- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Preterm survivors have increased risk for impaired cardiometabolic health. We assessed glucose regulation and cardiometabolic biomarkers in adult very low birth weight
(VLBW, <1500 g) survivors, using siblings as controls. METHODS VLBW-participants were matched with term-born, same-sex siblings. At mean age 29.2 years (SD 3.9), 74 VLBW-adults and 70
siblings underwent a 2-h 75 g oral glucose tolerance test and blood tests for assessment of cardiometabolic biomarkers. RESULTS Of participants, 23 (31%) VLBW and 11 (16%) sibling-controls
met World Health Organization criteria for impaired glucose regulation (OR adjusted for age and sex 2.5, 95% CI: 1.1 to 5.8). Adjusting for age and sex, VLBW-participants showed 9.2% higher
2-h glucose (95% CI: 0.4% to 18.8%) than their siblings. Also, fasting (13.4%, −0.3% to 29.0%) and 2-h free fatty acids (15.6%, −2.4% to 36.9%) were higher in VLBW-participants. These
differences were statistically significant only after further adjusting for confounders. No statistically significant differences were found regarding other measured biomarkers, including
insulin resistance, atherogenic lipid profiles or liver tests. CONCLUSIONS VLBW-adults showed more impaired fatty acid metabolism and glucose regulation. Differences in cardiometabolic
biomarkers were smaller than in previous non-sibling studies. This may partly be explained by shared familial, genetic, or environmental factors. IMPACT * At young adult age, odds for
impaired glucose regulation were 3.4-fold in those born at very low birth weight, compared to same-sex term-born siblings. * Taking into consideration possible unmeasured, shared familial
confounders, we compared cardiometabolic markers in adults born preterm at very low birth weight with term-born siblings. * Prematurity increased risk for impaired glucose regulation,
unrelated to current participant characteristics, including body mass index. * In contrast to previous studies, differences in insulin resistance were not apparent, suggesting that insulin
resistance may partially be explained by factors shared between siblings. Also, common cardiometabolic biomarkers were similar within sibling pairs. SIMILAR CONTENT BEING VIEWED BY OTHERS
LONG-TERM HEALTH IN INDIVIDUALS BORN PRETERM OR WITH LOW BIRTH WEIGHT: A COHORT STUDY Article Open access 04 July 2024 BODY COMPOSITION IN ADULTS BORN PRETERM WITH VERY LOW BIRTH WEIGHT
Article Open access 16 November 2023 CHILDHOOD OBESITY AND ADVERSE CARDIOMETABOLIC RISK IN LARGE FOR GESTATIONAL AGE INFANTS AND POTENTIAL EARLY PREVENTIVE STRATEGIES: A NARRATIVE REVIEW
Article 16 December 2021 INTRODUCTION Annually 14.8 million babies are born preterm (<37 weeks of gestation), constituting approximately 1/10th of all livebirths.1 The survival rate is
98% in high-income countries and 90% globally.1 Preterm birth may have life-long consequences on overall health and function including cognitive functioning, attention, neuromotor abilities,
physical fitness, lung function, and bone health.2,3,4,5,6,7 Moreover, prematurity is influencing later cardiovascular health and is associated with the components of metabolic syndrome and
cardiovascular disease in adult life.8,9 Prematurity has, for instance, been linked to insulin resistance, higher fat mass, blood pressure, cholesterol, fasting glucose and insulin levels
in comparisons between preterm and term-born adults.8,10,11,12 While many previous studies use carefully selected comparison groups and adjust for important confounders, such studies cannot
exclude residual confounding by, for example, unmeasured socio-economic, lifestyle or genetic factors that predispose both to preterm birth and adult cardiovascular outcomes. Efforts have
been made to overcome such confounding by utilizing sibling information from large register data. For example, in a retrospective national cohort study of 2.2 million adults, preterm birth
was associated with increased risk of lipid disorders in adulthood as compared with full-term birth.13 In that study, 84% of the cohort had a sibling, and based on co-sibling analyses, the
observed increase in risk of lipid disorders was largely explained by shared genetic or environmental factors in families.13 Another family-based study of 386485 singleton-born men,
conducted comparisons both between non-siblings and within siblings. The study found inverse associations of birth weight and gestational age with systolic blood pressure at ages 17 to 19
years.14 These results were not explained by family socioeconomic position or other factors shared by siblings. In a further Swedish population-based cohort study of 2.1 million
participants, which also included co-sibling analyses, preterm birth was associated with an increased risk for ischemic heart disease in adulthood, independently of shared familial genetic
or environmental factors.9 Despite evident benefits, the sibling study design has rarely been used in clinical birth cohort studies of adults born preterm. We examined associations of
preterm birth at very low birth weight (VLBW, birth weight ≤1500 g) with adult cardiometabolic biomarkers using same-sex siblings, born at term, as controls. We hypothesized that VLBW
affects cardiometabolic risk factors at adult age, and that possible differences compared with siblings born at term might be less pronounced, considering common genetic and background
factors. METHODS PARTICIPANTS VLBW participants, born between 1978 and 1989, were recruited from three sources: the Helsinki Study of Very Low Birth Weight Adults,10 the ESTER Preterm Birth
and Early-Life Programming of Adult Health and Disease Study11 and via the Finnish Medical Birth Register. The VLBW adults comprise a population-based sample as they were chosen among all
newborns in a defined geographic area. The Finnish Medical Birth Register includes data on all live and stillborn neonates in Finland since 1987, with birth weights ≥500 g or gestational age
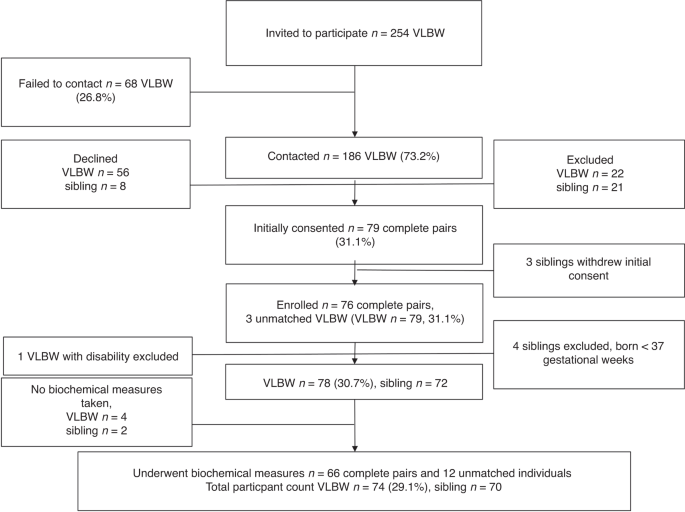
≥22 weeks. The recruitment (Fig. 1) has been described previously.15 In brief, between July 2014 and February 2017, 186 VLBW-adults were contacted, if population records showed that they
had a same-sex sibling with less than a 10-year age difference. Each VLBW-adult interested in participating, was asked to inquire whether also their sibling would be willing to participate.
All participating siblings were born at term and at least 18 years old. Pregnancy, cerebral palsy, intellectual disability, motor or sensory impairment, severe chronic disease, and endocrine
disorders, including type I and II diabetes, were exclusion criteria. As clinical study visits included detailed investigations, with for example magnetic resonance imagining16,17 and
tissue biopsies, we excluded individuals with manifest diabetes; thus, 1 VLBW and 2 sibling participants were excluded due to type 1 diabetes. Inhaled and topical glucocorticoids were
allowed while systemic use was an exclusion criterion. After initial assessment further exclusions were made due to participant related issues (pregnancy, compliance issues, declining
further participation) and four controls were found to be born preterm (Fig. 1). All excluded participants have previously been described in detail.15,16,17,18 Finally, 74 VLBW adults and 70
siblings underwent biochemical measurements (Fig. 1), of these 66 were complete sibling pairs and 12 unmatched participants. ETHICS Our study was performed in accordance with the
declaration of Helsinki and was approved by the ethics committee of the Hospital District of Helsinki and Uusimaa. All participants provided written informed consent. Because of individual
participant consent, these data are not freely available. Investigators requesting data access should contact the corresponding author (N.K.). Request could be subject to ethics review
and/or participant consent. MEASURES AND PROCEDURES Perinatal data was collected from maternity clinic and hospital records as previously described.15 Based on sex and age, we calculated
small for gestational age as birth weight < −2 standard deviations (SDs).19 Weight and height were measured by a trained study nurse during the clinical examinations, conducted in
2014–2017. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). All participants completed questionnaires on participant and parental health status, including
medical history and medications. Highest parental education served as indicator for childhood socioeconomic status and was categorized into three levels: lower secondary or less, higher
secondary and tertiary. Peripheral venous blood samples were collected and a 2-h, 75 g oral glucose tolerance test (OGTT) was performed after overnight fasting. After drinking the glucose
solution, blood samples were drawn at baseline, 30, 60, and 120 min, for analyzing glucose, insulin, and free fatty acids (FFA) levels. LABORATORY ANALYSES At the clinical visit, venous
blood samples were drawn in a sitting position with a light stasis into a fluoride-citrate tube (Venosafe, Terumo Europa, Leuven, Belgium) for glucose assays and into a tube containing clot
activator (Venosafe) for other assays. Fluoride-citrate plasma and serum were separated by centrifuging, frozen locally immediately after separation, and then transported frozen on dry ice
to the Biochemistry laboratory at the Finnish Institute for Health and Welfare (Helsinki, Finland). All analyses were performed on a clinical chemistry analyzer (Architect ci8200 Abbott
Laboratories, Abbott Park, Illinois). For standardizing measurements, the laboratory has taken part in the Lipid Standardization Program organized by Centers for Disease Control and
Prevention (CDC, Atlanta, Georgia) and External Quality Assessment Schemes organized by Labquality (Helsinki, Finland). The between-assay coefficient of variation (CV%, mean ± SD),
systematic error (Bias%, mean ± SD) and the principle of the methods in the biochemistry laboratory during the study are shown in Supplementary Table 1. STATISTICAL ANALYSIS Power
calculations performed before participant recruitment indicated that, with a power of 0.80 and an alpha value of 0.05, two-way paired comparisons within 75 sibpairs would allow us to detect
a 0.33 SD difference in continuous outcomes between VLBW adults and sibling-controls. For the actual number of 66 sibling pairs in this paper, the corresponding difference is 0.35 SD. All
analyses were performed using IBM SPSS Statistics versions 27 and 28 (IBM Corp. Armonk, NY). This was a cohort study, and the power of sample size is indicated by confidence intervals. The
95% CI reflects a significance level of 0.05. To estimate variation within each group of data we describe SDs. We used descriptive statistics to illustrate demographic and laboratory test
outcomes by group. Background variables were compared using t-test for continuous variables (described using means and SDs) and χ² test for categorical variables (described with counts and
percentages). P values were 2-sided and the significance level was set to 0.05. As laboratory outcomes were not normally distributed, we log-transformed them to attain normality prior to
performing statistical analyses. We used linear mixed effects models to assess the effect of VLBW-status on our outcomes, within-sibling analysis compared cardiometabolic biochemical
measures. The mixed model incorporates fixed and random effects and is useful for missing values, repeated measurements or when measuring clusters of related statistical units. We used the
following variables as fixed effects: crude model 1 adjusts for age and sex (if applicable), model 2 further adjusts for prenatal and environmental confounders, i.e., maternal gestational or
chronic hypertension, preeclampsia, maternal smoking during pregnancy and parental educational attainment, included because of potential effects on both preterm VLBW birth and later
offspring health, and model 3 additionally includes participant-related factors: BMI, height, and smoking status, included as possible mediators for the association between preterm VLBW
birth and cardiometabolic health. We used World Health Organization’s (WHO) criteria20 for type 2 diabetes (T2D, fasting plasma glucose ≥7.0 mmol/l or 2-h plasma glucose ≥11.1 mmol/l),
impaired glucose tolerance (IGT, fasting plasma glucose <7.0 mmol/l and 2-h plasma glucose ≥7.8 mmol/l and <11.1 mmol/l) and impaired fasting glucose (IFG, fasting plasma glucose 6.1
to 6.9 mmol/l and 2-h plasma glucose <7.8 mmol/l). Participants were compared by logistic regression model. RESULTS Study participants are described in Table 1. A total of 144
participants, consisting of 66 complete sibling pairs and 12 unmatched participants, provided blood samples and underwent OGTT. Mean age at participation was 29.2 (SD 3.9) years and 51.4%
were women. At adult age, VLBW participants were shorter than their siblings (Table 1). They were also more likely to have been born small for gestational age, with caesarean section and
from multiple pregnancy (Table 1). As our sample size is limited, we included all VLBW-individuals in the same group, both participants born small for gestational age (SGA: men _n_ = 10,
women _n_ = 18) and appropriate for gestational age (AGA: men _n_ = 26, women _n_ = 20). However, we reran the analyses separately comparing VLBW-SGA with controls and VLBW-AGA with controls
and present the results for additional information (Supplementary Table 2). This data should be interpreted with caution as our study is not powered to detect or exclude such associations,
especially in analyses stratified by sex. CARDIOMETABOLIC MARKERS Means of cardiometabolic biochemical measures for VLBW and sibling participants are shown in Table 2 and comparisons between
groups using linear mixed models are presented in Table 3. In the OGTT (Tables 3), 2-h glucose concentrations were higher (mean difference 9.2%, 95% CI [0.4, 18.8], _P_ = 0.04) in VLBW
participants as compared with siblings, while no differences were found in fasting glucose, fasting insulin or 2-h insulin concentrations. Both fasting FFA and 2-h FFA were higher in
VLBW-adults (models 1–3). 2 VLBW and 1 sibling met the WHO criteria20 for T2D, while 3 VLBW and 1 sibling had IFG, and IGT was found in 18 VLBW and 9 siblings (Table 4). When any form of
impaired glucose regulation, i.e., T2D, IFG, or IGT was studied as an outcome (Fig. 2), VLBW-adults showed a 2.5- to 3.4-fold increase compared with sibling participants (Table 4). Regarding
other cardiometabolic biomarkers, including testosterone, sex hormone binding globulin, lipid profiles, uric acid, ferritin, inflammatory markers and liver tests, findings were similar
between groups (Tables 2 and 3). 3 VLBW and 1 sibling participant had a high-sensitivity C-reactive protein greater than 10 mg/l and were excluded from high-sensitivity C-reactive protein
and ferritin analyses. CARDIOMETABOLIC BIOMARKERS IN COMPARISONS BETWEEN COMPLETE SIBLING PAIRS After excluding 12 unmatched participants, we reran all analyses to separately compare
complete sibling pairs. Most results remained similar. However, in the OGTT, the difference in 2-h glucose was somewhat attenuated: model 1 mean difference 8.3% (95% CI [−0.9, 18.2], _P_ =
0.08), model 2 10.7% (95% CI [1.5, 20.7], _P_ = 0.02), and model 3 9.0% (95% CI [−1.1, 20.2], _P_ = 0.08). In addition, compared with siblings, testosterone was 15.0–18.3% higher in
VLBW-women, reaching statistical significance only in the fully adjusted model 3 (mean difference 18.3%, 95% CI [0.3, 39.6], _P_ = 0.05). In men, no such difference was found. DISCUSSION
This cohort study compared cardiometabolic biomarkers in young adults born preterm at VLBW with their same-sex, term-born siblings. VLBW-adults had 3.4-fold odds for impaired glucose
regulation compared with their siblings. They also showed higher 2-h glucose concentrations, fasting and 2-h FFA concentrations. No differences were, however, seen in fasting glucose,
fasting insulin or 2-h insulin concentrations. Other cardiometabolic biomarkers, including lipid profiles, liver tests, inflammatory markers, and measures of hyperandrogenism were similar
between groups. Previous studies with unrelated controls have shown robust associations between preterm birth and increased risk for later chronic diseases, including cardiovascular disease,
metabolic syndrome and diabetes.8,21 In a Swedish cohort study, involving 674820 participants, each week of increased prematurity resulted in a 7% higher risk of dying in young adulthood
from cardiovascular disease.22 Another study, including one of our source cohorts, comparing VLBW with unrelated, term-born controls,10 showed 5.3% higher 2-h glucose, 12.6% higher fasting
insulin and 32.8% higher 2-h-insulin concentrations in VLBW individuals in their mid-twenties. In our study insulin concentrations were similar between VLBW and sibling participants, while
earlier studies, including two meta-analyses, have suggested point estimates ranging from 8% to 18.7%,8,10,23 which is beyond our 95% confidence interval for fasting insulin (−18.3%, 7.5%).
However, the point estimate of 2-h insulin, 32.8%, in the study with unrelated controls,10 was included in the confidence intervals (−17.8%, 34.9%) of our study, although it came close to
the upper limits. This suggests that shared family factors may partially explain differences in insulin sensitivity between VLBW adults and full-term controls. By contrast to lack of
difference in insulin sensitivity between groups in the current study, our finding of 9.2% higher 2-h glucose concentrations in VLBW-participants, corresponds to the previously reported
difference of 5.3% in the source cohort study with unrelated controls10 and approximately 1.4–2.4% in two meta-analyses8,23 that included also non-VLBW preterm-born adults. This points at
susceptibility for impaired glucose metabolism and increased risk of diabetes later in life in adults born preterm at VLBW. Our participants were relatively young, close to 30 years of age.
It is probable that the clinical implications of VLBW regarding cardiometabolic disorders will mostly become apparent at a later age. While only relatively few study participants fulfilled
the WHO criteria20 for T2D or IFG, risk of any category of impaired glucose regulation was 3.4-fold in VLBW-participants compared with siblings (Fig. 2). This is only slightly lower than the
4.0-fold risk in a cohort study of extremely low birth weight adults utilizing matched, normal birth weight controls.24 Similarly, in a recent study by Flahault et al., at mean age 23
years, glucose intolerance was 2.2-fold more common in former very preterm born (≤29 weeks) compared to full-term controls.25 That study attempted to mitigate bias caused by unmeasured
confounding factors regarding environment and lifestyle, by choosing relatives and friends of the preterm participants as controls. While not directly comparable to our study, Darlow et
al.26 found that VLBW adults, aged 26–30, displayed poorer physiological functioning than term-born controls. This was done by comparing an aggregate score consisting of 10 physiological
biomarkers, such as blood pressure and cholesterol. Although preterm birth is considered a risk factor for metabolic syndrome and cardiovascular disease in adult life,8 we found no evidence
on more atherogenic lipid profiles, signs of hyperandrogenism, elevated liver tests or inflammatory markers in VLBW participants. In our study, the largest group with impaired glucose
regulation (Fig. 2) was the one with IGT, which is characterized by insulin secretion inadequate for the glucose load.20 Insulin secretion plays a central role in the development of T2D as
most risk genes for T2D influence insulin secretion and not insulin resistance, and FFAs are known to influence insulin secretion.27 We did find higher fasting and 2-h FFA concentrations
after a 75 g glucose dose in VLBW vs sibling participants. FFAs play a central role in the body’s energy production. Fat is stored in adipose tissue as triglycerides, and FFAs are formed
when lipolysis breaks down triglycerides, a process inhibited by insulin. FFAs cause insulin resistance and inflammation, linking obesity, insulin resistance, inflammation, T2D, dyslipidemia
and atherosclerotic disease.28 Our findings of higher fasting and 2-h FFA concentrations in VLBW participants, might be a sign of insulin resistance of adipose tissue,29 i.e., insulin is
not suppressing FFA formation (lipolysis) after a glucose load. Higher fasting and 2-h FFA concentrations in VLBW participants could also indicate that prematurity influences energy
metabolism. Another indication of differences in metabolism was previously reported in one of our source cohorts, in which VLBW-adults had a higher than expected resting energy expenditure
based on lean body mass,30 suggesting the presence of more metabolically active tissue in VLBW individuals. Likewise, recently published data from our sibling study cohort, showed less
unsaturation in subcutaneous adipose tissue in VLBW adults,16 also suggesting differences in fat metabolism. Traditionally, in studies on long-term effects of preterm birth, the control
groups constitute of healthy term-born individuals. In our study, the lack of difference between VLBW and sibling controls regarding several common risk factors of cardiovascular disease
might be explained by shared genetic, socioeconomic, or environmental factors in the families. Our findings support this, the differences between groups in cardiometabolic risk factors seem
smaller than in studies utilizing full-term, unrelated study participants as controls. For instance, compared to our study, a previous non-sibling cohort study reported larger differences in
alanine aminotransferase levels (15.0% vs −2.2%), aspartate transaminase levels (11.7% vs −1.2%) and uric acid levels (20.1% vs 3.6%), with point estimates of that study not even included
in our study’s confidence intervals.11 This suggests that shared family factors may partly explain previously reported differences in these biomarkers between VLBW adults and controls born
at term. Further, the part not explained by shared family factors or pregnancy-related confounders could be explained by postnatal environment exposures encountered after VLBW birth.
Previous, non-sibling cohort studies on effects of preterm birth on cardiometabolic health also report differences regarding total cholesterol, low-density lipoprotein cholesterol and
apolipoprotein B concentrations31 although this is not found consistently in all studies.8,10,23 Similarly, in large systematic reviews and meta-analyses preterm born adults have exhibited
higher total cholesterol8 and low-density lipoprotein cholesterol levels,23 while in our study, the atherogenic lipid profiles did not differ between VLBW and siblings groups. Our sibling
design has inherent strengths and limitations. The most important strength is to circumvent many unmeasured familial confounders. Furthermore, we adjusted for many confounders that vary
within siblings, including maternal, pregnancy-related factors and current participant related mediators. The most important limitation is that we recruited VLBW participants with an
obliging same-sex sibling. As the protocol was extensive, there might be a bias towards sibling pairs who are more similar and closely connected, which could lead to more conservative
findings. It is also possible that childhood environmental exposures and family structure between siblings vary, as the maximum allowed age difference between participants was 10 years,
which we were not able to account for. Because our sample size is limited, we included all VLBW participants, both SGA and AGA, in the same group. Although we present these results to the
readers (Supplementary Table 2), results on SGA and AGA should be treated with caution as this study is not powered to detect or exclude such associations, especially in analyses stratified
by sex. Further, to make good use of all data collected, we included 12 unmatched participants in the analyses. In addition, our study population is Finnish, and the results may therefore
not be directly extrapolated globally to people with different genetic backgrounds. CONCLUSION Preterm birth at VLBW is a risk factor for impaired glucose metabolism. Already as young adults
the VLBW participants of our cohort displayed signs of IGT. In contrast to previous studies, differences in insulin resistance were not apparent, suggesting that insulin resistance may
partially be explained by factors shared between siblings. Further, common cardiometabolic biomarkers, including lipid profiles, signs of hyperandrogenism, liver tests and inflammatory
markers were similar within sibling pairs. Considering that 10% of the population is born preterm, the impact of perinatal history is relevant and significantly effects life-long public
health. DATA AVAILABILITY Deidentified individual participant data is not publicly available. Because of individual participant consent these data are not freely available. Investigators
requesting data access should contact the corresponding author (N.K.). Request could be subject to ethics review and/or participant consent. REFERENCES * Chawanpaiboon, S. et al. Global,
regional, and national estimates of levels of preterm birth in 2014: a systematic review and modelling analysis. _Lancet Glob. Health_ 7, e37–e46 (2019). Article PubMed Google Scholar *
Vollmer, B. & Stålnacke, J. Young adult motor, sensory, and cognitive outcomes and longitudinal development after very and extremely preterm birth. _Neuropediatrics_ 50, 219–227 (2019).
Article PubMed Google Scholar * Doyle, L. W. et al. Expiratory airflow in late adolescence and early adulthood in individuals born very preterm or with very low birthweight compared with
controls born at term or with normal birthweight: a meta-analysis of individual participant data. _Lancet Respir. Med._ 7, 677–686 (2019). Article PubMed Google Scholar * Tikanmäki, M. et
al. Physical fitness in young adults born preterm. _Pediatrics_ 137, e20151289 (2016). Article Google Scholar * Crump, C., Winkleby, M. A., Sundquist, J. & Sundquist, K. Risk of
asthma in young adults who were born preterm: a Swedish national cohort study. _Pediatrics_ 127, 913 (2011). Article Google Scholar * Hovi, P. et al. Decreased bone mineral density in
adults born with very low birth weight: a cohort study. _PLoS Med._ 6, e1000135 (2009). Article PubMed PubMed Central Google Scholar * Smith, C. M. et al. Very low birth weight survivors
have reduced peak bone mass and reduced insulin sensitivity. _Clin. Endocrinol. (Oxf.)._ 75, 443–449 (2011). Article CAS PubMed Google Scholar * Markopoulou, P., Papanikolaou, E.,
Analytis, A., Zoumakis, E. & Siahanidou, T. Preterm birth as a risk factor for metabolic syndrome and cardiovascular disease in adult life: a systematic review and meta-analysis. _J.
Pediatr._ 210, 69–80.e5 (2019). Article PubMed Google Scholar * Crump, C. et al. Association of preterm birth with risk of ischemic heart disease in adulthood. _JAMA Pediatr._ 173,
736–743 (2019). Article PubMed PubMed Central Google Scholar * Hovi, P. et al. Glucose regulation in young adults with very low birth weight. _N. Engl. J. Med._ 356, 2053–2063 (2007).
Article CAS PubMed Google Scholar * Sipola-Leppänen, M. et al. Cardiometabolic risk factors in young adults who were born preterm. _Am. J. Epidemiol._ 181, 861–873 (2015). Article
PubMed PubMed Central Google Scholar * de Jong, F., Monuteaux, M. C., van Elburg, R. M., Gillman, M. W. & Belfort, M. B. Systematic review and meta-analysis of preterm birth and later
systolic blood pressure. _Hypertension_ 59, 226–234 (2012). Article PubMed Google Scholar * Crump, C., Sundquist, J. & Sundquist, K. Association of preterm birth with lipid disorders
in early adulthood: a Swedish cohort study. _PLoS Med._ 16, e1002947 (2019). Article PubMed PubMed Central Google Scholar * Lawlor, D. A. et al. Associations of gestational age and
intrauterine growth with systolic blood pressure in a family-based study of 386 485 men in 331 089 families. _Circulation_ 115, 562–568 (2007). Article PubMed Google Scholar * Björkqvist,
J. et al. Chronotype in very low birth weight adults—a sibling study. _Chronobiol. Int._ 37, 1023–1033 (2020). Article PubMed Google Scholar * Kuula, J. et al. Abdominal adipose tissue
and liver fat imaging in very low birth weight adults born preterm: birth cohort with sibling-controls. _Sci. Rep._ 12, 9905 (2022). Article CAS PubMed PubMed Central Google Scholar *
Kuula, J. et al. Brain volumes and abnormalities in adults born preterm at very low birth weight. _J. Pediatr._ 246, 48 (2022). Article PubMed Google Scholar * Sandboge, S. et al. Bone
mineral density in very low birthweight adults—a sibling study. _Paediatr. Perinat. Epidemiol._ 36, 665–672 (2022). Article PubMed PubMed Central Google Scholar * Pihkala, J., Hakala,
T., Voutilainen, P. & Raivio, K. Characteristic of recent fetal growth curves in Finland. _Duodecim_ 105, 1540–1546 (1989). CAS PubMed Google Scholar * World Health Organization &
International Diabetes Federation. 2006. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia: report of a WHO/IDF consultation. World Health Organization.
https://apps.who.int/iris/handle/10665/43588 * Crump, C., Sundquist, J. & Sundquist, K. Preterm birth and risk of type 1 and type 2 diabetes: a national cohort study. _Diabetologia_ 63,
508–518 (2020). Article CAS PubMed Google Scholar * Crump, C., Sundquist, K., Sundquist, J. & Winkleby, M. A. Gestational age at birth and mortality in young adulthood. _JAMA: J. Am.
Med. Assoc._ 306, 1233–1240 (2011). Article CAS Google Scholar * Parkinson, J. R., Hyde, M. J., Gale, C., Santhakumaran, S. & Modi, N. Preterm birth and the metabolic syndrome in
adult life: a systematic review and meta-analysis. _Pediatrics_ 131, 1240 (2013). Article Google Scholar * Morrison, K. M. et al. Cardiometabolic health in adults born premature with
extremely low birth weight. _Pediatrics_ 138, e20160515 (2016). Article PubMed Google Scholar * Flahault, A. et al. Increased Incidence but lack of association between cardiovascular risk
factors in adults born preterm. _Hypertension_ 75, 796–805 (2020). Article CAS PubMed Google Scholar * Darlow, B. A. et al. Biomarkers of ageing in New Zealand VLBW young adults and
controls. _Pediatr. Res._ 89, 533–539 (2021). Article PubMed Google Scholar * Oberhauser, L. & Maechler, P. Lipid-induced adaptations of the pancreatic beta-cell to glucotoxic
conditions sustain insulin secretion. _Int. J. Mol. Sci._ 23, 324 (2021). Article PubMed PubMed Central Google Scholar * I S Sobczak, A., A Blindauer, C. & J Stewart, A. Changes in
plasma free fatty acids associated with type-2 diabetes. _Nutrients_ 11, 2022 (2019). Article PubMed PubMed Central Google Scholar * Ter Horst, K. W. et al. Methods for quantifying
adipose tissue insulin resistance in overweight/obese humans. _Int. J. Obes._ 41, 1288–1294 (2017). Article CAS Google Scholar * Sipola-Leppänen, M. et al. Resting energy expenditure in
young adults born preterm-the Helsinki study of very low birth weight adults. _PLoS One_ 6, e17700 (2011). Article PubMed PubMed Central Google Scholar * Sipola-Leppänen, M. et al.
Cardiovascular risk factors in adolescents born preterm. _Pediatrics_ 134, 1072 (2014). Article Google Scholar Download references FUNDING The study was supported by Finska
Läkaresällskapet (to N.K., S.S., and E.K.), Stiftelsen Dorothea Olivia, Karl Walter och Jarl Walter Perkléns minne (to N.K.), Yrjö Jahnsson Foundation (to N.K., J.K., and E.K.) Juho Vainio
Foundation (to N.K., J.K., and E.K.), Einar och Karin Stroems stiftelse för medicinsk forskning (to N.K. and S.S.), Academy of Finland (to E.K., K.H.P. grant numbers 335443, 314383, 272376,
266286), European Commission (733280 RECAP Research on Children and Adults Born Preterm to E.K.), Sigrid Juselius Foundation (to S.S., E.K., K.H.P.), Foundation for Pediatric Research (to
S.S. and E.K.), Signe and Ane Gyllenberg Foundation (to S.S., E.K., K.H.P.), Foundation for Cardiovascular Research (to E.K.), Diabetes Research Foundation (to E.K., K.H.P.), Novo Nordisk
Foundation (to E.K., K.H.P. grant numbers 335443, 314383, 272376, 266286), Jalmari and Rauha Ahokas Foundation (J.K.). Paulo Foundation (J.K.), Päivikki and Sakari Sohlberg Foundation
(J.K.), Doctoral Program in Clinical Research, University of Helsinki (J.K. and J.B.), Finnish Medical Foundation (to K.H.P.), Government Research Funds (K.H.P.), University of Helsinki
(K.H.P.). Open Access funding provided by Finnish Institute for Health and Welfare (THL). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Finnish Institute for Health and Welfare, Helsinki,
Finland Nina Kaseva, Juho Kuula, Samuel Sandboge, Helena Hauta-alus, Johan Björkqvist, Petteri Hovi, Terhi Vihervaara & Eero Kajantie * Department of Radiology, HUS Medical Imaging
Center, University of Helsinki and Helsinki University Hospital, Helsinki, Finland Juho Kuula * Psychology/Welfare Sciences, Faculty of Social Sciences, University of Tampere, Tampere,
Finland Samuel Sandboge * Children’s Hospital, and Pediatric Research Center, University of Helsinki and Helsinki University Hospital, Helsinki, Finland Helena Hauta-alus, Petteri Hovi &
Eero Kajantie * PEDEGO Research Unit, University of Oulu, Oulu, Finland Helena Hauta-alus & Eero Kajantie * Research Program for Clinical and Molecular Metabolism (CAMM), Faculty of
Medicine, University of Helsinki, Helsinki, Finland Helena Hauta-alus * Department of General Practice and Primary Health Care, University of Helsinki and Helsinki University Hospital,
Helsinki, Finland Johan G. Eriksson * Folkhälsan Research Center, Helsinki, Finland Johan G. Eriksson * Department of Obstetrics and Gynecology and Human Translational Research Programme,
Yong Loo Lin School of Medicine, National University of Singapore, Singapore, Singapore Johan G. Eriksson * Singapore Institute for Clinical Sciences (SICS), Agency for Science, Technology
and Research (A∗STAR), Singapore, Singapore Johan G. Eriksson * Obesity Research Unit, Research Program for Clinical and Molecular Metabolism, Faculty of Medicine, University of Helsinki,
Helsinki, Finland Kirsi H. Pietiläinen * Obesity Center, Abdominal Center, Helsinki University Hospital and University of Helsinki, Helsinki, Finland Kirsi H. Pietiläinen * Department of
Clinical and Molecular Medicine, Norwegian University of Science and Technology, Trondheim, Norway Eero Kajantie Authors * Nina Kaseva View author publications You can also search for this
author inPubMed Google Scholar * Juho Kuula View author publications You can also search for this author inPubMed Google Scholar * Samuel Sandboge View author publications You can also
search for this author inPubMed Google Scholar * Helena Hauta-alus View author publications You can also search for this author inPubMed Google Scholar * Johan Björkqvist View author
publications You can also search for this author inPubMed Google Scholar * Petteri Hovi View author publications You can also search for this author inPubMed Google Scholar * Johan G.
Eriksson View author publications You can also search for this author inPubMed Google Scholar * Terhi Vihervaara View author publications You can also search for this author inPubMed Google
Scholar * Kirsi H. Pietiläinen View author publications You can also search for this author inPubMed Google Scholar * Eero Kajantie View author publications You can also search for this
author inPubMed Google Scholar CONTRIBUTIONS N.K. participated in data cleaning, carried out data analyses, drafted the initial manuscript, and finalized the manuscript. J.K. recruited the
participants alongside a research nurse, was the research physician for the study visits, collected and cleaned data, and reviewed and revised the manuscript. S.S. and H.H. participated in
data cleaning and reviewed and revised the manuscript. J.B. collected data, participated in data cleaning and reviewed and revised the manuscript. P.H. conceptualized and designed the study,
and critically reviewed the manuscript for important intellectual content. J.G.E. conceptualized and designed the study, and critically reviewed the manuscript for important intellectual
content. T.V. designed and carried out laboratory analyses and reviewed and revised the manuscript. K.H.P. conceptualized and designed the study, and critically reviewed the manuscript for
important intellectual content. E.K. conceptualized and designed the study, coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual
content. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work. CORRESPONDING AUTHOR Correspondence to Nina Kaseva. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. INFORMED CONSENT Written informed consent was required and provided by all participants. ADDITIONAL INFORMATION
PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION SUPPLEMENTARY TABLES RIGHTS
AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in
any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The
images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not
included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly
from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Kaseva, N., Kuula,
J., Sandboge, S. _et al._ Cardiometabolic health in adults born with very low birth weight—a sibling study. _Pediatr Res_ 95, 316–324 (2024). https://doi.org/10.1038/s41390-023-02828-3
Download citation * Received: 02 June 2023 * Revised: 01 September 2023 * Accepted: 05 September 2023 * Published: 27 September 2023 * Issue Date: January 2024 * DOI:
https://doi.org/10.1038/s41390-023-02828-3 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not
currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative




:max_bytes(150000):strip_icc():focal(1064x349:1066x351)/backstreet-boys-nsync-1-675098ba87d84674ad2f62dbf2b919b0.jpg)



