- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT While pregnancy post-bariatric surgery has become increasingly common, little is known about whether and how maternal bariatric surgery affects the next generation. This scoping
review aimed to collate available evidence about the long-term health of offspring following maternal bariatric surgery. A literature search was conducted using three databases (PubMed,
PsycINFO, EMBASE) to obtain relevant human and animal studies. A total of 26 studies were included: 17 were ancillary reports from five “primary” studies (three human, two animal studies)
and the remaining nine were “independent” studies (eight human, one animal studies). The human studies adopted sibling-comparison, case-control, and single-group descriptive designs. Despite
limited data and inconsistent results across studies, findings suggested that maternal bariatric surgery appeared to (1) modify epigenetics (especially genes involved in immune, glucose,
and obesity regulation); (2) alter weight status (unclear direction of alteration); (3) impair cardiometabolic, immune, inflammatory, and appetite regulation markers (primarily based on
animal studies); and (4) not affect the neurodevelopment in offspring. In conclusion, this review supports that maternal bariatric surgery has an effect on the health of offspring. However,
the scarcity of studies and heterogenous findings highlight that more research is required to determine the scope and degree of such effects. IMPACT * There is evidence that bariatric
surgery modifies epigenetics in offspring, especially genes involved in immune, glucose, and obesity regulation. * Bariatric surgery appears to alter weight status in offspring, although the
direction of alteration is unclear. * There is preliminary evidence that bariatric surgery impairs offspring’s cardiometabolic, immune, inflammatory, and appetite regulation markers.
Therefore, extra care may be needed to ensure optimal growth in children born to mothers with previous bariatric surgery. You have full access to this article via your institution. Download
PDF SIMILAR CONTENT BEING VIEWED BY OTHERS MATERNAL ADIPOSITY AND PERINATAL AND OFFSPRING OUTCOMES: AN UMBRELLA REVIEW Article 11 October 2024 EVIDENCE FOR MATERNAL DIET-MEDIATED EFFECTS ON
THE OFFSPRING MICROBIOME AND IMMUNITY: IMPLICATIONS FOR PUBLIC HEALTH INITIATIVES Article 12 September 2020 MATERNAL OBESITY AND OFFSPRING CARDIOVASCULAR REMODELLING — THE EFFECT OF
PRECONCEPTION AND ANTENATAL LIFESTYLE INTERVENTIONS: A SYSTEMATIC REVIEW Article Open access 19 June 2024 INTRODUCTION Nearly 9% of US women enter pregnancy with severe obesity (body mass
index [BMI] ≥40 kg/m2).1 Maternal obesity is a well-established early life exposure that increases the risk for obstetrical complications during pregnancy, worsens birth outcomes, and
predisposes offspring to lifelong cardiometabolic, mental, and neurodevelopmental disorders.2 More concerningly, the degree of obesity progressively raises those risks in a dose-dependent
manner.3,4,5 To prevent or mitigate the deleterious consequences of severe obesity, women of reproductive age increasingly seek bariatric surgery—the most effective treatment for severe
obesity that results in significant and durable weight loss and comorbidity remission.6 Since 2015, more than 1.3 million bariatric operations have been performed in the US, and 40–50% were
in women of reproductive age.7,8 The weight loss, improved metabolic homeostasis, and restored hormonal balance resultant from bariatric surgery boost women’s reproductive efficiency, making
pregnancy post-surgery increasingly common. According to the most recent epidemiological data, the number of pregnancies after bariatric surgery in the US trended upward in recent years,
accounting for 0.3% of all pregnancies in 2016 and 0.5% in 2019.9 The expanded population of women who conceive following bariatric surgery has ignited interest in understanding whether and
how the surgery affects a subsequent pregnancy. Evidence is clear that preconception bariatric surgery brings both benefits and risks to mothers and neonates during the perinatal period
(e.g., reduced incidence of obstetrical complications, increased risk of small-for-gestational-age infants). However, a major gap remains in the transgenerational implications of maternal
bariatric surgery on the health of offspring in the long-term. Bariatric surgery in women before pregnancy theoretically produces lasting impacts on offspring through multiple mediators,
including the intrauterine environment, methods of delivery, birth outcomes, breastfeeding behaviors, and food-related parenting practices, all of which play a critical role in child growth.
In terms of the intrauterine environment, on the one hand, bariatric surgery reverses obesity-induced disruptions in neuroendocrine, inflammatory, and microbiota homeostasis in
mothers,10,11 exerting a positive programming effect on the fetus. Yet, on the other hand, the surgery increases the risk of maternal malnutrition, alters specific hormone expression and
signaling, and impairs placental function,12 which may collectively compromise fetal growth. Regarding methods of delivery, despite mixed evidence, there have been reports that women with
bariatric surgery were more likely to have cesarean delivery compared to those without bariatric surgery.13,14 For birth outcomes, while it is known that maternal bariatric surgery reduces
the incidence of large-for-gestational-age infants, the risk rises parallelly for intrauterine growth restriction and small-for-gestational infants,15,16 which would presumably influence the
growth trajectory in offspring. Concerning breastfeeding behaviors, a few human studies have shown that women with a history of bariatric surgery were less likely to initiate breastfeeding
and had lower median breastfeeding time than non-surgical counterparts with overweight/obesity or normal weight.17,18 Finally, patients who have undergone bariatric surgery often experience
certain eating restrictions (e.g., food intolerances) and are required to make profound eating modifications (e.g., high demand for protein intake). Those changes can potentially render
ripple effects on the home food environment and parenting practices and, subsequently, shaping the lifestyles and health of children living in the same household. Despite the theoretical
links, whether and how the various beneficial and detrimental effects derived from maternal bariatric surgery extend to the next generation, especially beyond the perinatal period, remains
poorly understood. Accordingly, the aim of this scoping review was to identify and map available literature characterizing the long-term health profiles of offspring born to mothers with
preconception bariatric surgery. METHODS STUDY DESIGN This study is a scoping review that followed the framework established by Arksey and O’Malley.19 The rationale for choosing a scoping
review instead of a systematic review is that the research on “long-term health of offspring following maternal bariatric surgery” is only recently emerging. The limited number and
heterogeneous nature of literature make it infeasible to formulate and answer a focused research question. Rather, a scoping review allows the embracement of diverse study designs with more
expansive inclusion criteria, which is particularly suitable for examining the potential size and scope of available literature pertaining to an emerging topic.20 Since this is a review
paper, approval from an Institutional Review Board is not required. INCLUSION/EXCLUSION CRITERIA Both human and animal studies were included. Studies that (1) described the long-term health
of offspring born to mothers with a history of bariatric surgery; or (2) compared the long-term health of offspring born to mothers with and without a history of bariatric surgery were
eligible. “Long-term” refers to an extended period of time beyond infancy, defined as age >1 year in human studies and >postnatal day 14 (weaning) in studies using rat models.
Single-group descriptive studies, case-control studies, case reports/series, and intervention studies were considered without limitation on the date of publication. Studies published in
non-English languages, and editorials, abstracts, book chapters, dissertation work, protocol papers, qualitative studies, and review papers were excluded. SEARCH STRATEGY The literature
search was conducted in November 2022 with three databases (PubMed, PsycINFO, and EMBASE) to obtain relevant studies. The search included the combination of the following keywords:
“children”, “adolescents”, “offspring”, “bariatric surgery”, “weight loss surgery”, “metabolic surgery”, “sleeve gastrectomy”, “gastric bypass”, “gastric banding”, and “biliopancreatic
diversion”. As an example, the search strategy for PubMed was (((children[tiab]) OR (adolescen*[tiab])) OR (offspring[tiab])) AND ((((((((bariatric surgery[tiab]) OR (sleeve
gastrectomy[tiab])) OR (gastric bypass[tiab])) OR (weight loss surgery[tiab])) OR (metabolic surgery[tiab])) OR (gastric banding[tiab])) OR (Biliopancreatic diversion[tiab])) OR (gastric
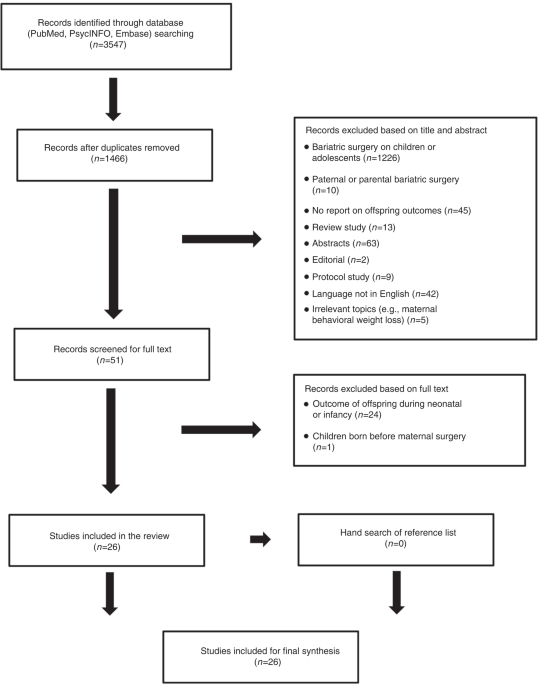
band[tiab])). The database search was complemented by a hand search of the reference lists obtained from the identified articles. The study selection flow is presented in Fig. 1. RESULTS
STUDY DESCRIPTION A total of 26 studies were included in the review, with 17 being ancillary reports21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 from five “primary” studies and nine
“independent” or “unrelated” studies38,39,40,41,42,43,44,45,46 (Table 1). Among the five “primary” studies (described below), there were two human sibling studies (seven ancillary
reports,28,29,30,31,35,36,37) one human case-control study (three ancillary reports,32,33,34) and two animal studies (seven ancillary reports.21,22,23,24,25,26,27) Among the nine
“independent” studies, there were eight human studies that adopted sibling-comparison,39,40 case-control,43,44 and retrospective or cross-sectional single-group designs,38,41,42,45 as well
as one animal study using rat model.46 Regarding offspring’s age groups, two “primary” (six ancillary reports32,33,34,35,36,37) and six “independent” human studies38,39,41,42,44,45 only
focused on childhood (1–12 years), one “independent” animal study46 on adulthood, and the remaining (one human “primary” [four ancillary reports,28,29,30,31] two animal “primary” [seven
ancillary reports,21,22,23,24,25,26,27] and two “independent” human studies40,43) entailed at least two developmental stages of childhood, adolescence, or early adulthood. DESCRIPTION OF THE
FIVE “PRIMARY” STUDIES A sibling study conducted in Canada (hereby referred to as the Canadian Sibling study)28,29,30,31 performed two surveys to compare the health of offspring born before
and after maternal biliopancreatic diversion. The first survey recruited all women who had given birth to children before (_n_ = 45) or after surgery (_n_ = 172) in a university-based
practice, and children’s weight patterns and neurodevelopment were reported (age range: 2 years to adolescence).28,29 A second survey was administered in a refined sample of mothers who had
given birth both before and after surgery, as well as additional age-matched mothers with children born before or after maternal surgery. In this later survey, weight status and metabolic
data were obtained from children born to mothers before (_n_ = 54, 16.0 ± 0.6 years) and after surgery (_n_ = 57, 10.7 ± 0.5 years).28 From the second survey cohort, a subset of 20 unrelated
mothers, along with 50 siblings born before and after surgery, were further extracted for epigenetic analysis.30,31 Another sibling study conducted in Sweden (hereby referred to as the
Swedish Sibling study)35,36,37 investigated the effects of maternal bariatric surgery on the weight patterns and epigenetics of offspring. Through linking several national registries, 164
children born before and 176 after maternal bariatric surgery were identified (including 71 sibling pairs), and their weight status at ages four, six, and ten were reported.36 A follow-up
analysis re-examined the weight status37 and characterized the methylation levels of genes associated with type 2 diabetes and obesity among 31 sibling peers.35 A case-control study
conducted in Belgium (hereby referred to as the Belgian case-control study)32,33,34 compared adiposity,33 neurodevelopment,33 and eating behaviors34 among three groups of children: 36 born
after maternal bariatric surgery (6.5 ± 1.3 years), 71 born to mothers with overweight/obesity (10.8 ± 0.3 years), and 36 born to mothers with a healthy weight (10.6 ± 0.2 years).
Additionally, in a subset of children, outcomes on endothelial function and a series of metabolic biomarkers were reported32 (_n_ = 26, 53, and 32 in the surgical, overweight/obesity, and
healthy weight groups, respectively). An animal study conducted in the US (hereby referred to as the US animal study)21,22,23,24 used a rat model of sleeve gastrectomy (SG) to examine the
impact of maternal SG on the physiology of offspring during childhood and adulthood. Female rats were placed on 4-week high-fat diets to induce obesity and were then divided into two groups
to receive SG or sham surgery. After a 5-week weight stabilization period, post-surgery female rats were mated with conventionally fed males, and pup litters were collected to assess
adiposity,24 immune,21 inflammation,21 leptin,22 and ghrelin functioning.23 Another animal study conducted in Brazil (hereby referred to as the Brazilian animal study)25,26,27 also adopted
rat models to evaluate the association between maternal Roux-en-Y Gastric Bypass (RYGB) and offspring outcomes. Following a cafeteria diet, female rats were submitted to RYGB or sham
surgery. The mating process began at five weeks post-surgery, and male offspring’s weight patterns,25,26 insulin,26 hepatic lipid metabolism,27 and brown adipose tissue thermogenesis25 were
assessed. The following sections qualitatively summarize the main findings from the 26 included papers, organized by different health outcomes that were examined. IMMUNE SYSTEM AND
INFLAMMATION The US animal study21 was the only study to examine the effects of maternal bariatric surgery on the immune system in offspring. Findings showed that at postnatal day 21,
offspring of SG vs sham dams had early deficits in certain immune cell populations, exemplified in reduced circulating CD45RA + B lymphocytes and reduced mRNA transcription for CD45 (a
marker for all hematopoietic cells) and Cd68 (a macrophage marker) in the spleen. Conversely, the immune cells in the neural tissue appeared to be stimulated as there was greater expression
of microglia markers in the hypothalamus. At postnatal day 60, most of those differences disappeared (except total and cytotoxic T-cell mRNA transcripts in the spleen were elevated in SG
relative to sham offspring), implying immune rebounds.21 Two human studies assessing inflammatory markers in the offspring of mothers with and without bariatric surgery failed to detect
significant differences. More specifically, the Canadian sibling study30 reported comparable C-reactive protein (CRP) levels in siblings born before and after maternal biliopancreatic
diversion (after adjustment for puberty and BMI percentile). The Belgian case-control study32 found no differences in high-sensitivity CRP levels among children born to mothers after
bariatric surgery, mothers with overweight/obesity, and mothers with a normal weight.32 In contrast to the human studies, the US animal study observed elevated expression of IL-1β (a
proinflammatory cytokine) in the hypothalamus and elevated mRNA expression of IL-1β in the spleen in SG compared with sham offspring, suggesting hyperactive inflammation in the central and
peripheral systems.21 EPIGENETICS Two human studies performed epigenetic analyses in siblings born pre- and post-maternal bariatric surgery, and both reported epigenetic variation within the
sibling peers. In the Canadian sibling study, the investigators applied DNA methylation, gene expression, and function and pathway analyses to quantify the impact of maternal
biliopancreatic diversion on epigenetic profiles in the offspring.30,31 Compared to children born before surgery, 3% of CpG probes were differently methylated in post-surgery siblings,
corresponding to biological functions related to disorders of glucose metabolism, pancreas disorders, autoimmune disease, and cardiovascular diseases.31 Further, genes involved in the type 1
diabetes signaling pathway were picked to validate the clinical implication of the methylation modification. The strong correlations between gene methylation levels, gene expression, and
plasma markers of insulin resistance (children born after surgery had improved insulin sensitivity)31 indicated that maternal bariatric surgery possibly modifies offspring’s cardiometabolic
health through epigenetic modification. Analogous to the Canadian sibling study, the Swedish sibling study35 evaluated the DNA methylation levels and the potentially altered gene functions
and pathways within 31 sibling peers born before and after bariatric surgery (e.g., gastric banding, gastric bypass). Distinct DNA methylation profiles were found between siblings,
especially in genes involved in type 2 diabetes, inflammation, and melanocyte signaling.35 WEIGHT STATUS Offspring’s weight status was examined in 13 studies, and the results were highly
mixed. Five studies (3 sibling-comparison, 1 cross-sectional, and 1 animal study) supported a lower weight status in offspring exposed to maternal bariatric surgery. In detail, several
ancillary reports28,29,30 from the Canadian sibling study documented lower mean BMI z-scores (boys only), lower waist circumference, and lower prevalence of overweight, obesity, and severe
obesity in children born after than those born before surgery. Concordantly, another two sibling studies39,40 similarly found a significantly decreased rate of overweight and obesity in
siblings born after vs before maternal bariatric surgery. Besides, a cross-sectional study conducted in Sweden41 also noted significantly lower body weights in 18-month boys born after
maternal RYGB compared to the national reference values. Finally, the Brazilian animal study reported that the offspring of RYGB dams had lower body weight and body weight gain throughout
life (at birth, weaning, and postnatal day 120) than offspring of sham dams.25,26 Five studies (1 sibling-comparison, 2 case-control, 1 cross-sectional, and 1 animal study) pointed in the
opposite direction. The Swedish sibling study36 documented a higher mean BMI in 10-year-old children (with a higher prevalence of obesity in girls) born after maternal surgery than those of
the same age born before surgery, after adjusting for important confounders such as maternal age. Two case-control studies,33,43 including one that used population-based design,43
demonstrated that children of mothers with a history of bariatric surgery had significantly higher body weight, BMI, excess fat percentage, waist circumference, and rates of obesity compared
to controls born to mothers with overweight/obesity or normal weight. Using the national value as a reference, a retrospective study in Italy42 reported a higher prevalence of overweight
(30.8% vs 22.4%) and obesity (23.1% vs 17%) in children born after maternal bariatric surgery (age: 19.0 years), although the statistical significance of the difference was not presented.
Lastly, the US animal study found at postnatal day 60, despite similar body weight,21 offspring of SG rats had greater adiposity than those of sham rats.24 One case-control study44 reported
nonsignificant findings. Each birth to mothers who underwent RYGB (_n_ = 32) was matched to two control births without bariatric surgery in women with prepregnancy BMI < 35 kg/m2 (_n_ =
32) and ≥35 kg/m2 (_n_ = 32). At seven years of age, no difference was observed in the prevalence of overweight or obesity among the three groups.44 OBESITY-ASSOCIATED METABOLIC DISEASES:
DIABETES/INSULIN SENSITIVITY, BLOOD PRESSURE, AND DYSLIPIDEMIA Five studies (1 sibling-comparison, 1 case-control, and 3 animal studies) characterized cardiometabolic profiles in offspring
following maternal bariatric surgery, and most found a certain degree of impairments. The only study that yielded favorable outcomes was the Canadian sibling study, which reported
improvements in all cardiometabolic indices in siblings born after than those born before maternal surgery, including improved insulin resistance,28,30,31 lower blood pressure30,31 and
triglycerides,28 and higher levels of high-density lipoprotein cholesterol.28 In contrast, the Belgian case-control study32 reported that compared to children of non-surgical mothers with
obesity or normal weight, those of mothers with prior bariatric surgery had higher diastolic blood pressure and worse endothelial function. There were however no differences in serum lipid
levels, and after adjusting for baseline differences, there was no difference in endothelial function. A population-based case-control study in Israel43 examined endocrine morbidity as a
whole (hypothyroidism, diabetes, hypoglycemia, obesity), and found that maternal bariatric surgery was an independent risk factor for developing pediatric endocrine morbidity in the
offspring, even after adjusting for important confounding variables such as maternal age, gestational week, and smoking.43 In line with findings from the Israel endocrine study, three animal
studies similarly found impaired metabolic profiles in offspring of bariatric surgery dams. Specifically, the US animal study found that SG dams’ pups had higher cholesterol levels at
weaning and greater glucose intolerance in adulthood compared to sham dams’ offspring.24 The Brazilian animal study noted that at postnatal day 120, the offspring of RYGB dams exhibited
insulin resistance in muscle and adipose tissue, and displayed lower β-cell responsiveness to glucose in pancreatic islets.26 Another animal study documented that at 120 days of age, the
livers of female offspring of RYGB dams displayed higher amounts of lipogenic genes and proteins than those of sham dams, indicating a deleterious modification in the hepatic morphology and
lipid metabolism.27 APPETITE-REGULATION HORMONES AND EATING BEHAVIORS Two studies (1 sibling-comparison, 1 animal study) assessed levels/functions of leptin and ghrelin—two of the most
important appetite-regulation hormones—in offspring following maternal bariatric surgery. The Canadian sibling study reported significantly lower levels of leptin (which inhibits hunger) and
higher levels of ghrelin (hunger hormone) in siblings born after maternal biliopancreatic diversion than those before surgery.28 The US animal study conducted a more sophisticated analysis
of the leptin and ghrelin system, including an examination of its circulating levels, sensitivity to exogenously administered leptin and ghrelin, and neural gene expression and activation.
Results suggested altered leptin and ghrelin signaling in offspring of SG dams, reflected in blunted responses to exogenously administered leptin22 and ghrelin (males only),23 elevated mRNA
expression of leptin receptor22 and ghrelin receptor23 in the hypothalamus, and elevated neural activation to leptin injection.22 Offspring’s eating behaviors were evaluated in two human
studies. Using the Food Frequency Questionnaire, the Belgian case-control study34 found similar meal patterns (frequency of consuming breakfast, lunch, and dinner) among children of mothers
who underwent bariatric surgery and children of mothers with overweight/obesity or normal weight. Nonetheless, few differences in food choices emerged. For example, children in the surgical
group reported consuming less poultry, fruit juices, and salty snacks, but more milk desserts than those in the overweight/obesity group.34 Based on three 24-hour dietary recalls with the
parents, a cross-sectional study in Brazil reported that among 13 children of women who underwent RYGB, 7.6% presented with carbohydrate intake and 30.7% with lipid intake lower than the
recommendation.45 NEURODEVELOPMENT Four human studies reported the neurodevelopmental outcomes in children born after maternal bariatric surgery: with one exception, all supported a normal
developmental trajectory. Specifically, the Canadian sibling study reported that among 123 school-age children whose mothers had previous biliopancreatic diversion, all performed normally in
terms of intellectual and social functioning.29 In a prospective case-control study conducted in Brazil44 no group differences were found in fluid intelligence among children of RYGB
mothers and children of non-surgical mothers with prepregnancy BMI < 35 kg/m2 or BMI ≥ 35 kg/m2. Finally, another study in Brazil40 assessed the neuropsychomotor development (indicated by
social and personal development, language, fine motor skills, and gross motor skills) in 23 children born after maternal bariatric surgery, and results showed that all components fell in
the normal range. The only study with negative findings was the Belgian case-control study,33 in which children’s neurodevelopmental data (indicated by internalizing problems, externalizing
problems, and prosocial behavior) were compared among three groups of children whose mothers had preconception bariatric surgery, had overweight/obesity without surgery, or had a healthy
weight without surgery. Results indicated that children in the surgical group had a significantly higher number of total difficulties and externalizing problems, meaning more aggressive and
rule-breaking behaviors in this cohort.33 A caveat to consider however is that the surgical group contained the lowest levels of maternal education and the children of post-bariatric mothers
were also significantly younger—these differences did not appear to be accounted for in the statistical analyses. OTHER HEALTH OUTCOMES MORPHOLOGY OF SKELETAL MUSCLE One animal study46
evaluated the effects of maternal RYGB on the morphology of skeletal muscle of the male offspring. Obese rats were submitted to either RYGB or sham surgery. Compared to offspring of sham
dams, those born to RYGB dams had altered homeostasis of the skeletal muscle, demonstrated in reductions in the muscle weight, area of muscle fibers, and the nucleus/fiber ratio.
Nonetheless, those reductions were accompanied by an increased number of fibers and capillary density, signifying an attempt to increase the oxygen supply to support metabolic activities.46
THERMOGENESIS IN BROWN ADIPOSE TISSUE The Brazilian animal study analyzed whether maternal RYGB promotes changes in the offspring’s brown adipose tissue, including the morphological profile
of lipids deposition and protein expressions involved in the thermogenic process. It was shown that offspring of RYGB vs sham dams exhibited reduced lipids deposition and adipocyte size, but
increased number of nuclei and Uncoupling Protein 1 expression in brown adipose tissue, suggesting restored thermogenesis from the impairment induced by maternal obesity.25 DISCUSSION To
our knowledge, this is the first study that reviewed and synthesized available evidence concerning the long-term effects of maternal bariatric surgery on the health of offspring. The
examined health outcomes spanned various domains, including immune and inflammation, epigenetics, weight status, cardiometabolic risk profiles, lifestyle behaviors, and neurodevelopment.
Although the number of studies devoted to each health domain is limited and results were not entirely consistent, maternal bariatric surgery appeared to have an effect on the offspring in
terms of (1) modified epigenetics; (2) altered weight status (albeit conflicting direction of the alteration); and (3) worsened cardiometabolic indices. Additionally, there was some evidence
derived from animal studies that the surgery produced anomalies in immune, inflammation, and appetite-regulation hormones in offspring. EPIGENETICS While only two human studies have
evaluated the epigenetic programming effect of maternal bariatric surgery, both demonstrated significant alterations in DNA methylation, particularly in genes involved in glucose, immune,
and obesity regulation, in siblings born after vs before surgery.30,31,35 Importantly, the altered methylome of genes was accompanied by coherent changes in gene expression patterns,
strengthening the clinical relevance of the epigenetic modification. Those findings were supported by previous research that bariatric surgery modulated global47 and
gene-specific48,49,50,51,52 methylation levels in the surgery recipient. The findings also aligned with prior animal reports using rat and sheep models, which showed that prepregnancy weight
loss resulting from calorie restriction or exercise led to epigenetic modification of genes functionally associated with insulin and lipid metabolism.53,54,55 A methodological strength of
the two epigenetic studies is that they adopted sibling-comparison designs, which substantially reduced confounding due to genetic, social, and lifestyle factors, thus yielding higher
confidence that the observed epigenetic alterations reflect intrauterine programming caused by maternal bariatric surgery. Nonetheless, the interpretation of results is still challenged by
various limitations such as residual confounders, the use of whole blood for analysis, and the lack of longitudinal assessment. For instance, in the Swedish sibling study,35 women were
significantly older, had lower gestational age at delivery, and had lower smoking rates during post-surgery than before-surgery pregnancy. Those differences are likely to affect the
methylation profiles in the offspring. Additionally, DNA methylation patterns are known to be tissue- and cell-specific. Hence, it is unclear whether findings from the whole-blood analysis
can be extrapolated to other tissues. Also, as both studies included a single assessment, it is unknown whether the epigenetic alterations during childhood can persist into later life, and
how those alterations influence downstream cardiometabolic consequences. Collectively, current research supports an epigenetic mechanism through which maternal bariatric surgery exerts
transgenerational effects. Still, more studies are needed to ascertain the causal effect and clarify the reproducibility, durability, and long-term implication of the epigenetic alterations.
WEIGHT STATUS Most studies supported an altered weight status in offspring born to mothers with a history of bariatric surgery; yet, results were inconsistent regarding the direction of the
alteration. The discrepancies can be explained by the methodological differences across studies, such as surgery procedure performed (e.g., RYGB, SG, biliopancreatic diversion), study
design (sibling-comparison, case-control, cross-sectional), and weight measurements (objectively measured, parent-reported, medical record). In addition to different methodologies, as
children’s weight is strongly influenced by the weight of their mothers, the mixed findings may, in part, reflect the variations in the maternal BMI classification. In detail, the human
studies that supported reduced weight in offspring born to operated mothers predominantly utilized sibling designs. In these studies, the comparison group consisted of siblings of mothers
with severe obesity who had not undergone surgery, thus had not yet achieved substantial weight loss. In contrast, studies that supported higher child weight status post-bariatric surgery
mainly involved a comparison of mothers with normal weight or weight of national reference. Future studies that incorporate a comparison group matched on prepregnancy BMI would be helpful to
better understand whether and how maternal bariatric surgery modifies the weight pattern of offspring. The discrepancies can also be attributed to variations in child characteristics, such
as age groups and gender composition. Among the reviewed studies, some recruited children over a wide age range29,40 (e.g., 2 years to adolescence), while others focused on a narrower age
group.36,39 Since each age group corresponds to a distinct growth rate, it is possible that the weight trajectories in children of mothers with and without bariatric surgery diverge
differently depending on their developmental stage. Two studies in this review support this hypothesis: the Canadian sibling study reported a lower prevalence of overweight and obesity in
siblings born after maternal bariatric surgery in the 7-year age group (not 11–18 years);29 the Swedish sibling study noted a higher mean BMI in post-surgery children, but only in the
10-year age group (not 4 or 6 years).36 Also, several studies included in this review suggested that maternal bariatric surgery affected offspring weight status in a gender-specific manner,
with the differences manifested in boys only.28,29 This observation agrees with previous research, which proposed that male fetuses are more sensitive to intrauterine environments than
female fetuses.56,57,58 Another point worth discussion is whether children born to mothers with bariatric surgery experience catch-up growth. Studies in this review repeatedly noted lower
birth weight in neonates post-surgery.28,37,39 However, following the early period of growth inhibition, some children maintained the lower weight status into later life stages, but others
surpassed their peers due to accelerated growth. Studies outside the bariatric field have uncovered various sociodemographic, environmental, and behavioral factors that may influence the
likelihood of catch-up growth, such as gestational age, education, family income, and breastfeeding.59 All of these factors likely play a role in the weight status of offspring post-maternal
bariatric surgery. Further research with longitudinal designs to assess catch-up growth will contribute to a better understanding of weight trajectories in offspring following maternal
bariatric surgery. OBESITY-ASSOCIATED METABOLIC DISEASES: DIABETES/INSULIN SENSITIVITY, BLOOD PRESSURE, AND DYSLIPIDEMIA While expectations that weight loss in mothers would reverse the
obesity-induced negative effects in offspring, our review found that prepregnancy bariatric surgery may negatively impact children’s cardiometabolic profiles. To further clarify this, we
searched literature that evaluated the offspring’s metabolic outcomes following prepregnancy behavioral weight loss. The only identified study reported that a 6-month preconception lifestyle
intervention in women with obesity had no effect on children’s insulin sensitivity or blood pressure at age 6.5 years.60 Catch-up growth, an independent predictor of cardiovascular disease
and its risk factors in later life,61,62 may account for the less favorable metabolic outcomes observed in children exposed to maternal bariatric surgery. Another explanation could be the
complex physiological changes that occur after surgery, such as disrupted glucose kinetics,63,64,65 endothelial dysfunction,66 and impaired hepatic lipid metabolism.27,67 Taking glucose
kinetics as an example, studies that used continuous glucose monitoring have consistently shown that patients after bariatric surgery have an exaggerated glucose variability, characterized
by a wide range between glucose peak and nadir, more time exposed to glucose levels out of normal range during the day and overnight, and postprandial glucose abnormalities.63,64,65 This
dysglycemia in the post-bariatric maternal body may be contributing to the altered glucose metabolism of their offspring. It is important to note that although studies have raised concern
about the potential risks of maternal bariatric surgery on children’s metabolic profiles, data supporting these claims were largely derived from animal studies. Due to distinct gut anatomy
and physiology, surgical techniques in animals cannot fully mimic human operations. For example, current mouse gastric bypass models either maintain or exclude the entire stomach, whereas
clinical procedure preserves a small pouch. The metabolic implications of the deviated surgical techniques are not fully understood. Yet, there has been evidence showing varying weight loss
rates and differences in mechanisms that mediate the weight loss (e.g., bile acids profiles, energy expenditure),68 indicating the need for caution when applying findings from animal models
to humans. NEURODEVELOPMENT Results of this review generally indicated that maternal bariatric surgery was not associated with neurodevelopmental delay in the offspring. Although one study33
described more externalizing problems in children of mothers with vs without preconception bariatric surgery, it should be noted that education level—one of the most prominent confounders
that predict offspring cognitive development—was significantly lower in surgical mothers. Even with promising findings, considering the limited number of studies and the various limitations
in those studies (e.g., cross-sectional design, small and non-generalizable samples, and reliance on parent-reported data), it is too early to conclude that maternal bariatric surgery is
absent of adverse effects on offspring’s neurofunction. Concerns still exist due to surgery-induced maternal malnutrition (especially folate and vitamin B12 deficiency), substance use, and
mental health disorders, all of which are potential threats to fetal brain development. Future longitudinal studies with larger sample sizes, longer follow-up, and objective cognitive
assessments would be necessary to clarify the effect of maternal bariatric surgery on offspring’s neurodevelopment. IMMUNE, INFLAMMATION, AND APPETITE-REGULATION HORMONES A series of
ancillary reports from the US animal study uncovered multiple anomalies related to immune, inflammation, and leptin and ghrelin regulation in the pups of SG dams,21,22,23 and the authors
proposed that those anomalies might contribute to downstream physiological impairments. Markedly, previous works from the same research team found corresponding impairments in the maternal
body and placenta following SG, including disrupted immune milieu,69 increased proinflammatory marker expression,69 elevated stress responsibility,70 and reduced leptin levels,69 reinforcing
the potentially harmful effects of maternal bariatric surgery. While results from the animal study may not be readily translated to humans, current evidence at least indicates the
importance of additional research. This scoping review has several limitations. First, data obtained were limited to three databases, and a gray literature search was not conducted;
therefore, important information presented elsewhere or unpublished may have been missed. Second, a focus on the statistical significance rather than effect size precludes understanding the
strength of the relationships between maternal bariatric surgery and long-term health outcomes in offspring. Third, some limitations are inherent in the included studies, including small
sample sizes and a lack of longitudinal studies that cover different developmental stages. Furthermore, beyond those examined in the literature, maternal obesity is associated with a wider
range of health outcomes in offspring, such as depression and allergic diseases. Future studies could include those outcomes for a more comprehensive understanding of the transgenerational
implications of maternal bariatric surgery. CONCLUSION Findings from this scoping review support an effect of maternal bariatric surgery on offspring’s epigenetic profiles, weight status,
and metabolism. Not all effects are advantageous given evidence (though mostly from animal studies) on impaired cardiometabolic indices, altered immune cell populations and inflammation, and
appetite regulation in the surgery-exposed offspring. Many questions are waiting to be answered in future studies, such as the downstream changes associated with epigenetic modification,
the shape of weight trajectory in children born after maternal surgery, and the surgical influence in a broader spectrum of health outcomes (e.g., lifestyle behaviors, mental health) in
offspring. REFERENCES * Stierman, B. et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected
Health Outcomes. _National Health Statistics Reports. no. 158_ (2021). * Sanchez, C. E. et al. Maternal pre-pregnancy obesity and child neurodevelopmental outcomes: a meta-analysis. _Obes.
Rev._ 19, 464–484 (2018). Article CAS PubMed Google Scholar * Lutsiv, O., Mah, J., Beyene, J. & McDonald, S. D. The effects of morbid obesity on maternal and neonatal health
outcomes: a systematic review and meta-analyses. _Obes. Rev._ 16, 531–546 (2015). Article CAS PubMed Google Scholar * Wu, H., Liu, F., Zhao, M., Liang, Y. & Xi, B. Maternal body mass
index and risks of neonatal mortality and offspring overweight and obesity: findings from 0.5 million samples in 61 low- and middle-income countries. _Pediatr. Obes._ 15, e12665 (2020).
Article PubMed Google Scholar * Kong, L., Nilsson, I. A. K., Brismar, K., Gissler, M. & Lavebratt, C. Associations of different types of maternal diabetes and body mass index with
offspring psychiatric disorders. _JAMA Netw. Open_ 3, e1920787 (2020). Article PubMed Google Scholar * Miras, A. D. & le Roux, C. W. Mechanisms underlying weight loss after bariatric
surgery. _Nat. Rev. Gastroenterol. Hepatol._ 10, 575–584 (2013). Article PubMed Google Scholar * Welbourn, R. et al. Bariatric surgery worldwide: baseline demographic description and
one-year outcomes from the Fourth IFSO Global Registry Report 2018. _Obes. Surg._ 29, 782–795 (2019). Article PubMed Google Scholar * American Society for Metabolic & Bariatric
Surgery. Estimate of bariatric surgery numbers, 2011–2020, accessed 12/5/2022. https://asmbs.org/resources/estimate-of-bariatric-surgery-numbers. * Youssefzadeh, A. C. et al. Pregnancy
characteristics and outcomes after bariatric surgery: national-level analysis in the United States. _Surg. Obes. Relat. Dis._ 19, 364–373 (2023). Article PubMed Google Scholar *
Khosravi-Largani, M. et al. Evaluation of all types of metabolic bariatric surgery and its consequences: a systematic review and meta-analysis. _Obes. Surg._ 29, 651–690 (2019). Article
PubMed Google Scholar * Liu, D. F. et al. The effects of bariatric surgery on dyslipidemia and insulin resistance in overweight patients with or without type 2 diabetes: a systematic
review and network meta-analysis. _Surg. Obes. Relat. Dis._ 17, 1655–1672 (2021). Article PubMed Google Scholar * Feichtinger, M. et al. Intrauterine fetal growth delay during late
pregnancy after maternal gastric bypass surgery. _Ultraschall Med._ 41, 52–59 (2020). Article PubMed Google Scholar * Youssefzadeh, A. C. et al. Cesarean delivery after bariatric surgery:
trends and outcomes in the United States. _AJOG_ 226, S341 (2022). Article Google Scholar * Różańska-Walędziak, A. et al. The influence of bariatric surgery on pregnancy and perinatal
outcomes-A case-control study. _J. Clin. Med._ 9, 1324 (2020). Article PubMed PubMed Central Google Scholar * Akhter, Z. et al. Pregnancy after bariatric surgery and adverse perinatal
outcomes: a systematic review and meta-analysis. _PLoS Med._ 16, e1002866 (2019). Article PubMed PubMed Central Google Scholar * Johansson, K. et al. Outcomes of pregnancy after
bariatric surgery. _NEJM_ 372, 814–824 (2015). Article CAS PubMed Google Scholar * Gascoin, G. et al. Risk of low birth weight and micronutrient deficiencies in neonates from mothers
after gastric bypass: a case control study. _Surg. Obes. Relat. Dis._ 13, 1384–1391 (2017). Article PubMed Google Scholar * Adsit, J. & Hewlings, S. J. Impact of bariatric surgery on
breastfeeding: a systematic review. _Surg. Obes. Relat. Dis._ 18, 117–122 (2022). Article PubMed Google Scholar * Arksey, H. & O’Malley, L. Scoping studies: towards a methodological
framework. _Int J. Soc. Res Methodol._ 8, 19–32 (2005). Article Google Scholar * Munn, Z. et al. Systematic review or scoping review? Guidance for authors when choosing between a
systematic or scoping review approach. _BMC Med. Res. Methodol._ 18, 143 (2018). Article PubMed PubMed Central Google Scholar * Spann, R. A., Taylor, E. B., Welch, B. A. & Grayson,
B. E. Altered immune system in offspring of rat maternal vertical sleeve gastrectomy. _Am. J. Physiol. Regul. Integr. Comp. Physiol._ 317, R852–r863 (2019). Article CAS PubMed PubMed
Central Google Scholar * Deer, E. M. et al. Dysregulated appetitive leptin signaling in male rodent offspring from post-bariatric dams. _Curr. Res Physiol._ 3, 50–58 (2020). Article
PubMed PubMed Central Google Scholar * Spann, R. A., Welch, B. A. & Grayson, B. E. Ghrelin signalling is dysregulated in male but not female offspring in a rat model of maternal
vertical sleeve gastrectomy. _J. Neuroendocrinol._ 33, e12913 (2021). Article CAS PubMed Google Scholar * Grayson, B. E., Schneider, K. M., Woods, S. C. & Seeley, R. J. Improved
rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. _Sci. Transl. Med._ 5, 199ra112 (2013). Article PubMed PubMed Central Google Scholar *
Ceglarek, V. M. et al. Maternal Roux-en-Y gastric bypass surgery reduces lipid deposition and increases UCP1 expression in the brown adipose tissue of male offspring. _Sci. Rep._ 11, 1158
(2021). Article CAS PubMed PubMed Central Google Scholar * Pietrobon, C. B. et al. Maternal Roux-en-Y gastric bypass impairs insulin action and endocrine pancreatic function in male F1
offspring. _Eur. J. Nutr._ 59, 1067–1079 (2020). Article CAS PubMed Google Scholar * Bertasso, I. M. et al. Pregnancy and lactation after Roux-en-Y gastric bypass worsen nonalcoholic
fatty liver disease in obese rats and lead to differential programming of hepatic de novo lipogenesis in offspring. _J. Dev. Orig. Health Dis._ 13, 263–273 (2022). Article CAS PubMed
Google Scholar * Smith, J. et al. Effects of maternal surgical weight loss in mothers on intergenerational transmission of obesity. _J. Clin. Endocrinol. Metab._ 94, 4275–4283 (2009).
Article CAS PubMed Google Scholar * Kral, J. G. et al. Large maternal weight loss from obesity surgery prevents transmission of obesity to children who were followed for 2 to 18 years.
_Pediatrics_ 118, e1644–e1649 (2006). Article PubMed Google Scholar * Guénard, F. et al. Methylation and expression of immune and inflammatory genes in the offspring of bariatric bypass
surgery patients. _J. Obes._ 2013, 492170 (2013). Article PubMed PubMed Central Google Scholar * Guénard, F. et al. Differential methylation in glucoregulatory genes of offspring born
before vs. after maternal gastrointestinal bypass surgery. _PNAS_ 110, 11439–11444 (2013). Article PubMed PubMed Central Google Scholar * Van De Maele, K., Devlieger, R., De Schepper, J.
& Gies, I. Endothelial function and its determinants in children born after maternal bariatric surgery. _Pediatr. Res_. 91, 699–704 (2022). Article PubMed Google Scholar * Van De
Maele, K. et al. Adiposity, psychomotor and behaviour outcomes of children born after maternal bariatric surgery. _Pediatr. Obes._ 16, e12749 (2021). Article PubMed Google Scholar * Van
De Maele, K., De Geyter, C., Vandenplas, Y., Gies, I. & Devlieger, R. Eating habits of children born after maternal bariatric surgery. _Nutrients_ 12, 2577 (2020). Article PubMed
Google Scholar * Berglind, D. et al. Differential methylation in inflammation and type 2 diabetes genes in siblings born before and after maternal bariatric surgery. _Obesity (Silver
Spring)_ 24, 250–261 (2016). Article CAS PubMed Google Scholar * Willmer, M. et al. Surgically induced interpregnancy weight loss and prevalence of overweight and obesity in offspring.
_PLoS One_ 8, e82247 (2013). Article PubMed PubMed Central Google Scholar * Berglind, D. et al. Differences in gestational weight gain between pregnancies before and after maternal
bariatric surgery correlate with differences in birth weight but not with scores on the body mass index in early childhood. _Pediatr. Obes._ 9, 427–434 (2014). Article CAS PubMed Google
Scholar * Malik, S. et al. Maternal and fetal outcomes of Asian pregnancies after bariatric surgery. _Surg. Obes. Relat. Dis._ 16, 529–535 (2020). Article PubMed Google Scholar *
Barisione, M., Carlini, F., Gradaschi, R., Camerini, G. & Adami, G. F. Body weight at developmental age in siblings born to mothers before and after surgically induced weight loss.
_Surg. Obes. Relat. Dis._ 8, 387–391 (2012). Article PubMed Google Scholar * Dell’Agnolo, C. M., Cyr, C., de Montigny, F., de Barros Carvalho, M. D. & Pelloso, S. M. Pregnancy after
bariatric surgery: obstetric and perinatal outcomes and the growth and development of children. _Obes. Surg._ 25, 2030–2039 (2015). Article PubMed Google Scholar * Larsson, L.,
Landin-Olsson, M. & Nilsson, C. Weight development in children after gastric bypass surgery. _J. Fam. Reprod. Health_ 13, 176–180 (2019). Google Scholar * Del Sordo, G. et al. Postnatal
health in children born to women after bariatric surgery. _Obes. Surg._ 30, 3898–3904 (2020). Article PubMed Google Scholar * Damti, P., Friger, M., Landau, D., Sergienko, R. &
Sheiner, E. Offspring of women following bariatric surgery and those of patients with obesity are at an increased risk for long-term pediatric endocrine morbidity. _Arch. Gynecol. Obstet._
300, 1253–1259 (2019). Article PubMed Google Scholar * Blume, C. A. et al. Association of maternal Roux-en-Y gastric bypass with obstetric outcomes and fluid intelligence in offspring.
_Obes. Surg._ 28, 3611–3620 (2018). Article PubMed Google Scholar * Gimenes, J. C. et al. Nutritional status of children from women with previously bariatric surgery. _Obes. Surg._ 28,
990–995 (2018). Article PubMed Google Scholar * Kuhn, C., Covatti, C., Ribeiro, L. F. C., Balbo, S. L. & Torrejais, M. M. Bariatric surgery induces morphological changes in the
extensor digitorum longus muscle in the offspring of obese rats. _Tissue Cell_ 72, 101537 (2021). Article CAS PubMed Google Scholar * Benton, M. C. et al. An analysis of DNA methylation
in human adipose tissue reveals differential modification of obesity genes before and after gastric bypass and weight loss. _Genome Biol._ 16, 8 (2015). Article CAS PubMed PubMed Central
Google Scholar * Fraszczyk, E. et al. The effects of bariatric surgery on clinical profile, DNA methylation, and ageing in severely obese patients. _Clin. Epigenetics_ 12, 14 (2020).
Article CAS PubMed PubMed Central Google Scholar * Nilsson, E. K. et al. Roux-en Y gastric bypass surgery induces genome-wide promoter-specific changes in DNA methylation in whole blood
of obese patients. _PLoS One_ 10, e0115186 (2015). Article PubMed PubMed Central Google Scholar * Garcia, L. A. et al. Weight loss after Roux-En-Y gastric bypass surgery reveals
skeletal muscle DNA methylation changes. _Clin. Epigenetics_ 13, 100 (2021). Article CAS PubMed PubMed Central Google Scholar * Pinhel, M. A. S. et al. Changes in DNA methylation and
gene expression of insulin and obesity-related gene PIK3R1 after Roux-en-Y gastric bypass. _Int J. Mol. Sci._ 21, 4476 (2020). Article CAS PubMed PubMed Central Google Scholar *
Wilhelm, J. et al. Promoter methylation of LEP and LEPR before and after bariatric surgery: a cross-sectional study. _Obes. Facts_ 14, 1–7 (2021). Article PubMed Google Scholar * Wei, Y.
et al. Enriched environment-induced maternal weight loss reprograms metabolic gene expression in mouse offspring. _J. Biol. Chem._ 290, 4604–4619 (2015). Article CAS PubMed PubMed Central
Google Scholar * Nicholas, L. M. et al. Differential effects of maternal obesity and weight loss in the periconceptional period on the epigenetic regulation of hepatic insulin-signaling
pathways in the offspring. _FASEB J._ 27, 3786–3796 (2013). Article CAS PubMed Google Scholar * Zhang, S. et al. Periconceptional undernutrition in normal and overweight ewes leads to
increased adrenal growth and epigenetic changes in adrenal IGF2/H19 gene in offspring. _FASEB J._ 24, 2772–2782 (2010). Article CAS PubMed Google Scholar * DiPietro, J. A. &
Voegtline, K. M. The gestational foundation of sex differences in development and vulnerability. _Neuroscience_ 342, 4–20 (2017). Article CAS PubMed Google Scholar * Powell, T. L. et al.
Sex-specific responses in placental fatty acid oxidation, esterification and transfer capacity to maternal obesity. _Biochim Biophys. Acta Mol. Cell Biol. Lipids_ 1866, 158861 (2021).
Article CAS PubMed Google Scholar * Wijenayake, S. et al. Maternal high-fat diet induces sex-specific changes to glucocorticoid and inflammatory signaling in response to corticosterone
and lipopolysaccharide challenge in adult rat offspring. _J. Neuroinflamm._ 17, 116 (2020). Article CAS Google Scholar * Pradeilles, R. et al. Factors associated with catch-up growth in
early infancy in rural Pakistan: a longitudinal analysis of the women’s work and nutrition study. _Matern Child Nutr._ 15, e12733 (2019). Article PubMed Google Scholar * den Harink, T. et
al. Preconception lifestyle intervention in women with obesity and echocardiographic indices of cardiovascular health in their children. _Int J. Obes. (Lond.)_ 46, 1262–1270 (2022). Article
Google Scholar * Goedegebuure, W. J., Van der Steen, M., Smeets, C. C. J., Kerkhof, G. F. & Hokken-Koelega, A. C. S. SGA-born adults with postnatal catch-up have a persistently
unfavourable metabolic health profile and increased adiposity at age 32 years. _Eur. J. Endocrinol._ 187, 15–26 (2022). Article CAS PubMed Google Scholar * Martin, A., Connelly, A.,
Bland, R. M., Reilly, J. J. Health impact of catch-up growth in low-birth weight infants: systematic review, evidence appraisal, and meta-analysis. _Matern Child Nutr_. 13,
https://doi.org/10.1111/mcn.12297 (2017). * Lee, D. et al. Glycemic patterns are distinct in post-bariatric hypoglycemia after gastric bypass (PBH-RYGB). _J. Clin. Endocrinol. Metab._ 106,
2291–2303 (2021). Article PubMed PubMed Central Google Scholar * Hanaire, H. et al. High glycemic variability assessed by continuous glucose monitoring after surgical treatment of
obesity by gastric bypass. _Diabetes Technol. Ther._ 13, 625–630 (2011). Article CAS PubMed Google Scholar * Capoccia, D. et al. Is type 2 diabetes really resolved after laparoscopic
sleeve gastrectomy? Glucose variability studied by continuous glucose monitoring. _J. Diabetes Res_. 2015, 674268 (2015). Article CAS PubMed PubMed Central Google Scholar * Borzì, A. M.
et al. Endothelial function in obese patients treated with bariatric surgery. _Diabetes Metab. Syndr. Obes._ 13, 247–256 (2020). Article PubMed PubMed Central Google Scholar * Cerreto,
M., Santopaolo, F., Gasbarrini, A., Pompili, M. & Ponziani, F. R. Bariatric surgery and liver disease: general considerations and role of the gut-liver axis. _Nutrients_ 13, 2649 (2021).
Article CAS PubMed PubMed Central Google Scholar * Lutz, T. A. & Bueter, M. The use of rat and mouse models in bariatric surgery experiments. _Front Nutr._ 3, 25 (2016). Article
PubMed PubMed Central Google Scholar * Spann, R. A. et al. Rodent vertical sleeve gastrectomy alters maternal immune health and fetoplacental development. _Clin. Sci. (Lond.)_ 132,
295–312 (2018). Article CAS PubMed Google Scholar * Himel, A. R., Cabral, S. A., Shaffery, J. P. & Grayson, B. E. Anxiety behavior and hypothalamic-pituitary-adrenal axis altered in
a female rat model of vertical sleeve gastrectomy. _PLoS One_ 13, e0200026 (2018). Article PubMed PubMed Central Google Scholar Download references FUNDING Funding V.L. was supported by
the National Institute of Health, Building Interdisciplinary Research Careers in Women’s Health at UC Davis through Grant Number: 5K12HD051958. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS *
School of Nursing, University of Rochester, 601 Elmwood Avenue, Rochester, NY, 14642, USA Yang Yu & Susan W. Groth * Department of Surgery, University of California Davis, Sacramento,
CA, 95817, USA Victoria Lyo * Center for Alimentary and Metabolic Science, University of California Davis, Sacramento, CA, 95817, USA Victoria Lyo Authors * Yang Yu View author publications
You can also search for this author inPubMed Google Scholar * Victoria Lyo View author publications You can also search for this author inPubMed Google Scholar * Susan W. Groth View author
publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS Y.Y.: conception and design; literature search and review; interpretation of results; drafting the
article; final approval. V.L.: interpretation of results; revising the article; final approval. S.W.G.: interpretation of results; revising the article; final approval CORRESPONDING AUTHOR
Correspondence to Yang Yu. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with
regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive
rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed
by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yu, Y., Lyo, V. & Groth, S.W. The impact of maternal bariatric
surgery on long-term health of offspring: a scoping review. _Pediatr Res_ 94, 1619–1630 (2023). https://doi.org/10.1038/s41390-023-02698-9 Download citation * Received: 30 March 2023 *
Revised: 12 May 2023 * Accepted: 15 May 2023 * Published: 20 June 2023 * Issue Date: November 2023 * DOI: https://doi.org/10.1038/s41390-023-02698-9 SHARE THIS ARTICLE Anyone you share the
following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer
Nature SharedIt content-sharing initiative