- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Maternal adversity (e.g., adverse childhood experiences, ACEs) and health (e.g., depressive symptoms and chronic illness) negatively impact offspring’s health. One possible
mechanism is via premature/accelerated biological aging, as indicated in telomere length. In this 3-year longitudinal study, we examined the association between maternal adversity and health
and children’s buccal telomere length (bTL) at age 3. Data from 122 mother-child dyads were analyzed. Maternal history of ACEs and chronic illness were collected at baseline (during 20–24
weeks of gestation). Their depressive symptoms across three periods (during pregnancy, 4 weeks after childbirth, and 3 years after childbirth) were also collected. Children’s TL were
extracted from their buccal swab samples at age 3. The children’s bTL was quantified using the quantitative PCR method and expressed in T/S ratio (the ratio of telomere repeats copy numbers
to single-copy gene numbers). Results showed pregnant women experienced distinctive trajectories of depressive symptoms over time. Children of mothers with relapsing/remitting depressive
symptoms had shorter bTL (_β_ = −0.19, 95% CI = −0.14 to −0.005) than mothers who had low-stable symptoms. This finding remained significant even after accounting for maternal ACEs and
chronic illness. Additionally, maternal ACEs, together with depressive symptoms, may affect children’s bTL. This study provides relatively comprehensive evidence on the effects of maternal
stressors, highlighting the relevance of maternal adversity and depressive symptom patterns as predictors of offspring telomere biology. SIMILAR CONTENT BEING VIEWED BY OTHERS MATERNAL
EXPERIENCE OF INTIMATE PARTNER VIOLENCE, MATERNAL DEPRESSION, AND PARENTAL STRESS ARE NOT ASSOCIATED WITH CHILD TELOMERE LENGTH IN BANGLADESH Article Open access 12 March 2025
PERICONCEPTIONAL ENVIRONMENT PREDICTS LEUKOCYTE TELOMERE LENGTH IN A CROSS-SECTIONAL STUDY OF 7–9 YEAR OLD RURAL GAMBIAN CHILDREN Article Open access 15 June 2020 MATERNAL STRESS OR SLEEP
DURING PREGNANCY ARE NOT REFLECTED ON TELOMERE LENGTH OF NEWBORNS Article Open access 19 August 2020 INTRODUCTION Prenatal exposure to maternal stress increases the risk of behavioral and
mental health problems in offspring [1]. Biological mechanisms associated with transgenerational health risks because of maternal stress have attracted increasing attention. One possible
mechanism is via premature/accelerated biological aging, as indicated in telomere length (TL). Shortened telomere length may explain long-term associations between maternal-fetal processes
and the future health of offspring [1]. Telomeres are composed of repetitive DNA sequences and play a crucial role in protecting the ends of chromosomes, ensuring the integrity of the genome
during replication [2]. TL at birth determines the initial length of an individual’s telomeres, and over time, telomeres naturally shorten due to cell replication and oxidative stress [2].
As telomeres become critically short, cells enter a state of senescence [3]. Shorter telomere length has been associated with early mortality [4], psychiatric disorders [5], and risk of
disease [6]. The fetal programming of the telomere biology hypothesis provides a conceptual framework that explains how maternal states and stress conditions during pregnancy can influence
the telomere biology of their offspring [7]. Several maternal demographic characteristics are associated with the TL of offspring, such as maternal age [8], BMI [9], and income [10]. Based
on this framework, our research primarily focuses on maternal adversity (i.e., adverse childhood experiences, ACEs) and mental (i.e., depressive symptoms) and physical (i.e., chronic
illness) health. By investigating these factors, we aim to gain insights into the development of prevention and intervention programs targeting different aspects of maternal conditions.
These insights can potentially contribute to improving the health outcomes of both mothers and their offspring. ACEs play a significant role in TL erosion as indicated by a meta-analysis
showing an association between childhood exposure to family violence and TL [11]. A longitudinal study involving 236 children also found that exposure to multiple forms of violence,
including domestic violence, bullying victimization, and maltreatment, was associated with significant telomere erosion [12]. In addition, findings regarding the relationship between
maternal ACEs and offspring biological aging are inconsistent. Some studies found that higher maternal ACEs were associated with shorter infant TL [13, 14], while others found children whose
mothers had 3+ ACEs had significantly longer DNAm telomere estimates than those whose mothers reported no ACEs [15]. These findings suggest that TL may serve as an emerging biomarker that
captures exposure to early-life adversity and predicts the risk of future psychopathology. Maternal psychological and physical health also have a critical relationship with children’s TL,
one of which is to determine whether maternal depressive symptoms during pregnancy predict TL in newborns [16]. The most common mental health problem among pregnant women is depressive
symptoms [17]. However, the literature on the association between maternal depressive symptoms and children’s TL has produced inconsistent findings. A review has summarized that maternal
depression is associated with shortened TL in children [10]. However, Ämmälä and colleagues conducted a study with 1405 infants and found that maternal depression was not a significant
factor associated with infants’ leukocyte TL [18]. Similar nonsignificant results were also found in other studies [19]. The inconsistent results in previous studies may be partly attributed
to methodological considerations. Most previous studies have investigated maternal depression at only one specific time (either during pregnancy or postpartum) and/or simply treated
depressive symptoms as a binary category (yes or no). This overly simplistic cross-sectional analysis approach generally cannot distinguish different trajectories from one another, nor can
it differentiate delayed dysfunction from chronic dysfunction, and this approach may miss relapsing/remitting trajectories altogether [20]. In contrast, growing research has emphasized the
importance of examining longitudinal trajectories of responses to stress over time [20, 21]. Trajectory analysis provides a more comprehensive understanding of the dynamic, long-term, and
individualized nature of depression, including the identification of key transition points or periods when depressive symptoms are most likely to change, compared to a single timepoint
assessment. This enables more targeted prevention and treatment approaches. Previous work has identified different individuals may exhibit distinct trajectories of depressive symptoms over
time [22, 23]. Moreover, the chronicity of prenatal psychological adversities may play a significant role in determining the magnitude of their effects on children’s TL [19, 24]. This is
consistent with the cumulative stress model, which posits that developmental exposure to stress accumulates to disrupt physical and mental health [25]. The unpredictability and instability
of prenatal psychological stressors may also be detrimental to individual health outcomes [26]. This notion is supported by the match-mismatch model, which suggests that adaptive development
is contingent upon the alignment of fetal predictions of the postnatal environment with actual postnatal environmental demands [27]. In other words, instability and unpredictability in
parental emotional states may lead to more adverse outcomes than stable levels of stress [28]. Echoing the match-mismatch model, a longitudinal study that tracked women from before
conception through pregnancy and the postpartum period found that fluctuations in maternal depressive symptoms, rather than consistent symptoms, were linked to less favorable developmental
outcomes in offspring [29]. These theoretical and empirical insights suggest that varying patterns of mental health can exert distinct influences, yet there is a paucity of research
investigating whether diverse trajectories of maternal depressive symptoms correlate with their offspring’s TL. The present study will conduct a comprehensive analysis that considers varying
patterns of maternal depressive symptoms and their distinct association with children’s TL, capturing changes in maternal depressive symptoms over time to inform more accurate interventions
targeting specific risk groups. In addition, the association of maternal chronic illness with children’s TL is inconsistent, as summarized in a review [30]. Some studies found that maternal
chronic illness was negatively associated with telomere length at birth [31], while others found no significant difference in cord blood telomere length in offspring from gestational
diabetes mellitus and normoglycemic pregnant women [32]. However, few studies have included multiple and varied types of maternal chronic illness to examine whether the severity of chronic
illness may be associated with children’s TL. This study aims to examine the association of maternal adversity, and physical and psychological health, with children’s TL. Specifically, we
assess the effects of the severity of maternal adverse childhood experiences and chronic illness. Additionally, we analyze the impact of different trajectories of maternal depression on
children’s TL 3 years after childbirth. We hypothesize that (a) more severe maternal adverse childhood experiences and chronic illness may be linked to offspring’s shorter TL; (b) distinct
trajectories of depressive symptoms among pregnant women, and those who experience a higher risk of depressive symptoms over time are more likely to have a negative impact on their
children’s TL. METHODS PARTICIPANTS Pregnant women were recruited from the antenatal clinic of Kwong Wah Hospital, a public hospital managed by the Hospital Authority in Hong Kong. Kwong Wah
Hospital has one of the city’s major obstetrics and gynaecology departments, providing services to ~5000 childbirths annually. A total of 340 pregnant women were followed from 20–24 weeks
of gestation (T1), then 4 weeks after childbirth (T2), and again 3 years after childbirth (T3). They provided information regarding demographic characteristics (marital status, employment
status, social security assistance, monthly household income) and depressive symptoms. Furthermore, buccal swab samples were provided by 122 of the 340 women’s children at age 3. The
relatively smaller sample size of children is primarily attributable to the ongoing COVID-19 pandemic at the time of data collection. During this period, caregivers were often hesitant to
permit buccal swab collection, fearing it could increase the risk of COVID-19 exposure and infection for their children. Informed consent was obtained from all participants. Details of
recruitment and inclusion criteria can be found in our previous papers [33, 34]. MEASURES OUTCOME MEASURE CHILDREN’S BUCCAL TELOMERE LENGTHS Samples of the children’s DNA were extracted from
buccal swab samples. Trained researchers helped to collect the children’s buccal swab samples following standardized instructions and procedures. Guided by the manufacturer’s instructions,
genomic DNA samples were isolated and extracted from the collected samples using the QIAamp DNA Mini kit (Qiagen). The isolated DNA samples were eluted into a buffer solution (10 mM Tris-HCl
and 1 mM ethylenediaminetetraacetic acid, pH 8.0) for quality checking and quantification. This was done using a spectrophotometer (NanoDrop 2000c, Thermo Scientific) to ensure that the DNA
quality and quantity were within an acceptable range for telomere length determination. Each DNA sample, determined to be of acceptable quality and quantity, was handled in triplicate for
the telomere length assay using quantitative polymerase chain reaction (qPCR). The qPCR was performed using a 7900HT Thermocycler (Applied Biosystems). After the telomere length assay, the
telomere length was determined by calculating the relative ratio of the telomere repeat copy number (T) to the single-copy gene 36B4 copy number (S). The formula used for this calculation
was T/S = 2(−ΔCt), where ΔCt represents the mean difference between the threshold cycle (Ct) value of the 36B4 gene and telomere repeats obtained from the qPCR. Children’s TL was
log-transformed. Details can be found elsewhere [33]. PREDICTOR MATERNAL DEPRESSIVE SYMPTOMS Pregnant women’s depressive symptoms were assessed using a 10-item Chinese Edinburgh Postnatal
Depression Scale (EPDS) [35]. Participants reported the presence of depressive symptoms experienced within the past week. Each item was rated from 0 (all the time) to 3 (not at all). All
items were summed to obtain a total score for depressive symptoms, with higher scores indicating a more severe level of depression. To screen for probable depression, we utilized a cut-off
score of ≥10 in this study. This cut-off value has been suggested as optimal for screening depressive symptoms during pregnancy and the postpartum period in Chinese mothers [36]. We used
continuous scores of depressive symptoms in the trajectories analyses. The Chinese version of the EPDS has been validated in prior studies which showed good psychometric properties [35]. In
the present study, Cronbach’s alphas for the EPDS were 0.84, 0.83, and 0.81 at T1, T2, and T3, respectively. MATERNAL ACES The Adverse Childhood Experiences (ACEs) Questionnaire by the World
Health Organization was utilized to identify childhood traumatic events [37]. Fourteen items were used to assess different domains of ACEs, such as childhood maltreatment, household
dysfunction, and exposure to war or collective violence before the age of 18 years. Participants were asked to report how frequently ACEs occurred. Each item was dichotomized into 1 =
exposed and 0 = not exposed. We summed all the ACE items to obtain a total score, which reflects the overall severity of childhood adversities experienced [34]. A higher total ACE score
indicates greater exposure to childhood adversities. Previous research has also examined these experiences in Chinese samples [38]. In the current study, the Cronbach’s alpha of the ACEs was
0.61. MATERNAL CHRONIC ILLNESS Pregnant women reported ten types of chronic illness at T1. These chronic illnesses include hypertension, heart disease, asthma, diabetes, nephropathy,
cataracts, pulmonary tuberculosis, peptic ulcer disease, skin disease, and others. Each illness was reported as 1 = yes and 0 = no. All items were summed to obtain a continuous score of
maternal chronic illness. These illnesses are the most common conditions in childbearing-age women and have also been evaluated in previous work [34]. DEMOGRAPHIC CHARACTERISTICS Demographic
characteristics about the mothers, such as maternal age, educational level, marital status, employment status, social security assistance, and monthly household income were collected. DATA
ANALYSIS The primary analyses included two steps. First, latent Class Growth Analysis (LCGA) in _Mplus_ 7.0 was conducted to identify latent classes of depressive symptoms. Data analysis and
results of LCGA have been shown in our accepted paper [39]. Specifically, intercepts and slopes for each latent class were estimated as in previous work [40]. One- to five-class
unconditional models were tested for depressive symptoms. Several criteria were used to determine the optimal class [41]: (a) lower information criteria fit indices including the Akaike’s
information criterion (AIC), the Bayesian information criterion (BIC), and the sample-size-adjusted Bayesian (SSBIC); (b) higher entropy values; (c) statistically significant _p_-values for
both the Lo-Mendell-Rubin likelihood ratio test (LRT) and the bootstrap likelihood ratio test (BLRT); and (d) the theoretical meaningfulness of group memberships. Moreover, latent classes
with less than 5% of the sample are not considered [42]. Additionally, Chi-square tests or _t_-tests were used to compare the distributions or scores of variables across different
categories. Further details regarding the data analysis and results of the depressive symptom trajectories can be found in our accepted paper [39]. Second, to test the association of
maternal ACEs, chronic illness, and trajectories of depressive symptoms with children’s TL at 3 years, linear regressions were conducted because children’s TL was a continuous variable. A
value of _p_ < 0.05 was considered to be of statistical significance. SENSITIVITY ANALYSIS On the one hand, conditional LCGA models with covariates were further conducted to adjust for
classification error and the effects of covariates. On the other hand, we conducted analyses using the BCH approach to explore whether there were differences between the trajectories related
to distal outcome variables (i.e., bTL). The BCH approach offers an omnibus test that includes differences between the two classes on the distal outcome. Based on a comparative analysis of
various methodologies, the BCH method has proven to be the most robust, consistently delivering unbiased estimates across all examined conditions [43]. ETHICAL APPROVAL The research protocol
was approved by the Institutional Review Board of the Hospital Authority Kowloon West Cluster Research Ethics Committee (Reference number: KW/FR-16-042(97-01)(1)). RESULTS The mean age of
women at baseline was 31.30 (_SD_ = 4.26). The mean of depressive symptoms was 6.98 (4.50), 4.31 (4.15), and 4.25 (4.46) at T1, T2, and T3, respectively. The prevalence of depressive
symptoms was 26.5%, 9.7%, and 12.6% at T1, T2, and T3, respectively. Latent Class Growth Analysis (LCGA) was used to identify different classes of women based on the dynamic changes in
depressive symptoms from pregnancy to 3 years after childbirth [39]. As shown in Table 1, the information criterion indices decreased from the one-class solution to the five-class solution,
indicating that models two to five are better than model one. However, models three to five had a very small group, comprising approximately 2% of the sample. This does not meet the criteria
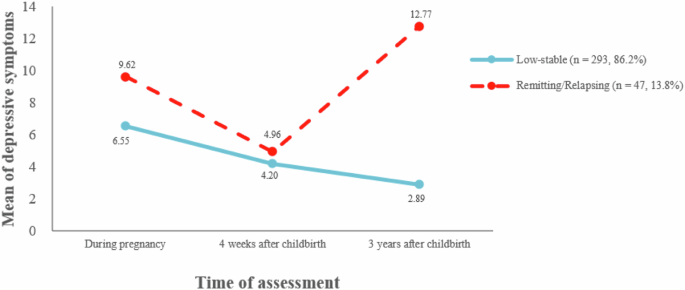
for determining the number of latent classes. Therefore, the two-class model was selected for further analysis. The two-class trajectory was [39]: The first class exhibited a trajectory
characterized by consistently low symptom ratings across all time points. This class was labeled as “the low-stable depressive symptoms” with 86.2% (_n_ = 293) of women classified in this
group. The second class displayed fluctuating depressive symptoms, demonstrating a cyclical course over time. This class was identified as “the relapsing/remitting depressive symptoms” with
13.8% (_n_ = 47) of women classified in this group. Figure 1 shows the trajectories of depressive symptoms [39]. As shown in Table 2, we compared any differences in demographic
characteristics, ACE, and chronic illness between the low-stable depressive group and the relapsing/remitting depressive group. We found no differences between these two groups in
demographic characteristics and chronic illness. However, women in the relapsing/remitting depressive symptoms group reported higher ACEs than women in the low-stable group (_p_ < 0.05).
Table 3 shows results of linear regression analyses on the association between maternal depressive symptoms, ACEs, and chronic illness and their children’s bTL. The first regression analysis
model showed the crude associations between different trajectories of depressive symptoms (or ACEs or chronic illness at T1) and children’s bTL. Results showed that children’s bTL became
shorter in those whose mothers had relapsing/remitting depressive symptoms (_β_ = −0.19, 95% CI = −0.14 to −0.005) when compared with those whose mothers were in the group of low-stable
depressive symptoms. There were no significant associations between ACEs or chronic illness at T1 and children’s bTL. In the adjusted models 2–4, when adjusting for the severity of chronic
illness, the relationship between the remitting/relapsing depressive group and shorter telomere length remained significant, while the association of chronic illness with children’s bTL was
not significant. Additionally, when adjusted for ACEs, the longitudinal associations of the remitting/relapsing depressive symptoms increased and the influence of ACEs became positively
associated with children’s bTL. These results remained consistent when adjusting for both ACEs and the severity of chronic illness. Results from the sensitivity analysis of the conditional
LCGA model (see Table S1) indicated that the 2-class model remains the most suitable for data fitting. Upon examination of the trajectories associated with the 2-class model, the same
patterns as the unconditional LCGA model were identified: a low-stable depressive symptoms group (_n_ = 292, 86%) and a relapsing/remitting depressive symptoms group (_n_ = 48, 14%). For the
results in bTL as assessed via the BCH method (see Table S2), it appears that children of mothers in the group with relapsing/remitting depressive symptoms may have shorter TL compared to
children of mothers in the low-stable depressive symptoms group. These findings align with our previously reported results as presented in Tables 1, 3. Consequently, we keep our initial
analytical approach and findings. DISCUSSION MAIN FINDINGS This study presents preliminary evidence that mothers exposed to a range of stressors, including adverse childhood experiences and
remitting/relapsing depressive symptoms, are associated with buccal telomere length (bTL) in their children at age 3. These findings expand on the concept of biological embedding [44] by
highlighting the importance of diverse stressors experienced by mothers in understanding the biological aging of their offspring. In this study, different women had different trajectories of
depressive symptoms over time. We identified about 13.8% experiencing relapsing/remitting depressive symptoms. The traditional approach of categorizing depression as a binary “yes” or “no”
overlooks the nuanced and variable nature of depressive symptoms, failing to capture the diverse ways in which individuals may present. In contrast, the person-centered approach employs
statistical techniques like cluster analysis or latent profile analysis to identify naturally occurring subgroups. These subgroups are defined by specific patterns of variables or shared
characteristics that differentiate them from other individuals or groups [45]. By identifying and analyzing these subgroups, researchers can customize interventions, policies, or programs to
better address the unique needs of different individuals or groups. Our successful categorization of depressive symptoms aligns with the literature in which diverse patterns of depressive
symptoms have also been observed [22, 23]. More importantly, our study revealed that the children of mothers with relapsing/remitting depressive symptoms had shorter bTL. This finding
remained significant even after accounting for maternal ACEs and chronic illness. Notably, in our current dataset, continuous depressive symptoms in mothers did not show a significant
correlation with their children’s bTL. Nevertheless, distinct trajectories of depressive symptoms were found to variously predict children’s bTL, underscoring the possibility that it is the
pattern of change in depressive symptoms that influences child bTL. Previous studies have reported inconsistent results regarding the effects of maternal depressive symptoms on children’s TL
[10, 18, 46]. To reconcile these inconsistencies, our current study highlights the importance of distinguishing between different trajectories of depressive symptoms. The significant impact
of maternal relapsing/remitting depressive symptoms on children’s telomere length, as compared to the low-stable group, provides valuable insights for the development of prevention and
intervention strategies targeting women at the highest risk (i.e., those experiencing relapsing/remitting depressive symptoms in our study). This information can guide efforts to identify
and provide support to women experiencing relapsing/remitting depressive symptoms, with the ultimate aim of mitigating the potential adverse effects on their children’s bTL. Two putative
biological mechanisms may explain the association between maternal depression trajectories and children’s TL. The first is through the offspring’s hypothalamic-pituitary-adrenal (HPA) axis
stress response. Early-life stressors like maternal mental health problems may activate the HPA axis, resulting in the secretion of cortisol. Elevated cortisol levels have been linked to
accelerated telomere shortening and cellular aging [4]. It concurs with a study that daughters of depressed mothers had shorter telomeres than daughters of never-depressed mothers and that
shorter telomeres were associated with greater cortisol reactivity to stress [47]. Another potential mechanism is through the offspring’s immune function. Fetal exposure to maternal
depression during pregnancy has been shown to have lasting effects on the child’s immune system later in life [48]. The chronic inflammatory responses stemming from these immune disturbances
may contribute to telomere attrition over time [49]. Importantly, we found that it may be the unpredictable, relapsing/remitting nature of maternal depressive symptoms that is particularly
detrimental. A study found that individuals dealing with this type of fluctuating mood and unpredictable depressive episodes often feel a heightened sense of hopelessness and lack of control
over their symptoms [26]. This pervasive uncertainty and instability may exacerbate the HPA axis dysregulation and immune system disturbances, thereby accelerating the shortening of the
child’s telomeres. In summary, our current findings, in conjunction with prior research [28, 29], underscore the even more severe consequences of the unpredictability of maternal mental
health on child health outcomes. These results support the match-mismatch model as delineated in the Introduction section. Unfortunately, our current dataset did not include the necessary
measures to empirically test these proposed mechanisms. Future research is needed to validate these explanations and provide a more conclusive understanding of the pathways linking maternal
relapsing/remitting depression and child telomere length. When considering the impact of the severity of maternal ACEs, the longitudinal associations of remitting/relapsing depressive
symptoms increased, while associations of ACEs became positive. The intergenerational link between maternal ACEs and the health of children (e.g., TL), may be elucidated by
neurodevelopmental programming. The transgenerational transmission of maternal preconception adversity, spanning prenatal and postnatal periods, is thought to be mediated through a complex
interplay of factors, including epigenetic modifications in the germline, changes to the intrauterine environment, and variations in postnatal caregiving practices, or more plausibly, a
combination of them [14, 50]. Indeed, the role of epigenetic mechanisms as a fundamental molecular mechanism has been extensively discussed. A meta-analysis has synthesized evidence
suggesting that DNA methylation likely contributes to the influence of prenatal maternal stress on adverse neurodevelopmental outcomes in offspring [51]. The literature has inconsistent
results about associations between maternal ACEs and epigenetic aging of their offspring [14, 15]. A study has found individuals with higher ACEs had greater TL [52]. The potential reason
might be that longer telomeres could serve as markers for survival, indicating a greater potential for a longer lifespan. The heightened survival potential conferred by longer telomeres may
have enabled these individuals to overcome life’s challenges more effectively. Without long telomeres at birth or a mechanism to maintain them, they would not have been able to overcome
those challenges as successfully [52]. In addition, the longitudinal associations of remitting/relapsing depressive symptoms increased. This may suggest that mothers with ACEs may be more
susceptible to experiencing depressive symptoms, which, in turn, could contribute to the shortening of their children’s TL. A study has revealed that ACEs increase the risk of depressive
symptoms in women during pregnancy and the postpartum period [53]. Furthermore, a national longitudinal cohort study demonstrated that mothers with incarcerated partners (adversity) were
more prone to experiencing depression when their children were between the ages of 9 and 15 years. This increased maternal depression was associated with accelerated telomere length
shortening in children [54]. The associations between maternal ACEs, depressive symptoms, and offspring’s TL were not tested in the current study due to limited sample sizes. It is thus
recommended that future studies address this topic to further explore these associations. In our study, we did not find a significant relationship between maternal chronic illness and
children’s bTL. However, it is important to consider the characteristics of our sample, as they may have influenced these results. Pregnant women included in our study were relatively young,
and there was a low prevalence of chronic illnesses among them, with most reporting having only one or fewer types of illness. Additionally, our study utilized non-clinical samples, which
may have limited our ability to detect the influence of chronic illness on telomere length. Further research is necessary to gain a more comprehensive understanding of the links of maternal
chronic illness to the telomere length of offspring. It would be valuable to investigate whether there are differences between clinical and non-clinical samples regarding the impact of
chronic illness, to provide more targeted support and better understand the potential effects on children’s telomere length. STRENGTHS Our current study has two notable strengths. Firstly,
we took a comprehensive approach by considering a wide range of factors related to maternal stressors, including adverse childhood experiences and health factors. By incorporating this
comprehensive set of information, we were able to provide a more integrated understanding of how various stressors experienced by mothers interact and influence the biological health of
their children. Secondly, we made a significant contribution to the conflicting literature by differentiating between different trajectories of depressive symptoms. This effort to identify
distinct patterns of depressive symptoms among women has provided more nuanced information for prevention and intervention efforts targeted toward women who may require the most support.
LIMITATIONS Several limitations should be acknowledged. Firstly, the measurements of independent variables were self-reported, which may introduce reporting bias. Secondly, we did not have
data on child sex, which may influence the children’s TL [16]. Thirdly, we only assessed buccal TL at 3 years after childbirth and did not have data on bTL at baseline. Also, we were unable
to adjust for cell types, although TL in different tissues is highly correlated [55]. Fourthly, we did not have data on maternal telomere length, which may affect the interpretation of our
findings. Previous studies have shown that telomere length is highly heritable [56]. The lack of maternal telomere length data means we cannot fully account for the potential
intergenerational influence on the telomere length of the individuals in our study. Fifth, the sample of children with available TL data is relatively small, with only 122 out of the 340
women’s children providing buccal swab samples. This limited sample size may affect the reliability and generalizability of the results. We encourage future studies to expand the sample size
to validate our findings further. Finally, we only recruited pregnant women at a single antenatal clinic of a public hospital in Hong Kong, which may limit our generalizability to other
samples. IMPLICATIONS This study has contributed to our understanding that women experience different trajectories of depressive symptoms, and it is more likely that the children of those in
the highest-risk group (i.e., the relapsing/remitting group in our study) would have shortened TL. While some studies have shown an association between maternal depressive symptoms and
offspring’s TL [10], in most studies it was assumed that women are homogeneous and experience the same changes in depression. Our findings highlight the considerable variation in maternal
depression from pregnancy through the postpartum period and the importance of routinely assessing maternal depression during this time to identify opportunities for better support. Moreover,
our study demonstrates that different trajectories of depressive symptoms have different associations with children’s future TL. This suggests that tailored treatments should be developed
to address the specific needs and levels of maternal risk within each trajectory. Nonpharmacological interventions such as cognitive-behavioral therapy [57] and physical exercise programs
[58] may enhance women’s motivation to cope with their depression. It would be valuable if future research were to evaluate whether these interventions have differential benefits for
decreasing remitting/relapsing depressive symptoms. In summary, our study highlights the importance of recognizing the heterogeneity of maternal depression trajectories and the need for
personalized interventions to support women at different levels of risk. Routine assessment of maternal depression and the evaluation of tailored interventions can contribute to improved
outcomes for both women and their children. In addition, we found maternal adverse childhood experiences may affect mothers’ depressive symptoms and children’s TL. This finding informs that
addressing maternal history of adversity together with mothers’ mental health problems could benefit children, even at the cellular level. Trauma-informed care is promising to respond to the
impacts of trauma appropriately. It is a comprehensive and multilevel approach that could help service providers and clients understand the impact of traumatic events on health indicators
and behaviors [59]. Perinatal care providers (e.g., perinatal nurses) are well-positioned to provide trauma-informed perinatal care, which could prevent or reduce the negative impact of ACEs
[60]. CONCLUSIONS This study provides evidence of an association between maternal relapsing/remitting depressive symptoms and the shortening of children’s TL, even when accounting for
maternal adverse childhood experiences and chronic illness. Furthermore, we found that maternal adverse childhood experiences, when combined with depressive symptoms, link to children’s TL.
These findings contribute to the concept of biological embedding, which suggests that early life experiences and maternal health can influence the biological processes and outcomes of
offspring. DATA AVAILABILITY Data used to support the findings of this study are available from the corresponding author upon reasonable request. CODE AVAILABILITY The data analysis
methodology for LCGA is well-established and widely adopted in the literature. The corresponding codes are publicly available on the official _Mplus_ website. REFERENCES * Entringer S, Buss
C, Wadhwa PD. Prenatal stress, development, health and disease risk: a psychobiological perspective. Psychoneuroendocrinology. 2015;62:366–75. Article PubMed PubMed Central Google Scholar
* Blackburn EH, Epel ES, Lin J. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science. 2015;350:1193–98. Article CAS PubMed
Google Scholar * Bernadotte A, Mikhelson VM, Spivak IM. Markers of cellular senescence. telomere shortening as a marker of cellular senescence. Aging. 2016;8:3–11. Article CAS PubMed
PubMed Central Google Scholar * Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, Lin J, et al. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology.
2013;38:1835–42. Article CAS PubMed PubMed Central Google Scholar * Malouff JM, Schutte NS. A meta-analysis of the relationship between anxiety and telomere length. Anxiety Stress
Coping. 2017;30:264–72. Article PubMed Google Scholar * Schneider CV, Schneider KM, Teumer A, Rudolph KL, Hartmann D, Rader DJ, et al. Association of telomere length with risk of disease
and mortality. JAMA Intern Med. 2022;182:291–300. Article CAS PubMed PubMed Central Google Scholar * Entringer S, de Punder K, Buss C, Wadhwa PD. The fetal programming of telomere
biology hypothesis: an update. Philos Trans R Soc Lond B Biol Sci. 2018;373:20170151. Article PubMed PubMed Central Google Scholar * Ly K, Walker C, Berry S, Snell R, Marks E, Thayer Z,
et al. Telomere length in early childhood is associated with sex and ethnicity. Sci Rep. 2019;9:10359. Article PubMed PubMed Central Google Scholar * Martens DS, Plusquin M, Gyselaers W,
De Vivo I, Nawrot TS. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med. 2016;14:148. Article PubMed PubMed Central Google Scholar * Coimbra BM, Carvalho CM,
Moretti PN, Mello MF, Belangero SI. Stress-related telomere length in children: a systematic review. J Psychiatr Res. 2017;92:47–54. Article PubMed Google Scholar * Chen XY, Lo CKM, Chan
KL, Leung WC, Ip P. Association between childhood exposure to family violence and telomere length: a meta-analysis. Int J Environ Res Public Health. 2022;19:12151. Article PubMed PubMed
Central Google Scholar * Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, Danese A, et al. Exposure to violence during childhood is associated with telomere erosion from 5–10 years of
age: a longitudinal study. Mol Psychiatry. 2013;18:576–81. Article CAS PubMed Google Scholar * Esteves KC, Jones CW, Wade M, Callerame K, Smith AK, Theall KP, et al. Adverse childhood
experiences: Implications for offspring telomere length and psychopathology. Am J Psychiatry. 2020;177:47–57. Article PubMed Google Scholar * Jones CW, Esteves KC, Gray SAO, Clarke TN,
Callerame K, Theall KP, et al. The transgenerational transmission of maternal adverse childhood experiences (ACEs): insights from placental aging and infant autonomic nervous system
reactivity. Psychoneuroendocrinology. 2019;106:20–27. Article PubMed PubMed Central Google Scholar * Nwanaji-Enwerem JC, Van Der Laan L, Kogut K, Eskenazi B, Holland N, Deardorff J, et
al. Maternal adverse childhood experiences before pregnancy are associated with epigenetic aging changes in their children. Aging. 2021;13:25653. Article CAS PubMed PubMed Central Google
Scholar * Enlow MB, Bollati V, Sideridis G, Flom JD, Hoxha M, Hacker MR, et al. Sex differences in effects of maternal risk and protective factors in childhood and pregnancy on newborn
telomere length. Psychoneuroendocrinology. 2018;95:74–85. Article PubMed Central Google Scholar * Sheeba B, Nath A, Metgud CS, Krishna M, Venkatesh S, Vindhya J, et al. Prenatal
depression and its associated risk factors among pregnant women in Bangalore: a hospital based prevalence study. Front Public Health. 2019;7:108. Article CAS PubMed PubMed Central Google
Scholar * Ämmälä A-J, Vitikainen EIK, Hovatta I, Paavonen J, Saarenpää-Heikkilä O, Kylliäinen A, et al. Maternal stress or sleep during pregnancy are not reflected on telomere length of
newborns. Sci Rep. 2020;10:13986. Article PubMed PubMed Central Google Scholar * Naudé PJ, Stein DJ, Lin J, Zar HJ. Investigating the association of prenatal psychological adversities
with mother and child telomere length and neurodevelopment. J Affect Disord. 2023;340:675–85. Article PubMed Google Scholar * Norris FH, Tracy M, Galea S. Looking for resilience:
understanding the longitudinal trajectories of responses to stress. Soc Sci Med. 2009;68:2190–8. Article PubMed Google Scholar * Bonanno GA, Mancini AD. Beyond resilience and PTSD:
mapping the heterogeneity of responses to potential trauma. Psychol Trauma. 2012;4:74–83. Article Google Scholar * Baron E, Bass J, Murray SM, Schneider M, Lund C. A systematic review of
growth curve mixture modelling literature investigating trajectories of perinatal depressive symptoms and associated risk factors. J Affect Disord. 2017;223:194–208. Article PubMed PubMed
Central Google Scholar * Santos H Jr., Tan X, Salomon R. Heterogeneity in perinatal depression: how far have we come? a systematic review. Arch Womens Ment Health. 2017;20:11–23. Article
PubMed Google Scholar * Rentscher KE, Carroll JE, Mitchell C. Psychosocial stressors and telomere length: A current review of the science. Annu Rev Public Health. 2020;41:223–45. Article
PubMed Google Scholar * Nederhof E, Schmidt MV. Mismatch or cumulative stress: Toward an integrated hypothesis of programming effects. Physiol Behav. 2012;106:691–700. Article CAS PubMed
Google Scholar * Chen XY, Zhou Y, Shi X, Ma Z, Fan F. Longitudinal associations between adolescents’ trajectory membership of depressive symptoms and suicidality in young adulthood: a
10-year cohort of Chinese Wenchuan earthquake survivors. Epidemiol Psychiatr Sci. 2020;29:e175. Article PubMed PubMed Central Google Scholar * Gluckman PD, Cutfield W, Hofman P, Hanson
MA. The fetal, neonatal, and infant environments-the long-term consequences for disease risk. Early Hum Dev. 2005;81:51–59. Article PubMed Google Scholar * Davis EP, Glynn LM. Annual
research review: the power of predictability - patterns of signals in early life shape neurodevelopment and mental health trajectories. J Child Psychol Psychiatry. 2024;65:508–34. Article
PubMed Google Scholar * Rinne GR, Davis EP, Mahrer NE, Guardino CM, Charalel JM, Shalowitz MU, et al. Maternal depressive symptom trajectories from preconception through postpartum:
associations with offspring developmental outcomes in early childhood. J Affect Disord. 2022;309:105–14. Article PubMed PubMed Central Google Scholar * Oerther S, Lorenz R. State of the
science: using telomeres as biomarkers during the first 1000 days of life. West J Nurs Res. 2018;41:305–25. Article PubMed Google Scholar * Schneper LM, Drake AJ, Dunstan T, Kotenko I,
Notterman DA, Piyasena C. Characteristics of salivary telomere length shortening in preterm infants. PLoS ONE. 2023;18:e0280184. Article CAS PubMed PubMed Central Google Scholar *
Pérez‑López FR, López‑Baena MT, Ulloque-Badaracco JR, Benites-Zapata VA. Telomere length in patients with gestational diabetes mellitus and normoglycemic pregnant women: A systematic review
and meta-analysis. Reprod Sci. 2024;31:45–55. * Chan KL, Lo CKM, Ho FK, Leung WC, Yee BK, Ip P. The association between intimate partner violence against women and newborn telomere length.
Transl Psychiatry. 2019;9:239. Article PubMed PubMed Central Google Scholar * Chen XY, Lo CKM, Ho FK, Leung WC, Ip P, Chan KL. Changing patterns of Iintimate partner violence against
pregnant women: a three-year longitudinal study. Int J Environ Res Public Health. 2022;19:14397. Article PubMed PubMed Central Google Scholar * Lee DTS, Yip SK, Chiu HFK, Leung TYS, Chan
KPM, Chau IOL, et al. Detecting postnatal depression in Chinese women: validation of the Chinese version of the Edinburgh Postnatal Depression Scale. Br J Psychiatry. 1998;172:433–37.
Article CAS PubMed Google Scholar * Sha T, Gao X, Chen C, Li L, Cheng G, Wu X, et al. A prospective study of maternal postnatal depressive symptoms with infant-feeding practices in a
Chinese birth cohort. BMC Pregnancy Childbirth. 2019;19:388. Article PubMed PubMed Central Google Scholar * WHO. Adverse childhood experiences international questionnaire (ACE-IQ). 2018.
https://www.who.int/publications/m/item/adverse-childhood-experiences-international-questionnaire-(ace-iq). Accessed date on 5 April 2024. * Ho GWK, Chan ACY, Chien WT, Bressington DT,
Karatzias T. Examining patterns of adversity in Chinese young adults using the adverse childhood experiences-international questionnaire (ACE-IQ). Child Abuse Negl. 2019;88:179–88. Article
PubMed Google Scholar * Chen XY, Lo CK, Wong RS, Tung KTS, Tso WWY, Ho FK, et al. Trajectories and predictors of depressive symptoms among pregnant women: a 3-year longitudinal study.
Psychol Trauma. 2024. https://doi.org/10.1037/tra0001750. * Shevlin M, Butter S, McBride O, Murphy J, Gibson-Miller J, Hartman TK, et al. Refuting the myth of a ‘tsunami’ of mental
ill-health in populations affected by COVID-19: evidence that response to the pandemic is heterogeneous, not homogeneous. Psychol Med. 2023;53:429–37. PubMed Google Scholar * Nylund KL,
Asparouhov T, Muthén BO. Deciding on the number of classes in latent class analysis and growth mixture modeling: A Monte Carlo simulation study. Struct Equ Modeling. 2007;14:535–69. Article
Google Scholar * Muthén B, Muthén LK. Integrating person‐centered and variable‐centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol Clin Exp Res.
2000;24:882–91. Article PubMed Google Scholar * Bakk Z, Vermunt JK. Robustness of stepwise latent class modeling with continuous distal outcomes. Struct Equ Modeling. 2015;23:20–31.
Article Google Scholar * Hertzman C. Putting the concept of biological embedding in historical perspective. Proc Natl Acad Sci USA. 2012;109:17160–67. Article CAS PubMed PubMed Central
Google Scholar * Howard MC, Hoffman ME. Variable-centered, person-centered, and person-specific approaches. Organ Res Methods. 2017;21:846–76. Article Google Scholar * Beijers R, Daehn
D, Shalev I, Belsky J, de Weerth C. Biological embedding of maternal postpartum depressive symptoms: the potential role of cortisol and telomere length. Biol Psychol. 2020;150:107809.
Article PubMed Google Scholar * Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, Joormann J, et al. Telomere length and cortisol reactivity in children of depressed mothers.
Mol Psychiatry. 2015;20:615–20. Article CAS PubMed Google Scholar * Plant DT, Pawlby S, Sharp D, Zunszain PA, Pariante CM. Prenatal maternal depression is associated with offspring
inflammation at 25 years: A prospective longitudinal cohort study. Transl Psychiatry. 2016;6:e936. Article CAS PubMed PubMed Central Google Scholar * Verhoeven JE, Revesz D, Wolkowitz
OM, Penninx BW. Cellular aging in depression: Permanent imprint or reversible process?: an overview of the current evidence, mechanistic pathways, and targets for interventions. Bioessays.
2014;36:968–78. Article PubMed Google Scholar * Bale TL. Epigenetic and transgenerational reprogramming of brain development. Nat Rev Neurosci. 2015;16:332–44. Article CAS PubMed
PubMed Central Google Scholar * Kotsakis Ruehlmann A, Sammallahti S, Cortés Hidalgo AP, Bakulski KM, Binder EB, Campbell ML, et al. Epigenome-wide meta-analysis of prenatal maternal
stressful life events and newborn DNA methylation. Mol Psychiatry. 2023;28:5090–100. Article CAS PubMed Google Scholar * Oliveira BS, Zunzunegui MV, Quinlan J, Batistuzzo de Medeiros SR,
Thomasini RL, Guerra RO. Lifecourse adversity and telomere length in older women from northeast Brazil. Rejuvenation Res. 2018;21:294–303. Article PubMed Google Scholar * Racine N,
Zumwalt K, McDonald S, Tough S, Madigan S. Perinatal depression: the role of maternal adverse childhood experiences and social support. J Affect Disord. 2020;263:576–81. Article PubMed
Google Scholar * Del Toro J, Fine A, Wang MT, Thomas A, Schneper LM, Mitchell C, et al. The longitudinal associations between paternal incarceration and family well-being: Implications for
ethnic/racial disparities in health. J Am Acad Child Adolesc Psychiatry. 2021;61:423–33. Article PubMed PubMed Central Google Scholar * Okuda K. Telomere length in the newborn. Pediatr
Res. 2002;52:377–81. Article PubMed Google Scholar * Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, Willemsen G, et al. Meta-analysis of telomere length in 19,713 subjects reveals high
heritability, stronger maternal inheritance and a paternal age effect. Eur J Hum Genet. 2013;21:1163–8. Article CAS PubMed PubMed Central Google Scholar * Shortis E, Warrington D,
Whittaker P. The efficacy of cognitive behavioral therapy for the treatment of antenatal depression: a systematic review. J Affect Disord. 2020;272:485–95. Article PubMed Google Scholar *
Vargas-Terrones M, Barakat R, Santacruz B, Fernandez-Buhigas I, Mottola MF. Physical exercise programme during pregnancy decreases perinatal depression risk: a randomised controlled trial.
Br J Sports Med. 2018;53:348–53. Article PubMed Google Scholar * Substance Abuse & Mental Health Services Administration. SAMHSA’s concept of trauma and guidance for a trauma-informed
approach. HHS Publication No. (SMA) 14-4884. Rockville, MD: Substance Abuse and Mental Health Services Administration. 2014. * Gillis BD, Parish AL. Group-based interventions for postpartum
depression: an integrative review and conceptual model. Arch Psychiatr Nurs. 2019;33:290–98. Article PubMed Google Scholar Download references ACKNOWLEDGEMENTS The work described in this
paper was supported by a fellowship award from the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. PolyU/SRFS2223-5H01) and APSS Research Fund
(P0046000). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * School of Psychology, Fujian Normal University, Fuzhou, China Xiao-Yan Chen * Department of Applied Social Sciences, The Hong Kong
Polytechnic University, Hung Hom, Hong Kong Camilla K. M. Lo, Qiqi Chen & Ko Ling Chan * School of Health and Wellbeing, University of Glasgow, Glasgow, UK Frederick K. Ho * Department
of Obstetrics & Gynaecology, Kwong Wah Hospital, Kowloon, Hong Kong Wing Cheong Leung Authors * Xiao-Yan Chen View author publications You can also search for this author inPubMed Google
Scholar * Camilla K. M. Lo View author publications You can also search for this author inPubMed Google Scholar * Qiqi Chen View author publications You can also search for this author
inPubMed Google Scholar * Frederick K. Ho View author publications You can also search for this author inPubMed Google Scholar * Wing Cheong Leung View author publications You can also
search for this author inPubMed Google Scholar * Ko Ling Chan View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS XYC: Conceptualization,
Investigation, Data analysis, Visualization, Writing—original draft, Writing— review and editing; CKML: Supervision, Writing— review and editing; QC, FKH, and WCL: Methodology,
Writing—review and editing; KLC: Conceptualization, Project administration, Funding acquisition, Investigation, Supervision, Writing—review and editing. CORRESPONDING AUTHORS Correspondence
to Qiqi Chen or Ko Ling Chan. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION TABLE S1, TABLE S2 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed
under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits any non-commercial use, sharing, distribution and reproduction in any medium or
format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if you modified the licensed material.
You do not have permission under this licence to share adapted material derived from this article or parts of it. The images or other third party material in this article are included in the
article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit
http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Chen, XY., Lo, C.K.M., Chen, Q. _et al._ Associations between maternal
adversity and health and children’s telomere length. _Transl Psychiatry_ 15, 106 (2025). https://doi.org/10.1038/s41398-025-03340-4 Download citation * Received: 05 April 2024 * Revised: 23
December 2024 * Accepted: 19 March 2025 * Published: 28 March 2025 * DOI: https://doi.org/10.1038/s41398-025-03340-4 SHARE THIS ARTICLE Anyone you share the following link with will be able
to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative





