- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Human postmortem studies suggest a major role for abnormalities in GABAergic interneurons in the prefrontal cortex in schizophrenia. Cortical interneurons differentiated from
induced pluripotent stem cells (iPSCs) of schizophrenia subjects showed significantly lower levels of glutamate decarboxylase 67 (GAD67), replicating findings from multiple postmortem
studies, as well as reduced levels of synaptic proteins gehpyrin and NLGN2. Co-cultures of the interneurons with excitatory cortical pyramidal neurons from schizophrenia iPSCs showed reduced
synaptic puncta density and lower action potential frequency. NLGN2 overexpression in schizophrenia neurons rescued synaptic puncta deficits while NLGN2 knockdown in healthy neurons
resulted in reduced synaptic puncta density. Schizophrenia interneurons also had significantly smaller nuclear area, suggesting an innate oxidative stressed state. The antioxidant
_N_-acetylcysteine increased the nuclear area in schizophrenia interneurons, increased NLGN2 expression and rescued synaptic deficits. These results implicate specific deficiencies in the
synaptic machinery in cortical interneurons as critical regulators of synaptic connections in schizophrenia and point to a nexus between oxidative stress and NLGN2 expression in mediating
synaptic deficits in schizophrenia. SIMILAR CONTENT BEING VIEWED BY OTHERS METABOLIC CONTRIBUTIONS TO NEURONAL DEFICITS CAUSED BY GENOMIC DISRUPTION OF SCHIZOPHRENIA RISK GENE SETD1A Article
Open access 29 December 2022 ANATOMICAL AND MOLECULAR CHARACTERIZATION OF PARVALBUMIN-CHOLECYSTOKININ CO-EXPRESSING INHIBITORY INTERNEURONS: IMPLICATIONS FOR NEUROPSYCHIATRIC CONDITIONS
Article Open access 13 July 2023 SCHIZOPHRENIA-ASSOCIATED _NRXN1_ DELETIONS INDUCE DEVELOPMENTAL-TIMING- AND CELL-TYPE-SPECIFIC VULNERABILITIES IN HUMAN BRAIN ORGANOIDS Article Open access
24 June 2023 INTRODUCTION Schizophrenia (SCZ) is a chronic and debilitating psychiatric disorder characterized by hallucinations, paranoid delusions, disordered thought processes, and
cognitive deficits1. The onset of psychosis is typically in adolescence or early adulthood and it follows a chronic course requiring treatment for the rest of a person’s life2,3. Patients
have an elevated risk of suicide compared to the general population, and suicide is the cause of over 10% of deaths in patients with psychotic disorders4. SCZ is a significant contributor to
the global burden of disease—SCZ is the 8th leading cause of disability-adjusted life year worldwide and psychosis is ranked as the 3rd most disabling condition5,6. The diagnosis and
treatment of SCZ is based only on clinical symptomatology, current treatments are only partly effective, and there are no biomarkers to aid in diagnosis, in guiding treatment decisions or in
monitoring treatment response. Despite the high prevalence and enormous impact, the disease biology of SCZ remains elusive7. There is an urgent need for understanding the cellular-molecular
underpinnings of SCZ that can be leveraged for the development of novel therapeutics that can bring about meaningful improvement in the functional outcomes for patients with SCZ8,9.
Postmortem studies and animal models indicate that the balance of excitatory and inhibitory (E-I) activity of cortical circuits is altered in SCZ10,11,12. One of the most replicated
postmortem findings in SCZ brains is evidence of GABAergic deficits in the prefrontal cortex that suggest a decrease in the activity of cortical interneurons13,14,15. Optogenetic studies in
animals show that elevated excitation, but not elevated inhibition, in the prefrontal cortex lead to impaired cognition and social behaviour16. Deficits in GABAergic transmission tip the E-I
balance in the cortex in this direction. In this study, we sought to develop ex vivo models of cortical interneuron cultures from human subjects in order to identify cellular and molecular
substrates of SCZ disease biology. To that end, we have generated iPSCs from 9 subjects each with SCZ and healthy controls (CON) and differentiated them into cortical interneurons in order
to examine disease-specific differences in the biology of inhibitory neurons. MATERIALS AND METHODS DIFFERENTIATION OF CORTICAL INTERNEURONS FROM HUMAN IPSCS iPSCs were cultured to 100%
confluency and media changed to N2/B27, along with addition of 10 μM SB431542 (Sigma S4317), 2 μM XAV939 (Sigma X3004) and 1 μM dorsomorphin (Sigma P5499). Media was changed daily for 7 days
and cells were split 1:1 onto Geltrex substrate on day 8. These neural progenitor cells were cultured in N2/B27 and split once cells were confluent. 1.5 μM purmorphamine (Sigma SML0868) was
added during day 10–20, cells transferred on day 21 to plates coated with 10 μg/ml poly-L-ornithine (Sigma P3655) and 10 μg/ml laminin (Sigma L2020) and then cultured in BrainPhys media
containing 10 μM DAPT (Sigma D5942). IMMUNOCYTOCHEMISTRY Cells were fixed with 4% paraformaldehyde at room temperature, washed with PBS, permeabilized in PBST (PBS + 0.1% Triton X) and
blocked with PBS plus 5% goat serum. Fixed cells were incubated with primary antibodies plus 1% goat serum overnight at 4 °C, followed by PBS washes, and incubation with secondary antibodies
plus 1% goat serum for 1 h at room temperature. Antibodies used are listed in Supplementary Table 1. WESTERN BLOTS Samples were lysed and protein concentration measured with a BSA assay. In
all, 10 μg of protein extract was run on each lane on a Criterion TGX Precast gel 4–20% (BIO-RAD 5671094). Gels were transferred to a Immobilon-P Transfer Membrane (Millipore IPVH00010,
pore size: 0.45 μm, PVDF), membrane blocked in Odyssey Blocking Buffer (Li-Cor 927-40,000) and probed overnight with primary antibodies at 4 °C. Following washes with 1x TBST, membrane was
incubated with secondary antibody using donkey anti-rabbit (Li-Cor 1:10,000, IRDye 680, 925-68073, lot #C70601-01) or anti-mouse (Li-Cor 1:10,000, IRDye 800, 925-32212, lot #C70502-03).
Images from Li-Cor Odyssey Clx Imaging System were analysed and quantified using Image Studio Version 5.2. Antibodies are listed in Supplementary Table 1. OXIDATIVE STRESS EXPERIMENTS We
used the ROS-ID® Total ROS/Superoxide detection kit (Enzo life ENZ-51010), which includes two fluorescent dyes—total reactive oxygen species (ROS) detection reagent (Green) and Superoxide
Detection Reagent (Orange). Neurons were imaged using Opera Phenix high-content imaging system (Perkin Elmer). IMAGE ANALYSIS Dissociated neurons were plated on poly-L-ornithine and
laminin-coated 24- or 96-well tissue culture plates. Cells were fixed and stained with neuronal marker MAP2. Quantitative image analyses of cortical interneuron cultures were conducted in
Opera Phenix at 20x magnification using Harmony software (Perkin Elmer). Cell soma area, nuclear area and neurite length from 10 randomly selected fields were quantified. QUANTIFICATION OF
SYNAPTIC PUNCTA Dissociated neurons were plated on poly-l-ornithine and laminin-coated glass bottom 24-well tissue culture plates. Cells were fixed and stained with neuronal markers MAP2,
gephyrin, synaptotagmin1/2 and Homer 1. Image analyses were conducted in Opera Phenix at 60x magnification using Harmony software. Neurites and synaptic puncta were identified in an
automated way based on synaptic marker staining along neurite length to calculate puncta density. QPCR RNA was extracted using the RNeasy Mini Kit (Qiagen 74104) and 1 μg RNA was converted
into cDNA using High Capacity cDNA Reverse Transcription Kit (ThermoFisher 4368814). SYBR green (Mangobio 08-25-00020) assay was run on Roche light cycler 480II. We used 96-well plates with
each well containing 4 μl of EvaGreen master mix, 2 μl primer (2 μM), 5 μlDNA (16 ng/μl) and 9 μl water. Three technical replicates per sample were tested. Analysis was performed on Excel
and graphs prepared with GraphPad version 8.0. Primers are listed in Supplementary Table 2. NLGN2 KNOCKDOWN Knockdown NLGN2 GFP shRNA lentiviral particles (Origene TL302944V) or scrambled
GFP lentiviral particles were transduced in a co-culture of excitatory and inhibitory neurons from a control iPSC line at day 85 of differentiation. Knockdown was considered successful when
NLGN2 antibody staining did not co-localize with GFP-positive cells. GFP-positive cells were analysed for synaptic puncta quantification. NLGN2 OVEREXPRESSION Overexpression NLGN2 GFP
lentiviral particles (Origene RC222544L2V) or scrambled GFP lentiviral particles were transduced in a co-culture of excitatory and inhibitory neurons from a SCZ iPSC line at day 85 of
differentiation. Overexpression was considered successful when NLGN2 antibody staining co-localized with GFP-positive cells. MICROELECTRODE ARRAY EXPERIMENTS Microelectrode array (MEA)
experiments were performed using a MED64 Presto. Co-cultured neurons at day 90 were plated on MEA 24-well plates, which has 16 electrodes per well. Spontaneous activity was recorded for
1-min periods and data analysed using MEA symphony software. CALCIUM IMAGING AND ANALYSIS We imaged interneurons at 37 °C in 1 μM Fluo-4AM solution for 60 min. The glass dish with the cells
was mounted on stage of the Leica TCS SP8. Exposures (ex/em 494/506 nm) were captured every two seconds for 90 s before and after addition of 30 mM GABA. To record responses from specific
neurons, the experiment was “replayed” using ImageJ and regions of interest (ROIs) drawn around neurons to measure fluorescence intensity. Collected data was used to calculate magnitude of
fluorescence change by dividing fluorescence intensity after addition of GABA (F) with baseline fluorescence intensity (F0). DATA COLLECTION AND STATISTICS All experiments were repeated at
least three times. Only neuronal cultures with >80% MAP2 positive cells were used. Cultures were randomly selected for assays in a blinded manner. Statistical analyses used are reported
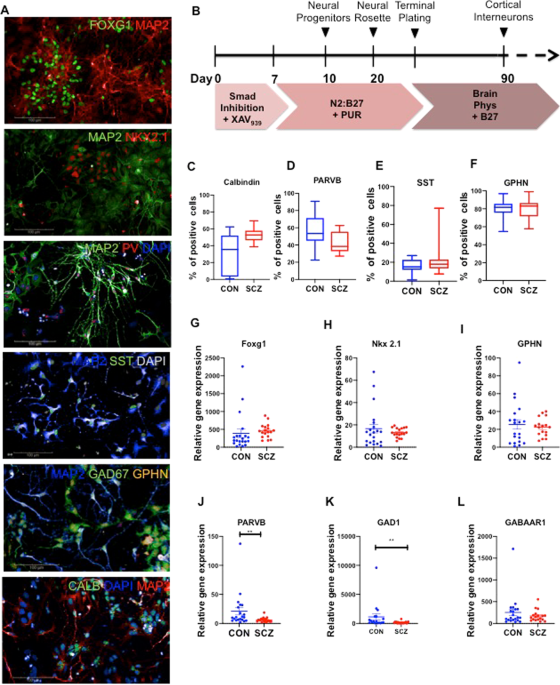
in figure legends. Normal distribution was checked using the Kolmogorov-Smirnov’s test. Statistical analyses were done with Prism8. RESULTS GENERATION OF CORTICAL INTERNEURONS FROM IPSCS
With approval from the Institutional Review Boards (IRB), we recruited age- and sex-matched SCZ and CON subjects to obtain fibroblasts and reprogramme them into iPSCs through induction with
modified mRNA17,18. Supplementary Table 3 describes the iPSC lines used in this study, along with details of the subjects’ diagnoses, age, antipsychotic treatment history and medical
co-morbidites. We validated reprogrammed iPSCs using standard protocols (Supplementary Fig. S1) and differentiated them to generate cortical interneurons using established protocols19. We
confirmed cortical interneuron identity using immunocytochemistry and gene expression (Fig. 1). At day 90 of differentiation, the cultures were positive for neurons expressing FOXG1, Nkx2.1,
parvalbumin (PARVB), somatostatin (SST), GAD67, gephyrin (GPHN) and calbindin (CALB). We quantified the these markers in all lines and ~80% of the cells expressed gephyrin, confirming their
inhibitory nature (Fig. 1a, f). >40% of the cells were positive for calbindin, a calcium-binding protein found in inhibitory interneurons (Fig. 1a, c). Somatostatin was expressed in ~20%
of neurons, while parvalbumin was expressed in 40–50% of the cells (Fig. 1a, d, e). Gene expression analysis with qPCR showed presence of FOXG1, Nkx2.1, GPHN, PARVB, GAD1, GABAAR1, and SST
in all lines (Fig. 1g–m). While the fraction of cells positive for parvalbumin was similar in CON and SCZ (Fig. 1d), expression level of PARVB mRNA was significantly lower in SCZ compared to
CON (Fig. 1j). A similar pattern has been reported in SCZ brains—density of parvalbumin expressing neurons was not different in SCZ but PARVB mRNA levels were significantly lower in the
prefrontal cortex15,20. We found that GAD1 levels were significantly lower in SCZ interneurons (Fig. 1k), which recapitulates well-replicated postmortem findings of reduced levels of GAD1
expression in the prefrontal cortex in SCZ21,22. EFFECTS OF OXIDATIVE STRESS IN CORTICAL INTERNEURONS We compared SCZ and CON cortical interneurons for cell soma area, nuclear area and
neurite length (Fig. 2). The nuclear area in SCZ interneurons was significantly smaller when compared to CON (Fig. 2b) but cell soma area and neurite lengths did not vary between the groups
(Fig. 2c, d). There is a large body of research indicating the presence of oxidative stress in SCZ, specifically in cortical interneurons23,24,25,26,27. Interneurons are highly susceptible
to oxidative stress28 and oxidative stress affects the structure of the nuclear extracellular matrix and the nuclear area29. Our results suggested that SCZ interneurons have an innate
oxidative stressed state at baseline. We examined the effect of oxidative stress with pyocyanin on cortical interneurons by measuring reactive oxygen species (ROS) and super oxygen species
(SO). Pyocyanin (0.1 mM) increased ROS and SO species in both CON and SCZ cultures (Fig. 2a), which led to significant reduction in nuclear area in CON neurons but not in SCZ neurons (Fig.
2e). This suggests that SCZ interneurons have an innately stressed biology at baseline compared to CON interneurons and SCZ interneurons are restricted in their ability to modulate their
nuclear area in response to oxidative stress. GAD67 AND GEPHYRIN LEVELS ARE REDUCED IN SCZ CORTICAL INTERNEURONS Postmortem studies in SCZ have shown significant alterations of key enzymes
involved in synthesis of gamma-aminobutyric acid (GABA)30. It has not been clear whether these findings in postmortem brains were contributing to the disease biology of SCZ or whether they
were downstream consequences of disease processes or treatment. iPSC-derived SCZ cortical interneurons showed significantly lower levels of GAD67 protein, an enzyme that decarboxylates
glutamate to GABA (Fig. 2f, h), as well as reduced levels of GAD1 mRNA (Fig. 1k), consistent with postmortem SCZ findings30,31. These results indicate that the complex genetic background of
SCZ predisposes interneurons to decreased expression of GAD67. SCZ cortical interneurons also expressed significantly lower levels of gephyrin, a postsynaptic protein specific to inhibitory
neurons (Fig. 2f, g). SYNAPTIC DEFICITS IN EX VIVO CO-CULTURES OF SCZ CORTICAL EXCITATORY NEURONS AND INTERNEURONS Since gephyrin is a neuronal assembly protein that anchors inhibitory
neurotransmitter receptors to the postsynaptic cytoskeleton32, we examined whether there were differences in measures of synaptic connectivity in SCZ. Postmortem studies in SCZ show
reduction in postsynaptic elements in the cortical but not in subcortical tissue33. We generated excitatory cortical neurons from the same iPSC lines using dual SMAD inhibition34,35 and
co-cultured them with inhibitory cortical interneurons (Fig. 3). We quantified gephyrin (GPHN), Homer 1, and synaptotagmin1/2 (SYT1/2) in the co-cultures (Fig. 3a, b). SCZ co-cultures showed
significantly lower density of GPHN, Homer 1, and SYT1/2 puncta when compared to CON co-cultures (Fig. 3c–e). We then “cross-cultured” SCZ and CON excitatory and inhibitory neurons to
investigate how co-culturing SCZ interneurons with CON excitatory neurons, and vice versa, would affect synaptic puncta density (Fig. 3a, b). SCZ(e)-CON(i) co-cultures had synaptic puncta
density similar to CON(e)-CON(i) while CON(e)-SCZ(i) densities were in the range seen with SCZ(e)-SCZ(i) co-cultures (Fig. 3c–e). These experiments showed that the decreased synaptic puncta
density in SCZ co-cultures result from a deficiency inherent in SCZ interneurons. DECREASED SYNAPTIC ADHESION PROTEINS IN SCZ CORTICAL INTERNEURONS Changes in genes expressing synaptic
adhesion proteins such as neural cell adhesion molecules and neuroligins have been implicated in SCZ36. In light of lower levels of GAD67 and gephyrin and reduced synaptic puncta density in
SCZ interneurons, we examined whether synaptic adhesion and cytoskeletal proteins involved in synapse formation and maintenance were altered in SCZ interneurons. We found significant
reduction in levels of Neural Cell Adhesion Molecule 1 (NCAM1) and Neuroligin 2 (NLGN2) in SCZ interneurons when compared to CON (Fig. 2f, i, j). NCAM1 plays an important role in molecular
organization of the synaptic terminal and interacts with gephyrin to stabilize glycine and GABAA receptors at inhibitory synapse36,37. NLGN2 is expressed exclusively in inhibitory synapses,
in contrast to NLGN1 and NLGN338. These findings indicate that SCZ interneurons have innate deficiencies in the machinery for creating and maintaining the inhibitory synapse that could lead
to decreased levels of synaptic connections and neuronal connectivity. NLGN2 LOSS-OF-FUNCTION IN HEALTHY NEURONS LEADS TO REDUCED SYNAPTIC PUNCTA DENSITY WHILE NLGN2 OVEREXPRESSION RESCUES
SYNAPTIC DEFICITS IN SCZ NLGN2 has a pivotal role in the inhibitory synaptic structure38. Since decreased NLGN2 accompanied reduced synaptic puncta density in SCZ interneurons, we sought to
to determine whether differential expression of NLGN2 was mediating the reduction in synaptic puncta density. We carried out loss-of-function studies of NLGN2 in CON cultures, utilizing
shRNA lentivirus against NLGN2 (Fig. 4). NLGN2 knockdown in CON cultures resulted in significant reduction of Homer 1, GPHN and SYT1/2 puncta (Fig. 4a, b), mirroring results observed in SCZ
interneurons (Fig. 2f, i). We then carried out the converse experiment by overexpressing NLGN2 in SCZ neurons, which led to a significant increase in Homer 1, GPHN and SYT1/2 puncta (Fig.
4a, c). These results show that NLGN2, which is decreased in SCZ interneurons, mediates synaptic puncta density reduction in SCZ neurons. SCZ INTERNEURONS SHOW REDUCED SPONTANEOUS ACTIVITY
In light of the reduced synaptic density in SCZ interneurons, we sought to characterize functional activity in SCZ and CON neuronal cultures. We measured Ca2+ oscillations under baseline
conditions and in setting of GABA exposure (Fig. 5). CON interneurons had a much more robust response to GABA compared to the SCZ interneurons (Fig. 5a, b). We also used a microelectrode
array (MEA) to record extracellular action potential waveforms and quantify the frequency of spontaneous action potential spikes in a real-time and in a label-free manner. To verify that MEA
was accurately recording neuronal activity, we confirmed that 1 μM tetrodotoxin (TTX) abolished neuronal electrical activity (Supplementary Figure S2F, G). In MEA experiments, SCZ
co-cultures had significantly lower frequency of spontaneous activity compared to CON neurons (Fig. 5c, d). We then cross-cultured SCZ and CON interneurons and excitatory neurons and
recorded extracellular action potential waveforms. Co-culturing CON interneurons with SCZ excitatory neurons resulted in a frequency of spontaneous activity similar to that observed with the
CON co-cultures while co-culturing SCZ interneurons with CON excitatory neurons resulted in a frequency of spontaneous activity similar to that observed with the SCZ co-cultures. These
results are consistent with results of the synaptic puncta co-culture studies (Fig. 3) and indicate that differences in the electrical activity between the SCZ and CON neuronal cultures are
attributable to deficits in SCZ cortical interneurons. N-ACETYL CYSTEINE RESCUES MORPHOLOGICAL AND SYNAPTIC PUNCTA DEFICITS IN SCZ INTERNEURONS _N_-acetyl cysteine (NAC) is an antioxidant
with efficacy in treating symptoms relevant to schizophrenia, both in preclinical models and in clinical studies39,40,41,42,43. Since our studies indicated that SCZ interneurons have an
innately stressed biology at baseline, reflected in the reduced nuclear area (Fig. 2b), we examined the effect of NAC on the nuclear area in SCZ. We carried out a dose-response experiment
and found that exposure to 0.25 mM NAC for 24 h resulted in significant increase in nuclear area of SCZ interneurons (Fig. 5f). Exposure to 0.25 mM NAC in SCZ co-cultures also led to
significant increase in the density of Homer 1, GPHN and SYT1/2 puncta (Fig. 5g, h). This suggests that decreasing oxidative stress with NAC in SCZ cultures leads to increased expression of
synaptic markers. Since we had earlier found that differences in NLGN2 expression modulated levels of synaptic puncta density in the co-cultures, we examined whether NAC affected NLGN2
levels in mediating the increase in synaptic puncta density. SCZ interneurons exposed to 0.25 mM NAC showed a significant increase in NLGN2 levels (Fig. 5i, j). Collectively, these results
showed that ameliorating oxidative stress with NAC in SCZ leads to increased NLGN2 expression in interneurons and restores synaptic deficits in SCZ neuronal cultures. DISCUSSION Advances in
our understanding of the disease biology of SCZ have been hindered by the difficulty in generating cell types relevant to the disease and in identifying disease-specific abnormalities in
cells from patients with complex psychiatric disorders44. Cellular reprogramming methods enable generation of human iPSCs, which can be differentiated to neuronal subtypes implicated in the
biology of psychiatric disorders45,46,47,48,49,50,51. Recent methodological advances in human iPSC differentiation enable generation of cortical interneurons implicated in SCZ disease
biology19,52. We differentiated iPSCs to cortical interneurons from 18 individual subjects to examine disease-specific differences in specific neuronal subtypes. We show here that SCZ
interneurons express lower levels of GAD67 and gephyrin and have a reduced density of synaptic puncta in co-cultures with excitatory neurons. In doing so, we show that a well-replicated
post-mortem finding in SCZ, that of lower levels of GAD67, can be recapitulated in neuronal cultures generated from iPSCs of SCZ subjects. By cross-culturing inhibitory and excitatory
neurons, we show that the decreased synaptic puncta in SCZ result from deficiencies in SCZ cortical interneurons. We further show that lower levels of NLGN2 accompany the decreased synaptic
puncta density in SCZ. With knockdown and overexpression experiments, we show that NLGN2 expression mediates the reduction in synaptic puncta density in SCZ. Ca2+ imaging and MEA studies
revealed that the functional decifits in SCZ neurons arise from deficits inherent in the interneurons. Taken together, our findings implicate deficiencies in the synaptic machinery in
cortical interneurons as a critical regulator of excitatory and inhibitory (E-I) activity imbalance in SCZ. We also discovered a heretofore-unknown connection between oxidative stress and
synaptic connections in SCZ mediated by NLGN2. SCZ interneurons, which have a smaller nuclear area indicative of an innate oxidative stressed state, do not modulate their nuclear area in
response to oxidative stress, unlike CON interneurons. There is a large body of literature on the role of oxidative stress in schizophrenia23,24,25,26,27,28 and the potential of NAC in
treating symptoms relevant to schizophrenia39,40,41,42,43. We found that NAC increased nuclear area in SCZ interneurons in a dose-dependent manner to values in observed in CON interneurons.
Futhermore, NAC increased NLGN2 levels in SCZ interneurons and led to increased synaptic puncta density. While previous literature had reported deficits in oxidative stress in SCZ, we show
for the first time that ameliorating oxidative stress with NAC leads to significant effects on synaptic biology in SCZ. There are notes of caution in generalizing results from cellular
models of idiopathic complex brain disorders such as SCZ. There are continuing debates about whether SCZ constitutes one major disease entity that has the same underlying biology or whether
it is a syndrome that comprises of multiple disorders with different causes53. It is possible that there may be different biological processes involved in the development of the disease in
different subsets of patients. Nevertheless, the clinical presentation of SCZ is rather stereotyped and most cases of this condition may share substantial mechanisms at the genomic, cellular
and circuitry levels. The GABAergic hypothesis tested here was based on postmortem findings from patients who had been subjected to the physiological stress of psychotic episodes as well as
that of the effects of medications over the years20,21,30,31. The iPSC reprogramming process results in erasure of much of the epigenetics and hence, it only enables us to capture the risks
and features accorded by the underlying complex genetics and not from epigenetic differences54. Since our ex vivo findings recapitulate the postmortem results, this suggests that the
postmortem findings of GABAergic deficits in SCZ reflect underlying genetic differences. Pursuit of studies like the ones reported here might lead to a better characterization of those
underlying mechanisms and the identification of targets for new therapeutic approaches. REFERENCES * Mueser, K. T. & McGurk, S. R. Schizophrenia. _Lancet_ 363, 2063–2072 (2004). Article
PubMed Google Scholar * Harrow, M., Sands, J. R., Silverstein, M. L. & Goldberg, J. F. Course and outcome for schizophrenia versus other psychotic patients: a longitudinal study.
_Schizophr. Bull._ 23, 287–303 (1997). Article CAS PubMed Google Scholar * Selemon, L. D. & Zecevic, N. Schizophrenia: a tale of two critical periods for prefrontal cortical
development. _Transl. Psychiatry_ 5, e623 (2015). Article CAS PubMed PubMed Central Google Scholar * Saha, S., Chant, D. & McGrath, J. A systematic review of mortality in
schizophrenia: is the differential mortality gap worsening over time? _Arch. Gen. Psychiatry_ 64, 1123–1131 (2007). Article PubMed Google Scholar * Jablensky, A. Epidemiology of
schizophrenia: the global burden of disease and disability. _Eur. Arch. Psychiatry Clin. Neurosci._ 250, 274–285 (2000). Article CAS PubMed Google Scholar * Rössler, W., Salize, H. J.,
van Os, J. & Riecher-Rössler, A. Size of burden of schizophrenia and psychotic disorders. _Eur. Neuropsychopharmacol._ 15, 399–409 (2005). Article PubMed CAS Google Scholar * Millan,
M. J., Goodwin, G. M., Meyer-Lindenberg, A. & Ögren, S. O. 60 years of advances in neuropsychopharmacology for improving brain health, renewed hope for progress. _Eur.
Neuropsychopharmacol._ 25, 591–598 (2015). Article CAS PubMed Google Scholar * Millan, M. J., Goodwin, G. M., Meyer-Lindenberg, A. & Ove Ögren, S. Learning from the past and looking
to the future: emerging perspectives for improving the treatment of psychiatric disorders. _Eur. Neuropsychopharmacol._ 25, 599–656 (2015). Article CAS PubMed Google Scholar * Kesby, J.
P., Eyles, D. W., McGrath, J. J. & Scott, J. G. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. _Transl. Psychiatry_ 8, 30 (2018).
Article CAS PubMed PubMed Central Google Scholar * Gao, R. & Penzes, P. Common mechanisms of excitatory and inhibitory imbalance in schizophrenia and autism spectrum disorders.
_Curr. Mol. Med._ 15, 146–67. (2015). Article CAS PubMed PubMed Central Google Scholar * Selten, M., van Bokhoven, H. & Nadif Kasri, N. Inhibitory control of the
excitatory/inhibitory balance in psychiatric disorders. _F1000Res_ 7, 23 (2018). Article PubMed PubMed Central Google Scholar * Kehrer, C., Maziashvili, N., Dugladze, T. & Gloveli,
T. Altered excitatory-inhibitory balance in the NMDA-hypofunction model of schizophrenia. _Front. Mol. Neurosci._ 1, 6 (2008). Article PubMed PubMed Central Google Scholar * Inan, M.,
Petros, T. J. & Anderson, S. A. Losing your inhibition: linking cortical GABAergic interneurons to schizophrenia. _Neurobiol. Dis._ 53, 36–48 (2013). Article CAS PubMed Google Scholar
* Benes, F. M. & Berretta, S. GABAergic interneurons: implications for understanding schizophrenia and bipolar disorder. _Neuropsychopharmacology_ 25, 1–27 (2001). Article CAS PubMed
Google Scholar * Lewis, D. A., Curley, A. A., Glausier, J. R. & Volk, D. W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. _Trends Neurosci._ 35, 57–67
(2012). Article CAS PubMed Google Scholar * Ferguson, B. R. & Gao, W. J. PV interneurons: critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric
disorders. _Front Neural Circuits_ 12, 37 (2018). Article PubMed PubMed Central CAS Google Scholar * Warren, L. & Lin, C. mRNA-based genetic reprogramming. _Mol. Ther._ 27, 729–34.
(2019). Article CAS PubMed Google Scholar * Okano, H. & Yamanaka, S. iPS cell technologies: significance and applications to CNS regeneration and disease. _Mol. Brain_ 7, 22 (2014).
Article PubMed PubMed Central CAS Google Scholar * Liu, Y. et al. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. _Nat. Protoc._ 8, 1670–1679
(2013). Article CAS PubMed PubMed Central Google Scholar * Hashimoto, T. et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with
schizophrenia. _J. Neurosci._ 23, 6315–6326 (2003). Article CAS PubMed PubMed Central Google Scholar * Akbarian, S. et al. Gene expression for glutamic acid decarboxylase is reduced
without loss of neurons in prefrontal cortex of schizophrenics. _Arch. Gen. Psychiatry_ 52, 258–266 (1995). Article CAS PubMed Google Scholar * Volk, D. W., Austin, M. C., Pierri, J. N.,
Sampson, A. R. & Lewis, D. A. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with
schizophrenia. _Arch. Gen. Psychiatry_ 57, 237–245 (2000). Article CAS PubMed Google Scholar * Do, K. Q., Cuenod, M. & Hensch, T. K. Targeting oxidative stress and aberrant critical
period plasticity in the developmental trajectory to schizophrenia. _Schizophr. Bull._ 41, 835–846 (2015). Article PubMed PubMed Central Google Scholar * O’Donnell, P. Cortical
interneurons, immune factors and oxidative stress as early targets for schizophrenia. _Eur. J. Neurosci._ 35, 1866–1870 (2012). Article PubMed Google Scholar * Steullet, P. et al. Redox
dysregulation, neuroinflammation, and NMDA receptor hypofunction: a “central hub” in schizophrenia pathophysiology? _Schizophr. Res._ 176, 41–51 (2016). Article CAS PubMed Google Scholar
* Maas, D. A., Vallès, A. & Martens, G. J. M. Oxidative stress, prefrontal cortex hypomyelination and cognitive symptoms in schizophrenia. _Transl. Psychiatry_ 7, e1171 (2017). Article
CAS PubMed PubMed Central Google Scholar * Kim, S. Y. et al. Redox dysregulation in schizophrenia revealed by in vivo NAD+/NADH measurement. _Schizophr. Bull._ 43, 197–204 (2017).
Article PubMed Google Scholar * Sullivan, E. M. & O’Donnell, P. Inhibitory interneurons, oxidative stress, and schizophrenia. _Schizophr. Bull._ 38, 373–376 (2012). Article PubMed
PubMed Central Google Scholar * Barascu, A. et al. Oxydative stress alters nuclear shape through lamins dysregulation: a route to senescence. _Nucleus_ 3, 411–417 (2012). Article PubMed
PubMed Central Google Scholar * Curley, A. A. et al. Cortical deficits of glutamic acid decarboxylase 67 expression in schizophrenia: clinical, protein, and cell type-specific features.
_Am. J. Psychiatry_ 168, 921–929 (2011). Article PubMed PubMed Central Google Scholar * Woo, T. U., Walsh, J. P. & Benes, F. M. Density of glutamic acid decarboxylase 67 messenger
RNA-containing neurons that express the N-methyl-D-aspartate receptor subunit NR2A in the anterior cingulate cortex in schizophrenia and bipolar disorder. _Arch. Gen. Psychiatry_ 61, 649–657
(2004). Article CAS PubMed Google Scholar * Groeneweg, F. L., Trattnig, C., Kuhse, J., Nawrotzki, R. A. & Kirsch, J. Gephyrin: a key regulatory protein of inhibitory synapses and
beyond. _Histochem. Cell Biol._ 150, 489–508 (2018). Article CAS PubMed Google Scholar * Berdenis van Berlekom A. et al. Synapse pathology in schizophrenia: a meta-analysis of
postsynaptic elements in postmortem brain studies. Schizophr Bull. pii: sbz060 2019. https://doi.org/10.1093/schbul/sbz060. * Shi, Y., Kirwan, P. & Livesey, F. J. Directed
differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. _Nat. Protoc._ 7, 1836–1846 (2012). Article CAS PubMed Google Scholar * Watmuff, B. et al.
Disease signatures for schizophrenia and bipolar disorder using patient-derived induced pluripotent stem cells. _Mol. Cell. Neurosci._ 73, 96–103 (2016). Article CAS PubMed PubMed
Central Google Scholar * Maćkowiak, M., Mordalska, P. & Wędzony, K. Neuroligins, synapse balance and neuropsychiatric disorders. _Pharmacol. Rep._ 66, 830–835 (2014). Article PubMed
CAS Google Scholar * Sullivan, C. S., Kümper, M., Temple, B. S. & Maness, P. F. The neural cell adhesion molecule (NCAM) promotes clustering and activation of EphA3 receptors in
GABAergic interneurons to induce ras homolog gene family, member A (RhoA)/Rho-associated protein kinase (ROCK)-mediated growth cone collapse. _J. Biol. Chem._ 291, 26262–72. (2016). Article
CAS PubMed PubMed Central Google Scholar * Nguyen Q. A., Horn M. E., Nicoll R. A. Distinct roles for extracellular and intracellular domains in neuroligin function at inhibitory
synapses. Elife. 5, e19236. (2016). * Conus, P. et al. N-acetylcysteine in a double-blind randomized placebo-controlled trial: toward biomarker-guided treatment in early psychosis.
_Schizophr. Bull._ 44, 317–27. (2018). Article PubMed Google Scholar * Girgis, R. R. et al. Effects of acute N-acetylcysteine challenge on cortical glutathione and glutamate in
schizophrenia: a pilot in vivo proton magnetic resonance spectroscopy study. _Psychiatry Res._ 275, 78–85 (2019). Article CAS PubMed PubMed Central Google Scholar * Kulak, A. et al.
Redox dysregulation in the pathophysiology of schizophrenia and bipolar disorder: insights from animal models. _Antioxid. Redox Signal._ 18, 1428–1443 (2013). Article CAS PubMed Google
Scholar * Klauser, P. et al. N-acetylcysteine add-on treatment leads to an improvement of fornix white matter integrity in early psychosis: a double-blind randomized placebo-controlled
trial. _Transl. Psychiatry_ 8, 220 (2018). Article PubMed PubMed Central CAS Google Scholar * Mullier, E. et al. N-acetyl-cysteine supplementation improves functional connectivity
within the cingulate cortex in early psychosis: a pilot study. _Int. J. Neuropsychopharmacol._ 22, 478–87. (2019). Article PubMed PubMed Central Google Scholar * Karmacharya, R. &
Haggarty, S. J. Stem cell models of neuropsychiatric disorders. _Mol. Cell. Neurosci._ 73, 1–2 (2016). Article CAS PubMed Google Scholar * Sellgren, C. M. et al. Increased synapse
elimination by microglia in schizophrenia patient-derived models of synaptic pruning. _Nat. Neurosci._ 22, 374–85. (2019). Article CAS PubMed PubMed Central Google Scholar * Chen, H. M.
et al. Transcripts involved in calcium signaling and telencephalic neuronal fate are altered in induced pluripotent stem cells from bipolar disorder patients. _Transl. Psychiatry_ 4, e375
(2014). Article CAS PubMed PubMed Central Google Scholar * O’Shea, K. S. & McInnis, M. G. Neurodevelopmental origins of bipolar disorder: iPSC models. _Mol. Cell. Neurosci._ 73,
63–83 (2015). Article PubMed CAS Google Scholar * Grunwald, L. M. et al. Comparative characterization of human induced pluripotent stem cells (hiPSC) derived from patients with
schizophrenia and autism. _Transl. Psychiatry_ 9, 179 (2019). Article PubMed PubMed Central CAS Google Scholar * Watmuff, B., Liu, B. & Karmacharya, R. Stem cell-derived neurons in
the development of targeted treatment for schizophrenia and bipolar disorder. _Pharmacogenomics_ 18, 471–479 (2017). Article CAS PubMed Google Scholar * Lin, M., Lachman, H. M. &
Zheng, D. Transcriptomics analysis of iPSC-derived neurons and modeling of neuropsychiatric disorders. _Mol. Cell. Neurosci._ 73, 32–42 (2015). Article PubMed PubMed Central CAS Google
Scholar * McPhie, D. L. et al. Oligodendrocyte differentiation of induced pluripotent stem cells derived from subjects with schizophrenias implicate abnormalities in development. _Transl.
Psychiatry_ 8, 230 (2018). Article PubMed PubMed Central CAS Google Scholar * Ni P. et al. iPSC-derived homogeneous populations of developing schizophrenia cortical interneurons have
compromised mitochondrial function. Molecular psychiatry. https://doi.org/10.1038/s41380-019-0423-3 (2019). * Arnedo, J. et al. Uncovering the hidden risk architecture of the schizophrenias:
confirmation in three independent genome-wide association studies. _Am. J. Psychiatry_ 172, 139–153 (2015). Article PubMed Google Scholar * Watanabe, A., Yamada, Y. & Yamanaka, S.
Epigenetic regulation in pluripotent stem cells: a key to breaking the epigenetic barrier. _Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci._ 368, 20120292 (2013). Article CAS Google Scholar
Download references ACKNOWLEDGEMENTS This work was supported by a National Institute of Mental Health Biobehavioral Research Awards for Innovative New Scientists (BRAINS) Award R01MH113858
(to R.K.), National Institute of Mental Health Clinical Scientist Development Award K08MH086846 (to R.K.), the Doris Duke Charitable Foundation Clinical Scientist Development Award (to
R.K.), the Ryan Licht Sang Bipolar Foundation (to R.K.), the Harvard Stem Cell Institute (to R.K.), the Phyllis & Jerome Lyle Rappaport Foundation (to R.K.) and by Steve Willis and
Elissa Freud (to R.K.). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Center for Genomic Medicine, Massachusetts General Hospital, Boston, MA, USA Annie Kathuria, Kara Lopez-Lengowski,
Bradley Watmuff & Rakesh Karmacharya * Chemical Biology and Therapeutic Science Program, Broad Institute of MIT & Harvard, Cambridge, MA, USA Annie Kathuria, Kara Lopez-Lengowski,
Bradley Watmuff & Rakesh Karmacharya * Department of Psychiatry, Harvard Medical School, Boston, MA, USA Annie Kathuria, Bradley Watmuff, Donna McPhie, Bruce M. Cohen & Rakesh
Karmacharya * Schizophrenia and Bipolar Disorder Program, McLean Hospital, Belmont, MA, USA Donna McPhie, Bruce M. Cohen & Rakesh Karmacharya * Graduate Program in Chemical Biology,
Harvard University, Cambridge, MA, USA Rakesh Karmacharya * Program in Neuroscience, Harvard University, Cambridge, MA, USA Rakesh Karmacharya Authors * Annie Kathuria View author
publications You can also search for this author inPubMed Google Scholar * Kara Lopez-Lengowski View author publications You can also search for this author inPubMed Google Scholar * Bradley
Watmuff View author publications You can also search for this author inPubMed Google Scholar * Donna McPhie View author publications You can also search for this author inPubMed Google
Scholar * Bruce M. Cohen View author publications You can also search for this author inPubMed Google Scholar * Rakesh Karmacharya View author publications You can also search for this
author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Rakesh Karmacharya. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict of interest.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation,
distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and
indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to
the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will
need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE
CITE THIS ARTICLE Kathuria, A., Lopez-Lengowski, K., Watmuff, B. _et al._ Synaptic deficits in iPSC-derived cortical interneurons in schizophrenia are mediated by NLGN2 and rescued by
_N_-acetylcysteine. _Transl Psychiatry_ 9, 321 (2019). https://doi.org/10.1038/s41398-019-0660-x Download citation * Received: 08 August 2019 * Revised: 06 September 2019 * Accepted: 20
October 2019 * Published: 28 November 2019 * DOI: https://doi.org/10.1038/s41398-019-0660-x SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative






