival, and this might be the reason for the insensitivity of CML stem cells to TKIs [16]. These leukemia stem
cell-like populations may have different immunophenotypes (CD34+CD38+, CD34+CD38−, CD34−). CD34+CD38− stem cells seem to be the most resistant to therapy and the least immunogenic [17]. We
isolated CD34+ hematopoietic progenitor cells from primary CML cells (sample #1) by magnetic–activated cell sorting (MACS) (Fig. 6f, g). Following magnetic separation, CD34+ cells were
collected. After treatment with 0, 10, and 15 μM of LW-213, cells treated with LW-215 had a higher percentage of cells with higher CFSE intensity than did untreated CD34+ cells (Fig. 6h, i).
These results indicated that LW-213 inhibited the proliferation of CD34+ cells. The high dose (15 μM) of LW-213 induced 58% apoptosis in CD34+ cells, thus supporting the ability of LW-213
to target CD34+ cells (Fig. 6j, k). The cytotoxic effects of LW-213 on normal CD34+ cells, which were isolated and purified from umbilical cord blood, were also determined. Cell viability
decreased by 9.56% after treatment with 15 μM LW-213, and the IC50 value was 26.73 ± 1.47 μM at 24 h (Fig. S1a). LW-213 SUPPRESSES THE PROLIFERATION OF CML CELLS IN VIVO We next established
a NOD/SCID mouse model engrafted with K562 cells via tail vein injection to examine the antileukemia effects of LW-213 in vivo (Fig. 7a). Mice were equally distributed into three groups,
which received 2.5 mg/kg LW-213, 5 mg/kg LW-213 or saline. The results showed that mice in the control group expressed huCD45+ in the spleen, and LW-213 significantly decreased the number of
huCD45+ cells at both low and high doses. A significant reduction in MCL-1 expression in huCD45+ cells after treatment with LW-213 was observed, showing that LW-213 effectively blocks the
activity of MCL-1 in vivo (Fig. 7c). LW-213 also extended the survival of K562 cell-bearing mice compared with mice in the control group. The median survival of the control mice injected
with K562 cells was 15.5 ± 19.9 day. In the LW-213 groups, the median survival was 45.5 ± 16.0 and 48.5 ± 19.2 days at low and high doses, respectively (Fig. 7b). These results demonstrated
that LW-213 suppresses the proliferation of CML cells in vivo. DISCUSSION Flavonoids are well known for their physiological anti-inflammatory and antitumor activities [18]. To date, several
flavonoids, such as wogonin, baicalein and oroxylin A, have been found to inhibit the proliferation of several human cancers [11, 19, 20]. LW-213, a newly synthesized flavonoid, could induce
G2/M cell cycle arrest in human breast cancer cells via the AKT/GSK3β/β-catenin signaling pathway [10]. In this study, we examined the antitumor effects of LW-213 on CML cell lines and
primary CML cells. Our studies were performed both in vitro and in vivo. LW-213 reduced cell viability in a concentration- and time-dependent manner in K562 and K562r cells. The LW-213
cytotoxic effects correlated with a significant induction of apoptosis and cleavage of caspase-3 and -9. In agreement with previous findings in other cancers, LW-213 induced cell cycle
arrest at G2/M phase in CML cell lines, suggesting impairment in mechanisms involved in cell cycle progression and mitosis regulation. Due to the remarkable apoptosis effects of LW-213, we
then explored its mechanism. LW-213 is a derivative of wogonin. Wogonin and other structurally related natural flavones, such as apigenin, chrysin, and luteolin, are inhibitors of CDK9 and
block phosphorylation of the carboxyl-terminal domain of RNA polymerase II at Ser2. This effect leads to reduced RNA synthesis and subsequent rapid downregulation of the short-lived
antiapoptotic protein MCL-1, resulting in apoptosis induction in cancer cells [5]. MCL-1, an antiapoptotic protein in the BCL-2 family, has been found to be a therapeutic target due to its
upregulation in numerous hematological malignancies and solid tumors and acts as an important factor in resistance to apoptosis [8, 21]. Decreased levels of p-CDK9, p-RNAPII-S2/S5 and MCL-1
were observed after treatment with LW-213. This highlighted the fact that LW-213 induced apoptosis via the CDK9/RNAPII/MCL-1 axis. Our data also showed that LW-213 decreased the expression
of CDK9. The ubiquitin-proteasome pathway and lysosomal degradation are two intracellular systems that degrade proteins [22]. We presumed that LW-213-induced apoptosis is triggered by
protein degradation. The lysosome inhibitor NH4Cl rescued the cells after LW-213 treatment and reversed the decreases in CDK9 expression. LW-213-induced apoptosis and protein degradation
were lysosome-dependent. However, the downregulation of Cyclin B1 and G2/M phase arrest could not be reversed by NH4Cl (Fig. S1b–e), and overexpression of CDK9 could not rescue cells from
LW-213-induced G2/M arrest (Fig. S1f–i). The downstream proteins of BCR-ABL1, such as STAT5, AKT, and STAT3, could also be decreased after LW-213 treatment, while the expression of BCR-ABL1
was not influenced by LW-213 (Fig. S1j–k). Thus, LW-213 could inhibit signaling downstream of BCR-ABL1 and induce apoptosis in CML cells. LW-213-induced apoptosis could be reversed by a
lysosome inhibitor. This might indicate the involvement of autophagy in LW-213-induced apoptosis and degradation. LW-213 increased the expression of p62 and LC3-II and the ratio of
LC3-II/LC3-I, but the effect was modest (Fig. S2a–c). Additionally, 3-MA could not inhibit the apoptosis effects of LW-213 (Fig. S2d–e). 3-MA decreased the expression of LC3-II induced by
LW-213, while the reduction in the expression of CDK9, MCL-1 and the proteins downstream of BCR-ABL1 as well as the levels of p-RNAPII-S2 could not be reversed by 3-MA (Fig. S3a–d). It is
suggested that LW-213 had modest autophagy effects that were not associated with LW-213-induced protein degradation and apoptosis. CML is a clonal myeloproliferative disorder characterized
by a _BCR-ABL_ oncogene encoding a constitutively active kinase [23]. Although the majority of patients are treated with TKIs, the disease in CML patients was well controlled, and overall
survival was improved significantly [3], a large proportion of CML patients treated with TKIs develop drug resistance due to the inability of TKIs to kill leukemia stem cells [23]. CML stem
cells that are responsible for the initiation, drug resistance and relapse of CML [23]. Therefore, there is an urgent need for potent therapies against leukemia stem cells in order to cure
CML. We found that LW-213 induced apoptosis in CD34+ cells isolated from primary CML cells by MACS in a concentration- and time-dependent manner. It is suggested that the combination of
LW-213 and TKIs might be useful for CML treatment. This finding could be of significance in the clinical setting in the future. Taken together, the data from our study provided evidence of
the effectiveness of LW-213 as a CML treatment. The agent showed high antitumor activity against CML cell lines and primary CML cells from patients. In conclusion, LW-213 may be a promising
treatment option for CML therapy. REFERENCES * Apperley JF. Chronic myeloid leukaemia. Lancet. 2015;385:1447–59. Article Google Scholar * Aichberger KJ, Mayerhofer M, Krauth MT, Skvara H,
Florian S, Sonneck K, et al. Identification of mcl-1 as a BCR/ABL-dependent target in chronic myeloid leukemia (CML): evidence for cooperative antileukemic effects of imatinib and mcl-1
antisense oligonucleotides. Blood. 2005;105:3303–11. Article CAS Google Scholar * Holyoake TL, Vetrie D. The chronic myeloid leukemia stem cell: stemming the tide of persistence. Blood.
2017;129:1595–606. Article CAS Google Scholar * Coppo P, Flamant S, De Mas V, Jarrier P, Guillier M, Bonnet ML, et al. BCR-ABL activates STAT3 via JAK and MEK pathways in human cells. Br
J Haematol. 2006;134:171–9. Article CAS Google Scholar * Polier G, Ding J, Konkimalla BV, Eick D, Ribeiro N, Kohler R, et al. Wogonin and related natural flavones are inhibitors of CDK9
that induce apoptosis in cancer cells by transcriptional suppression of Mcl-1. Cell Death Dis. 2011;2:e182. Article CAS Google Scholar * Gressel S, Schwalb B, Decker TM, Qin W, Leonhardt
H, Eick D, et al. CDK9-dependent RNA polymerase II pausing controls transcription initiation. Elife. 2017;6:e29736. Article Google Scholar * Thomas D, Powell JA, Vergez F, Segal DH, Nguyen
NY, Baker A, et al. Targeting acute myeloid leukemia by dual inhibition of PI3K signaling and Cdk9-mediated Mcl-1 transcription. Blood. 2013;122:738–48. Article CAS Google Scholar *
Akgul C. Mcl-1 is a potential therapeutic target in multiple types of cancer. Cell Mol Life Sci. 2009;66:1326–36. Article CAS Google Scholar * Hussain SRA, Cheney CM, Johnson AJ, Lin TS,
Grever MR, Caligiuri MA, et al. Mcl-1 is a relevant therapeutic target in acute and chronic lymphoid malignancies: down-regulation enhances rituximab-mediated apoptosis and
complement-dependent cytotoxicity. Clin Cancer Res. 2007;13:2144–50. Article CAS Google Scholar * Zhao L, Miao HC, Li WJ, Sun Y, Huang SL, Li ZY, et al. LW-213 induces G2/M cell cycle
arrest through AKT/GSK3beta/beta-catenin signaling pathway in human breast cancer cells. Mol Carcinog. 2016;55:778–92. Article CAS Google Scholar * Chen Y, Hui H, Yang H, Zhao K, Qin Y,
Gu C, et al. Wogonoside induces cell cycle arrest and differentiation by affecting expression and subcellular localization of PLSCR1 in AML cells. Blood. 2013;121:3682–91. Article CAS
Google Scholar * Lonetti A, Antunes IL, Chiarini F, Orsini E, Buontempo F, Ricci F, et al. Activity of the pan-class I phosphoinositide 3-kinase inhibitor NVP-BKM120 in T-cell acute
lymphoblastic leukemia. Leukemia. 2014;28:1196–206. Article CAS Google Scholar * Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood.
2000;96:3343–56. Article CAS Google Scholar * Khalil R. Ubiquitin-Proteasome Pathway and Muscle Atrophy. Adv Exp Med Biol. 2018;1088:235–48. Article CAS Google Scholar * Hall CP,
Reynolds CP, Kang MH. Modulation of glucocorticoid resistance in pediatric T-cell acute lymphoblastic leukemia by increasing BIM expression with the PI3K/mTOR inhibitor BEZ235. Clin Cancer
Res. 2016;22:621–32. Article CAS Google Scholar * Yang K, Fu LW. Mechanisms of resistance to BCR-ABL TKIs and the therapeutic strategies: a review. Crit Rev Oncol Hematol. 2015;93:277–92.
Article Google Scholar * Zeijlemaker W, Grob T, Meijer R, Hanekamp D, Kelder A, Carbaat-Ham JC, et al. CD34(+)CD38(−) leukemic stem cell frequency to predict outcome in acute myeloid
leukemia. Leukemia. 2019;33:1102–12. * Kidd PM. Bioavailability and activity of phytosome complexes from botanical polyphenols: the silymarin, curcumin, green tea, and grape seed extracts.
Alter Med Rev. 2009;14:226–46. Google Scholar * Yang Y, Liu K, Yang L, Zhang G. Bladder cancer cell viability inhibition and apoptosis induction by baicalein through targeting the
expression of anti-apoptotic genes. Saudi J Biol Sci. 2018;25:1478–82. Article CAS Google Scholar * Hu Y, Yang Y, You QD, Liu W, Gu HY, Zhao L, et al. Oroxylin A induced apoptosis of
human hepatocellular carcinoma cell line HepG2 was involved in its antitumor activity. Biochem Biophys Res Commun. 2006;351:521–7. Article CAS Google Scholar * Warr MR, Shore GC. Unique
biology of Mcl-1: therapeutic opportunities in cancer. Curr Mol Med. 2008;8:138–47. Article CAS Google Scholar * Zhan J, He J, Zhou Y, Wu M, Liu Y, Shang F, et al. Crosstalk between the
autophagy-lysosome pathway and the ubiquitin-proteasome pathway in retinal pigment epithelial cells. Curr Mol Med. 2016;16:487–95. Article CAS Google Scholar * Zhou H, Xu R. Leukemia stem
cells: the root of chronic myeloid leukemia. Protein Cell. 2015;6:403–12. Article CAS Google Scholar Download references ACKNOWLEDGEMENTS This work was supported by the National Natural
Science Foundation of China [No. 81673461, 81873046 and 81830105], the Drug Innovation Major Project [2017ZX09301014, 2018ZX09711001-003-007], the Project Program of State Key Laboratory of
Natural Medicines, China Pharmaceutical University [No. SKLNMZZCX201823], the Social Development Project of Jiangsu Provincial Science and Technology Department [NO. BE2018711], and the
“Double First-Class” University project [CPU 2018GF11, CPU2018GF05]. AUTHOR INFORMATION Author notes * These authors contributed equally: Xiao Liu, Po Hu AUTHORS AND AFFILIATIONS * State Key
Laboratory of Natural Medicines, Jiangsu Key Laboratory of Carcinogenesis and Intervention, China Pharmaceutical University, Nanjing, 210009, China Xiao Liu, Po Hu, Hui Li, Xiao-xuan Yu,
Xiang-yuan Wang, Ying-jie Qing, Zhan-yu Wang, Hong-zheng Wang, Meng-yuan Zhu, Qing-long Guo & Hui Hui Authors * Xiao Liu View author publications You can also search for this author
inPubMed Google Scholar * Po Hu View author publications You can also search for this author inPubMed Google Scholar * Hui Li View author publications You can also search for this author
inPubMed Google Scholar * Xiao-xuan Yu View author publications You can also search for this author inPubMed Google Scholar * Xiang-yuan Wang View author publications You can also search for
this author inPubMed Google Scholar * Ying-jie Qing View author publications You can also search for this author inPubMed Google Scholar * Zhan-yu Wang View author publications You can also
search for this author inPubMed Google Scholar * Hong-zheng Wang View author publications You can also search for this author inPubMed Google Scholar * Meng-yuan Zhu View author
publications You can also search for this author inPubMed Google Scholar * Qing-long Guo View author publications You can also search for this author inPubMed Google Scholar * Hui Hui View
author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS XL designed and performed the research; PH analyzed the data and wrote the paper; HL and XXY
performed the research and analyzed the data; XYW and YJQ performed the research; ZYW collected data and performed the statistical analysis; HZW and MYZ collected and analyzed data; and QLG
and HH conceptualized the project and directed the experimental design and data analysis. CORRESPONDING AUTHORS Correspondence to Qing-long Guo or Hui Hui. ETHICS DECLARATIONS COMPETING
INTERESTS The authors declare no competing interests. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURE 1 SUPPLEMENTARY FIGURE 2 SUPPLEMENTARY FIGURE 3 RIGHTS AND PERMISSIONS Reprints and
permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Liu, X., Hu, P., Li, H. _et al._ LW-213, a newly synthesized flavonoid, induces G2/M phase arrest and apoptosis in chronic myeloid leukemia.
_Acta Pharmacol Sin_ 41, 249–259 (2020). https://doi.org/10.1038/s41401-019-0270-4 Download citation * Received: 13 March 2019 * Accepted: 08 June 2019 * Published: 17 July 2019 * Issue
Date: February 2020 * DOI: https://doi.org/10.1038/s41401-019-0270-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry,
a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative KEYWORDS * chronic myeloid leukemia *
LW-213 * G2/M phase arrest * lysosome-dependent apoptosis * CDK9 * MCL-1