- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT The long-known resistance to pathogens provided by host-associated microbiota fostered the notion that adding protective bacteria could prevent or attenuate infection. However, the
identification of endogenous or exogenous bacteria conferring such protection is often hindered by the complexity of host microbial communities. Here, we used zebrafish and the fish pathogen
_Flavobacterium columnare_ as a model system to study the determinants of microbiota-associated colonization resistance. We compared infection susceptibility in germ-free, conventional and
reconventionalized larvae and showed that a consortium of 10 culturable bacterial species are sufficient to protect zebrafish. Whereas survival to _F. columnare_ infection does not rely on
host innate immunity, we used antibiotic dysbiosis to alter zebrafish microbiota composition, leading to the identification of two different protection strategies. We first identified that
the bacterium _Chryseobacterium massiliae_ individually protects both larvae and adult zebrafish. We also showed that an assembly of 9 endogenous zebrafish species that do not otherwise
protect individually confer a community-level resistance to infection. Our study therefore provides a rational approach to identify key endogenous protecting bacteria and promising
candidates to engineer resilient microbial communities. It also shows how direct experimental analysis of colonization resistance in low-complexity in vivo models can reveal unsuspected
ecological strategies at play in microbiota-based protection against pathogens. You have full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS
PROBIOTICS: THEIR ACTION AGAINST PATHOGENS CAN BE TURNED AROUND Article Open access 24 June 2021 HOST DEVELOPMENT OVERWHELMS ENVIRONMENTAL DISPERSAL IN GOVERNING THE ECOLOGICAL SUCCESSION
OF ZEBRAFISH GUT MICROBIOTA Article Open access 19 January 2021 UNRAVELLING THE TEMPORAL AND SPATIAL VARIATION OF FUNGAL PHYLOTYPES FROM EMBRYO TO ADULT STAGES IN ATLANTIC SALMON Article
Open access 10 January 2024 INTRODUCTION Animal resident microbial consortia form complex and long-term associations with important community-level functions essential for host development
and physiology [1, 2]. Microbial ecosystems also provide protection against exogenous pathogens by inhibition of pathogen settlement and growth and/or stimulation of the host immune system
[3,4,5,6,7,8]. From the perspective of microbial community composition, a shift or reduction in resident microbial diversity, a phenomenon generally referred to as dysbiosis, is often
associated with increased susceptibility to infection due to the loss or change in abundance of key microbial community members [3, 9]. These observations early supported the notion that
addition or promotion of individually or communally protective bacteria (such as probiotics) could minimize microbiota dysbiosis or directly prevent infection to restore host health
[10,11,_–_12]. Although the efficacy of probiotics has been shown in animals and humans, their mechanisms of action are poorly understood and low throughput experimental models often offer
limited information on the individual contribution of probiotic species to community functions [1, 6, 7, 13, 14]. Moreover, characterization of bacterial strains improving colonization
resistance is still hindered by the complexity of host-commensal ecosystems. Zebrafish have recently emerged as a powerful tool to study microbe-microbe and host-microbe interactions
[15,16,17,18,_–_19]. Zebrafish can be easily reared germ-free or gnotobiotically in association with specific bacterial species [15, 20]. Moreover, zebrafish bacterial communities are
increasingly well characterized and a number of phylogenetically distinct zebrafish gut bacteria can be cultured, making this model system directly amenable to microbiota manipulation and
assessment of probiotic effect on host infection resistance [21,22,23,_–_24]. Several studies have used zebrafish to evaluate the effect of exogenous addition of potential probiotics on host
resistance to infection by various pathogens [22,23,24,25,26,27,28,_–_29]. However, the role of the endogenous microbial community in protecting against invasive pathogens was rarely
assessed and the reported protections were often partial, illustrating the difficulty in identifying fully protective exogenous probiotics. One major fish pathogen causing such problematic
seasonal outbreaks is _Flavobacterium columnare_, a ubiquitously distributed freshwater bacterium that is the etiological agent of columnaris disease [30]. This disease affects a broad range
of wild and cultured species including carp, channel catfish, goldfish, eel, salmonids and tilapia [30,31,32,33,_–_34]. Different _F. columnare_ strains exhibit different degrees of
virulence but relatively similar infection phenotypes [30, 31, 35,36,37]. The symptoms primarily associated with strains with low virulence are gross tissue damages of gills, skin, fins, and
tail, whilst such damages are not observed in highly virulent strains, leading to mortality within hours [31, 38]. Although _F. columnare_ infection causes important losses in aquaculture,
there is no consensus on the determinants of its virulence. Recently, however, type IX secretion system (T9SS) was shown to be involved in _F. columnare_ pathogenesis in adult zebrafish, but
the nature of the secreted virulence factors remains unclear [39]. Here we used germ-free and conventional zebrafish larvae to mine the indigenous commensal microbiota for bacterial species
protecting against _F. columnare_. We identified two distinct infection resistance strategies preventing mortality caused by _F. columnare_, mediated either by an individual member of the
microbiota, the _Bacteroidetes Chryseobacterium massiliae_ or by an assembly of 9 individually non-protecting bacterial species that formed a protective community. Our results demonstrated
that mining host microbiota constitutes a powerful approach to identify key mediators of intrinsic colonization resistance, providing insight into how to engineer ecologically resilient and
protective microbial communities. MATERIALS AND METHODS BACTERIAL STRAINS AND GROWTH CONDITIONS Bacterial strains isolated from zebrafish microbiota are listed in Table 1. _F. columnare_
strains (Supplementary Table S1) were grown at 28 °C in tryptone yeast extract salts (TYES) broth [0.4% (w/v) tryptone, 0.04% yeast extract, 0.05% (w/v) MgSO4 7H2O, 0.02% (w/v) CaCl2 2H2O,
0.05% (w/v) D-glucose, pH 7.2]. _F. columnare_ strains were assigned into four genomovar groups using 16S rRNA restriction fragment length polymorphism analysis, including genomovar I, I/II,
II, and III [40]. All 10 strains of the core zebrafish microbiota species were grown in TYES or LB at 28 °C. GENERAL HANDLING OF ZEBRAFISH Wild-type AB fish, originally purchased from the
Zebrafish International Resource Center (Eugene, OR, USA), or _myd88_-null mutants (_myd88__hu3568/hu3568_) [41], kindly provided by A.H. Meijer, (Leiden University, the Netherlands), were
raised in our facility. A few hours after spawning, eggs were collected, rinsed, and sorted under a dissecting scope to remove faeces and unfertilized eggs. All following procedures were
performed in a laminar microbiological cabinet with single-use disposable plasticware. Fish were kept in sterile 25 cm3 vented cap culture flasks containing 20 mL of water (0-6 days post
fertilization (dpf), 15 fish per flask) or 24-well microtiter plates (6-15 dpf,1 fish per 2 mL well) in autoclaved mineral water (Volvic) at 28 °C. Fish were fed 3 times a week from 4 dpf
with germ-free _Tetrahymena thermophila_ protozoans [22]. Germ-free zebrafish were produced after sterilizing the egg chorion protecting the otherwise sterile egg, with antibiotic and
chemical treatments (see below), whereas conventional larvae (with facility-innate microbiota) were directly reared from non-sterilized eggs and then handled exactly as the germ-free larvae.
STERILIZATION OF ZEBRAFISH EGGS Egg sterilization was performed as previously described with some modifications [22]. Freshly fertilized zebrafish eggs were first bleached (0.003%) for 5
min, washed 3 times in sterile water under gentle agitation and maintained overnight in groups of 100 eggs per 75 cm3 culture flasks with vented caps containing 100 mL of autoclaved Volvic
mineral water supplemented with methylene blue solution (0.3 µg/mL). Afterwards, eggs were transferred into 50 mL Falcon tubes (100 eggs per tube) and treated with a mixture of antibiotics
(500 μL of penicillin G: streptomycin, 10,000 U/ml: 10 mg/mL GIBCO #P4333), 200 μL of filtered kanamycin sulfate (100 mg/mL, SERVA Electrophoresis #26899) and antifungal drug (50 μL of
amphotericin B solution Sigma-Aldrich (250 μg/mL) #A2942) for 2 h under agitation at 28 °C. Eggs were then washed 3 times in sterile water under gentle agitation and bleached (0.003%) for 15
min, resuspending the eggs every 3 min by inversion. Eggs were washed again 3 times in water and incubated 10 min with 0.01% Romeiod (COFA, Coopérative Française de l’Aquaculture). Finally,
eggs were washed 3 times in water and transferred into 25 cm3 culture flasks with vented caps containing 20 mL of water. After sterilization, eggs were transferred with approximately 30 to
35 eggs / flasks and were transferred into new flasks at 4 dpf before reconventionalization with 10 to 15 fish / flask. We monitored sterility at several points during the experiment by
spotting 50 μL of water from each flask on LB, TYES and on YPD agar plates, all incubated at 28 °C under aerobic conditions. Plates were left for at least 3 days to allow slow-growing
organisms to multiply. Spot checks for bacterial contamination were also carried out by PCR amplification of water samples with the 16S rRNA gene primers and procedure detailed further
below. If a particular flask was contaminated, those fish were removed from the experiment. PROCEDURE FOR RAISING GERM-FREE ZEBRAFISH After hatching, fish were fed with germ-free _T.
thermophila_ 3 times per week from 4 dpf onwards. (i) _T. thermophila stock_. A germ-free line of _T. thermophila_ was maintained at 28 °C in 20 mL of PPYE (0.25% proteose peptone BD Bacto
#211684, 0.25% yeast extract BD Bacto #212750) supplemented with penicillin G (10 unit/mL) and streptomycin (10 µg/mL). Medium was inoculated with 100 μL of the preceding _T. thermophila_
stock. After one week of growth, samples were taken, tested for sterility on LB, TYES, and YPD plates and restocked again. (ii) _Growth. T. thermophila_ were incubated at 28 °C in MYE broth
(1% milk powder, 1% yeast extract) inoculated from stock suspension at a 1:50 ratio. After 24 h of growth, _T. thermophila_ were transferred to Falcon tubes and washed (4400 rpm, 3 min at 25
°C) 3 times in 50 mL of autoclaved Volvic water. Finally, _T. thermophila_ were resuspended in sterile water and added to culture flasks (500 µL in 20 mL) or 24-well plates (50 µL / well).
Sterility of _T. thermophila_ was tested by plating and 16S rRNA PCR as described in the section above. (iii) Fine-powder feeding. When indicated, fish were fed with previously
γ-ray-sterilized fine-powdered food suitable for an early first feeding gape size (ZM-000 fish feed, ZM Ltd) every 48 h [42]. RECONVENTIONALIZATION OF GERM-FREE ZEBRAFISH At 4 dpf, just
after hatching, zebrafish larvae were reconventionalized with a single bacterial population or a mix of several. The 10 bacterial strains constituting the core protective microbiota were
grown for 24 h in suitable media (TYES or LB) at 28 °C. Bacteria were then pelleted and washed twice in sterile water, and all adjusted to the same cell density (OD600 = 1 or 5.107 colony
forming units (cfu)/mL) (i) Reconventionalization with individual species. Bacteria were resuspended and transferred to culture flasks containing germ-free fish at a final concentration of
5.105 cfu/mL. (ii) Reconventionalization with bacterial mixtures. For the preparation of Mix10, Mix9, Mix8 and all other mixes used, equimolar mixtures were prepared by adding each bacterial
species at initial concentration to 5.107 cfu/mL. Each bacterial mixture suspension was added to culture flasks containing germ-free fish at a final concentration of 5.105 cfu/mL. INFECTION
CHALLENGES _F. columnare_ strains (Supplementary Table S1) were grown overnight in TYES broth at 28 °C. Then, 2 mL of culture were pelleted (10,000 rpm for 5 min) and washed once in sterile
water. GF zebrafish were brought in contact with the tested pathogens at 6 dpf for 3 h by immersion in culture flasks with bacterial doses ranging from 5.102 to 5.107 cfu/mL. Fish were then
transferred to individual wells of 24-well plates, containing 2 mL of water and 50 μL of freshly prepared GF _T. thermophila_ per well. Mortality was monitored daily as described in [22],
and measured in days post infection (dpi), with 0 dpi corresponding to the infection day, _i.e_. 6 dpf-old larvae. As few as 54 ± 9 cfu/larva of _F. columnare_ were recovered from infected
larvae. All zebrafish experiments were stopped at day 9 post-infection and zebrafish were euthanized with tricaine (MS-222) (Sigma-Aldrich #E10521). Each experiment was repeated at least 3
times and between 10 and 15 larvae were used per condition and per experiment. COLLECTION OF EGGS FROM OTHER ZEBRAFISH FACILITIES Conventional zebrafish eggs were collected in 50 mL Falcon
tubes from the following facilities: Facility 1 - zebrafish facility in Hospital Robert Debré, Paris; Facility 2 - Jussieu zebrafish facility A2, University Paris 6; Facility 3 - Jussieu
zebrafish facility C8 (UMR7622), University Paris 6; Facility 4- AMAGEN commercial facility, Gif sur Yvette; Larvae were treated with the same rearing conditions, sterilization and infection
procedures used in the Institut Pasteur facility. DETERMINATION OF FISH BACTERIAL LOAD USING CFU COUNT Zebrafish were euthanized with tricaine (MS-222) (Sigma-Aldrich #E10521) at 0.3 mg/mL
for 10 min. Then they were washed in 3 different baths of sterile PBS-0.1% Tween to remove bacteria loosely attached to the skin. Finally, they were transferred to tubes containing
calibrated glass beads (acid-washed, 425 μm to 600 μm, SIGMA-ALDRICH #G8772) and 500 μL of autoclaved PBS. They were homogenized using FastPrep Cell Disrupter (BIO101/FP120 QBioGene) for 45
s at maximum speed (6.5 m/s). Finally, serial dilutions of recovered suspension were spotted on TYES agar and cfu were counted after 48 h of incubation at 28 °C. CHARACTERIZATION OF
ZEBRAFISH MICROBIAL CONTENT Over 3 months, the experiment was run independently 3 times and 3 different batches of eggs were collected from different fish couples in different tanks. Larvae
were reared as described above. GF and Conv larvae were collected at 6 dpf and 11 dpf for each batch. Infected Conv larvae were exposed to _F. columnare_ALG for 3 h by immersion as described
above. For each experimental group, triplicate pools of 10 larvae (one in each experimental batch) were euthanized, washed and lysed as above. Lysates were split into 3 aliquots, one for
culture followed by 16S rRNA gene sequencing (A), one for 16S rRNA gene clone library generation and Sanger sequencing (B), and one for Illumina metabarcoding-based sequencing (C). BACTERIAL
CULTURE FOLLOWED BY 16S RRNA GENE-BASED IDENTIFICATION Lysates were serially diluted and immediately plated on R2A, TYES, LB, MacConkey, BHI, BCYE, TCBS and TSB agars and incubated at 28 oC
for 24-72 h. For each agar, colony morphotypes were documented, and colonies were picked and restreaked on the same agar in duplicate. In order to identify the individual morphotypes,
individual colonies were picked for each identified morphotype from each agar, vortexed in 200 μL DNA-free water and boiled for 20 min at 90 oC. Five μL of this bacterial suspension were
used as template for colony PCR to amplify the 16S rRNA gene with the universal primer pair for the Domain bacteria 8 f (5’-AGA GTT TGA TCC TGG CTC AG-3’) and 1492r (5’-GGT TAC CTT GTT ACG
ACT T-3’). Each primer was used at a final concentration of 0.2 μM in 50 μL reactions. PCR cycling conditions were - initial denaturation at 94 °C for 2 min, followed by 32 cycles of
denaturation at 94 °C for 1 min, annealing at 56 °C for 1 min, and extension at 72 °C for 2 min, with a final extension step at 72 °C for 10 min. 16S rRNA gene PCR products were verified on
1% agarose gels, purified with the QIAquick® PCR purification kit and two PCR products for each morphotype were sent for sequencing (Eurofins, Ebersberg, Germany). 16S rRNA sequences were
manually proofread, and sequences of low quality were removed from the analysis. Primer sequences were trimmed, and sequences were compared to GenBank (NCBI) with BLAST, and to the Ribosomal
Database Project with SeqMatch. For genus determination a 95% similarity cut-off was used, for Operational Taxonomic Unit determination, a 98% cut-off was used. 16S RRNA GENE CLONE LIBRARY
GENERATION Total DNA was extracted from the lysates with the Mobio PowerLyzer® Ultraclean® kit according to manufacturer’s instructions. Germ-free larvae and DNA-free water were also
extracted as control samples. Extracted genomic DNA was verified by Tris-acetate-EDTA-agarose gel electrophoresis (1%) stained with GelRed and quantified by applying 2.5 μL directly to a
NanoDrop® ND-1000 Spectrophotometer. The 16S rRNA gene was amplified by PCR with the primers 8 f and 1492r, and products checked and purified as described above. Here, we added 25–50 ng of
DNA as template to 50 μL reactions. Clone libraries were generated with the pGEM®-T Easy Vector system (Promega) according to manufacturer’s instructions. Presence of the cloned insert was
confirmed by colony PCR with vector primers gemsp6 (5’-GCT GCG ACT TCA CTA GTG AT-3’) and gemt7 (5’-GTG GCA GCG GGA ATT CGA T-3’). Clones with an insert of the correct size were purified as
above and sent for sequencing (Eurofins, Ebersberg, Germany). Blanks using DNA-free water as template were run for all procedures as controls. For the three independent runs of the
experiment, 10 Conv fish per condition (6 and 11 dpf, exposed or not to _F. columnare_) and per repeat were pooled. Each pool of 10 fish was sequenced separately, generating 3 replicates for
each condition (_n_ = 12), resulting in a total of 857 clones. Clone library coverage was calculated with the following formula [1-(n1/N2)] x 100, where n1 is the number of singletons
detected in the clone library, and N2 is the total number of clones generated for this sample. Clone libraries were generated to a minimum coverage of 95%. Sequence analysis and
identification was carried out as above. BY 16S RRNA V3V4 AMPLICON ILLUMINA SEQUENCING To identify the 16S rRNA gene diversity in our facility and fish collected from 4 other zebrafish
facilities, fish were reared as described above. GF fish were sterilized as above, and uninfected germ-free and conventional fish were collected at 6 dpf and 11 dpf. Infection was carried
out as above with _F. columnare_ALG for 3 h by bath immersion, followed by transfer to clean water. Infected conventional fish were collected at 6 dpf 6 h after infection with _F. columnare_
and at 11 dpf, the same as uninfected fish. GF infected larvae were only collected at 6 dpf 6 h post infection, as at 11 dpf all larvae had succumbed to infection. Triplicate pools of 10
larvae were euthanized, washed and lysed as above. Total DNA was extracted with the Mobio PowerLyzer® Ultraclean® kit as described above and quantified with a NanoDrop® ND-1000
Spectrophotometer and sent to IMGM Laboratories GmbH (Germany) for Illumina sequencing. Primers Bakt_341F (5’-CCTACGGGNGGCWGCAG-3’) and Bakt_805R (5’-GACTACHVGGGTATCTAATCC-3’), amplifying
variable regions 3 and 4 of the 16S gene were used for amplification [43]. Each amplicon was purified with solid phase reversible immobilization (SPRI) paramagnetic bead-based technology
(AMPure XP beads, Beckman Coulter) with a Bead:DNA ratio of 0.7:1 (v/v) following manufacturers instructions. Amplicons were normalized with the Sequal-Prep Kit (Life Technologies), so each
sample contained approximately 1 ng/μl DNA. Samples, positive and negative controls were generated in one library. The High Sensitivity DNA LabChip Kit was used on the 2100 Bioanalyzer
system (both Agilent Technologies) to check the quality of the purified amplicon library. For cluster generation and sequencing, MiSeq® reagents kit 500 cycles Nano v2 (Illumina Inc.) was
used. Before sequencing, cluster generation by two-dimensional bridge amplification was performed, followed by bidirectional sequencing, producing 2 × 250 bp paired-end (PE) reads. MiSeq®
Reporter 2.5.1.3 software was used for primary data analysis (signal processing, demultiplexing, trimming of adapter sequences). CLC Genomics Workbench 8.5.1 (Qiagen) was used for
read-merging, quality trimming, and QC reports and OTU definition were carried out in the CLC plugin Microbial Genomics module. COMPARISON OF WHOLE LARVAE VS INTESTINAL BACTERIAL CONTENT
Larvae reconventionalized with Mix10 and infected with _F. columnare_ALG at 6 dpf for 3 h were euthanized and washed. DNA was extracted from pools of 10 whole larvae or of pools of 10
intestinal tubes dissected with sterile surgical tweezer and subjected to Illumina 16S rRNA gene sequencing. GF larvae and dissected GF intestines were sampled as controls. As dissection of
the larval guts involved high animal loss and was a potential important contamination source, we proceeded with using entire larvae for the rest of the study. WHOLE GENOME SEQUENCING
Chromosomal DNA of the ten species composing the core of zebrafish larvae microbiota was extracted using the DNeasy Blood & Tissue kit (QIAGEN) including RNase treatment. DNA quality and
quantity were assessed on a NanoDrop ND-1000 spectrophotometer (Thermo Scientific). DNA sequencing libraries were made using the Nextera DNA Library Preparation Kit (Illumina Inc.) and
library quality was checked using the High Sensitivity DNA LabChip Kit on the Bioanalyzer 2100 (Agilent Technologies). Sequencing clusters were generated using the MiSeq reagents kit v2 500
cycles (Illumina Inc.) according to manufacturer’s instructions. DNA was sequenced at the Helmholtz Centre for Infection Research by bidirectional sequencing, producing 2 × 250 bp paired-end
reads. Between 1,108,578 and 2,914,480 reads per sample were retrieved with a median of 1,528,402. Reads were quality filtered, trimmed and adapters removed with trimmomatic 0.39 [44] and
genomes assembled using SPAdes 3.14 [45]. BACTERIAL SPECIES IDENTIFICATION Whole genome-based bacterial species identification was performed by the TrueBac ID system (v1.92, DB:20190603)
[46]. Species-level identification was performed based on the algorithmic cut-off set at 95% ANI when possible or when the 16S rRNA gene sequence similarity was >99 %. MONITORING OF
BACTERIAL DYNAMICS Three independent experiments were run over 6 weeks with eggs collected from different fish couples from different tanks to monitor establishment and recovery. Larvae were
reared, sterilized and infected as above with the only difference that 75 cm3 culture flasks with vented caps (filled with 50 mL of sterile Volvic) were used to accommodate the larger
number of larvae required, as in each experiment. Larvae for time course Illumina sequencing were removed sequentially from the experiment that monitored the survival of the larvae. Animals
were pooled (10 larvae for each time point/condition), euthanized, washed and lysed as described above and stored at −20o C until the end of the survival monitoring, and until all
triplicates had been collected. COMMUNITY ESTABLISHMENT In order to follow the establishment of the 10 core strains in the larvae, GF larvae were reconventionalized with an equiratio Mix10
as above. Re-convMix10 larvae were sampled at 4 dpf immediately after addition of the 10 core species and then 20 min, 2 h, 4 h and 8 h after. Germ-free, conventional larvae and the inoculum
were also sampled as controls. INDUCTION OF DYSBIOSIS Different doses of kanamycin (dose 1 = 200 µg/mL; dose 2 = 50 µg/mL; dose 3 = 25 µg/mL) and a penicillin/streptomycin antibiotic mix
dose 1 = 250 µg/mL; dose 2 = 15.6 µg/mL were tested on re-convMix10 4 dpf zebrafish larvae by adding them to the flask water to identify antibiotic treatments that were non-toxic to larvae
but that caused dysbiosis. After 16 h of treatment, antibiotics were extensively washed off with sterile water and larvae were challenged with _F. columnare_ALG, leading to the death of all
larvae – e.g. successful abolition of colonization resistance with best results in all repeats with 250 µg/mL penicillin/streptomycin and 50 µg/mL kanamycin as antibiotic treatment.
COMMUNITY RECOVERY As above, after 8 h of incubation, 4 dpf re-convMix10 larvae were treated with 250 µg/mL penicillin/streptomycin and 50 µg/mL kanamycin for 16 h. Antibiotics were
extensively washed off and larvae were now left to recover in sterile water for 24 h to assess resilience of the bacterial community. Samples (pools of 10 larvae) were taken at 3 h, 6 h, 12
h, 18 h, and 24 h during recovery and sent for 16S rRNA Illumina sequencing. Larvae were then challenged at 6 dpf with _F. columnare_ALG for 3 h and survival was monitored daily for 9 days
post-infection. All time course samples were sequenced by IMGM Laboratories GmbH, as described above. STATISTICAL ANALYSIS OF METATAXONOMIC DATA 16S RNA analysis was performed with SHAMAN
[47]. Library adapters, primer sequences, and base pairs occurring at 5’ and 3’ends with a Phred quality score <20 were trimmed off by using Alientrimmer (v0.4.0). Reads with a positive
match against zebrafish genome (mm10) were removed. Filtered high-quality reads were merged into amplicons with Flash (v1.2.11). Resulting amplicons were clustered into operational taxonomic
units (OTU) with VSEARCH (v2.3.4) [48]. The process includes several steps for de-replication, singletons removal, and chimera detection. The clustering was performed at 97% sequence
identity threshold, producing 459 OTUs. The OTU taxonomic annotation was performed against the SILVA SSU (v132) database [49] completed with 16S sequence of 10 bacterial communities using
VSEARCH and filtered according to their identity with the reference [50]. Annotations were kept when the identity between the OTU sequence and reference sequence is ≥78.5% for taxonomic
Classes, ≥82% for Orders, ≥86.5% for Families, ≥94.5% for Genera and ≥98% for species. Here, 73.2% of the OTUs set was annotated and 91.69% of them were annotated at genus level. The input
amplicons were then aligned against the OTU set to get an OTU contingency table containing the number of amplicon associated with each OTU using VSEARCH global alignment. The matrix of OTU
count data was normalized for library size at the OTU level using a weighted non-null count normalization. Normalized counts were then summed within genera. The generalized linear model
(GLM) implemented in the DESeq2 R package [51] was then applied to detect differences in abundance of genera between each group. We defined a GLM that included the treatment (condition) and
the time (variable) as main effects and an interaction between the treatment and the time. Resulting _P_ values were adjusted according to the Benjamini and Hochberg procedure [45]. The
statistical analysis can be reproduced on SHAMAN by loading the count table, the taxonomic results with the target and contrast files that are available on figshare
https://doi.org/10.6084/m9.figshare.11417082.v2. DETERMINATION OF CYTOKINE LEVELS Total RNA from individual zebrafish larvae were extracted using RNeasy kit (Qiagen), 18 h post pathogen
exposure (12 h post-wash). Oligo(dT17)-primed reverse transcriptions were carried out using M-MLV H- reverse- transcriptase (Promega). Primer specificity was initially tested by sequencing
the amplicons from a positive control template. At the end of each real-time qPCR assay, a denaturation step was conducted to determine the melt curve of the amplicon, for comparison with a
positive control sample systematically included. Quantitative PCRs were performed using Takyon SYBR Green PCR Mastermix (Eurogentec) on a StepOne thermocycler (Applied Biosystems). Primers
for _ef1a_ (housekeeping gene, used for cDNA amount normalization), _il1b_, _il10_ and _il22_ are described in [22]. Data were analyzed using the ∆∆Ct method. Four larvae were analyzed per
condition. Zebrafish genes and proteins mentioned in the text: _ef1a_ NM_131263_; il1b_ BC098597_; il22_ NM_001020792_; il10_ NM_001020785_; myd88_ NM_212814. HISTOLOGICAL COMPARISONS OF GF,
CONV AND RE-CONV FISH GF INFECTED OR NOT WITH _F. COLUMNARE_ Fish were collected 24 h after infection (7 dpf) and were fixed for 24 h at 4 °C in Trump fixative (4% methanol-free
formaldehyde, 1% glutaraldehyde in 0.1 M PBS, pH 7.2) and sent to the PIBiSA Microscopy facility services (https://microscopies.med.univ-tours.fr/) in the Faculté de Médecine de Tours
(France), where whole fixed animals were processed, embedded in Epon. Semi-thin sections (1 µm) were cut using a X ultra-microtome and then either dyed with toluidine blue for observation by
light microscopy and imaging or processed for Transmission electron microscopy. ADULT ZEBRAFISH PRE-TREATMENT WITH _C. MASSILIAE_ The zebrafish line AB was used. Fish were reared at 28 °C
in dechlorinated recirculated water, then transferred into continuous flow aquaria when aging 3–4 months for infection experiments. _C. massiliae_ was grown in TYES broth at 150 rpm and 28
°C until stationary phase. This bacterial culture was washed twice in sterile water and adjusted to OD600nm = 1. Adult fish reconventionalization was performed by adding _C. massiliae_
bacterial suspension directly into the fish water (1 L) at a final concentration of 2.106 cfu/mL. Bacteria were maintained in contact with fish for 24 h by stopping the water flow then
subsequently removed by restoring the water flow. _C. massiliae_ administration was performed twice after water renewal. In the control group, the same volume of sterile water was added.
ADULT ZEBRAFISH INFECTION CHALLENGE _F. columnare_ infection was performed just after fish reconventionalization with _C. massiliae_. The infection was performed as previously described by
Li and co-workers with few modifications [39]. Briefly, _F. columnare_ strain ALG-00-530 was grown in TYES broth at 150 rpm and 28 °C until late-exponential phase. Then, bacterial cultures
were diluted directly into the water of aquaria (200 mL) at a final concentration of 5.106 cfu/mL. Bacteria were maintained in contact with fish for 1 h by stopping the water flow then
subsequently removed by restoring the water flow. Sterile TYES broth was used for the control group. Bacterial counts were determined at the beginning of the immersion challenge by plating
serial dilutions of water samples on TYES agar. Water was maintained at 28 °C and under continuous oxygenation for the duration of the immersion. Groups were composed of 10 fish. Virulence
was evaluated according to fish mortality 10 days post-infection. STATISTICAL METHODS Statistical analyses were performed using unpaired, nonparametric Mann–Whitney test or unpaired t-tests.
Analyses were performed using Prism v8.2 (GraphPad Software). Evenness: The Shannon diversity index was calculated with the formula (HS = −Σ[P(ln(P)])) where P is the relative species
abundance. Total evenness was calculated for the Shannon index as E = HS/Hmax. The less evenness in communities between the species (and the presence of a dominant species), the lower this
index is. RESULTS _FLAVOBACTERIUM COLUMNARE_ KILLS GERM-FREE BUT NOT CONVENTIONAL ZEBRAFISH To investigate microbiota-based resistance to infection in zebrafish, we compared the sensitivity
of germ-free (GF) and conventional (Conv) zebrafish larvae to _F. columnare_, an important fish pathogen affecting carp, channel catfish, goldfish, eel, salmonids and tilapia and previously
shown to infect and kill adult zebrafish [12, 30, 33, 39, 52]. We used bath immersion to expose GF and Conv zebrafish larvae at 6 days post-fertilization (dpf), to a collection of 28 _F.
columnare_ strains, belonging to four different genomovars for 3 h at the chosen median infection dose of 5.105 colony forming units (cfu)/mL (see Fig. 1). Daily monitoring showed that 16
out of 28 _F. columnare_ strains killed GF larvae in less than 48 h (Supplementary Fig. S1A), whereas Conv larvae survived exposure to all tested virulent _F. columnare_ strains
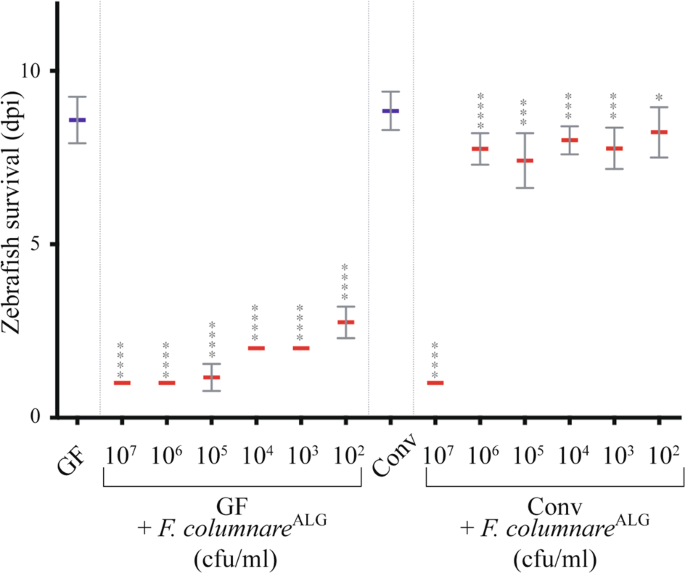
(Supplementary Fig. S1B). Exposure to the highly virulent strain ALG-00–530 (hereafter _F. columnare_ALG) also showed that GF mortality was fast (1 day post-infection -dpi) and
dose-dependent and that Conv zebrafish survived all but the highest dose (107 cfu/mL) (Fig. 1). Similar survival of infected Conv larvae was obtained with zebrafish AB strain eggs obtained
from 4 different zebrafish facilities (Supplementary Fig. S2), suggesting that conventional zebrafish microbiota could provide protection against _F. columnare_ infection. A COMMUNITY OF 10
CULTURABLE BACTERIAL STRAINS PROTECTS AGAINST _F. COLUMNARE_ INFECTION In our rearing conditions, the conventional larval microbiota is acquired after hatching from microorganisms present on
the egg chorion and in fish facility water. To test the hypothesis that microorganisms associated with conventional eggs provided protection against _F. columnare_ALG, we exposed sterilized
eggs to either fish facility tank water or to non-sterilized conventional eggs at 0 or 4 dpf (before or after hatching, respectively). In both cases, these reconventionalized (re-Conv)
zebrafish survived _F. columnare_ALG infection as well as Conv zebrafish (Supplementary Fig. S3). To determine the composition of conventional zebrafish microbiota, we generated 16S rRNA
gene clone libraries from homogenate pools of Conv larvae aged 6 and 11 dpf exposed or not to _F. columnare_ALG, sampled over 3 months from 3 different batches of larvae (_n_ = 10). A total
of 857 clones were generated for all samples. We identified 15 operational taxonomical units (OTUs), 10 of which were identified in all experiments (Supplementary Table S2, in Table 1 the
16S rRNA gene similarity is shown). Two OTUs (belonging to an _Ensifer_ sp. and a _Hydrogenophaga_ sp.) were only detected once, and a _Delftia_ sp., a _Limnobacter_ sp. and a
_Novosphingobium_ sp. were detected more than once (2, 3, and 2 times, respectively), but not consistently in all batches of fish (Supplementary Table S2). Moreover, deep-sequencing of the
16S rRNA V3-V4 region of gDNA retrieved from larvae originating from the other four zebrafish facilities described above, revealed that OTUs for all of these 10 species were also detected in
Conv larvae, with the exception of _A. veronii_ 2 that was not detected in all samples (Supplementary Table S3). To isolate culturable zebrafish microbiota bacteria, we plated dilutions of
homogenized 6 dpf and 11 dpf larvae pools on various growth media and we identified 10 different bacterial morphotypes. 16S-based analysis followed by full genome sequencing identified 10
bacteria corresponding to 10 strains of 9 different species that were also consistently detected by culture-free approaches (Table 1 shows the average nucleotide identity value for the
culture isolates). To assess the potential protective role of these 10 strains, we reconventionalized GF zebrafish at 4 dpf with a mix of all 10 identified culturable bacterial species
(hereafter called Mix10), each at a concentration of 5.105 cfu/mL and we monitored zebrafish survival after exposure to _F. columnare_ALG at 6 dpf. We showed that zebrafish
reconventionalized with the Mix10 (Re-ConvMix10) displayed a strong level of protection against all identified highly virulent _F. columnare_ strains (Supplementary Fig. S4). These results
demonstrated that the Mix10 constitutes a core protective bacterial community providing full protection of zebrafish larvae against _F. columnare_ infection. COMMUNITY DYNAMICS UNDER
ANTIBIOTIC-INDUCED DYSBIOSIS REVEAL A KEY CONTRIBUTOR TO RESISTANCE TO _F. COLUMNARE_ INFECTION To further analyze the determinants of Mix10 protection against _F. columnare_ALG infection,
we inoculated 4 dpf germ-free larvae with an equal-ratio mix of the 10 bacteria (at 5.105 cfu/mL each) and monitored their establishment over 8 h. We first verified that whole larvae
bacterial content (OTU abundance) was not significantly different from content of dissected intestinal tubes (_p_ = 0.99, two-tailed _t_ test) (Supplementary Fig. S5) and proceeded to use
entire larvae to monitor bacterial establishment and recovery in the rest of the study. We then collected pools of 10 larvae immediately after reconventionalization (t0), and then at 20 min,
2 h, 4 h, and 8 h in three independent experiments. Illumina sequencing of the 16S rRNA gene was used to follow bacterial relative abundance. At t0, all species were present at >4% in
the zebrafish, apart from _A. veronii_ strains 1 (0.2%) and 2 (not detected) (Supplementary Fig. S6). _Aeromonas caviae_ was detected as the most abundant species (33%), followed by
_Stenotrophomonas maltophilia_ (23%) and _Chryseobacterium massiliae_ (12%), altogether composing 68% of the community (Supplementary Fig. S6). The relative species abundance, possibly
reflecting initial colonization ability, was relatively stable for most species during community establishment, with similar species evenness at t0 (_E_ = 0.84) and t8h (_E_ = 0.85). Whereas
both Conv and Re-ConvMix10 larvae were protected against _F. columnare_ALG infection, the global structure of the reconstituted Mix10 population was different from the conventional one at 4
dpf (Supplementary Fig. S6). To test the sensitivity to disturbance and the resilience of the protection provided by Mix10 bacterial community, we determined the minimal inhibitory
concentration to penicillin/streptomycin and kanamycin of the strains composing the Mix10 microbiota (Supplementary Fig. S7A) and tested different dose of penicillin/streptomycin and
kanamycin treatment on zebrafish survival to _F. columnare_ infection (Supplementary Fig. S7B). We then subjected Re-ConvMix10 zebrafish to identify a non-toxic antibiotic treatment at 4 dpf
using either 250 µg/mL penicillin/streptomycin combination (all members of the Mix10 bacteria are sensitive to penicillin/streptomycin) or 50 µg/mL kanamycin (affecting all members of the
Mix10 bacteria except _C. massiliae_, _P. myrsinacearum_ and _S. maltophilia_) (Supplementary Fig. S7A). At 5 dpf, after 16 h of exposure, antibiotics were washed off and zebrafish were
immediately exposed to _F. columnare_ALG. Both antibiotic treatments resulted in complete loss of the protection against _F. columnare_ALG infection observed in Re-ConvMix10 (Fig. 2a). We
then used the same antibiotic treatments but followed by a 24 h recovery period after washing off the antibiotics at 5 dpf, therefore only performing the infection at 6 dpf (Fig. 2b). Whilst
Re-ConvMix10 larvae treated with penicillin/streptomycin showed similar survival to infected GF larvae, kanamycin-treated Re-ConvMix10 zebrafish displayed restored protection after 24 h
recovery and survived similarly to untreated conventionalized fish (Fig. 2b). Sampling and 16S gene analysis during recovery experiments at different time points showed that bacterial
community evenness decreased after antibiotic administration for both treatments (_E_ = 0.85 for 4 dpf control, _E_ = 0.72 for t0 kanamycin and _E_ = 0.7 for t0 penicillin/streptomycin), and
continued to decrease during recovery (_E_ = 0.6 and 0.64 for kanamycin and penicillin/streptomycin treatment after 24 h recovery, respectively). Although _C. massiliae_ remained detectable
immediately after both antibiotic treatments, penicillin/streptomycin treatment led to a significant reduction in its relative abundance (0.21%) (Fig. 2c). By contrast, _C. massiliae_
relative abundance rebounded 6 h after cessation of kanamycin treatment and was the dominant member (52%) of the reconstituted microbiota after 24 h recovery period (Fig. 2d), suggesting
that the protective effect observed in the kanamycin-treated larvae might be due to the recovery of _C. massiliae_. RESISTANCE TO _F. COLUMNARE_ INFECTION IS PROVIDED BY BOTH INDIVIDUAL AND
COMMUNITY-LEVEL PROTECTION To test the potential key role played by _C. massiliae_ in protection against _F. columnare_ALG infection, we exposed 4 dpf GF zebrafish to _C. massiliae_ only and
showed that it conferred individual protection at doses as low as 5.102 cfu/mL (Fig. 3a). Whereas none of the 9 other species composing the Mix10 were protective individually (Fig. 3a),
their equiratio combination (designated as Mix9) conferred protection to zebrafish, although not at doses lower than 5.104 cfu/mL (Fig. 3b) and not as reproducibly as with _C. massiliae_. To
identify which association of species protected Re-ConvMix9 zebrafish against _F. columnare_ALG infection, we tested all 9 combinations of 8 species (Mix8), as well as several combinations
of 7, 6, 4, or 3 species and showed no protection (Supplementary Fig. S8A and Supplementary Table S4). We then tested whether lack of protection of Mix8 compared to Mix9 could rely on a
density effect by doubling the concentration of any of the species within the nonprotective Mix8a (Supplementary Fig. S8B) and showed no protection. Interestingly, monitoring _C. massiliae_
and the bacteria composing the Mix9 in conventional fish challenged by _F. columnare_ only resulted (after 1 day) in an increase of _P. sediminis_ and _P. nitroreducens_ (_p_ = <0.0001)
and reduction of _Phyllobacterium myrsinacearum_, but no change in _C. massiliae_ (Supplementary Fig. S9). Evenness also increased after infection from 0.65 in unchallenged Conv larvae to
0.82 for larvae infected with _F. columnare_. These results therefore also indicate that microbiota-based protection against _F. columnare_ALG infection can rely on either _C.
massiliae-_dependent membership effect or on a community-structure-dependent effect mediated by the Mix9 consortium. PRO- AND ANTI- INFLAMMATORY CYTOKINE PRODUCTION DOES NOT CONTRIBUTE TO
MICROBIOTA-MEDIATED PROTECTION AGAINST _F. COLUMNARE_ ALG INFECTION To test the contribution of the immune response of zebrafish larvae to resistance to _F. columnare_ infection, we used
qRT-PCR to measure cytokine mRNA expression in GF and Conv zebrafish exposed or not to _F. columnare_ALG. We also tested the impact of reconventionalization with _C. massiliae_
(re-Conv_Cm_), Mix10 (re-ConvMix10) or with Mix4 (_A. caviae_, both _A. veronii_ spp., _P. mossellii_) as a nonprotective control (Supplementary Table S4). We tested genes encoding IL1β
(pro-inflammatory), IL22 (promoting gut repair), and IL10 (anti-inflammatory) cytokines. While we observed some variation in _il10_ expression among noninfected reconventionalized larvae,
this did not correlate with protection. Furthermore, _il10_ expression was not modulated by infection in any of the tested conditions (Fig. 4a). By contrast, we observed a strong induction
of _il1b_ and _il22_ in GF zebrafish exposed to _F. columnare_ALG (Fig. 4b, c). However, although this induction was reduced in protected Conv, Re-ConvCm and Re-ConvMix10, it was also
observed in nonprotective Re-ConvMix4 larvae, indicating that down-modulation of the inflammatory response induced by _F. columnare_ does not correlate with resistance to infection.
Consistently, the use of a _myd88_ mutant, a key adapter of IL-1 and toll-like receptor signaling deficient in innate immunity [41, 53], showed that Conv or Re-ConvMix10, but not GF _myd88_
mutants survived _F. columnare_ as well as wild-type zebrafish (Fig. 4d). Moreover, _il1b_ induction by _F. columnare_ infection was observed only in GF larvae and was _myd88_-independent
(Supplementary Fig. S10). These results therefore indicated that the tested cytokine responses do not play a significant role in the microbiota-mediated protection against _F. columnare_
infection. _C. MASSILIAE_ AND MIX9 PROTECT ZEBRAFISH FROM INTESTINAL DAMAGES UPON _F. COLUMNARE_ ALG INFECTION Histological analysis of GF larvae fixed 24 h after exposure to _F.
columnare_ALG revealed extensive intestinal damage (Fig. 5a) prior to noticeable signs in other potential target organs such as gills or skin. To test the requirement for gut access in _F.
columnare_ALG infection process, we modified our standard rearing protocol of GF fish, which involves feeding with live germ-free _T. thermophila_. We found that, if left unfed, GF zebrafish
did not die after _F. columnare_ALG exposure, while feeding with either _T. thermophila_ or another food source such as sterile fish food powder, restored sensitivity to _F. columnare_ALG
infection (Supplementary Fig S11), suggesting that successful infection requires feeding and ingestion. Histological sections consistently showed severe disorganization of the intestine with
blebbing in the microvilli and vacuole formation in _F. columnare_ALG-infected GF larvae (Fig. 5). In contrast, zebrafish pre-incubated with either _C. massiliae_ or Mix9 consortium at 4
dpf, and then exposed to _F. columnare_ALG at 6 dpf showed no difference compared to noninfected larvae or conventional infected larvae (Fig. 5), confirming full protection against _F.
columnare_ALG at the intestinal level. _C. MASSILIAE_ PROTECTS LARVAE AND ADULT ZEBRAFISH AGAINST _F. COLUMNARE_ The clear protection provided by _C. massiliae_ against _F. columnare_ALG
infection prompted us to test whether exogenous addition of this bacterium could improve microbiota-based protection towards this widespread fish pathogen. We first showed that zebrafish
larvae colonized with _C. massiliae_ were fully protected against all virulent _F. columnare_ strains identified in this study (Fig. 6a). To test whether _C. massiliae_ could also protect
adult zebrafish from _F. columnare_ infection, we pre-treated conventional 3–4-month-old Conv adult zebrafish with _C. massiliae_ for 48 h before challenging them with a high dose (5.106
cfu/mL) of _F. columnare_ALG. Monitoring of mortality rate showed that pre-treatment with _C. massiliae_ significantly increased the survival rate of adult zebrafish upon _F. columnare_ALG
infection compared to non-treated conventional fish (_p_ = 0.0084 Mann–Whitney test, Fig. 6b). Taken together, these results show that _C. massiliae_ is a promising probiotic protecting
zebrafish against columnaris disease caused by _F. columnare_. DISCUSSION In this study, we used gnotobiotic zebrafish reconventionalized with relevant but relatively simple zebrafish larval
microbiota in order to identify communities involved in colonization resistance against the fish pathogen _F. columnare_. We chose to work on larvae instead of adult fish because zebrafish
microbiotas complexity increases when shifting from larval to later developmental stages [15, 54], while avoiding the important husbandry challenges associated with rearing germ-free adult
zebrafish [20]. Using reconventionalization of otherwise germ-free zebrafish larvae we showed that conventional-level protection against infection by a broad range of highly virulent _F.
columnare_ strains is provided by a set of 10 culturable bacterial strains, belonging to 9 different species, isolated from the indigenous standard laboratory zebrafish microbiota. With the
exception of the Bacteroidetes _C. massiliae_, this protective consortium was dominated by Proteobacteria such as _Pseudomonas_ and _Aeromonas_ spp., bacteria commonly found in aquatic
environments [55, 56]. Despite the relative permissiveness of zebrafish larvae microbiota to environmental variations and inherent variability between samples [54], we showed that these ten
bacteria were consistently identified in four different zebrafish facilities, suggesting the existence of a core microbiota assemblage with important colonization resistance functionality.
Use of controlled combinations of these 10 bacterial species enabled us to show a very robust species-specific protection effect in larvae mono-associated with _C. massiliae_. We also
identified a community-level protection provided by the combination of the 9 other species that were otherwise unable to protect against _F. columnare_ when provided individually. This
protection was however less reproducible and required a minimum inoculum of 5.104 cfu/mL, compared to 5.102 cfu/mL with _C. massiliae_. These results therefore suggest the existence of two
distinct microbiota-based protection scenarios: one based on a membership effect provided by _C. massiliae_, and the other mediated by the higher-order activity of the Mix9 bacterial
community. Although protection against _F. columnare_ infection does not seem to rely on microbiota-based immuno-modulation, we cannot exclude that, individually, some members of the studied
core zebrafish microbiota could induce pro- or anti-inflammatory responses masked in presence of the full Mix10 consortium [1]. Whereas the identification of the mechanisms involved in the
community-level Mix9 protection will require further studies, reconventionalization and dysbiosis and recovery experiments demonstrated the key role of _C. massiliae_ in resistance against
_F. columnare_. The mechanisms underlying this protection may be multi-factorial. First, these two phylogenetically close _Bacteroidetes_ bacteria could compete for similar resources and
directly antagonize each other [6, 13]. For example, we identified a cluster of 11 genes in the genome of _C. massiliae_ (_tssB_, _tssC_, _tssD_, _tssE_, _tssF_, _tssG_, _tssH_, _tssI_,
_tssK_, _tssN_, and _tssP_) encoding a putative contact-dependent type VI secretion system (T6SS) potentially injecting toxins [57]. Second, we also identified a gene encoding a putative
pore-forming toxin of the Membrane Attack Complex/Perforin superfamily, which has been shown to contribute to interbacterial competition that occurs between phylogenetically close
Bacteroidetes species [57,58,59,_–_60]. Finally, all the genes associated with a functional T9SS involved in gliding motility as well as secretion of carbohydrate-active enzymes and other
toxin or virulence factors are also conserved in _C. massiliae_ and could contribute to its protective activity [61]. We cannot, however, exclude other mechanisms of protection such as
nutrient depletion or pathogen exclusion upon direct competition for adhesion to host tissues [11, 22, 26], and experiments are currently underway to identify nonprotective _C. massiliae_
mutants to uncover the bases of its activity against _F. columnare_. Interestingly, infected larvae reconventionalized with either C. _massiliae_ or Mix9 showed no signs of the intestinal
damage displayed by germ-free larvae, suggesting that both _C. massiliae_ and Mix9 provide similar intestinal resistance to _F. columnare_ infection. Whereas microbial colonization
contributes to gut maturation and stimulates the production of epithelial passive defenses such as mucus [62, 63], lack of intestinal maturation is unlikely to be contributing to _F.
columnare_-induced mortality, as mono-colonized larvae or larvae reconventionalized with nonprotective mixes died as rapidly as germ-free larvae. Several studies have monitored the long-term
assembly and development of the zebrafish microbiota from larvae to sexually mature adults, however little is known about the initial colonization establishment of the larvae after hatching
[64, 65]. Neutral (stochastic) and deterministic (host niche-based) processes [66,67,_–_68] lead to microbial communities that are mostly represented by a limited number of highly abundant
species with highly diverse low-abundant populations. In our experiments, the Mix10 species inoculum corresponded to an equiratio bacterial mix, thus starting from an engineered and assumed
total evenness (_E_ = 1) [69, 70]. Evenness was still relatively high (0.84) and remained similar up until 8 h in our study, indicating that most of the ten species were able to colonize the
larvae. From the perspective of community composition, a loss of diversity is often associated with decreased colonization resistance, but it remains unclear whether this increased
susceptibility is due to the loss of certain key member species of the microbial community and/or a change in their prevalence [3, 9]. We further investigated resistance to infection by
exposing established bacterial communities to different antibiotic perturbations, followed by direct challenge with _F. columnare_ (to study core microbiota sensitivity to disturbance) or
after recovery (to study its resilience) [12, 71]. Antibiotics are known to shift the composition and relative abundances of the microbiota according to their spectrum [13, 72]. We observed
that penicillin/streptomycin treatment that would affect most of the core species, reduced the abundance of all but two species (_A. veronii_ 1 and _P. myrsinacearum)_ that became relatively
dominant during recovery, but failed to provide protection against _F. columnare_. With the kanamycin treatment, colonization resistance was fully restored at the end of the 24 h recovery
period, indicative of a resilience that could result from species recovering quickly to their pre-perturbation levels due to fast growth rates, physiological flexibility or mutations [73].
Interestingly, even taking into account potential biases associated with the use of the 16S rRNA as a proxy index to determine relative abundance [74, 75], evenness was similarly reduced
during recovery for both treatments, but abundance at phylum level changed to 48% for Proteobacteria, and 52% for Bacteroidetes compared to the >98% of Proteobacteria with the
penicillin/streptomycin treatment. Furthermore, _C. massiliae_ was detected as rare (<1%) in conventional larvae, suggesting that it could have a disproportionate effect on the community
or that community-level protection provided by the nine other bacteria was also responsible for the protection of conventional larvae to _F. columnare_ infection. We showed that germ-free
zebrafish larvae are highly susceptible to a variety of different _F. columnare_ genomovars isolated from different hosts, demonstrating that they are a robust animal model for the study of
its pathogenicity. Recently, _F. columnare_ mutants in T9SS were shown to be avirulent in adult zebrafish, suggesting that proteins secreted by the T9SS are likely to be key, but still
largely unidentified, _F. columnare_ virulence determinants [39]. Body skin, gills, fins and tail are also frequently damaged in salmonid fish, whereas severe infection cases are associated
with septicemia [38]. We could not identify such clear _F. columnare_ infection sites in zebrafish larvae, perhaps due to the very low dose of infection used, with less than 100 cfu
recovered from infected moribund larvae. However, several lines of evidence suggest that the gut is the main target of _F. columnare_ infection in our model: (i) unfed germ-free larvae
survived exposure, (ii) histology analysis showing severe disruption of the intestinal region just hours after infection in germ-free larvae, and (iii) induction of _il22_ in germ-free
larvae exposed to _F. columnare_, since a major function of IL-22 is to promote gut repair [76]. This induction appears to be a consequence of the pathogen-mediated damage, as there was no
observed induction in conventional or reconventionalized larvae. The very rapid death of larvae likely caused by this severe intestinal damage may explain why other common target organs of
columnaris disease showed little damage. In this study, we showed that _C. massiliae_ is a promising probiotic candidate that could contribute to reduce the use of antibiotics to prevent
columnaris diseases in research and aquaculture settings. Whereas _C. massiliae_ provided full and robust protection against all tested virulent _F. columnare_ genomovars and significantly
increased survival of exposed adult conventional zebrafish, further studies are needed to elucidate _C. massiliae_ protection potential in other teleost fish. However, the endogenous nature
of _C. massiliae_ suggests that it could establish itself as a long-term resident of the zebrafish larval and adult microbiota, an advantageous trait when seeking a stable modulation of the
bacterial community over long periods [43]. In conclusion, the use of a simple and tractable zebrafish larval model to mine indigenous host microbial communities allowed us to identify two
independent protection strategies against the same pathogen. Whereas further study will determine how these strategies may contribute to protection against a wider range of pathogens, this
work also provides insights into how to engineer stable protective microbial communities with controlled colonization resistance functions. DATA AVAILABILITY The raw sequences generated for
the study can be found in the NCBI Short Read Archive under BioProject No. PRJNA649696. Bacterial genome sequences obtained in the present study are available at the European Nucleotide
Archive with the project number PRJEB36872, under accession numbers ERS4385993 (_Aeromonas veronii 1_); ERS4386000 (_Aeromonas veronii 2_); ERS4385996 (_Aeromonas caviae_); ERS4385998
(_Chryseobacterium massiliae_); ERS4385999 (_Phyllobacterium myrsinacearum_); ERS4406247 (_Pseudomonas sediminis_); ERS4385994 (_Pseudomonas mossellii_) ERS4386001 (_Pseudomonas
nitroreducens_); ERS4385997 (_Pseudomonas peli_); ERS4385995 (_Stenotrophomas maltophilia_). REFERENCES * Rolig AS, Parthasarathy R, Burns AR, Bohannan BJ, Guillemin K. Individual members of
the microbiota disproportionately modulate host innate immune responses. Cell Host Microbe. 2015;18:613–20. Article CAS PubMed PubMed Central Google Scholar * McFall-Ngai MJ. Unseen
forces: the influence of bacteria on animal development. Dev Biol. 2002;242:1–14. Article CAS PubMed Google Scholar * van der Waaij D, Berghuis-de Vries JM, Lekkerkerk-van der Wees JEC.
Colonization resistance of the digestive tract and the spread of bacteria to the lymphatic organs in mice. J Hyg. 1972;70:335–42. Article PubMed PubMed Central Google Scholar * Cani PD,
Delzenne NM. The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des. 2009;15:1546–58. Article CAS PubMed Google Scholar * Stecher B, Hardt WD. The role
of microbiota in infectious disease. Trends Microbiol. 2008;16:107–14. Article CAS PubMed Google Scholar * Stecher B, Hardt WD. Mechanisms controlling pathogen colonization of the gut.
Curr Opin Microbiol. 2011;14:82–91. Article CAS PubMed Google Scholar * Falcinelli S, Rodiles A, Unniappan S, Picchietti S, Gioacchini G, Merrifield DL, et al. Probiotic treatment
reduces appetite and glucose level in the zebrafish model. Sci Rep. 2016;6:18061. Article CAS PubMed PubMed Central Google Scholar * Olsan EE, Byndloss MX, Faber F, Rivera-Chavez F,
Tsolis RM, Baumler AJ. Colonization resistance: The deconvolution of a complex trait. J Biol Chem. 2017;292:8577–81. Article CAS PubMed PubMed Central Google Scholar * Littman DR, Pamer
EG. Role of the commensal microbiota in normal and pathogenic host immune responses. Cell Host Microbe. 2011;10:311–23. Article CAS PubMed PubMed Central Google Scholar * Heselmans M,
Reid G, Akkermans LM, Savelkoul H, Timmerman H, Rombouts FM. Gut flora in health and disease: potential role of probiotics. Curr Issues Intest Microbiol. 2005;6:1–7. CAS PubMed Google
Scholar * Boirivant M, Strober W. The mechanism of action of probiotics. Curr Opin Gastroenterol. 2007;23:679–92. Article PubMed Google Scholar * Robinson CJ, Bohannan BJ, Young VB. From
structure to function: the ecology of host-associated microbial communities. Microbiol Mol Biol Rev. 2010;74:453–76. Article CAS PubMed PubMed Central Google Scholar * Vollaard EJ,
Clasener HA. Colonization resistance. Antimicrob Agents Chemother. 1994;38:409–14. Article CAS PubMed PubMed Central Google Scholar * Gill HS. Probiotics to enhance anti-infective
defences in the gastrointestinal tract. Best Pr Res Clin Gastroenterol. 2003;17:755–73. Article CAS Google Scholar * Rawls JF, Samuel BS, Gordon JI. Gnotobiotic zebrafish reveal
evolutionarily conserved responses to the gut microbiota. Proc Natl Acad Sci USA. 2004;101:4596–601. Article CAS PubMed PubMed Central Google Scholar * Milligan-Myhre K, Charette JR,
Phennicie RT, Stephens WZ, Rawls JF, Guillemin K, et al. Study of host-microbe interactions in zebrafish. Methods cell Biol. 2011;105:87–116. Article PubMed PubMed Central Google Scholar
* Burns AR, Guillemin K. The scales of the zebrafish: host-microbiota interactions from proteins to populations. Curr Opin Microbiol. 2017;38:137–41. Article CAS PubMed PubMed Central
Google Scholar * Douglas AE. Simple animal models for microbiome research. Nat Rev Microbiol. 2019;17:764–75. Article CAS PubMed Google Scholar * Flores EM, Nguyen AT, Odem MA,
Eisenhoffer GT, Krachler AM. The zebrafish as a model for gastrointestinal tract-microbe interactions. Cell Microbiol. 2020;22:e13152. Article CAS PubMed PubMed Central Google Scholar *
Melancon E, Gomez De La Torre Canny S, Sichel S, Kelly M, Wiles TJ, Rawls JF, et al. Best practices for germ-free derivation and gnotobiotic zebrafish husbandry. Methods cell Biol.
2017;138:61–100. Article CAS PubMed PubMed Central Google Scholar * Cantas L, Sorby JR, Alestrom P, Sorum H. Culturable gut microbiota diversity in zebrafish. Zebrafish. 2012;9:26–37.
Article CAS PubMed PubMed Central Google Scholar * Rendueles O, Ferrieres L, Fretaud M, Begaud E, Herbomel P, Levraud JP, et al. A new zebrafish model of oro-intestinal pathogen
colonization reveals a key role for adhesion in protection by probiotic bacteria. PLoS Pathog. 2012;8:e1002815. Article CAS PubMed PubMed Central Google Scholar * Caruffo M, Navarrete
NC, Salgado OA, Faundez NB, Gajardo MC, Feijoo CG, et al. Protective Yeasts Control V. anguillarum Pathogenicity and Modulate the Innate Immune Response of Challenged Zebrafish (Danio rerio)
Larvae. Front Cell Infect Microbiol. 2016;6:127. Article PubMed PubMed Central Google Scholar * Perez-Ramos A, Mohedano ML, Pardo MA, Lopez P. Beta-glucan-producing pediococcus parvulus
2.6: test of probiotic and immunomodulatory properties in zebrafish models. Front Microbiol. 2018;9:1684. Article PubMed PubMed Central Google Scholar * Chu W, Zhou S, Zhu W, Zhuang X.
Quorum quenching bacteria Bacillus sp. QSI-1 protect zebrafish (Danio rerio) from Aeromonas hydrophila infection. Sci Rep. 2014;4:5446. Article PubMed PubMed Central CAS Google Scholar
* Wang Y, Ren Z, Fu L, Su X. Two highly adhesive lactic acid bacteria strains are protective in zebrafish infected with Aeromonas hydrophila by evocation of gut mucosal immunity. J Appl
Microbiol. 2016;120:441–51. Article CAS PubMed Google Scholar * Qin C, Zhang Z, Wang Y, Li S, Ran C, Hu J, et al. EPSP of L. casei BL23 Protected against the Infection Caused by
Aeromonas veronii via Enhancement of Immune Response in Zebrafish. Front Microbiol. 2017;8:2406. Article PubMed PubMed Central Google Scholar * Girija V, Malaikozhundan B, Vaseeharan B,
Vijayakumar S, Gobi N, Del Valle Herrera M, et al. In vitro antagonistic activity and the protective effect of probiotic Bacillus licheniformis Dahb1 in zebrafish challenged with GFP tagged
Vibrio parahaemolyticus Dahv2. Microb Pathogenesis. 2018;114:274–80. Article Google Scholar * Lin YS, Saputra F, Chen YC, Hu SY. Dietary administration of Bacillus amyloliquefaciens R8
reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio). Fish Shellfish Immunol.
2019;86:410–9. Article CAS PubMed Google Scholar * Declercq AM, Haesebrouck F, Van den Broeck W, Bossier P, Decostere A. Columnaris disease in fish: a review with emphasis on
bacterium-host interactions. Vet Res. 2013;44:27. Article PubMed PubMed Central Google Scholar * Decostere A, Haesebrouck F, Devriese LA. Characterization of four Flavobacterium
columnare (Flexibacter columnaris) strains isolated from tropical fish. Vet Microbiol. 1998;62:35–45. Article CAS PubMed Google Scholar * Figueiredo HC, Klesius PH, Arias CR, Evans J,
Shoemaker CA, Pereira DJ Jr, et al. Isolation and characterization of strains of Flavobacterium columnare from Brazil. J fish Dis. 2005;28:199–204. Article CAS PubMed Google Scholar *
Soto E, Mauel MJ, Karsi A, Lawrence ML. Genetic and virulence characterization of Flavobacterium columnare from channel catfish (Ictalurus punctatus). J Appl Microbiol. 2008;104:1302–10.
Article CAS PubMed Google Scholar * Suomalainen LR, Bandilla M, Valtonen ET. Immunostimulants in prevention of columnaris disease of rainbow trout, Oncorhynchus mykiss (Walbaum). J fish
Dis. 2009;32:723–6. Article PubMed Google Scholar * Pacha RE, Ordal EJ Myxobacterial infections of salmonids. American Fisheries Society, Diseases of Fishes and Shellfishes 1970:12. *
Amin NE, Abdallah IS, Faisal M, Easa Me-S, Alaway T, Alyan SA. Columnaris infection among cultured Nile tilapia Oreochromis niloticus. Antonie Van Leeuwenhoek. 1988;54:509–20. Article CAS
PubMed Google Scholar * Decostere A, Haesebrouck F, Charlier G, Ducatelle R. The association of Flavobacterium columnare strains of high and low virulence with gill tissue of black mollies
(Poecilia sphenops). Vet Microbiol. 1999;67:287–98. Article CAS PubMed Google Scholar * Bernardet J-F, Bowman JP. The genus flavobacterium. Prokaryotes. 2006;7:481–531. Article Google
Scholar * Li N, Zhu Y, LaFrentz BR, Evenhuis JP, Hunnicutt DW, Conrad RA, et al. The type IX secretion system is required for virulence of the fish pathogen flavobacterium columnare. Appl
Environ Microbiol. 2017;83:e01769–17. PubMed PubMed Central Google Scholar * Garcia JC, LaFrentz BR, Waldbieser GC, Wong FS, Chang SF. Characterization of atypical Flavobacterium
columnare and identification of a new genomovar. J Fish Dis. 2018;41:1159–64. Article CAS PubMed Google Scholar * van der Vaart M, van Soest JJ, Spaink HP, Meijer AH. Functional analysis
of a zebrafish _myd88_ mutant identifies key transcriptional components of the innate immune system. Dis Models Mech. 2013;6:841–54. Google Scholar * Pham LN, Kanther M, Semova I, Rawls
JF. Methods for generating and colonizing gnotobiotic zebrafish. Nat Protoc. 2008;3:1862–75. Article CAS PubMed PubMed Central Google Scholar * Lemon KP, Armitage GC, Relman DA,
Fischbach MA. Microbiota-targeted therapies: an ecological perspective. Sci Transl Med. 2012;4:137rv5. Article PubMed PubMed Central CAS Google Scholar * Bolger AM, Lohse M, Usadel B.
Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinforma. 2014;30:2114–20. Article CAS Google Scholar * Benjamini Y, Hochberg Y. Controlling the false discovery rate: a
practical and powerful approach to multiple testing. J R Stat Soc, Ser B,57, 289-300.1995;57:289–300. Google Scholar * Ha SM, Kim CK, Roh J, Byun JH, Yang SJ, Choi SB, et al. Application of
the whole genome-based bacterial identification system, TrueBac ID, using clinical isolates that were not identified with three matrix-assisted laser desorption/ionization time-of-flight
mass spectrometry (MALDI-TOF MS) systems. Ann Lab Med. 2019;39:530–6. Article CAS PubMed PubMed Central Google Scholar * Volant S, Lechat P, Woringer P, Motreff L, Campagne P, Malabat
C, et al. SHAMAN: a user-friendly website for metataxonomic analysis from raw reads to statistical analysis. BMC Bioinforma. 2020;21:345. Article Google Scholar * Rognes T, Flouri T,
Nichols B, Quince C, Mahe F. VSEARCH: a versatile open source tool for metagenomics. PeerJ. 2016;4:e2584. Article PubMed PubMed Central Google Scholar * Quast C, Pruesse E, Yilmaz P,
Gerken J, Schweer T, Yarza P, et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 2012;41:D590–D6. Article PubMed PubMed
Central CAS Google Scholar * Yarza P, Yilmaz P, Pruesse E, Glockner FO, Ludwig W, Schleifer KH, et al. Uniting the classification of cultured and uncultured bacteria and archaea using 16S
rRNA gene sequences. Nat Rev Microbiol. 2014;12:635–45. Article CAS PubMed Google Scholar * Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq
data with DESeq2. Genome Biol. 2014;15:550. Article PubMed PubMed Central CAS Google Scholar * Olivares-Fuster O, Bullard SA, McElwain A, Llosa MJ, Arias CR. Adhesion dynamics of
Flavobacterium columnare to channel catfish Ictalurus punctatus and zebrafish Danio rerio after immersion challenge. Dis Aquat Org. 2011;96:221–7. Article Google Scholar * Cheesman SE,
Neal JT, Mittge E, Seredick BM, Guillemin K. Epithelial cell proliferation in the developing zebrafish intestine is regulated by the Wnt pathway and microbial signaling via Myd88. Proc Natl
Acad Sci USA. 2011;108:4570–7. Article CAS PubMed Google Scholar * Stephens WZ, Burns AR, Stagaman K, Wong S, Rawls JF, Guillemin K, et al. The composition of the zebrafish intestinal
microbial community varies across development. ISME J. 2016;10:644–54. Article PubMed CAS Google Scholar * Mena KD, Gerba CP. Risk assessment of pseudomonas aeruginosa in water. Rev
Environ contamination Toxicol. 2009;201:71–115. CAS Google Scholar * Goncalves Pessoa RB, de Oliveira WF, Marques DSC, Dos Santos Correia MT, de Carvalho E, Coelho L. The genus Aeromonas:
a general approach. Microb pathogenesis. 2019;130:81–94. Article CAS Google Scholar * Russell AB, Wexler AG, Harding BN, Whitney JC, Bohn AJ, Goo YA, et al. A type VI secretion-related
pathway in Bacteroidetes mediates interbacterial antagonism. Cell Host Microbe. 2014;16:227–36. Article CAS PubMed PubMed Central Google Scholar * Chatzidaki-Livanis M, Coyne MJ,
Comstock LE. An antimicrobial protein of the gut symbiont Bacteroides fragilis with a MACPF domain of host immune proteins. Mol Microbiol. 2014;94:1361–74. Article CAS PubMed PubMed
Central Google Scholar * Roelofs KG, Coyne MJ, Gentyala RR, Chatzidaki-Livanis M, Comstock LE. Bacteroidales secreted antimicrobial proteins target surface molecules necessary for gut
colonization and mediate competition in vivo. mBio. 2016;7:e01055–16. Article CAS PubMed PubMed Central Google Scholar * Jacobson A, Lam L, Rajendram M, Tamburini F, Honeycutt J, Pham
T, et al. A gut commensal-produced metabolite mediates colonization resistance to salmonella infection. Cell host microbe. 2018;24:296–307.e7. Article CAS PubMed PubMed Central Google
Scholar * Larsbrink J, McKee LS. Bacteroidetes bacteria in the soil: glycan acquisition, enzyme secretion, and gliding motility. Adv Appl Microbiol. 2020;110:63–98. Article PubMed CAS
Google Scholar * Kamada N, Seo SU, Chen GY, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–35. Article CAS PubMed Google Scholar
* Ganz J, Melancon E, Eisen JS. Zebrafish as a model for understanding enteric nervous system interactions in the developing intestinal tract. Methods cell Biol. 2016;134:139–64. Article
CAS PubMed Google Scholar * Bates JM, Mittge E, Kuhlman J, Baden KN, Cheesman SE, Guillemin K. Distinct signals from the microbiota promote different aspects of zebrafish gut
differentiation. Dev Biol. 2006;297:374–86. Article CAS PubMed Google Scholar * Yan Q, van der Gast CJ, Yu Y. Bacterial community assembly and turnover within the intestines of
developing zebrafish. PLoS ONE. 2012;7:e30603. Article CAS PubMed PubMed Central Google Scholar * Costello EK, Stagaman K, Dethlefsen L, Bohannan BJ, Relman DA. The application of
ecological theory toward an understanding of the human microbiome. Science. 2012;336:1255–62. Article CAS PubMed PubMed Central Google Scholar * Rogers GB, Hoffman LR, Carroll MP, Bruce
KD. Interpreting infective microbiota: the importance of an ecological perspective. Trends Microbiol. 2013;21:271–6. Article CAS PubMed Google Scholar * Burns AR, Stephens WZ, Stagaman
K, Wong S, Rawls JF, Guillemin K, et al. Contribution of neutral processes to the assembly of gut microbial communities in the zebrafish over host development. ISME J. 2016;10:655–64.
Article CAS PubMed Google Scholar * Hibbing ME, Fuqua C, Parsek MR, Peterson SB. Bacterial competition: surviving and thriving in the microbial jungle. Nat Rev Microbiol. 2010;8:15–25.
Article CAS PubMed PubMed Central Google Scholar * Shafquat A, Joice R, Simmons SL, Huttenhower C. Functional and phylogenetic assembly of microbial communities in the human microbiome.
Trends Microbiol. 2014;22:261–6. Article CAS PubMed PubMed Central Google Scholar * Shade A, Peter H, Allison SD, Baho DL, Berga M, Burgmann H, et al. Fundamentals of microbial
community resistance and resilience. Front Microbiol. 2012;3:417. Article PubMed PubMed Central Google Scholar * Willing BP, Russell SL, Finlay BB. Shifting the balance: antibiotic
effects on host-microbiota mutualism. Nat Rev Microbiol. 2011;9:233–43. Article CAS PubMed Google Scholar * Brugman S, Liu KY, Lindenbergh-Kortleve D, Samsom JN, Furuta GT, Renshaw SA,
et al. Oxazolone-induced enterocolitis in zebrafish depends on the composition of the intestinal microbiota. Gastroenterology. 2009;137:1757–67.e1. Article CAS PubMed Google Scholar *
Salonen A, Nikkila J, Jalanka-Tuovinen J, Immonen O, Rajilic-Stojanovic M, Kekkonen RA, et al. Comparative analysis of fecal DNA extraction methods with phylogenetic microarray: effective
recovery of bacterial and archaeal DNA using mechanical cell lysis. J Microbiol Methods. 2010;81:127–34. Article CAS PubMed Google Scholar * Kunin V, Engelbrektson A, Ochman H,
Hugenholtz P. Wrinkles in the rare biosphere: pyrosequencing errors can lead to artificial inflation of diversity estimates. Environ Microbiol. 2010;12:118–23. Article CAS PubMed Google
Scholar * Shabgah AG, Navashenaq JG, Shabgah OG, Mohammadi H, Sahebkar A. Interleukin-22 in human inflammatory diseases and viral infections. Autoimmun Rev. 2017;16:1209–18. Article CAS
PubMed Google Scholar Download references ACKNOWLEDGEMENTS We thank Mark McBride, Pierre Boudinot, and Rebecca Stevick for critical reading of the manuscript. We are grateful to the late
Covadonga Arias for the gift of _F. columnare_ ALG 00–530, to Mark McBride for _F. columnare_ C#2 strain and to Jean-François Bernardet for all other _F. columnare_ strains. Prof. Annemarie
Meijer (Leiden University) kindly provided the _myd88_ mutant zebrafish line. We thank Chloé Baron for her help, Julien Burlaud-Gaillard and Rustem Uzbekov from the IBiSA Microscopy
facility, Tours University, France and the following zebrafish facility teams for providing eggs: José Perez and Yohann Rolin (Institut Pasteur), Nadia Soussi-Yanicostas (INSERM Robert
Debré), Sylvie Schneider-Manoury and Isabelle Anselme (UMR7622, University Paris 6) and Frédéric Sohm (AMAGEN Gif-sur-Yvette). FUNDING This work was supported by the Institut Pasteur, the
French Government’s _Investissement d’Avenir_ program: _Laboratoire d’Excellence_ ‘Integrative Biology of Emerging Infectious Diseases’ (grant no. ANR-10-LABX-62-IBEID to J-MG.), the
Fondation pour la Recherche Médicale (grant no. DEQ20180339185 to J-MG). FS was the recipient of a post-doctoral Marie Curie fellowship from the EU-FP7 program, JBB was the recipient of a
long-term post-doctoral fellowship from the Federation of European Biochemical Societies (FEBS) and by the European Union’s Horizon 2020 research and innovation programme under the Marie
Skłodowska-Curie grant agreement No 842629. DP-P was supported by an Institut Carnot MS Postdoctoral fellowship. The funders had no role in study design, data collection and analysis,
decision to publish, or preparation of the manuscript. AUTHOR INFORMATION Author notes * Franziska A. Stressmann Present address: Department of Chemical Analytics and Biogeochemistry,
Leibniz-Institute of Freshwater Ecology and Inland Fisheries, Müggelseedamm 310, 12587, Berlin, Germany * Joaquín Bernal-Bayard Present address: Departamento de Genética, Facultad de
Biología, Universidad de Sevilla, Apartado 1095, 41080, Sevilla, Spain * Olaya Rendueles Present address: Microbial Evolutionary Genomics Laboratory, Institut Pasteur, UMR3525, 75015, Paris,
France * Sebastian Bruchmann Present address: Department of Veterinary Medicine, University of Cambridge, Madingley Road, Cambridge, CB3 0ES, UK * Susanne Häussler Present address:
Department of Clinical Microbiology, Rigshospitalet, 2100, Copenhagen, Denmark * These authors contributed equally: Franziska A. Stressmann, Joaquín Bernal-Bayard AUTHORS AND AFFILIATIONS *
Genetics of Biofilms Laboratory, Institut Pasteur, UMR CNRS2001, 75015, Paris, France Franziska A. Stressmann, Joaquín Bernal-Bayard, David Perez-Pascual, Bianca Audrain, Olaya Rendueles
& Jean-Marc Ghigo * Macrophages and Development of Immunity Laboratory, Institut Pasteur, UMR3738 CNRS, 75015, Paris, France Valérie Briolat & Jean-Pierre Levraud * Department of
Molecular Bacteriology, Helmholtz Centre for Infection Research, Braunschweig, Germany Sebastian Bruchmann & Susanne Häussler * Hub de Bioinformatique et Biostatistique – Département
Biologie Computationnelle, Institut Pasteur, USR 3756 CNRS, Paris, France Stevenn Volant & Amine Ghozlane * Unité VIM, INRAE, Université Paris-Saclay, 78350, Jouy-en-Josas, France Eric
Duchaud Authors * Franziska A. Stressmann View author publications You can also search for this author inPubMed Google Scholar * Joaquín Bernal-Bayard View author publications You can also
search for this author inPubMed Google Scholar * David Perez-Pascual View author publications You can also search for this author inPubMed Google Scholar * Bianca Audrain View author
publications You can also search for this author inPubMed Google Scholar * Olaya Rendueles View author publications You can also search for this author inPubMed Google Scholar * Valérie
Briolat View author publications You can also search for this author inPubMed Google Scholar * Sebastian Bruchmann View author publications You can also search for this author inPubMed
Google Scholar * Stevenn Volant View author publications You can also search for this author inPubMed Google Scholar * Amine Ghozlane View author publications You can also search for this
author inPubMed Google Scholar * Susanne Häussler View author publications You can also search for this author inPubMed Google Scholar * Eric Duchaud View author publications You can also
search for this author inPubMed Google Scholar * Jean-Pierre Levraud View author publications You can also search for this author inPubMed Google Scholar * Jean-Marc Ghigo View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS FAS, JBB, DP-P, J-PL, and J-MG designed the experiments. OR contributed to the initial experiments. VB
and J-PL provided zebrafish material and advice. FAS, JBB, DP-P, BA, VB, and J-PL performed the experiments. SB, SH performed bacterial genome sequencing and analysis, AG, SV, FAS, and DP-P
performed the bioinformatic and sequence analyses. FAS, JBB, DP-P, J-PL, and J-MG analysed the data and wrote the paper with significant contribution from OR and ED. CORRESPONDING AUTHOR
Correspondence to Jean-Marc Ghigo. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors of this manuscript have the following conflict of interest: a provisional patent application has been
filed: “bacterial strains for use as probiotics, compositions thereof, deposited strains and method to identify probiotic bacterial strains” by J-MG, FAS, DP-P, and JBB The other authors
declare no conflict of interest in relation to the submitted work. ETHICS All animal experiments described in the present study were conducted at the Institut Pasteur (larvae) or at INRA
Jouy-en-Josas (adults) according to European Union guidelines for handling of laboratory animals (http://ec.europa.eu/environment/chemicals/lab_animals/home_en.htm) and were approved by the
relevant institutional Animal Health and Care Committees. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and
institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES AND TABLES RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Stressmann, F.A.,
Bernal-Bayard, J., Perez-Pascual, D. _et al._ Mining zebrafish microbiota reveals key community-level resistance against fish pathogen infection. _ISME J_ 15, 702–719 (2021).
https://doi.org/10.1038/s41396-020-00807-8 Download citation * Received: 02 May 2020 * Revised: 30 September 2020 * Accepted: 05 October 2020 * Published: 19 October 2020 * Issue Date: March
2021 * DOI: https://doi.org/10.1038/s41396-020-00807-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative