- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Obsessive-compulsive disorder (OCD) is associated with a high disease burden, and treatment options are limited. We used intensified electrical stimulation in two dosages to target
a main circuitry associated with the pathophysiology of OCD, left dorsolateral prefrontal cortex (l-DLPFC), and pre-supplementary motor area (pre-SMA) and assessed clinical outcomes,
neuropsychological performance, and brain physiology. In a double-blind, randomized controlled trial, thirty-nine patients with OCD were randomly assigned to three groups of sham, 2-mA, or
1-mA transcranial direct current stimulation (tDCS) targeting the l-DLPFC (F3) and pre-SMA (FC2) with anodal and cathodal stimulation respectively. The treatment included 10 sessions of
20-minute stimulation delivered twice per day with 20-min between-session intervals. Outcome measures were reduction in OCD symptoms, anxiety, and depressive states, performance on a
neuropsychological test battery (response inhibition, working memory, attention), oscillatory brain activities, and functional connectivity. All outcome measures except EEG were examined at
pre-intervention, post-intervention, and 1-month follow-up times. The 2-mA protocol significantly reduced OCD symptoms, anxiety, and depression states and improved quality of life after the
intervention up to 1-month follow-up compared to the sham group, while the 1-mA protocol reduced OCD symptoms only in the follow-up and depressive state immediately after and 1-month
following the intervention. Both protocols partially improved response inhibition, and the 2-mA protocol reduced attention bias to OCD-related stimuli and improved reaction time in working
memory performance. Both protocols increased alpha oscillatory power, and the 2-mA protocol decreased delta power as well. Both protocols increased connectivity in higher frequency bands at
frontal-central areas compared to the sham. Modulation of the prefrontal-supplementary motor network with intensified tDCS ameliorates OCD clinical symptoms and results in beneficial
cognitive effects. The 2-mA intensified stimulation resulted in larger symptom reduction and improved more converging outcome variables related to therapeutic efficacy. These results support
applying the intensified prefrontal-SMA tDCS in larger trials. SIMILAR CONTENT BEING VIEWED BY OTHERS EFFICACY AND SAFETY OF TRANSCRANIAL DIRECT CURRENT STIMULATION AS AN ADD-ON TREATMENT
FOR OBSESSIVE-COMPULSIVE DISORDER: A RANDOMIZED, SHAM-CONTROLLED TRIAL Article 15 January 2021 INVESTIGATING THE EFFECTS OF BRAIN STIMULATION ON THE NEURAL SUBSTRATES OF INHIBITION IN
PATIENTS WITH OCD: A SIMULTANEOUS TDCS – FMRI STUDY Article Open access 19 May 2025 EFFICACY AND TOLERABILITY OF REPETITIVE TRANSCRANIAL MAGNETIC STIMULATION FOR THE TREATMENT OF
OBSESSIVE-COMPULSIVE DISORDER IN ADULTS: A SYSTEMATIC REVIEW AND NETWORK META-ANALYSIS Article Open access 28 May 2021 INTRODUCTION With a lifetime prevalence of 2–3%, Obsessive-compulsive
disorder (OCD) is one of the most disabling psychiatric disorders [1], with substantial functional impairment and increased risk of early mortality [2, 3]. Individuals with OCD have unwanted
and distressing thoughts (obsessions) and repetitive behaviors that the individual feels driven to perform (compulsions) [4]. While cognitive-behavioral therapy with exposure/response
prevention and serotonin reuptake inhibitor medication are considered first-line treatments for OCD, up to 40% of patients fail to respond to these treatments [5]. Non-invasive brain
stimulation techniques provide unique opportunities to not only study brain functions but also to modify core physiological parameters of human behavior and cognition (e.g., neuroplasticity)
in both healthy and clinical populations [6, 7]. Some non-invasive brain stimulation techniques, such as repetitive transcranial magnetic stimulation (rTMS), are Food and Drug
Administration (FDA)-approved for the treatment of several major neuropsychiatric disorders, including OCD [8], suggesting that other forms of techniques may be considered as a potential
intervention for patients with OCD. Transcranial direct current stimulation (tDCS) is a non-invasive brain stimulation technique that uses a weak direct electrical current to modulate brain
activity and excitability [9]. The exact mechanisms by which tDCS works are not fully understood, but its primary mechanism of action, which emerges immediately during stimulation, involves
subthreshold de- or hyperpolarization of neuronal membrane potentials, resulting in excitability-enhancing effects by anodal and excitability-reducing effects by cathodal stimulation in
conventional protocols [10, 11]. In neuropsychiatric disorders that are characterized by functional brain abnormalities (i.e., hyper- or hypoactivity of specific brain region/s and
network/s), it is possible to modify altered brain functions with tDCS and affect target behavior or cognition [12,13,14,15,16]. In OCD, results of tDCS studies have been mixed so far, and
knowledge is still limited about optimal stimulation parameters and efficacy of interventions, such as in other clinical non-invasive brain stimulation scenarios [12, 17,18,19,20].
Functional abnormalities of the dorsolateral prefrontal cortex (DLPFC) are documented in OCD [21]. Specifically, response inhibition, a core cognitive ability that is severely impaired in
OCD is linked to several regions of the prefrontal cortex, including the DLPFC and inferior frontal gyrus [22,23,24]. Another cortical region that is consistently shown to be involved in the
pathophysiology of OCD is the pre-supplementary Motor Area (pre-SMA), which is important for inhibitory control, especially of ongoing actions [25,26,27]. In OCD patients, the pre-SMA is
hyperactive, especially during cognitive task performance that requires attentional and inhibitory control [28, 29], and is, therefore, a major target of non-invasive brain stimulation
treatment [17, 30, 31]. Although the left DLPFC and pre-SMA have been targeted in previous tDCS studies, targeting both regions with anodal and cathodal stimulation respectively has not been
reported so far [19, 32]. Applying a protocol that can modulate the prefrontal-SMA network and presumably restore physiological abnormalities can have therapeutic effects. Beyond the choice
of the target region, stimulation parameters (e.g., stimulation intensity and repetition) are critical for the efficacy of the neurostimulation intervention, and recent work stress on
optimizing and/or individualizing the intervention [33, 34]. Physiological findings in healthy humans have shown that repeated stimulation with a short interval (e.g., two consecutive
stimulation sessions with a 20 min interval) can induce long-lasting LTP-like plasticity in the brain [35]. This has implications for the clinical application of tDCS. We recently showed
that such a stimulation protocol, which we refer to as “intensified” protocol, has stronger and longer therapeutic effects on social anxiety disorder [36]. In the present study, we adopted
the same stimulation protocol and furthermore, included different outcome variables to evaluate treatment efficacy. In addition to primary clinical symptoms, we assessed core cognitive
deficits in OCD patients (e.g., response inhibition, working memory) [37] and monitored changes in the oscillatory power spectrum and functional connectivity of the brain, which are
abnormally changed in OCD, such as reduced and raised alpha and delta power respectively and reduced functional connectivity [38,39,40,41]. Accordingly, in this registered, randomized,
double-blind, sham-controlled clinical trial we aimed to (1) investigate the effect of intensified stimulation over prefrontal and pre-SMA regions on primary and secondary clinical variables
in patients with OCD, (2) explore the stimulation dosage-dependency (1-mA vs 2-mA) of treatment efficacy, (3) and examine the effects of these interventions on cognitive (response
inhibition, attention bias) and electrophysiological (oscillatory power, functional connectivity) correlates of the psychopathology of OCD. This is the first tDCS RCT in OCD to explore the
effects of a novel intensified tDCS intervention at two different stimulation intensities on symptom reduction and neurocognitive correlates of OCD. METHODS PARTICIPANTS This study had a
randomized, double-blind, parallel-group design to prevent blinding failure and carry-over effects. Thirty-nine individuals diagnosed with OCD (mean age = 31.59, SD = 8.24, 26 females) were
recruited from several neuropsychiatric clinics in Ardabil, Iran from August 2020 to January 2022. Patients were randomly assigned to the active and sham stimulation groups by the block
randomization method (supplementary content). The sample size was calculated a priori based on a medium effect size suggested for tDCS studies [42] (_f_ = 0.30, _α_ = 0.05, power = 0.95, _N_
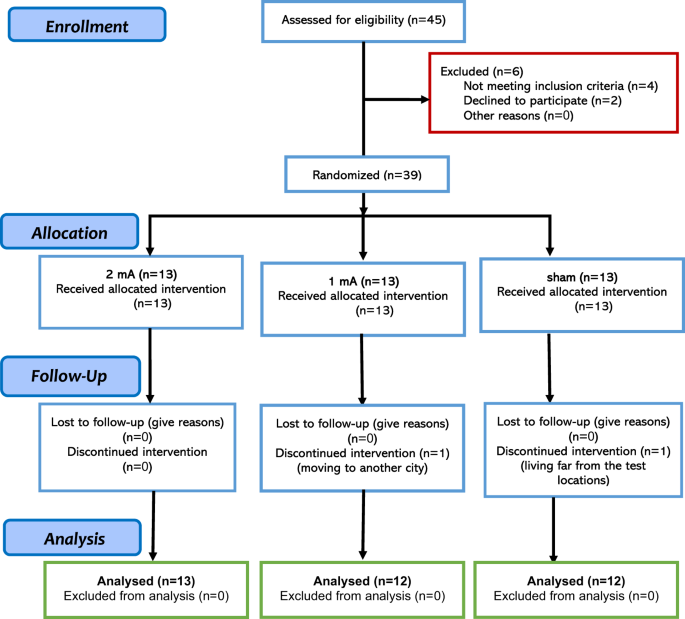
= 39, mixed-model ANOVA with 3 measurements). Two patients from the 1-mA and sham groups did not complete the whole treatment, and final analysis was conducted on 37 participants (1 mA tDCS
_N_ = 12, 2 mA tDCS _N_ = 13, sham tDCS _N_ = 12) (Fig. 1). The inclusion criteria were: (1) diagnosis of OCD according to DSM-5, (2) being 18–50 years old, (3) being non-smoker, (4) no
previous history of neurological diseases, brain surgery, epilepsy, seizures, brain damage, head injury, or metal brain implants, and (5) absence of other psychiatric disorders. Those
patients taking anxiolytic (_N_ = _6_) and/or SSRI (_N_ = 22) medication were receiving stable doses for 6 weeks before the experiment up to the follow-up. All participants were native
speakers and had normal or corrected-to-normal vision. This was a registered clinical trial (ClinicalTrials.gov Identifier: NCT05501132) approved by the Ethics Committee of the Ardabil
University of Medical Science (Ethics code: IR.ARUMS.REC.1399.102). Participants gave their written informed consent before participation (see Table 1 for demographics). OUTCOME MEASURES
(PRIMARY AND SECONDARY CLINICAL MEASURES, COGNITIVE DEFICITS, AND BRAIN PHYSIOLOGY) PRIMARY AND SECONDARY CLINICAL MEASURES The primary outcome measure to examine the effects of the
intervention on OCD symptoms was the Yale-Brown Obsessive-Compulsive Scale (Y-BOCS) [43]. Additionally, anxiety and depressive states were tested by the Beck Anxiety Inventory (BAI) [44] and
the Beck Depression Inventory (BDI-II) [45], respectively, and quality of life was assessed with the WHO Quality of Life Questionnaire (WHOQUL) [46]. These measures were used to evaluate
the clinical efficacy of the intervention. The Y-BOCS is the most widely used clinician-rated interview for assessing OCD symptom severity and is a reliable measure of treatment-based
reduction of symptoms [47]. The BAI is also well suited to monitor treatment outcomes [48], and the evaluated anxiety state is correlated with OCD symptoms [49, 50]. Similarly, BDI-II scores
are associated with OCD symptoms [50], in line with the fact that around one-third of OCD patients suffer from comorbid depression [51]. A detailed description of these measures can be
found in the supplementary information. COGNITIVE ASSESSMENT AND BRAIN PHYSIOLOGY We used a battery of neuropsychological tests that are sensitive to the cognitive deficit profile of OCD
affected by interventions. Deficits of inhibitory control, working memory performance, and attention (e.g., sustained attention, set-shifting) are among the most well-documented cognitive
deficits in OCD [37, 52, 53]. Importantly, these cognitive deficits are associated with frontal–striatal and frontal dysfunctions [29, 54,55,56], which are targeted by the intervention in
this experiment. We examined response inhibition with the Go/No-Go task and Flanker test, working memory with the n-back task, and attention bias to OCD-related stimuli with an adapted
dot-probe task. A detailed description of these measures is provided in the supplementary information. Finally, we monitored resting EEG to see how power spectrum and functional connectivity
change after the intervention, specifically in frequency bands of interest (e.g., alpha, delta, gamma) [38,39,40,41]. A detailed description of the measures and EEG data preprocessing and
analysis are in the supplementary content. TDCS Direct currents were generated by an electrical stimulator (Oasis Pro, Mind Alive, Canada), and applied through a pair of saline-soaked sponge
electrodes (7 × 5 cm) for two periods of 20 min and 20 min intervals between each stimulation period [36]. Stimulation was delivered on 5 consecutive days (two stimulations per day). In
both active (1-mA, 2-mA) and sham conditions, anodal and cathodal electrodes were placed over the left DLPFC (F3), and right pre-SMA (FC2), respectively, to keep a minimum 6 cm distance
between the edges of the electrodes [57]. To localize the right pre-SMA first, the pre-SMA was identified using the EEG 10–20 system for electrode positioning (i.e., 15% cm anterior to Cz)
[17, 58]. In sham stimulation, the electrical current was ramped up and down for 30 seconds to generate the same sensation as in the active condition and then turned off [59]. To guarantee
blinding, tDCS was applied by independent investigators who were not involved in outcome measures rating [60]. A side-effect survey was done after each tDCS session. Blinding efficacy was
not explored among patients and experimenters. A 3D model of the current flow in the head was created to determine induced electrical fields in the brain for the above-mentioned tDCS
protocol (Fig. 2). PROCEDURE Prior to the experiment, participants completed a brief questionnaire to evaluate their suitability for brain stimulation. All participants received 10 sessions
of stimulation (2 sessions daily, 5 days in total) with 20-minute intervals between the sessions. To avoid confounding effects of the intervention at circadian non-preferred time, which can
significantly affect neuroplasticity induction [61], all stimulation sessions took place between 11:00–14:00, and participants were not under sleep pressure [62]. Clinical cognitive measures
were evaluated before the first intervention (pre-intervention), right after the end of the last intervention (post-intervention), and 1-month following the last stimulation session
(follow-up). EEG measurements took place only before and after the intervention. Patients were instructed about the tasks before the beginning of the experiment. None of the patients
received any kind of psychotherapy during the study. Participants were blind to the study hypotheses and stimulation conditions. The experimenter who conducted the outcome measures was
blinded to the tDCS conditions (Fig. 2). STATISTICAL ANALYSIS Data analyses were conducted with the statistical package SPSS, version 26.0 (IBM, SPSS, Inc., Chicago, IL), and the GraphPad
Prism 8.2.1 (GraphPad Software, San Diego, California). The normality and homogeneity of data distribution, and variance were confirmed by Shapiro-Wilk and Levin tests, respectively.
Between-group differences in demographic variables were explored by Chi-square tests or Fisher’s exact test for categorical variables and F-tests for continuous variables. A multivariate
Analysis of Variance (MANOVA) was first performed on the post-intervention and follow-up means of all outcome variables with group as the fixed factor and pre-intervention measures as
covariates. This was to help protect against inflating the Type 1 error rate in the follow-up ANOVAs and post-hoc comparisons. A series of one-way ANOVA’s on each dependent variable was
conducted as follow-up tests to the MANOVA. Finally, a series of post-hoc analyses were calculated using Dunnett’s multiple comparisons to examine individual mean difference comparisons
across groups (active 1 mA, active 2 mA, sham) and time points (pre-intervention, post-intervention, follow-up). The critical level of significance was 0.05 for all statistical analyses.
RESULTS SIDE EFFECTS AND BASELINE ASSESSMENT Participants tolerated the stimulation well, and no adverse effects were reported during and after stimulation, replicating the safety of the
intervention [63, 64]. No significant difference was found between the group ratings of tDCS side effects (Supplementary Table S1). No significant between-group differences emerged in the
pre-intervention measurements (Supplementary Table S2). PRIMARY CLINICAL OUTCOME: REDUCTION OF OCD SYMPTOMS AND ANXIETY A statistically significant MANOVA effect was seen for both
post-intervention (Pillais’ Trace = 1.64, _F_(24, 24) = 4.63, _p_ < 0.001) and follow-up measurements (Pillais’ Trace = 2.98, _F_(24, 24) = 4.63, _p_ = 0.005). The results of the
follow-up ANOVAs revealed a significant main effect of group on both Y-BOCS scores (post-intervention: _F_(2, 22) = 7.14, _p_ = 0.004, _ηp__2_ = 0.394; follow-up: _F_(2, 22) = 13.54, _p_
< 0.004, _ηp__2_ = 0.552) and BAI scores (post-intervention_: F_(2, 22) = 8.78, _p_ = 0.002, _ηp__2_ = 0.423; follow-up: _F_(2, 22) = 5.78, _p_ = 0.010, _ηp__2_ = 0.345). Next, Dunnett’s
multiple comparisons were performed on individual mean difference and showed a significant decrease in Y-BOCS scores at the post-intervention time in the 2-mA group (_p_ = 0.021, _d_ =
0.98), at the 1-month follow-up in both the 2-mA (_p_ = 0.004, _d_ = 1.01) and 1-mA (_p_ = 0.013, _d_ = 1.23) groups as compared to pre-intervention time, but no significant changes were
seen in the sham group (Fig. 3A). When compared to the sham group, reduced Y-BOCS scores were significant in both active groups only at the follow-up (1-mA: _p_ = 0.044, _d_ = 1.16; 2-mA:
_p_ = 0.045, _d_ = 0.82) (Fig. 3B). For the BAI scores, Dunnett’s multiple comparisons showed a significant decrease in BAI scores from pre-intervention to both post-intervention (_p_ =
0.025, _d_ = 0.85) and 1-month follow-up (_p_ = 0.009, _d_ = 0.97) only in the 2-mA group (Fig. 3C). When compared to the sham group, both active groups showed a non-significant trendwise
reduction of BAI scores at the post-intervention assessment (1-mA: _p_ = 0.074; 2-mA: _p_ = 0.083) (Fig. 3D). SECONDARY CLINICAL OUTCOMES: MOOD AND QUALITY OF LIFE The results of the
follow-up ANOVAs revealed a significant main effect of group on BDI-II scores (post-intervention: _F_(2, 22) = 7.13, _p_ = 0.004, _ηp__2_ = 0.394; follow-up: _F_(2, 22) = 10.47, _p_ <
0.001, _ηp__2_ = 0.488) and quality of life (post-intervention: _F_(2, 22) = 3.58, _p_ = 0.045, _ηp__2_ = 0.246; follow-up: _F_(2, 22) = 7.33, _p_ = 0.004, _ηp__2_ = 0.400). Dunnett’s post
hoc tests showed that BDI-II scores were reduced from the pre-intervention to both post-intervention and 1-month follow-up assessment in both, 2-mA (post-intervention: _p_ = 0.002, _d_ =
1.15; follow-up: _p_ < 0.001, _d_ = 1.34) and 1-mA (post-intervention: _p_ < 0.001, _d_ = 1.65; follow-up: _p_ = 0.001, _d_ = 1.49) groups, but not in the sham group, and reduced
depressive state at each time point was significant in both active groups vs. the sham group (Fig. 3E, F). No significant individual mean differences were found across groups in
quality-of-life scores (Fig. 3G). However, we calculated the changes in quality of life scores from the baseline to post-intervention and follow-up. Dunnett’s post hoc test of score changes
across groups showed that quality of life scores significantly improved after the intervention only in the 2 mA group (_p_ = 0.025) (Fig. 3H). COGNITIVE FUNCTIONS: IMPROVED INHIBITORY
CONTROL IN BOTH ACTIVE TDCS GROUPS In the Flanker test, the follow-up ANOVAs revealed a significant main effect of group on both, congruent (post-intervention: _F_(2, 22) = 10.08, _p_ <
0.001, _ηp__2_ = 0.478; follow-up: _p_ = 0.901) and incongruent trials (post-intervention: _F_(2, 22) = 8.01, _p_ = 0.002, _ηp__2_ = 0.422; follow-up: _p_ = 0.445) only after the
intervention and not follow-up. Dunnett’s multiple-test comparisons revealed a significant _pre_ vs _post_-intervention RT reduction of incongruent stimuli (_p_ = 0.015, _d_ = 1.35) only in
the 1-mA group, which, however, was not significant vs the sham (Fig. 4A, B). In the Go/No-Go task, the results of the follow-up ANOVAs revealed a significant main effect of group for No-Go
trials reaction time (post-intervention: _F_(2, 22) = 7.11, _p_ = 0.004, _ηp__2_ = 0.393; follow-up: _F_(2, 22) = 4.34, _p_ = 0.026, _ηp__2_ = 0.283) and a marginally significant effect on
No-Go trials accuracy at the post-intervention measurement (_F_(2, 22) = 3.39, _p_follow-up = 0.054, _ηp__2_ = 0.233). Dunnett’s multiple-test comparisons showed increased accuracy from the
pre-intervention to the follow-up measurement in 2-mA (_p_ = 0.022, _d_ = 0.87) and 1-mA (_p_ = 0.032, _d_ = 0.27) groups (Fig. 4C). The 2-mA protocol significantly reduced RT from pre vs
post-intervention (_p_ = 0.027, _d_ = 1.92) and pre vs follow-up (_p_ = 0.037, _d_ = 1.95) as well (Fig. 4E) and here the performance speed on the No-Go trials was significantly faster in
the 2-mA group vs the sham after the intervention (_p_ = 0.014, _d_ = 1.73) (Fig. 4F). COGNITIVE FUNCTIONS: WORKING MEMORY AND ATTENTION BIAS In working memory performance, the follow-up
ANOVAs revealed a significant main effect of group only on performance speed after the intervention (_F_(2, 22) = 16.76, _p_ < 0.001, _ηp__2_ = 0.604) and 1-month follow-up (_F_(2, 22) =
13.87, _p_ < 0.001, _ηp__2_ = 0.558). Dunnett’s multiple comparisons showed a significantly faster pre- vs post-intervention RT (_p_ < 0.001, _d_ = 1.92) and pre vs follow-up RT (_p_
< 0.001, _d_ = 1.95) in the 2-mA and a significant pre vs follow-up RT reduction (_p_ = 0.003, _d_ = 1.01) in the 1-mA group. In the 2-mA group, this RT reduction was furthermore larger
than that of the sham group at post-intervention (_p_ = 0.007, _d_ = 1.73) and follow-up (_p_ = 0.002, _d_ = 1.91) measurements (Fig. 4G, H). Finally, in the attention bias of patients to
OCD-related stimuli, the follow-up ANOVAs showed a significant main effect of group for both, OCD-related (post-intervention: _F_(2, 22) = 8.82, _p_ = 0.002, _ηp__2_ = 0.445; follow-up:
_F_(2, 22) = 5.53, _p_ = 0.011, _ηp__2_ = 0.335) and unrelated stimuli (post-intervention: _F_(2, 22) = 5.82, _p_ = 0.009, _ηp__2_ = 0.346; follow-up: _F_(2, 22) = 9.45, _p_ = 0.001, _ηp__2_
= 0.462). Dunnett’s multiple-test comparisons showed a significantly faster pre- vs post-intervention RT for both, OCD-related (_p_ < 0.026, _d_ = 0.70) and unrelated (_p_ < 0.007,
_d_ = 0.93) stimuli in the 2-mA group (Fig. 4I, K). No significant between-group RT differences were, however found for the post-intervention and follow-up measurements. INTERVENTION-RELATED
CHANGES IN EEG POWER SPECTRUM DENSITY AND FUNCTIONAL CONNECTIVITY PSD Analysis with a cluster-based permutation test (post vs pre) revealed a significant increase in relative alpha power in
the left frontal region (cluster-level statistic = 16, _p_ < 0.01) and the occipital region (cluster-level statistic = 11, _p_ < 0.05) in the 2-mA group compared to the sham group.
Additionally, a significant decrease in relative delta power was observed in a cluster located in the occipital region (cluster-level statistic = −11, _p_ < 0.05) in the 2-mA group
compared to the sham group. In the 1-mA group, a significant increase in relative alpha power was observed in the right frontal region (cluster-level statistic = 11, _p_ < 0.001) (Fig.
5C). Topographical plots of the relative power changes (post power – pre power) in all frequency bands after each intervention are in supplementary information (Fig. S1). Regarding
functional connectivity, comparative analysis of post-intervention Phase Locking Value (PLV) matrices showed a general trend of increased connectivity in higher frequency bands in both
active groups as compared to the sham group (Fig. 5A, B and Fig. S2). When we compared both groups with each other, the 2-mA group generally decreased functional connectivity across most
frequency bands (EEG connectivity results are fully described in the supplementary information). We did not see any relevant correlation between EEG parameters and clinical/cognitive
measures. DISCUSSION In this randomized, double-blind, sham-controlled, parallel-group clinical trial, we investigated the impact of an intensified tDCS protocol (stimulation twice per day
with 20-min intervals) over the prefrontal-supplementary motor network, in two dosages (1-mA vs. 2-mA) on primary clinical symptoms, neuropsychological performance, and electrophysiological
correlates in patients with OCD. The 2-mA stimulation dosage significantly reduced OCD symptoms and anxiety after the intervention and in the follow-up. Both active stimulation protocols
significantly reduced depressive symptoms. At the neuropsychological level, both active protocols partially improved response inhibition, and the 2-mA protocol reduced attention bias to
threat-related stimuli and improved working memory performance as well. Both protocols increased alpha, and the 2-mA protocol decreased delta oscillatory power too. Both protocols increased
connectivity in higher frequency bands at frontal-central areas compared to the sham. No significant changes were observed in the sham group for any outcome measures. These findings can be
explained from neurophysiological and neuropsychological perspectives. The hallmark finding of neuroimaging studies refers to lateral hypoconnectivity (including the DLPFC) and medial
hyperconnectivity (including the pre-SMA) in OCD [37, 65], which was the rationale for applying our stimulation protocol and is in line with findings from rTMS studies [66]. We applied
anodal stimulation over the left DLPFC to increase the activity of this region and cathodal stimulation of the pre-SMA to downregulate activity. With causal modulation of cerebral
excitability with tDCS [6], we expected to restore functional abnormalities in the OCD-relevant brain circuitry, and in principal accordance, this intervention was associated with behavioral
and clinical improvement in this study. In further accordance, the intervention, especially after 2-mA stimulation, restored altered alpha and delta oscillatory power in patients [38], and
both protocols increased connectivity in the prefrontal regions, which is reduced in OCD patients, that can be likely an appropriate treatment cortical target [21, 66]. In addition to
neurophysiological changes, neuropsychological accounts could also explain our findings. The most well-known psychological mechanisms underlying OCD psychopathology include impaired
cognitive control (the inability to regulate compulsive behavior) [67], impaired cognitive flexibility (the inability to regulate thinking) [68], and impaired balance between goal-directed
behavior and more automatic habit learning [69, 70]. Importantly, these cognitive abilities are related to lateral and medial prefrontal cortices [23, 37, 71]. The behavioral tasks we used
are primarily related to cognitive control and cognitive flexibility (e.g., response inhibition, working memory), and the performance of these tasks was significantly improved after
intervention, more obviously in the 2-mA group. Here, it should however be noted that the effects of both protocols on response inhibition were smaller than expected, which could be due to
the higher relevance of the right prefrontal region in cognitive inhibition [72]. That said, anodal stimulation of the left DLPFC was also shown to improve executive functions in
neuropsychiatric patients in previous studies [13, 36, 73, 74], and might explain treatment effects in OCD patients. One major rationale of this study was to identify the effect of different
stimulation dosages on treatment efficacy, specifically in the intensified protocol, which we had already applied in another study with promising results [36]. This protocol has not been
applied in OCD to the best of our knowledge. Our results in this study show that the 2-mA intensified tDCS protocol was overall more effective than both, sham stimulation and the 1-mA
stimulation, especially for the clinical variables, and it improved more outcome measures including measures of behavioral performance, compared to the 1-mA protocol (e.g., working memory,
attention bias). The rationale behind the protocol comes from a study showing that twice-stimulation with 20-minute intervals leads to longer aftereffects on cortical excitability compared
to non-repeated stimulation or stimulation with long intervals and resembles features of late-phase LTP [35, 75]. This finding has at least two important clinical implications. First, the
2-mA stimulation is associated with higher clinical efficacy in OCD, and probably in other anxiety disorders, as shown in our previous work in patients with social anxiety disorder [36].
Second, the intensified stimulation (twice per day with a 20 min interval), has significant clinical efficacy for treatment-related variables. This is in line with physiological studies that
have shown that repeated tDCS sessions induce larger increases in excitability [76] and more importantly suggest that the intensified protocol (repetition of two 20-minute stimulation with
a 20-minute interval between) can be promising for clinical application in other neuropsychiatric diosders. Our protocol was different from other commonly applied protocols in other aspects.
First and to the best of our knowledge, none of the previous tDCS randomized trials targeted the prefrontal-SMA network by stimulating both left DLPFC and pre-SMA [77, 78]. Additionally,
this is also the first randomized-controlled trial that compared the efficacy of two stimulation dosages which is typically needed for establishing clinical efficacy of an intervention.
Finally, in comparison to other protocols used in previous studies, a recent metanalysis of tDCS RCTs in OCD showed that protocols that applied cathodal stimulation over the pre-SMA with an
extracephalic return electrode delivered stronger electric fields to the circuity involved in OCD in comparison to the other montages [19]. None of these tDCS studies targeted the left DLPFC
with anodal tDCS. This metanalysis, however, did not find significant differences between active vs sham tDCS in contrast to our study. Our study had several limitations. First, the
intrinsically limited focality of tDCS can result in a relatively diffuse stimulation. Neuroimaging methods can help to more accurately identify the regions directly affected by tDCS in
future studies. Furthermore, we did not examine blinding efficacy in patients and could not measure EEG in the follow-up due to COVID-19-related restrictions. With respect to blinding
efficacy, the 2-mA intensity typically results in more sensations over the skin as compared to the sham and 1-mA protocol, which may affect patients’ blinding. However, there was no
significant difference in reported ratings of tDCS side effects between groups (see supplementary information, Table S1). Taken together, our findings suggest that the intensified
prefrontal-supplementary motor cortex tDCS protocol introduced for the treatment of OCD is promising and might be effective in other neuropsychiatric disorders. Both primary OCD symptoms and
secondary treatment-related variables (anxiety, depressive state, quality of life) and cognitive functions (response inhibition, working memory, and attentional bias) improved after the
intervention, especially in the 2-mA group. Partial effects of the intervention on response inhibition might suggest further optimizing the protocol by targeting the right prefrontal cortex,
which was not the primary target here, and the sessions were relatively low. Both protocols also significantly restored brain oscillatory power in frequency bands introduced as biomarkers
of OCD. In line with rTMS intervention [8, 79], tDCS may also hold the potential to serve as a therapeutic intervention in OCD treatment. Future larger trials with longer follow-up
assessments are needed to support the clinical efficacy of this intervention. REFERENCES * American Psychiatric Association. Diagnostic and statistical manual of mental disorders (DSM-5®).
American Publishing, Inc. 2013. * Meier SM, Mattheisen M, Mors O, Schendel DE, Mortensen PB, Plessen KJ. Mortality among persons with obsessive-compulsive disorder in Denmark. JAMA
Psychiatry. 2016;73:268–74. Article PubMed PubMed Central Google Scholar * Wayne K, Goodman MD, Eric A. Harmonizing the neurobiology and treatment of obsessive-compulsive disorder. Am J
Psychiatry. 2021;178:17–29. Article Google Scholar * Stein DJ, Costa DLC, Lochner C, Miguel EC, Reddy YCJ, Shavitt RG, et al. Obsessive–compulsive disorder. Nat Rev Dis Prim. 2019;5:52.
Article PubMed Google Scholar * Romanelli RJ, Wu FM, Gamba R, Mojtabai R, Segal JB. Behavioral therapy and serotonin reuptake inhibitor pharmacotherapy in the treatment of
obsessive-compulsive disorder: a systematic review and meta-analysis of head-to-head randomized controlled trials. Depress Anxiety. 2014;31:641–52. Article PubMed Google Scholar * Polanía
R, Nitsche MA, Ruff CC. Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci. 2018;21:174–87. Article PubMed Google Scholar * Fregni F, Pascual-Leone
A. Technology Insight: noninvasive brain stimulation in neurology[mdash]perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. 2007;3:383–93. Article PubMed
Google Scholar * Carmi L, Tendler A, Bystritsky A, Hollander E, Blumberger DM, Daskalakis J, et al. Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive
disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry. 2019;176:931–8. Article PubMed Google Scholar * Nitsche M, Paulus W. Excitability
changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–9. Article CAS PubMed PubMed Central Google Scholar * Stagg CJ,
Nitsche MA. Physiological basis of transcranial direct current stimulation. Neuroscientist. 2011;17:37–53. Article PubMed Google Scholar * Polania R, Kuo M-F, Nitsche MA. Physiology of
transcranial direct and alternating current stimulation. In: Brunoni AR, Nitsche MA, Loo CK (eds). Transcranial direct current stimulation in neuropsychiatric disorders: clinical principles
and management. Springer International Publishing: Cham, 2021, 29–47. * Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, et al. Evidence-based guidelines and
secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol. 2020;24:256–313. Article PubMed Central
Google Scholar * Alizadehgoradel J, Nejati V, Sadeghi Movahed F, Imani S, Taherifard M, Mosayebi-Samani M, et al. Repeated stimulation of the dorsolateral-prefrontal cortex improves
executive dysfunctions and craving in drug addiction: a randomized, double-blind, parallel-group study. Brain Stimul. 2020;13:582–93. Article PubMed Google Scholar * Salehinejad MA,
Ghanavati E, Glinski B, Hallajian A-H, Azarkolah A. A systematic review of randomized controlled trials on efficacy and safety of transcranial direct current stimulation in major
neurodevelopmental disorders: ADHD, autism, and dyslexia. Brain Behav. 2022;12:e2724. Article CAS PubMed PubMed Central Google Scholar * Nikolin S, Moffa A, Razza L, Martin D, Brunoni
AR, Palm U, et al. Time-course of the tDCS antidepressant effect: an individual participant data meta-analysis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2023;125:110752. Article CAS
Google Scholar * Azarkolah A, Noorbala AA, Ansari S, Hallajian A-H, Salehinejad MA. Efficacy of transcranial direct current stimulation on pain level and disability of patients with
fibromyalgia: a systematic review of randomized controlled trials with parallel-group design. Brain Sci. 2024;14:26. Article Google Scholar * Silva RdMFd, Brunoni AR, Goerigk S, Batistuzzo
MC, Costa DLdC, Diniz JB, et al. Efficacy and safety of transcranial direct current stimulation as an add-on treatment for obsessive-compulsive disorder: a randomized, sham-controlled
trial. Neuropsychopharmacology. 2021;46:1028–34. Article CAS PubMed PubMed Central Google Scholar * da Silva RdMF, Batistuzzo MC, Shavitt RG, Miguel EC, Stern E, Mezger E, et al.
Transcranial direct current stimulation in obsessive-compulsive disorder: an update in electric field modeling and investigations for optimal electrode montage. Expert Rev Neurother.
2019;19:1025–35. Article PubMed Google Scholar * Pinto BS, Cavendish BA, da Silva PHR, Suen PJC, Marinho KAP, Valiengo LDCL, et al. The effects of transcranial direct current stimulation
in obsessive–compulsive disorder symptoms: a meta-analysis and integrated electric fields modeling analysis. Biomedicines. 2022;11:80. Article PubMed PubMed Central Google Scholar *
Rostami R, Kazemi R, Jabbari A, Madani AS, Rostami H, Taherpour MA, et al. Efficacy and clinical predictors of response to rTMS treatment in pharmacoresistant obsessive-compulsive disorder
(OCD): a retrospective study. BMC Psychiatry. 2020;20:372. Article PubMed PubMed Central Google Scholar * Li H, Hu X, Gao Y, Cao L, Zhang L, Bu X, et al. Neural primacy of the
dorsolateral prefrontal cortex in patients with obsessive-compulsive disorder. NeuroImage Clin. 2020;28:102432. Article PubMed PubMed Central Google Scholar * Aron AR, Robbins TW,
Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8:170–7. Article PubMed Google Scholar * Aron AR, Robbins TW, Poldrack RA. Inhibition and the right
inferior frontal cortex: one decade on. Trends Cogn Sci. 2014;18:177–85. Article PubMed Google Scholar * Hung Y, Gaillard SL, Yarmak P, Arsalidou M. Dissociations of cognitive inhibition,
response inhibition, and emotional interference: voxelwise ALE meta-analyses of fMRI studies. Hum Brain Mapp. 2018;39:4065–82. Article PubMed PubMed Central Google Scholar *
Johansen-Berg H, Behrens TEJ, Robson MD, Drobnjak I, Rushworth MFS, Brady JM, et al. Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex.
Proc Natl Acad Sci USA. 2004;101:13335–40. Article CAS PubMed PubMed Central ADS Google Scholar * Hampshire A, Sharp DJ. Contrasting network and modular perspectives on inhibitory
control. Trends Cogn Sci. 2015;19:445–52. Article PubMed Google Scholar * Sharp DJ, Bonnelle V, De Boissezon X, Beckmann CF, James SG, Patel MC, et al. Distinct frontal systems for
response inhibition, attentional capture, and error processing. Proc Natl Acad Sci. 2010;107:6106–11. Article CAS PubMed PubMed Central ADS Google Scholar * Stella J, de Wit MD,
Froukje E, de Vries MD, Ysbrand van der Werf D, et al. Presupplementary motor area hyperactivity during response inhibition: a candidate endophenotype of obsessive-compulsive disorder. Am J
Psychiatry. 2012;169:1100–8. Article Google Scholar * Norman LJ, Taylor SF, Liu Y, Radua J, Chye Y, De Wit SJ, et al. Error processing and inhibitory control in obsessive-compulsive
disorder: a meta-analysis using statistical parametric maps. Biol Psychiatry. 2019;85:713–25. Article PubMed Google Scholar * Gowda SM, Narayanaswamy JC, Hazari N, Bose A, Chhabra H,
Balachander S, et al. Efficacy of pre-supplementary motor area transcranial direct current stimulation for treatment resistant obsessive compulsive disorder: A randomized, double blinded,
sham controlled trial. Brain Stimul. 2019;12:922–9. Article PubMed Google Scholar * Rehn S, Eslick GD, Brakoulias V. A meta-analysis of the effectiveness of different cortical targets
used in repetitive transcranial magnetic stimulation (rTMS) for the treatment of obsessive-compulsive disorder (OCD). Psychiatr Q. 2018;89:645–65. Article PubMed Google Scholar * Acevedo
N, Bosanac P, Pikoos T, Rossell S, Castle D. Therapeutic neurostimulation in obsessive-compulsive and related disorders: a systematic review. Brain Sci. 2021;11:948. Article PubMed PubMed
Central Google Scholar * Salehinejad MA, Nejati V, Mosayebi-Samani M, Mohammadi A, Wischnewski M, Kuo M-F, et al. Transcranial direct current stimulation in ADHD: a systematic review of
efficacy, safety, and protocol-induced electrical field modeling results. Neurosci Bull. 2020;36:1191–212. Article PubMed PubMed Central Google Scholar * Jog MV, Wang DJJ, Narr KL. A
review of transcranial direct current stimulation (tDCS) for the individualized treatment of depressive symptoms. Pers Med Psychiatry. 2019;17-18:17–22. PubMed PubMed Central Google
Scholar * Monte-Silva K, Kuo M-F, Hessenthaler S, Fresnoza S, Liebetanz D, Paulus W, et al. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain
stimulation. Brain Stimul. 2013;6:424–32. Article PubMed Google Scholar * Jafari E, Alizadehgoradel J, Pourmohseni Koluri F, Nikoozadehkordmirza E, Refahi M, Taherifard M, et al.
Intensified electrical stimulation targeting lateral and medial prefrontal cortices for the treatment of social anxiety disorder: A randomized, double-blind, parallel-group, dose-comparison
study. Brain Stimul. 2021;14:974–86. Article PubMed Google Scholar * Robbins TW, Vaghi MM, Banca P. Obsessive-compulsive disorder: puzzles and prospects. Neuron. 2019;102:27–47. Article
CAS PubMed Google Scholar * Perera MPN, Mallawaarachchi S, Bailey NW, Murphy OW, Fitzgerald PB. Obsessive-compulsive disorder (OCD) is associated with increased electroencephalographic
(EEG) delta and theta oscillatory power but reduced delta connectivity. J Psychiatr Res. 2023;163:310–7. Article PubMed Google Scholar * Velikova S, Locatelli M, Insacco C, Smeraldi E,
Comi G, Leocani L. Dysfunctional brain circuitry in obsessive–compulsive disorder: Source and coherence analysis of EEG rhythms. Neuroimage. 2010;49:977–83. Article PubMed Google Scholar
* Wong M, Woody EZ, Schmidt LA, Ameringen MV, Soreni N, Szechtman H. Frontal EEG alpha activity and obsessive-compulsive behaviors in non-clinical young adults: a pilot study. Front Psychol.
2015;6:1480. Article PubMed PubMed Central Google Scholar * Buot A, Karachi C, Lau B, Belaid H, Fernandez-Vidal S, Welter M-L, et al. Emotions modulate subthalamic nucleus activity: new
evidence in obsessive-compulsive disorder and Parkinson’s disease patients. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2021;6:556–67. PubMed Google Scholar * Minarik T, Berger B,
Althaus L, Bader V, Biebl B, Brotzeller F, et al. The importance of sample size for reproducibility of tDCS effects. Front Hum Neurosci. 2016;10:453. Article PubMed PubMed Central Google
Scholar * Goodman WK, Price LH, Rasmussen SA, et al. The yale-brown obsessive compulsive scale: I. development, use, and reliability. Arch Gen Psychiatry. 1989;46:1006–11. Article CAS
PubMed Google Scholar * Steer RA, Beck AT. Beck anxiety inventory. Evaluating stress: a book of resources. Scarecrow Education: Lanham, MD, US, 1997, 23–40. * Beck AT, Ward CH, Mendelson
MM, Mock JJ, Erbaugh JJ. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. Article CAS PubMed Google Scholar * Skevington SM, Lotfy M, O’Connell KA. The World
Health Organization’s WHOQOL-BREF quality of life assessment: Psychometric properties and results of the international field trial. A report from the WHOQOL group. Qual Life Res.
2004;13:299–310. Article CAS PubMed Google Scholar * Maust D, Cristancho M, Gray L, Rushing S, Tjoa C, Thase ME. Chapter 13 - Psychiatric rating scales. In: Aminoff MJ, Boller F, Swaab
DF (eds). Handbook of Clinical Neurology, 106. Elsevier 2012, 227–37. * Leyfer OT, Ruberg JL, Woodruff-Borden J. Examination of the utility of the beck anxiety inventory and its factors as a
screener for anxiety disorders. J Anxiety Disord. 2006;20:444–58. Article PubMed Google Scholar * Reuman L, Jacoby RJ, Blakey SM, Riemann BC, Leonard RC, Abramowitz JS. Predictors of
illness anxiety symptoms in patients with obsessive compulsive disorder. Psychiatry Res. 2017;256:417–22. Article PubMed Google Scholar * Velloso P, Piccinato C, Ferrão Y, Aliende Perin
E, Cesar R, Fontenelle L, et al. The suicidality continuum in a large sample of obsessive–compulsive disorder (OCD) patients. Eur Psychiatry. 2016;38:1–7. Article CAS PubMed Google
Scholar * Overbeek T, Schruers K, Vermetten E, Griez E. Comorbidity of obsessive-compulsive disorder and depression: prevalence, symptom severity, and treatment effect. J Clin Psychiatry.
2002;63:1106–12. Article PubMed Google Scholar * Shin NY, Lee TY, Kim E, Kwon JS. Cognitive functioning in obsessive-compulsive disorder: a meta-analysis. Psychol Med. 2013;44:1121–30.
Article PubMed Google Scholar * Benzina N, Mallet L, Burguière E, N’Diaye K, Pelissolo A. Cognitive dysfunction in obsessive-compulsive disorder. Curr Psychiatry Rep. 2016;18:80. Article
PubMed Google Scholar * Norman LJ, Carlisi C, Lukito S, Hart H, Mataix-Cols D, Radua J, et al. Structural and functional brain abnormalities in attention-deficit/hyperactivity disorder
and obsessive-compulsive disorder: a comparative meta-analysis. JAMA Psychiatry. 2016;73:815–25. Article PubMed Google Scholar * Heinzel S, Kaufmann C, Grützmann R, Hummel R, Klawohn J,
Riesel A, et al. Neural correlates of working memory deficits and associations to response inhibition in obsessive compulsive disorder. NeuroImage Clin. 2018;17:426–34. Article PubMed
Google Scholar * van den Heuvel OA, Veltman DJ, Groenewegen HJ, Witter MP, Merkelbach J, Cath DC, et al. Disorder-specific neuroanatomical correlates of attentional bias in
obsessive-compulsive disorder, panic disorder, and hypochondriasis. Arch Gen Psychiatry. 2005;62:922–33. Article PubMed Google Scholar * Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni
AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. 2016;127:1031–48. Article CAS PubMed Google Scholar * D’Urso G,
Brunoni AR, Mazzaferro MP, Anastasia A, de Bartolomeis A, Mantovani A. Transcranial direct current stimulation for obsessive–compulsive disorder: a randomized, controlled, partial crossover
trial. Depress Anxiety. 2016;33:1132–40. Article PubMed Google Scholar * Brunoni AR, Schestatsky P, Lotufo PA, Benseñor IM, Fregni F. Comparison of blinding effectiveness between sham
tDCS and placebo sertraline in a 6-week major depression randomized clinical trial. Clin Neurophysiol. 2014;125:298–305. Article PubMed Google Scholar * Gandiga PC, Hummel FC, Cohen LG.
Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117:845–50. Article PubMed Google Scholar *
Salehinejad MA, Wischnewski M, Ghanavati E, Mosayebi-Samani M, Kuo M-F, Nitsche MA. Cognitive functions and underlying parameters of human brain physiology are associated with chronotype.
Nat Commun. 2021;12:4672. Article CAS PubMed PubMed Central ADS Google Scholar * Salehinejad MA, Ghanavati E, Reinders J, Hengstler JG, Kuo M-F, Nitsche MA. Sleep-dependent upscaled
excitability, saturated neuroplasticity, and modulated cognition in the human brain. eLife. 2022;11:e69308. Article CAS PubMed PubMed Central Google Scholar * Salehinejad MA,
Siniatchkin M. Safety of noninvasive brain stimulation in children. Curr Opin Psychiatry. 2024;37:1–9. Google Scholar * Salehinejad MA, Nikolin S, Vicario CM, Nitsche MA, Loo CK, Brunoni
AR, Brunoni AR, et al. Safety and Tolerability. Transcranial Direct Current Stimulation in Neuropsychiatric Disorders: Clinical Principles and Management. Cham: Springer International
Publishing; 2021. pp. 667–76. Chapter Google Scholar * van den Heuvel OA, van Wingen G, Soriano-Mas C, Alonso P, Chamberlain SR, Nakamae T, et al. Brain circuitry of compulsivity. Eur
Neuropsychopharmacol. 2016;26:810–27. Article PubMed Google Scholar * Kar SK, Agrawal A, Silva-dos-Santos A, Gupta Y, Deng Z-D. The Efficacy of Transcranial Magnetic Stimulation in the
Treatment of Obsessive-Compulsive Disorder: An Umbrella Review of Meta-Analyses. CNS Spectr. 2023,1–10. https://doi.org/10.1017/S1092852923006387. * Menzies L, Achard S, Chamberlain SR,
Fineberg N, Chen C-H, del Campo N, et al. Neurocognitive endophenotypes of obsessive-compulsive disorder. Brain. 2007;130:3223–36. Article PubMed Google Scholar * Chamberlain SR, Fineberg
NA, Menzies LA, Blackwell AD, Bullmore ET, Robbins TW, et al. Impaired cognitive flexibility and motor inhibition in unaffected first-degree relatives of patients with obsessive-compulsive
disorder. Am J Psychiatry. 2007;164:335–8. Article PubMed PubMed Central Google Scholar * Graybiel AM, Rauch SL. Toward a neurobiology of obsessive-compulsive disorder. Neuron.
2000;28:343–7. Article CAS PubMed Google Scholar * Everitt BJ, Robbins TW. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 2016;67:23–50.
Article PubMed Google Scholar * Salehinejad MA, Ghanavati E, Rashid MHA, Nitsche MA. Hot and cold executive functions in the brain: a prefrontal-cingular network. Brain Neurosci Adv.
2021;5:23982128211007769. Article PubMed PubMed Central Google Scholar * Schroeder PA, Schwippel T, Wolz I, Svaldi J. Meta-analysis of the effects of transcranial direct current
stimulation on inhibitory control. Brain Stimul. 2020;13:1159–67. Article PubMed Google Scholar * Molavi P, Aziziaram S, Basharpoor S, Atadokht A, Nitsche MA, Salehinejad MA. Repeated
transcranial direct current stimulation of dorsolateral-prefrontal cortex improves executive functions, cognitive reappraisal emotion regulation, and control over emotional processing in
borderline personality disorder: a randomized, sham-controlled, parallel-group study. J Affect Disord. 2020;274:93–102. Article PubMed Google Scholar * Li Q, Fu Y, Liu C, Meng Z.
Transcranial direct current stimulation of the dorsolateral prefrontal cortex for treatment of neuropsychiatric disorders. Front Behav Neurosci. 2022;16:893955. Article PubMed PubMed
Central Google Scholar * Agboada D, Mosayebi-Samani M, Kuo M-F, Nitsche MA. Induction of long-term potentiation-like plasticity in the primary motor cortex with repeated anodal
transcranial direct current stimulation – better effects with intensified protocols? Brain Stimul. 2020;13:987–97. Article PubMed Google Scholar * Ho K-A, Taylor JL, Chew T, Gálvez V,
Alonzo A, Bai S, et al. The effect of transcranial direct current stimulation (tDCS) electrode size and current intensity on motor cortical excitability: evidence from single and repeated
sessions. Brain Stimul. 2016;9:1–7. Article CAS PubMed Google Scholar * Gao T, Du J, Tian S, Liu W. A meta-analysis of the effects of non-invasive brain stimulation on
obsessive-compulsive disorder. Psychiatry Res. 2022;312:114530. Article PubMed Google Scholar * Camacho-Conde JA, del Rosario Gonzalez-Bermudez M, Carretero-Rey M, Khan ZU. Therapeutic
potential of brain stimulation techniques in the treatment of mental, psychiatric, and cognitive disorders. CNS Neurosci Ther. 2023;29:8–23. Article PubMed Google Scholar * Steuber ER,
McGuire JF. A meta-analysis of transcranial magnetic stimulation in obsessive-compulsive disorder. Biol Psychiatry: Cogn Neurosci Neuroimaging. 2023;8:1145–55. PubMed Google Scholar *
Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? Proceedings of the 2015 37th Annual International Conference of the IEEE
Engineering in Medicine and Biology Society (EMBC); 25–29 Aug. 2015. * Symmetric Atlasing and Model Based Segmentation: an application to the hippocampus in older adults. 2006. Springer,
Berlin, Heidelberg. Download references ACKNOWLEDGEMENTS Michael Nitsche is supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—Project Number 316803389—SFB
1280, project A6, and by the German Centre of Mental Health (Project Number 01EE2302D). The datasets generated and/or analyzed during the current study are publicly available at
https://osf.io/dfm2j/ and in the supplementary content. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Psychology, Faculty of Humanities, University of Zanjan, Zanjan, Iran
Jaber Alizadehgoradel * Department of Psychiatry and Psychology, School of Medicine, Ardabil University of Medical Sciences, Ardabil, Iran Behnam Molaei * Department of Psychiatry, School of
Medicine, Hacettepe University, Ankara, Turkey Khandan Barzegar Jalali * Department of Family Health, Social Determinants of Health Research Center, Ardabil University of Medical Sciences,
Ardabil, Iran Asghar Pouresmali * Sharif Brain Center, Department of Electrical Engineering, Sharif University of Technology, Tehran, Iran Kiomars Sharifi * School of Cognitive Sciences,
Institute for Research in Fundamental Sciences, Tehran, Iran Kiomars Sharifi & Mohammad Ali Salehinejad * Department of Psychology and Educational Sciences, University of Tehran, Tehran,
Iran Amir-Homayun Hallajian * Department of Psychology, Shahid Beheshti University, Tehran, Iran Vahid Nejati * Department of Psychology and Neurosciences, Leibniz Research Centre for
Working Environment and Human Factors, Dortmund, Germany Benedikt Glinski, Michael A. Nitsche & Mohammad Ali Salehinejad * Dipartimento di Scienze Cognitive, Psicologiche, Pedagogiche e
degli studi culturali, Università di Messina, Messina, Italy Carmelo M. Vicario * Bielefeld University, University Hospital OWL, Protestant Hospital of Bethel Foundation, University Clinic
of Psychiatry and Psychotherapy and University Clinic of Child and Adolescent Psychiatry and Psychotherapy, Bielefeld, Germany Michael A. Nitsche * German Centre for Mental Health (DZPG),
Bochum, Germany Michael A. Nitsche Authors * Jaber Alizadehgoradel View author publications You can also search for this author inPubMed Google Scholar * Behnam Molaei View author
publications You can also search for this author inPubMed Google Scholar * Khandan Barzegar Jalali View author publications You can also search for this author inPubMed Google Scholar *
Asghar Pouresmali View author publications You can also search for this author inPubMed Google Scholar * Kiomars Sharifi View author publications You can also search for this author inPubMed
Google Scholar * Amir-Homayun Hallajian View author publications You can also search for this author inPubMed Google Scholar * Vahid Nejati View author publications You can also search for
this author inPubMed Google Scholar * Benedikt Glinski View author publications You can also search for this author inPubMed Google Scholar * Carmelo M. Vicario View author publications You
can also search for this author inPubMed Google Scholar * Michael A. Nitsche View author publications You can also search for this author inPubMed Google Scholar * Mohammad Ali Salehinejad
View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS JA: conceptualization, investigation, data curation, validation. BM: resources, project
administration, data curation. KBJ & AP: investigation, data curation. A-HH & KS: EEG analysis, 3D modeling, visualization, writing—original draft (EEG part). VN: software. BG: data
analysis. CMV: writing—review & editing. MAN: supervision, methodology, writing—review & editing. MAS: conceptualization, methodology, supervision, writing—original draft,
writing—review & editing, visualization, formal analysis (clinical/behavioral part). CORRESPONDING AUTHORS Correspondence to Behnam Molaei or Mohammad Ali Salehinejad. ETHICS
DECLARATIONS COMPETING INTERESTS Michael Nitsche is a member of the Scientific Advisory Boards of Neuroelectrics and NeuroDevice. All other authors declare no competing interests. ADDITIONAL
INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY
INFORMATION FIGURE S1 FIGURE S2 RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,
adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons
license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a
credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted
use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT
THIS ARTICLE CITE THIS ARTICLE Alizadehgoradel, J., Molaei, B., Barzegar Jalali, K. _et al._ Targeting the prefrontal-supplementary motor network in obsessive-compulsive disorder with
intensified electrical stimulation in two dosages: a randomized, controlled trial. _Transl Psychiatry_ 14, 78 (2024). https://doi.org/10.1038/s41398-024-02736-y Download citation * Received:
28 July 2023 * Revised: 04 January 2024 * Accepted: 05 January 2024 * Published: 05 February 2024 * DOI: https://doi.org/10.1038/s41398-024-02736-y SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative