- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Acute cholangitis is an ominous complication in biliary atresia (BA) patients. We investigated the prevalence of small intestine bacterial overgrowth (SIBO) in BA
patients and its role in predicting acute cholangitis. METHODS There are 69 BA patients with native liver recruited into this study prospectively. They received hydrogen and methane-based
breath testing (HMBT) to detect SIBO after recruitment and were followed prospectively in our institute. RESULTS There are 16 (23.19%) subjects detected to have SIBO by HMBT. BA subjects
with SIBO were noted to have higher serum alanine aminotransferase levels than others without SIBO (_P_ = 0.03). The risk of acute cholangitis is significantly higher in BA patients with
SIBO than in others without SIBO (62.50% vs. 15.09%, _P_ < 0.001). The logistic regression analysis demonstrated that BA subjects with SIBO have a higher risk of acute cholangitis than
others without SIBO (odds ratio = 9.38, _P_ = 0.001). Cox’s proportional hazard analysis further confirmed the phenomena in survival analysis (hazard ratio = 6.43, _P_ < 0.001).
CONCLUSIONS The prevalence of SIBO in BA patients is 23.19% in this study. The presence of SIBO is associated with the occurrence of acute cholangitis in BA patients. IMPACT * What is the
key message of your article? Acute cholangitis is common in BA, and is associated with SIBO after hepatoportoenterostomy in this study. * What does it add to the existing literature? This
study demonstrated that SIBO is common in BA after hepatoportoenterostomy, and is predictive of acute cholangitis and elevated serum ALT levels in BA. * What is the impact? This prospective
cohort study provides data regarding the significance of SIBO on the risk of acute cholangitis in BA patients. You have full access to this article via your institution. Download PDF SIMILAR
CONTENT BEING VIEWED BY OTHERS BACTERIAL BILE DUCT COLONIZATION IN PERIHILAR CHOLANGIOCARCINOMA AND ITS CLINICAL SIGNIFICANCE Article Open access 03 February 2021 BIOMARKERS FOR THE
DIAGNOSIS AND POST-KASAI PORTOENTEROSTOMY PROGNOSIS OF BILIARY ATRESIA: A SYSTEMATIC REVIEW AND META-ANALYSIS Article Open access 03 June 2021 BILE ACID PROFILES IN ADULT PATIENTS WITH
BILIARY ATRESIA WHO ACHIEVE NATIVE LIVER SURVIVAL AFTER PORTOENTEROSTOMY Article Open access 30 January 2024 INTRODUCTION Biliary atresia (BA), a progressive fibro-inflammatory
cholangiopathy, is a severe cholestatic liver disease in early infancy. BA is currently the leading cause of chronic liver insufficiency in children and the major indication for pediatric
liver transplants in the world.1,2,3 Early hepatoportoenterostomy is the gold standard of treatment to restore bile flow and improve native liver survival.4,5,6,7 However, around 30% of BA
patients suffered from acute cholangitis after hepatoportoenterostomy.8,9,10 BA patients with recurrent cholangitis usually have a greater risk of persistent cholestasis, liver cirrhosis,
chronic liver insufficiency, portal hypertension, esophageal and gastric varices, and liver transplantation in the future.9 Among the mechanisms for postoperative cholangitis, ascending
infection through the bilioenteric conduit has been widely accepted, but the precise mechanism of remains unclear.9,11 Dysbiosis of the small bowel is a potential mechanism of the disease
progression and cholangitis of BA patients, but the data remains controversial in published data.11,12,13,14 Small intestinal bacterial overgrowth (SIBO), indicating excessive bacteria in
the small bowel, is a consequence of the failure of intestinal clearance of bacteria, which may be due to dysmotility, immune deficit, or altered anatomy.15,16 Small bowel aspirate and
culture is the gold standard of the diagnostic test to confirm SIBO, but it is invasive.15 Hydrogen and methane-based breath test (HMBT) is regarded as a non-invasive alternative for the
diagnosis of SIBO in current practice guidelines and expert opinions.16,17,18 An elevation in hydrogen ≥20 ppm within 90 min or methane ≥10 ppm of glucose or lactulose HMBT was considered
positive for SIBO.17 The presence of SIBO has been reported to be associated with various kinds of liver diseases, including liver cirrhosis, spontaneous bacterial peritonitis, and
nonalcoholic steatohepatitis.19,20,21,22 The relationship between SIBO and the disease progression in BA children remains unclear at large. We aimed to elucidate the relationship between
SIBO, biliary cirrhosis, and the risk of acute cholangitis in BA patients in this study. METHODS STUDY DESIGN AND INCLUSION CRITERIA In this prospectively designed study, we recruited and
followed 72 BA patients (35 males and 37 females) consecutively from the Department of Pediatrics of National Taiwan University Hospital (NTUH) between December 2019 and October 2022 in the
outpatient clinic. Three BA children (2.5, 2.6 and 3 years of age) failed to complete the HBMT, and were not included in this analysis. Finally, 69 BA patients (33 males and 36 females) were
recruited in this analysis. The diagnosis of BA was confirmed by intraoperative cholangiography in all subjects, all of whom underwent hepatoportoenterostomy. Regular blood tests for
hemogram, biochemical parameters (total and direct bilirubin, alanine aminotransferase [ALT], and gamma-glutamyl transferase [GGT]), transient elastography, and abdominal ultrasounds were
performed during the follow-up after operation every 3–6 months. The majority of our study subjects (_n_ = 44, 63.77%) had a previous medical history of acute cholangitis after
hepatoportoenterostomy, and 23 (52.27%, 6 with neomycin and another 17 with sulfamethoxazole + trimethoprim) of them received prophylactic antibiotics after the previous event of
cholangitis. The diagnosis of acute cholangitis after the hepatoportoenterostomy operation was made based on clinical and laboratory findings (including fever with elevated serum
GGT/bilirubin/ALT levels, elevated serum C reactive protein, tea-colored urine, clay-colored stool with/without the presence of bacteremia) in this study. In our institute, we prescribed
prophylactic antibiotics (neomycin or sulfamethoxazole + trimethoprim) in BA patients for 2 years after their acute cholangitis events. Acute cholangitis events that occurred after HMBT in
the study cohort were recorded prospectively for statistical analysis. The inclusion criteria of this study are confirmed diagnosis of BA, signed approved informed consent, regular follow-up
in our clinics, and ability to perform the HMBT. Subjects with a liver transplant, unable to perform the HMBT, irregular follow-up, unwilling to participate in the study, cholangitis within
3 months, or under systemic intravenous antibiotics were excluded from this study. LIVER FIBROSIS MEASUREMENT BY TRANSIENT ELASTOGRAPHY We assessed liver fibrosis measurement (LSM) by using
transient elastography (Fibroscan® 502 Touch, Echosens, Paris, France) for the BA cohort during the study period. We used the S probe and M probe for LSM assessment in this study. Ten shots
within 5 min were performed on each study subject. A median over interquartile range (IQR) LSM value less than 30% was defined as a valid test. The LSM data within 1 month of HMBT was
applied for analysis. The transient elastography evaluation was done by WJF with the same instrument. The LSM >16 kPa after hepatoportoenterostomy was considered to be associated with
METAVIR F4 liver fibrosis and cholestatic complications, according to previous reports.23,24 HYDROGEN AND METHANE-BASED BREATH TESTING (HMBT) We performed the HMBT based on the latest North
American Consensus.17 All BA subjects were asked to do nothing per os at least 8 h before HMBT. The dose of lactulose was 10 g for HMBT, and then breath samples were collected at 15-min
intervals for 150 min. The GastroCH4ECK Gastrolyzer system (Bedfont Scientific Ltd., Salzburg, Austria) was applied for the analysis of hydrogen and methane in this study. The criteria of
SIBO was an elevation in hydrogen ≥20 ppm within 90 min or methane ≥10 ppm of HMBT.17 STATISTICAL ANALYSIS MedCalc (version 20.118; MedCalc Software, Ostend, Belgium) and STATA (version 17;
StataCorp, College Station, TX) software packages were applied for statistical analyses. The primary outcome of this study was the prevalence of SIBO in BA patients and its’ impacts on the
risk of acute cholangitis that occurred after HMBT. We applied the nonparametric Mann–Whitney _U_ test to assess the differences in medians and IQR for continuous variables between the two
groups. For the analysis of categorical variables, Fisher’s exact test or the _χ_2 test was performed to assess the differences in incidence between the two groups. Logistic regression was
used to assess the odds ratio (OR) and 95% confidence interval (95% CI) for predicting acute cholangitis in BA patients. The Kaplan–Meier plot, Cox’s proportional hazard analysis, and the
log-rank test were used for the survival analysis of the occurrence of acute cholangitis after HMBT. A _P_ value <0.05 was considered statistically significant in this analysis. RESULTS
GENERAL CHARACTERISTICS OF THE STUDY SUBJECTS A total of 69 BA patients (33 males and 36 females) were recruited into this prospective study at the median age of 9.35 years (IQR, 4.27–19.93
years of age). They received hepatoportoenterostomy surgery at the median age of 47 days (IQR, 36–69 days of age). Of them, 44 (63.77%) had a history of acute cholangitis before HMBT, 12
(17.39%) of them with serum total bilirubin ≧2 mg/dL, 8 (11.59%) with serum direct bilirubin >1 mg/dL, and 18 (26.09%) with LSM >16 kPa at the time of HMBT. Sixteen (23.19%) of the
study subjects were in preparation for liver transplantation due to hepatic encephalopathy, intractable esophageal varices bleeding, chronic liver insufficiency, spontaneous bacterial
peritonitis, and hepatopulmonary syndrome. Sixteen (23.19%) of them were diagnosed to have SIBO by HMBT examination. At the median follow-up time of 2.67 years, 18 (26.09%) of the whole
cohort have acute cholangitis events detected after HMBT (Table 1). There is no significant statistical difference in the age of HMBT between subjects with and without SIBO (Table 2).
Subjects with SIBO (_n_ = 16) were noted to have higher serum ALT levels, serum bile acid levels, and APRI scores than others without SIBO (_P_ = 0.03, 0.02, and 0.03, respectively). The
prevalence of acute cholangitis is significantly higher in BA subjects with SIBO than in others without SIBO (62.50% vs. 15.09%, _P_ < 0.001). The presence of SIBO is significantly
correlated with the occurrence of acute cholangitis (correlation coefficient = 0.47, _P_ < 0.001). There is no obvious correlation between SIBO, the use of prophylactic antibiotics, bile
lake, previous history of cholangitis, and other cholestatic complications (including hepatic encephalopathy, esophageal varices, ascites, spontaneous bacterial peritonitis, and
hepatopulmonary syndrome) in this cohort. PREDICTORS OF ACUTE CHOLANGITIS IN BA PATIENTS In the whole cohort (_n_ = 69), the logistic regression analysis demonstrated that BA subjects with
SIBO have a higher risk of acute cholangitis than others without SIBO (OR = 9.38, _P_ = 0.001) during follow-up, and the significance persisted in the multivariate model (OR = 10.25, _P_ =
0.001, Table 3). In the sub-cohort without prophylactic antibiotics (_n_ = 46), we further confirmed the significance of SIBO for the prediction of acute cholangitis (OR = 14.47 and 15.13,
_P_ = 0.002 and 0.002, respectively, in univariate and multivariate models, Table 3). The Kaplan–Meier plot with the log-rank test in survival analysis in the whole cohort demonstrated that
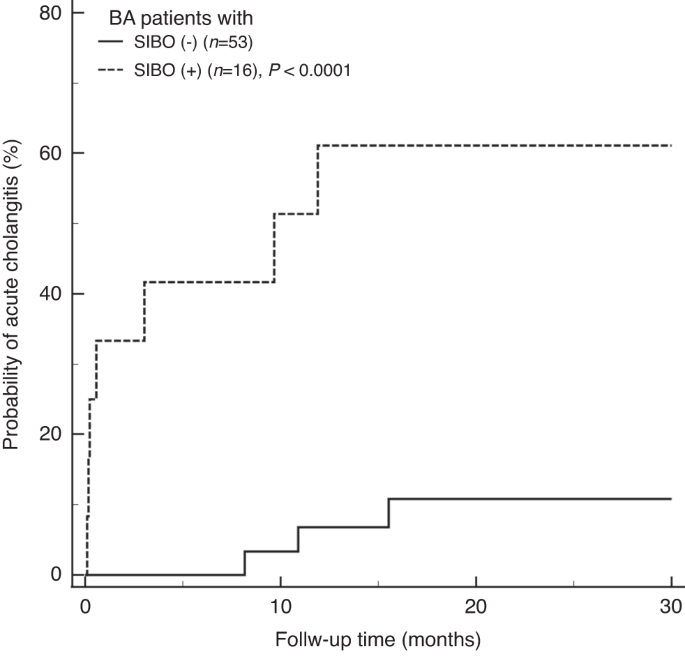
the presence of SIBO is associated with the occurrence of acute cholangitis (_P_ < 0.001, Fig. 1). The Cox’s proportional hazard analysis further showed a high hazard ratio (HR) of SIBO
for the prediction of acute cholangitis (HR = 6.43, _P_ < 0.001), and the prediction persisted after adjusting the sex, usage of prophylactic antibiotics, previous history of cholangitis,
presence of bile lake, and liver cirrhosis status in multivariate Cox’s model (HR = 5.98, _P_ < 0.001) (Table 4). In the sub-cohort without the treatment of prophylactic antibiotics (_n_
= 46), the Kaplan–Meier plot with the log-rank test further confirmed the presence of SIBO is predictive of acute cholangitis (_P_ < 0.001, Fig. 2). The Cox’s proportional hazard
analysis also demonstrated the significance of SIBO for the prediction of acute cholangitis (HR = 10.50, _P_ = 0.001), and the prediction persisted after adjusting the sex, usage of
prophylactic antibiotics, previous history of cholangitis, presence of bile lake, and liver cirrhosis status in multivariate Cox’s model (HR = 9.28, _P_ = 0.002) (Table 4). DISCUSSION
Ascending cholangitis is believed as a mechanism of acute cholangitis in BA after hepatoportoenterostomy, and the presence of SIBO is logical to predispose the ascending of bacterial
pathogens through the hepatoportoenterostomy. Our novel study demonstrated that the prevalence of SIBO in our BA cohort is 23.19%, while 63.77% of our cohort had experience cholangitis
before enrollment. The presence of SIBO in BA patients is associated with higher serum ALT and bile acid levels and is also predictive of the risk of acute cholangitis in both logistic
regression and survival analysis at the median follow-up time of 2.67 years. The significance of SIBO in the prediction of acute cholangitis persisted after adjusting the sex, usage of
prophylactic antibiotics, presence of bile lake, previous history of cholangitis and liver cirrhosis in this study. In the sub-cohort without prophylactic antibiotics, the significance of
SIBO in predicting acute cholangitis persisted. A recent smaller retrospective cohort in Japan (_n_ = 14) reported that the prevalence of SIBO in BA patients is 28.57% (4/14), which further
supports our observation in a larger prospective BA cohort in Taiwan (_n_ = 69).12 The prevalence of SIBO in BA patients is not statistically different between the Japanese cohort and our
cohort (_P_ = 0.73, Fisher’s exact test). The prevalence of SIBO in BA in our cohort (23.19%) and Japanese cohort (28.57%) are both significantly higher than the previous reports in healthy
children (2.4–9.3%).25,26,27 The presence of SIBO is associated with higher serum ALT and bile acid levels in our study. The presence of high SIBO prevalence in BA may be associated with
insufficient bile flow and altered gastrointestinal anatomy after hepatoportoenterostomy. Elevated serum ALT and bile acid levels are both correlated with SIBO in our study population. The
relationship between SIBO and elevated liver aminotransferase is also observed in patients with nonalcoholic steatohepatitis.21,22,28 There is increasing evidence that SIBO increases
intestine permeability and bacterial translocation, alters the gut-liver axis hormones, and results in a systemic inflammatory state.21,22,28 The liver exposed to bacterial endotoxins and
lipopolysaccharide, may trigger inflammatory responses in the liver and possibly result in the elevation of serum ALT levels.29 The phenomena are also consistent with frequent bacterial
translocation, bacteremia, and endotoxemia observed in BA patients during acute cholangitis. The observation is very important, which implies the potential diagnostic role of HMBT and the
therapeutic benefit of eradicating SIBO in BA patients. We also demonstrated the correlation between SIBO and serum bile acid levels in this study. The elevation of serum bile acid in
cholestatic patients indicates the impairment of bile flow through the biliary tree.30 The previous studies reported the relationship between SIBO and the lack of gut bile acid is supportive
of our observation.14,31,32 Our prospective designed study demonstrated a significant correlation between SIBO and acute cholangitis in BA patients. The novel finding highlighted the
significant role of SIBO in the pathogenesis of acute cholangitis in BA patients. Since SIBO is reported to result in a leaky gut, increased gut permeability, and the chance of bacteria and
endotoxins translocation into the portal venous blood flow, and is logical to result in an increased risk of acute cholangitis in BA.28,29 Hence, the identification of SIBO in BA patients is
of potential benefit in the precision care of BA patients, guiding physicians to modify the disease management and improve the native liver survival of BA patients. Several limitations
existed in this study. First, the sample size of the current study to perform HMBT in BA patients with the native liver is limited but is the largest one in reported data to date. This is
because part of our BA patients received a liver transplant due to liver insufficiency and other cholestatic complications before the start of this study. And some of our young BA children
are unable to perform HMBT by the GastroCH4ECK Gastrolyzer system. The significant results of the current study indicate adequate static sample size and statistical power for the analysis
between SIBO and acute cholangitis. Second, previous studies demonstrated the relationship between hepatitis-related liver cirrhosis and SIBO in the adult population, but the correlation is
insignificant in our study cohort with biliary cirrhosis.19,20 Our study only demonstrated the relationship between APRI score and SIBO, but not the LSM assessed by the transient
elastography. Third, our study showed no correlation between the presence of bile lakes and cholangitis in our cohort (Table 3). The lack of significance may result from the small sample
size and inadequate statistical power in our study. Fourth, due to the lack of gold standard for the diagnosis of cholangitis in BA patients, the diagnosis was made based on clinical and
laboratory findings in this study. Hence, larger-scale studies or the application of serum fibrosis biomarkers may help to further elucidate the relationship. In conclusion, we report a high
prevalence of SIBO in BA patients and the significant role of SIBO to correlate with acute cholangitis in this novel prospective study. HMBT for the diagnosis of SIBO in BA patients and the
eradication of SIBO with effective antibiotics are of clinical significance. DATA AVAILABILITY Data are available on request and the approval of the local ethic committee. REFERENCES *
Hartley, J. L., Davenport, M. & Kelly, D. A. Biliary atresia. _Lancet_ 374, 1704–1713 (2009). Article PubMed Google Scholar * Hoofnagle, J. H. Biliary Atresia Research Consortium
(BARC). _Hepatology_ 39, 891 (2004). Article PubMed Google Scholar * Sokol, R. J. et al. Screening and outcomes in biliary atresia: summary of a National Institutes of Health workshop.
_Hepatology_ 46, 566–581 (2007). Article CAS PubMed Google Scholar * Venkat, V. et al. Modeling outcomes in children with biliary atresia with native liver after 2 years of age.
_Hepatol. Commun._ 4, 1824–1834 (2020). Article CAS PubMed PubMed Central Google Scholar * Lien, T. H. et al. Effects of the infant stool color card screening program on 5-year outcome
of biliary atresia in Taiwan. _Hepatology_ 53, 202–208 (2011). Article PubMed Google Scholar * Sokol, R. J., Mack, C., Narkewicz, M. R. & Karrer, F. M. Pathogenesis and outcome of
biliary atresia: current concepts. _J. Pediatr. Gastroenterol. Nutr._ 37, 4–21 (2003). PubMed Google Scholar * Ohi, R. Biliary atresia. A surgical perspective. _Clin. Liver Dis._ 4,
779–804 (2000). Article CAS PubMed Google Scholar * Oh, M., Hobeldin, M., Chen, T., Thomas, D. W. & Atkinson, J. B. The Kasai procedure in the treatment of biliary atresia. _J.
Pediatr. Surg._ 30, 1077–1080 (1995). Article CAS PubMed Google Scholar * Chung, P. H. Y. et al. Life long follow up and management strategies of patients living with native livers after
Kasai portoenterostomy. _Sci. Rep._ 11, 11207 (2021). Article CAS PubMed PubMed Central Google Scholar * Pietrobattista, A. et al. Does the treatment after Kasai procedure influence
biliary atresia outcome and native liver survival? _J. Pediatr. Gastroenterol. Nutr._ 71, 446–451 (2020). Article CAS PubMed Google Scholar * Jain, V., Alexander, E. C., Burford, C.,
Verma, A. & Dhawan, A. Gut Microbiome: a potential modifiable risk factor in biliary atresia. _J. Pediatr. Gastroenterol. Nutr._ 72, 184–193 (2021). Article CAS PubMed Google Scholar
* Sakamoto, S., Fukahori, S., Hashizume, N. & Yagi, M. Measuring small intestinal bacterial overgrowth using the hydrogen breath test among postoperative patients with biliary atresia.
_Asian J. Surg._ 43, 1130–1131 (2020). Article PubMed Google Scholar * Hitch, D. C. & Lilly, J. R. Identification, quantification, and significance of bacterial growth within the
biliary tract after Kasai’s operation. _J. Pediatr. Surg._ 13, 563–569 (1978). Article CAS PubMed Google Scholar * Yamamoto, T., Hamanaka, Y. & Suzuki, T. Bile acids and
microorganisms in the jejunal lumen after biliary reconstruction in dogs. _J. Am. Coll. Surg._ 181, 525–529 (1995). CAS PubMed Google Scholar * Rao, S. S. C. & Bhagatwala, J. Small
intestinal bacterial overgrowth: clinical features and therapeutic management. _Clin. Transl. Gastroenterol._ 10, e00078 (2019). Article PubMed PubMed Central Google Scholar * Quigley,
E. M. M., Murray, J. A. & Pimentel, M. AGA clinical practice update on small intestinal bacterial overgrowth: expert review. _Gastroenterology_ 159, 1526–1532 (2020). Article CAS
PubMed Google Scholar * Rezaie, A. et al. Hydrogen and methane-based breath testing in gastrointestinal disorders: The North American Consensus. _Am. J. Gastroenterol._ 112, 775–784
(2017). Article CAS PubMed PubMed Central Google Scholar * Ghoshal, U. C. How to interpret hydrogen breath tests. _J. Neurogastroenterol. Motil._ 17, 312–317 (2011). Article PubMed
PubMed Central Google Scholar * Pande, C., Kumar, A. & Sarin, S. K. Small-intestinal bacterial overgrowth in cirrhosis is related to the severity of liver disease. _Aliment Pharm.
Ther._ 29, 1273–1281 (2009). Article CAS Google Scholar * Lakshmi, C. P. et al. Frequency and factors associated with small intestinal bacterial overgrowth in patients with cirrhosis of
the liver and extra hepatic portal venous obstruction. _Dig. Dis. Sci._ 55, 1142–1148 (2010). Article CAS PubMed Google Scholar * Abu-Shanab, A. & Quigley, E. M. The role of the gut
microbiota in nonalcoholic fatty liver disease. _Nat. Rev. Gastroenterol. Hepatol._ 7, 691–701 (2010). Article PubMed Google Scholar * Quigley, E. M., Abu-Shanab, A., Murphy, E. F.,
Stanton, C. & Monsour, H. P. Jr. The metabolic role of the microbiome: implications for NAFLD and the metabolic syndrome. _Semin Liver Dis._ 36, 312–316 (2016). Article CAS PubMed
Google Scholar * Wu, J. F. et al. Transient elastography is useful in diagnosing biliary atresia and predicting prognosis after hepatoportoenterostomy. _Hepatology_ 68, 616–624 (2018).
Article PubMed Google Scholar * Shen, Q. L. et al. Assessment of liver fibrosis by Fibroscan as compared to liver biopsy in biliary atresia. _World J. Gastroenterol._ 21, 6931–6936
(2015). Article PubMed PubMed Central Google Scholar * Belei, O., Olariu, L., Dobrescu, A., Marcovici, T. & Marginean, O. The relationship between non-alcoholic fatty liver disease
and small intestinal bacterial overgrowth among overweight and obese children and adolescents. _J. Pediatr. Endocrinol. Metab._ 30, 1161–1168 (2017). Article CAS PubMed Google Scholar *
Cares, K. et al. Risk of small intestinal bacterial overgrowth with chronic use of proton pump inhibitors in children. _Eur. J. Gastroenterol. Hepatol._ 29, 396–399 (2017). Article CAS
PubMed Google Scholar * Wang, L. et al. Hydrogen breath test to detect small intestinal bacterial overgrowth: a prevalence case–control study in autism. _Eur. Child Adolesc. Psychiatry_
27, 233–240 (2018). Article PubMed Google Scholar * Augustyn, M., Grys, I. & Kukla, M. Small intestinal bacterial overgrowth and nonalcoholic fatty liver disease. _Clin. Exp.
Hepatol._ 5, 1–10 (2019). Article PubMed PubMed Central Google Scholar * Ferolla, S. M., Armiliato, G. N. A., Couto, G. A. & Ferrari, T. C. A. The role of intestinal bacteria
overgrowth in obesity-related nonalcoholic fatty liver disease. _Nutrients_ 6, 5583–5599 (2014). Article CAS PubMed PubMed Central Google Scholar * Wu, J. F. et al. Serum bile acid
levels assist the prediction of biliary stricture and survival after liver transplantation in children. _Eur. J. Pediatr._ 180, 2539–2547 (2021). Article CAS PubMed Google Scholar *
Ramírez-Pérez, O., Cruz-Ramón, V., Chinchilla-López, P. & Méndez-Sánchez, N. The role of the gut microbiota in bile acid metabolism. _Ann. Hepatol._ 16, S21–S26 (2017). Article PubMed
Google Scholar * Larsen, H. M. et al. Chronic loose stools following right-sided hemicolectomy for colon cancer and the association with bile acid malabsorption and small intestinal
bacterial overgrowth. _Colorectal Dis._ 25, 600–607 (2023). Article CAS PubMed Google Scholar Download references FUNDING This work is supported by grants from the National Taiwan
University Hospital (NTUH.110-T05, NTUH.111-T0009, NTUH. 112-E0009). The funding agencies had no role in the design, and conduct of the study; in the collection, analysis and interpretation
of the data; or in the preparation, review, or approval of the manuscript. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatrics, National Taiwan University Hospital,
Taipei, Taiwan Jia-Feng Wu, Hsiu-Hao Chang, Che-Ming Chiang & Mei-Hwei Chang * Department of Internal Medicine, National Taiwan University Hospital, Taipei, Taiwan Ping-Huei Tseng *
Department of Pediatrics, Tri‐Service General Hospital, National Defense Medical Center, Taipei, Taiwan Che-Ming Chiang * Department of Surgery, National Taiwan University Hospital, Taipei,
Taiwan Wen-Hsi Lin & Wen-Ming Hsu Authors * Jia-Feng Wu View author publications You can also search for this author inPubMed Google Scholar * Ping-Huei Tseng View author publications
You can also search for this author inPubMed Google Scholar * Hsiu-Hao Chang View author publications You can also search for this author inPubMed Google Scholar * Che-Ming Chiang View
author publications You can also search for this author inPubMed Google Scholar * Wen-Hsi Lin View author publications You can also search for this author inPubMed Google Scholar * Wen-Ming
Hsu View author publications You can also search for this author inPubMed Google Scholar * Mei-Hwei Chang View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS J.-F.W., the first author of the study, is responsible for the study design, data management, and manuscript writing. P.-H.T., H.-H.C., C.-M.C., W.-H.L., W.-M.H., and M.-H.C.
are responsible for long-term patient follow, recruitment, and critical review of the manuscript. P.-H.T. is responsible for the study design, hydrogen breath test interpretation, and
critical review of the manuscript. M.-H.C., the corresponding author, is responsible for patient recruitment, study design, critical review of the article, and is the principal investigator
of this study. All authors have seen and approved the final version. The corresponding author had full access to all the data in this study and had final responsibility for the decision to
submit it for publication. CORRESPONDING AUTHOR Correspondence to Mei-Hwei Chang. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ETHICS APPROVAL AND
CONSENT TO PARTICIPATE The study protocol was approved by the institutional review board of National Taiwan University Hospital. All participants or their parents signed the informed consent
approved by the institutional review board. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional
affiliations. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s)
or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints
and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Wu, JF., Tseng, PH., Chang, HH. _et al._ The prevalence and impact of small intestine bacterial overgrowth in biliary atresia patients.
_Pediatr Res_ 95, 302–307 (2024). https://doi.org/10.1038/s41390-023-02818-5 Download citation * Received: 25 April 2023 * Revised: 04 August 2023 * Accepted: 30 August 2023 * Published: 19
September 2023 * Issue Date: January 2024 * DOI: https://doi.org/10.1038/s41390-023-02818-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative