- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND This study provides reference values for cardiovascular modulation at rest, during maximal exercise test and recovery after exercise in Caucasian children according to
weight status and cardiorespiratory fitness (CRF) level. Additionally, the current study analyzed several correlations between autonomic cardiovascular modulation, cardiorespiratory
performance and cardiometabolic risk. The principal goal of this study was to analyze cardiac function at rest, during maximum exercise, and during the recovery phase in children grouped
according to weight status and CRF level. METHODS One hundred and fifty-two healthy children (78 girls) 10–16 years of age were divided into three groups: soccer and basketball players
(SBG), endurance group (EG), and sedentary people with overweight and obesity (OOG). A cardiac RR interval monitor recorded the cardiac data and specific software analyzed the cardiac
autonomic response through heart rate (HR) and HR variability. The study analyzed resting HR (RHR), HRpeak, and HR recovery (HRR). RESULTS OOG showed significant poorer performance in the
Léger test lower V̇O2 max and higher values of blood pressure at rest and post-exercise than sport groups. The EG presented the best results in CRF and cardiometabolic risk (CMR) in relation
to SBG and OOG. The OOG showed higher percentage of HR values, compatible with an unhealthy cardiovascular autonomic modulation than the sport groups, with significant differences in
bradycardia, HR reserve, and HRR 5 min. CONCLUSIONS Aerobic performance, vagal activity, blood pressure, chronotropic competence, and HRR have significant associations with CMR parameters.
IMPACT * The current study presents reference values of autonomic cardiac function in Caucasian children according to weight status and cardiorespiratory fitness level. * Aerobic
performance, vagal activity, blood pressure, chronotropic competence, and heart rate during the recovery period after exercise have significant associations with cardiometabolic risk
parameters. * Children with overweight and obesity show signs of autonomic dysfunction reflected as low cardiac vagal activity and poor chronotropic competence. You have full access to this
article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS COMPREHENSIVE CARDIAC EVALUATION TO MAXIMAL EXERCISE IN A CONTEMPORARY POPULATION OF PREPUBERTAL CHILDREN
Article 30 October 2021 PREDICTIVE APPROACH OF HEALTH INDICATORS FROM THE PHYSICAL ACTIVITY HABITS OF ACTIVE YOUTH Article Open access 06 June 2024 PHASE ANGLE AND ITS DETERMINANTS AMONG
ADOLESCENTS: INFLUENCE OF BODY COMPOSITION AND PHYSICAL FITNESS LEVEL Article Open access 13 June 2024 INTRODUCTION Currently, the relevance of physical activity (PA) for health cannot be
questioned, indeed, children have shown both physical and psychological benefits following PA.1 However, it has been shown that larger levels of inactive lifestyles are related with the
following health problems in children and adolescents: cardiometabolic risk (CMR), low cardiopulmonary fitness (CRF), minor functioning of the cardiac autonomic nervous system and increased
adiposity.2 In this regard, the predominance of obesity (OB) between children and adolescents (aged 2–18 years) is an a major public health issue,3 leading to malnutrition with lower levels
of PA.4 Obese children show breathing difficulties, hypertension, early markers of cardiovascular disease, and insulin resistance.4 Especially, autonomic dysfunction, in combination with
poor physical fitness, may be a mechanism associated with early glucose dysmetabolism and the development of diabetes.5 It seems that the development of autonomic dysfunction starts at an
early age, and is related to metabolic syndrome prevalence that is growing in younger populations.6 Adolescents and young adults with severe OB have a more adverse cardiovascular risk
profile and poorer cardiac and vascular structure and function.7 One of the diseases that has the greatest influence on morbidity and mortality trends worldwide is cardiovascular disease,
and indeed, some of it may originate in early childhood.8 Throughout development, from infancy to adulthood, there are changes in cardiac autonomic neural regulation that affect heart
rhythm. Indeed, these modifications could be used as a diagnostic method in those individuals at greater risk of suffering some type of alteration that could lead to a severe cardiovascular
disorder.9 As a matter of fact, several childhood diseases, such as OB10 and diabetes, are related to cardiac autonomic dysfunction.11 PA is a protective factor for autonomic dysfunction,
above all, vigorous PA is most strongly related with cardiac vagal modulation in young adults.12 Regardless of sex, a physically active life was associated with better cardiovascular
autonomic modulation in adolescents.13 Therefore, engaging in sports practice should be encouraged to improve cardiac autonomic function in obese adolescent boys.14 Similarly, physical
fitness is recognized as an essential marker of health across the life stages, because it has been shown to be a powerful marker of health in early childhood and later in life.15,16 It seems
important to assess the function of the respiratory systems, since negative CRF in these stages is associated with adverse cardiovascular indicators (e.g., hypertension).17 Indeed, the
evidence have established cut points of CRF to avoid cardiovascular disease risk in children and adolescents, identifying CRF as an important indicator of cardiometabolic health.18 From
early childhood, heart rate (HR) could be a helpful further measure to detect subtle alterations in cardiovascular health during PA through simple clinical measurements.8 It should be noted
that the balance of the autonomic nervous system could be determined by analyzing various values of the cardiopulmonary stress trial, such as the rest period, HR response to dynamic
exercise, HR recovery (HRR) from the exercise test, and HR variability (HRV).19 Indeed, CRF evaluation could supply significant evidence about the state of health and way of life of the
child population, difficult to identify at rest.20 While positive relationships between PA and CRF with resting HRV have been established in childhood, additional understanding about the
possible relationships of PA and CRF to cardiac autonomic function during recovery following exercise is required. Moreover, the effects of PA intensity on HRV and HRR is not clear, when
measures of cardiac autonomic function with CMR factors are analyzed.21 Additional studies are needed to clarify the role of PA and CRF on HRV in children and adolescents,22 especially, in
children with overweight (OW) and OB. Therefore, to propose realistic and medically safe exercise interventions for obese adolescents, it remains to be determined whether exercise tolerance
is altered and whether anomalous cardiopulmonary responses during maximal exercise testing are present.23 Since exercise dysfunction precedes resting abnormalities, exercise testing could be
a tool for early detection of cardiac dysfunction in children.24 Therefore, studies are needed to examine the cardiopulmonary response during a maximal exercise test in OW and OB children.
For clinical purposes, cardiovascular autonomic function has been studied in depth in children25; however, limited studies22,26,27 have considered the effect that CRF and weight status have
on HR response to maximal exercise and following recovery. Therefore, the principal goal was to examine cardiac function at rest, during maximum exercise, and during the recuperation stage
to define sex reference values of RHR, HRV, HRpeak, and HRR in child population considering weight status and CRF level. METHODS PARTICIPANTS This cross-sectional research required a group
of 152 healthy children (78 girls) aged 10–16 years (age=12.71 ± 1.72 years old) and they were selected by convenience from several schools and athletic clubs in southern Spain. Any physical
or intellectual disability was taken as a criterion for exclusion; indeed, it was necessary to receive from the parents of the children included in the study a certificate declaring that
their children were free of physical and intellectual disabilities. The participants were divided into three groups: soccer and basketball players (SBG) (_n_ = 76, 46.1% girls), endurance
group (EG) (_n_ = 45, 57.8% girls), and those who did not participate in any sport and showed OW and OB (OOG) (_n_ = 31, 54.8% girls). Both EG and SBG made 4 weekly training sessions. For
this study, an informed consent form was voluntarily signed by the parents so that their children could participate. The study was carried out respecting the standard of the Declaration of
Helsinki (2013 version) and was consented by the Ethics Committee of the University of Jaen (SEPT.20/9.TES). MATERIALS AND TESTING ANTHROPOMETRIC VARIABLES The body mass index (BMI) was
calculated by dividing body mass (kg) by body height2 (in meters). The 85th and 95th percentiles of the study by Sobradillo et al.28 were considered in classifying children as OW or OB,
respectively, in relation to BMI. Waist circumference (WC) and waist-to-height ratio (WHR) were measured as previously described by Latorre-Román.29 CARDIORESPIRATORY FITNESS The 20-m
shuttle run test (20Msrt) was used to measure the CRF.30 A high value in the number of stages performed, indicates a better result, which showed a high CRF. Additionally, the maximum volume
of oxygen uptake (V̇O2 max) was calculated with the equation below: V̇O2 max = (31.025) + (3.238×_V_) − (3.248×_A_) + (0.1536×_A_×_V_), where _V_ = speed in km/h and _A_ = age in years.30
Moreover, to gain more evidence about the perceived exertion after the completion of the Léger test, the rate of perceived exertion (RPE) was documented on a scale from 0 to10.31 HEART RATE
RECORDING In terms of HR control, we used a Firstbeat Bodyguard 2 of Firstbeat Technologies Ltd. (Jyväskylä, Finland) to record the RR intervals. The monitor measures at a sample rate of
1000 Hz and has been calibrated with standard electrocardiogram apparatus.32,33 The program includes the HRV algorithms supplied by the European Society of Cardiology34 (Firstbeat Uploader,
Firsbeat Technologies Ltd. Yliopistonkatu, Jyväskylä; Version 3.3.4.0) and a signal filter which is based on the artifact compensation algorithm defined in Saalasti’s study.35 The frequency
domain of HRV involves the measurement of the high-frequency (HF) (0.15–0.4 Hz) and low-frequency (LF) spectrum in ms2 (0.04–0.15 Hz), and also allows for an LF/HF ratio. Parasympathetic
activity may be reflected by HF, in addition a combination of sympathetic and parasympathetic input may be indicated by LF and sympathovagal balance by the ratio of LF/HF.36 Furthermore, the
time domain analysis comprises statistical metrics that reflect parasympathetic activity, such as the root-mean-square differences of successive heartbeat intervals (RMSSD) and the average
standard deviation of the NN interval (normal RR) (SDNN).34,37 The maximal predicted HR of reserve (HRr) attained was indicated as the chronotropic index (CI); HRr is the difference between
HRpeak and HR at rest. Particularly, the CI was consequently explained as CI = (HRpeak − RHR)/[(220 − age) − RHR)]38 and chronotropic incompetence was said to be show when amounts of the
chronotropic index were <0.80.38 Additionally, based on earlier studies,39,40 HR was also registered at 1 (HRR1) and 5 min (HRR5) after the end of the test and the HRR was determined as
the difference among the HRpeak and HR at these recuperation spots, represent the fast and slow points of recovery time, correspondingly.41 According to earlier studies, we used HR
references at rest, during exercise, and in the recovery time related to an enhance in the risk of death.42,43 Mostly showed in people with a RHR that was more than 75 beats per minute
(bpm); individuals with a raise in HRr during exercise that was less than 89 bpm; and in subjects with a HRR1 and HRR5 of less than 25 beats and 75 beats per minute after the end of test
respectively.42,43 Also, an OMRON® digital electronic monitor model HEM 7114 (Illinois) was used to analyze blood pressure (BP). PROCEDURE In the morning time, this study was carried out in
the school’s athletic installations, at a minimum of 3 h after the last feeding. In addition, participants were asked to avoid any strenuous PA the day before the test. Before stress test,
it was registered BP, RHR and HRV together with anthropometric variables, which were determined after 10 min in a sitting posture and with spontaneous breathing according to Young et al.44
Prior to the measurements, participants were commanded that talking or moving during the test was strictly forbidden. Seated short-term resting HRV determinations can be performed in the
field (i.e., outside lab-controlled settings) in children.45 Successively, the children completed a standard warm-up. Using the Léger test, HR was constantly monitored in order to determine
the HRpeak. The HR was also recorded at the first (HRR1) and the fifth (HRR5) minute after the end of the test). Based on Buchheit et al.,46 at the end of the Léger test, the participants
were instantly seated passively on a chair next to the track. Time duration among the end of exercise and sitting was less than 5 s. Consequently, pre- and post-exercise measures of HR were
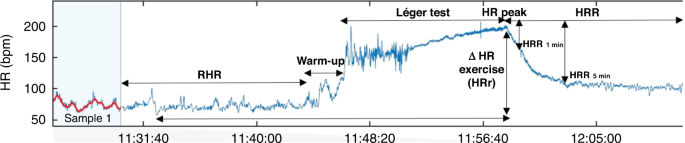
performed in the sitting posture for at least 10 min (Participants had to remain seated, breathe naturally and not join in any dialog) in conformity with Bentley et al.47 Figure 1 shows an
example of HR profile at rest, during exercise, and recovery times. STATISTICAL ANALYSIS The results were examined using SPSS software., v.22.0 for Windows (SPSS Inc, Chicago. Tests of
normal distribution and homogeneity (Kolmogorov–Smirnov and Levene’s test, respectively) were conducted on all data periods after the analyses. Descriptive data are described in terms of
means and standard deviations (SD) and percentage (%). The differences in the different parameters between sex and groups were analyzed using an analysis of covariance adjusted for age, and
post hoc analysis by Bonferroni test was performed. Moreover, in nonparametric variables the Kruskal–Wallis test and post hoc analysis by the Mann–Whitney _U_-test were used. Furthermore, to
validate the association between anthropometric variables, HR parameters, and CRF, partial correlation analysis and a simple linear regression analysis (adjusted by age and sex) were used.
The size of correlation between outcome variables was denoted as: <0.1 (trivial), 0.1–0.3 (small), 0.3–0.5 (moderate), 0.5–0.7 (large), 0.7–0.9 (very large), and 0.9–1.0 (almost
perfect).48 The significance level was set at _p_ < 0.05. RESULTS Figure 2 indicated the weight status with respect to the groups analyzed. Table 1 illustrates anthropometric
characteristics, CMR factor, and Léger test performance according to sex and groups. OOG showed significantly poorer performance in the Léger test (_p_ < 0.001), lower V̇O2 max (_p_ <
0.001) than sport groups, and higher values of WtHR and BP at rest and post-exercise than EG. EG presented the best results in CRF and CMR in relation to SBG and OOG. Table 2 indicates HR
and HRV at rest, HR during the Léger test and during the recovery period after exercise testing relating to sex and groups. OOG exhibit higher values of RHR and lower values of HRpeak, HRr,
RMSSD and HRR5 than EG (_p_ < 0.001, _p_ = 0.005, _p_ < 0.001, _p_ = 0.004, _p_ = 0.006, respectively) and SBG (_p_ < 0.001, _p_ < 0.001, _p_ < 0.001, _p_ = 0.002, _p_ <
0.001, respectively). In addition, EG showed the highest CI values with significant differences compared to the OOG (_p_ = 0.004). Controlling for age and sex, it is noteworthy that we found
a very large and inverse correlations between Léger performance and BMI and WtHR. In additions, a very large positive correlation between HRR5 and HRr, and a large and inverse correlation
between Léger performance and WC, between RHR and HRR5, between RHR and RMSSD. Finally, a large positive correlation between HRR5 and HRpeak (Table 3). Figure 3 shows the percentage of
children within each HR references values (at rest, during maximal exercise, and at recovery time) associated with a healthy cardiovascular system. In all cardiovascular risk parameters, OOG
showed significant differences with respect to EG and SBG in bradycardia, HRr, and HRR5. Finally, simple linear regression analysis revealed that RHR and HRr were the main variables that
showed influence on V̇O2 max and CMR parameters (Table 4). DISCUSSION The principal goal was to examine cardiac autonomic function at rest, during maximum exercise, and during the recovery
phase to determine sex reference values of RHR, HRV, HRpeak, and HRR in children ‘according to weight status and CRF level. Based on our information, this is the first study that conducted
this comparison between children trained in endurance sports, in soccer and basketball, and children with OW and OB who do not train in any sport. The principal outcomes of this study show
that there are significant differences between these groups in cardiac autonomic modulation and in the cardiac response to maximal exercise. Children who practice competitive sports
displayed low RHR, and higher values of RMSSD, chronotropic competence, and HRR than OOG. These values are associated with the highest performance in the Léger test by sport groups and lower
values of CMR. CARDIAC AUTONOMIC FUNCTION AT REST With respect to RHR, we reported that weight status and aerobic capacity influences RHR; thereby, high RHR is associated with low aerobic
performance and OW and OB. Furthermore, no sex difference was found in RHR. In this regard, RHR shows significant correlations with BMI, WC and WtHR. As regards weight status, the outcomes
of this study, align with those of Kwok et al.,49 which detected an association between RHR and OB. In addition, Kwok et al.,49 indicated that high RHR is related, among other factors to
elevated BP and physical inactivity in children, moreover, RHR is more closely linked to obesity than body size, and with increased WC in boys but not girls.49 In addition, previous studies
showed that a RHR ≥ 86 bpm is associated with an increased likelihood for high BP values in both non-obese and obese children,50 furthermore, school children with a higher RHR (91 bpm)
displayed higher values of LDL cholesterol.51 Finally, a longitudinal study showed a causal association between RHR and myocardial dysfunction, this association was independent of other risk
factors, including BP, BMI, and fitness level.52 Regarding aerobic capacity, these results match those observed in earlier studies that have reported strong correlations between RHR and
aerobic performance in children and adolescents.53,54 Concerning sex, these outcomes are contrary to those examined in prior to studies that propose that girls present higher RHR values than
boys.49,54,55 Moreover, bradycardia, a RHR < 60 bpm, may be joined by exercise intolerance in older children.56 However, regular PA stimulates bradycardia57,58 showing that the RHR of
endurance athletes children is 11 bpm lower than age-matched non-athletes.59 This event would suggest that it is caused to cardiac adaptations led by a vagal predominance, sinus bradycardia,
and increased HRV,60 and is not linked with cardiovascular risk factors. In the current study, the prevalence of bradycardia was 11.1%, 22.4%, and 0% in EG, SBG and OOG groups,
respectively. The RHR of children who play sports was 16 bpm lower than age-matched non-athlete. In the EG and SBG, the RHR values found in the present study were in accordance with the
values of international references, however, the OOG showed high values in relation to these references.61 CARDIAC AUTONOMIC FUNCTION AT MAXIMUM EXERCISE Overall, in the current study, no
sex difference was found in HRpeak. This finding is in agreement with previous studies,62,63,64 in the OW and OB group, girls present lower HRpeak values than boys, which could indicate that
obese girls do not perform maximum effort or show intolerance to exercise. In fact, according to clinical guidelines,65 a usual measures to conclude whether the child gave a maximum effort
is a peak HR approaching 200 bpm (may not be attained in children with chronotropic or other limitations to exercise). OB girls achieved a HRpeak ~ 191 bpm. Additionally, in our study is
interesting to observe that HRpeak exhibited a low correlation with age. This outcome is in accord with Van Brussel et al.,66 which shows that average HRpeak remains relatively stable,
around 200 bpm (treadmill) in children and adolescents. Contemplating the narrow range of HRpeak in youth, Gelbart et al.,67 proposed using 197 bpm as the mean HRpeak in children and
adolescents, with 180 bpm as the minimum threshold value. The current findings agree with the earlier work regardless of the group analyzed. In addition, chronotropic incompetence has proven
to be a useful predictive instrument for patients with coronary heart disease and an effective indicator of all-cause mortality, and has been reported in various heart diseases in the
pediatric community.68 However, data involving chronotropic response during exercise in children and adolescents is reduced.38 In the present research, chronotropic competence analyzed by CI
and HRr was better in children who practice sport than children with OW and OB; 100% vs. 93% achieve a CI > 0.80, which could indicate a healthy HR response to exercise. Therefore,
according to a recent report,69 chronotropic incompetence was found to be highly prevalent in OOG and related to poor cardiometabolic health as well as exercise intolerance. Likewise,
Nikolaidis et al.70 observed lower HRpeak among OW and OB children. Nevertheless, Von Scheidt et al.38 showed values of CI < 0.80 in healthy children related with children with congenital
heart diseases, so it is advisable not to use the threshold of 0.8 for the identification of chronotropic incompetence using treadmill exercise testing in children, most likely due to an
overestimation of the maximal HR using the Karvonen HRmax formula (220 − age of subject).71 However, HRr is also higher in child athletes and these parameters showed significant correlations
with parameters of metabolic risk. In this sense, other significant outcome of the present study was that BMI, WC and WtHR were negatively correlated not only with the HRr, also with the
V̇O2 max and performance in Léger test. In this regard, Delgado-Flody et al.72 noted that children aged 11 to 13 years with OB showed a lower V̇O2 max, recorded by Léger’s test, than
children with normal weight and OW. Therefore, chronotropic incompetence is more frequent in obese adolescents compared to their lean counterparts and is related to exercise intolerance,69
although, in a review, HRpeak was not reduced in obese adolescents.73 More research on this topic needs to be undertaken before the association between HRpeak and OB in children is more
clearly understood. CARDIAC AUTONOMIC FUNCTION IN RECOVERY AFTER EXERCISE Metabolic risks are inversely associated with HRR in healthy children and adolescents.74 In the current study, it is
noteworthy that HRR5 was more sensitive than HRR1, revealing differences between groups and showing several associations with other cardiac variables such as RHR, HRV, HRr and HRpeak. In
accordance with Lin et al.,74 a simple linear regression analysis, indicates that WC and WtHR were the only parameters associated with HRR parameters, in particular with HRR5. However, these
findings are not coherent with those showed by Easley et al.,39 who note that OB alone does not seem to considerably impact HRR. Similarly, a current study noted that there are no
differences in autonomic function during recovery (HRR1) from maximal exercise in lean and obese 8- to 12-year-old children.75 Conversely, Laguna et al.76 observed that HRR was inversely
associated with OB traits and related CMR factors mainly in healthy boys. Concerning sex differences, girls displayed high values of HRR5 than boys in SBG and OOG. These outcomes are
contrary to those detected by Guilkey et al.,77 who observed no significant differences in HRR following maximal and submaximal exercise between boys and girls, reporting that
parasympathetic modulation was related between boys and girls at rest and during recovery from exercise. Furthermore, HRR is associated with some cardiovascular fitness indices such as V̇O2
max.78 However, the present study has been unable to demonstrate strong relationships between V̇O2 max and HRR. Similarly, Fernando et al.79 showed that no relationship between aerobic
fitness and HRR1 after a three minutes modified Harvard’s step test in children 7 to 11 years of age. HEART RATE VARIABILITY BMI and PA are the main factors that can influence HRV between 12
and 17 years.80 Thereby, the obese children displayed modifications in HRV, characterized by a reduction in both sympathetic and parasympathetic activity.81 In this regard, another outcome
of this research is that HRV differs between groups; OOG showed the lowest values of RMSSD at rest, in particular in boys; furthermore, only in this parameter did the boys show higher values
than the girls in the groups that participated in sports. This outcome is in concert, in part, with the outcomes from Jarrin et al.,82 which show significantly greater HRV values in boys in
comparison to girls. These differences can be partly explained by lower HR in boys,83 differences in level of training,80 more in boys (which we have not measured), or that the kind of
training may have different influence on girls and boys (which requires further research). Nonetheless, more recently, bibliography has developed that offers inconsistent outcomes concerning
the sex influence on HRV.84 In this sense, Sharma et al.,80 found significant differences in HRV between girls and boys in non-athletes, but not in athletes, which lead to the hypothesis
that physical training in boys could have improved their HRV similar to that of girls, therefore, irrespective of sex, athletic level training had positive influence on HRV in terms of
increased resting parasympathetic activity and decreased sympathetic activity.80 Furthermore, there is no correlation between HRV with BMI, WC and WtHR. The current outcomes appear to be
consistent with other study that has not found a clear association between weight status and CMR with HRV.75 Likewise, Kaufman et al.,85 showed no significant differences in HRV measures
between the normal weight and OW children; the most prominent differences were observed between normal weight children and children at the extreme levels of OB (BMI 95th percentile).85
Although, a recent study indicated that higher CMR was associated with smaller HRV, mainly indicating lower parasympathetic activity in young children.86 In this regard, in the current
study, simple linear regression showed that RMSSD is inversely associated with WC and WtHR. Additionally, taking into account CRF, the findings of the current study further support the idea
that there is an association between CRF and HRV,22 simple linear regression showed that both RMSSD and SDNN are positively associated with V̇O2 max. The current outcomes appear to be
coherent with other study that indicated that there was a trend for higher baroreflex sensitivity in athletes, thus HRV (total power and SDNN) was higher in athletes. In the same way, the
parasympathetic tone was higher in terms of higher RMSSD, and higher HF power.87 In this same way, a recent review indicated that high PA level was associated with significant cardiac
autonomic control in children and adolescent, in particular, PA and HRV were significantly positive correlated.88 However, it was recently noted that HR is a better predictor of CRF than HRV
parameters and the complex concept of HRV might not provide additional information to the prediction of CRF in OW and OB children.27 In the same way, Grant et al.89 emphasize that during an
investigation of aerobic capacity, quantification of HRV may not add significant value. Gamelin et al. reported that after 7 weeks of high intermittent exercise training increased aerobic
fitness. Conversely, this training did not generate substantial changes in HRV in prepubertal children.90 Furthermore, Da Silva et al.91 support the theory that physical training does not
enhance HRV in healthy children. Therefore, future studies, which take these variables into account, will need to be undertaken. RELATIONSHIP BETWEEN THE DIFFERENT HR BEHAVIORS Significant
correlations were found between aerobic performance with RHR and HRr. In addition, following the present results, an earlier study has shown that RHR is adversely correlated to HRR.92 In
turn, we found significant correlation between HRR and HRr, in this regard, a previous study noted that the main portion of the abnormality in HRR after exercise can be explained by CI.93
Moreover, these findings add to past research that observes no relation between aerobic fitness and HRR after a 20-m shuttle test in children aged 7–11 years.79 Moreover, in the current
study, lower values of HRr and higher values of RHR and BP could be indicators of CMR. In this sense, in accordance with the present results, previous studies have demonstrated that children
and adolescents with high total or central OB had higher BP at rest.94,95 Finally, according to Proudfoot et al.96 engagement in more intense PA programs as they were performed in EG and
SBG, results in greater cardiovascular fitness, better autonomic function and provides additional benefits because it is associated with lower values of CMR. The present study has some
limitations that should be mentioned. First, we used a cross-sectional design, therefore, both cardiac function at rest and during maximum exercise and recovery in growing children should
have been measured in a long-term longitudinal study. Second, Since the sample included Caucasian Spaniards, we should be cautious when generalizing these results to other populations.
Third, the HR response must take into account the mode of the stress test (i.e., treadmill vs. cycling). Fourth, in our study, the mestrual cycle was not tracked. In spite of these
limitations, this current study is the first study to observe the correlations of PA and CRF with cardiac function along with traditional CMR risk factors in this population. Therefore, our
results present some insights into cardiovascular autonomic modulation in children and adolescent populations during typical field tests, such Léger test, reinforcing the ecological validity
of this study. From a practical point of view and considering the lack of reference values for assessing the autonomic cardiac function during maximal exercise and recovery in children
concerning weight status and CRF level, the values obtained in this study might play a key role for teachers, coaches, and physicians who work with children aged 10–16 years. It will enable
the development of individualized health programs based on measurable values of HR. In addition, assessing HR has a minimal cost and its ease of use allows the test to be employed in both
sports and clinical practice. CONCLUSIONS In conclusion, aerobic performance, vagal activity, BP, chronotropic competence, and HRR have significant associations with CMR. In accordance with
Dangardt et al.,97 children with OW and OB show signs of autonomic dysfunction reflected as low cardiac vagal activity and poor chronotropic competence. Therefore, weight reduction and
increased PA may, in concert or independently of each other, restore cardiac autonomic control back to normal operating balance in children who are OW and OB, and possibly helping to
maintain their normal cardiac autonomic function long term.97 The age- and sex-specific reference range at rest, during and post-exercise HR, determined by Léger test, provides the
assessment and monitoring of exercise-induced adaptations in the cardiovascular system and the potential to detect children with poor exercise tolerance or abnormal hemodynamic responses to
exercise. DATA AVAILABILITY The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request. REFERENCES * Ahn, S.
& Fedewa, A. L. A meta-analysis of the relationship between children’s physical activity and mental health. _J. Pediatr. Psychol._ 36, 385–397 (2011). Article PubMed Google Scholar *
Martínez-Gómez, D. et al. Sedentary behavior, adiposity, and cardiovascular risk factors in adolescents. The AFINOS study. _Rev Española Cardiol. (Engl. Ed.)_ 63, 277–285 (2010). Article
Google Scholar * Lee, E. Y. & Yoon, K.-H. Epidemic obesity in children and adolescents: risk factors and prevention. _Front. Med._ 12, 658–666 (2018). Article PubMed Google Scholar *
World Health Organization. _Levels and Trends in Child Malnutrition: UNICEF/WHO/The World Bank Group Joint Child Malnutrition Estimates: Key Findings of the 2021 Edition_ (WHO, 2021). *
Carnethon, M. R., Jacobs, D. R. Jr, Sidney, S. & Liu, K. Influence of autonomic nervous system dysfunction on the development of type 2 diabetes: the CARDIA study. _Diabetes Care_ 26,
3035–3041 (2003). Article PubMed Google Scholar * Soares‐Miranda, L. et al. Metabolic syndrome, physical activity and cardiac autonomic function. _Diabetes Metab. Res. Rev._ 28, 363–369
(2012). Article PubMed Google Scholar * Shah, A. S. et al. Severe obesity in adolescents and young adults is associated with subclinical cardiac and vascular changes. _J. Clin.
Endocrinol. Metab._ 100, 2751–2757 (2015). Article CAS PubMed PubMed Central Google Scholar * Bongers-Karmaoui, M. N., Jaddoe, V. W. V., Roest, A. A. W. & Gaillard, R. The
cardiovascular stress response as early life marker of cardiovascular health: applications in population-based pediatric studies—a narrative review. _Pediatr. Cardiol._ 41, 1739–1755 (2020).
Article PubMed PubMed Central Google Scholar * Billman, G. E., Sacha, J., Werner, B., Jelen, P. J. & Gąsior, J. S. Editorial: Heart rate variability and other autonomic markers in
children and adolescents. _Front. Physiol._ 11, 1265 (2019). Article Google Scholar * Evans, C. A., Selvadurai, H., Baur, L. A. & Waters, K. A. Effects of obstructive sleep apnea and
obesity on exercise function in children. _Sleep_ 37, 1103–1110 (2014). Article PubMed PubMed Central Google Scholar * Metwalley, K. A., Hamed, S. A. & Farghaly, H. S. Cardiac
autonomic function in children with type 1 diabetes. _Eur. J. Pediatr._ 177, 805–813 (2018). Article PubMed Google Scholar * Soares-Miranda, L. et al. Vigorous physical activity and vagal
modulation in young adults. _Eur. J. Prev. Cardiol._ 16, 705–711 (2009). Article Google Scholar * Nascimento, R. D. et al. Sedentary lifestyle in adolescents is associated with impairment
in autonomic cardiovascular modulation. _Rev. Bras. Med. Esport._ 25, 191–195 (2019). Article Google Scholar * Farah, B. Q. et al. Association between sedentary recreational time and
cardiac autonomic modulation in adolescent boys: cross-sectional study. _Sport Sci. Health_ 16, 677–683 https://doi.org/10.1007/s11332-020-00641-7 (2020). * Latorre-Román, P.Á.,
Navarro-Martínez, A. V. & García-Pinillos, F. The effectiveness of an indoor intermittent training program for improving lung function, physical capacity, body composition and quality of
life in children with asthma. _J. Asthma_ 51, 544–551 (2014). * Zaqout, M. et al. Determinant factors of physical fitness in European children. _Int. J. Public Health_ 61, 573–582 (2016).
Article PubMed Google Scholar * Álvarez, C. et al. Associations of cardiorespiratory fitness and obesity parameters with blood pressure: fitness and fatness in youth Latin-American ethnic
minority. _Ethn. Health_ 27, 1058–1074 (2022). * Buchan, D. S., Knox, G., Jones, A. M., Tomkinson, G. R. & Baker, J. S. Utility of international normative 20 m shuttle run values for
identifying youth at increased cardiometabolic risk. _J. Sports Sci._ 37, 507–514 (2019). Article PubMed Google Scholar * Freeman, J. V., Dewey, F. E., Hadley, D. M., Myers, J. &
Froelicher, V. F. Autonomic nervous system interaction with the cardiovascular system during exercise. _Prog. Cardiovasc. Dis._ 48, 342–362 (2006). Article PubMed Google Scholar * Akdur,
H. et al. The evaluation of cardiovascular response to exercise in healthy Turkish children. _Turk. J. Pediatr._ 51, 472–477 (2009). PubMed Google Scholar * Oliveira, R. S., Barker, A. R.
& Williams, C. A. Cardiac autonomic function, cardiovascular risk and physical activity in adolescents. _Int. J. Sports Med._ 39, 89–96 (2018). Article PubMed Google Scholar *
Oliveira, R. S., Barker, A. R., Wilkinson, K. M., Abbott, R. A. & Williams, C. A. Is cardiac autonomic function associated with cardiorespiratory fitness and physical activity in
children and adolescents? A systematic review of cross-sectional studies. _Int. J. Cardiol._ 236, 113–122 (2017). Article PubMed Google Scholar * Franssen, W. et al. Cardiac function in
adolescents with obesity: cardiometabolic risk factors and impact on physical fitness. _Int. J. Obes._ 43, 1400–1410 (2019). Article Google Scholar * Schuster, I. et al. Cardiac function
during exercise in obese prepubertal boys: effect of degree of obesity. _Obesity_ 17, 1878–1883 (2009). Article PubMed Google Scholar * Gasior, J. S. et al. Normative values for heart
rate variability parameters in school-aged children: simple approach considering differences in average heart rate. _Front. Physiol._ 24, 1495 (2018). Article Google Scholar * Veijalainen,
A. et al. Associations of physical activity, sedentary time, and cardiorespiratory fitness with heart rate variability in 6- to 9-year-old children: the PANIC study. _Eur. J. Appl.
Physiol._ 119, 2487–2498 (2019). Article CAS PubMed PubMed Central Google Scholar * Plaza-Florido, A. et al. Heart rate is a better predictor of cardiorespiratory fitness than heart
rate variability in overweight/obese children: the Activebrains project. _Front. Physiol._ 10, 510 (2019). Article PubMed PubMed Central Google Scholar * Sobradillo, B. et al. Curvas y
tablas de crecimiento (estudio longitudinal y transversal) [Internet]. Fundación Faustino Orbegozo Eizaguirre. http://www.aepap.org/pdf/f_orbegozo_04.pdf (2004). * Latorre-Román, P. A. et
al. Comprehensive cardiac evaluation to maximal exercise in a contemporary population of prepubertal children. _Pediatr. Res_. 92, 526–535 https://doi.org/10.1038/s41390-021-01809-8 (2021).
* Léger, L. A., Mercier, D., Gadoury, C. & Lambert, J. The multistage 20 metre shuttle run test for aerobic fitness. _J. Sports Sci._ 6, 93–101 (1988). Article PubMed Google Scholar *
Borg, G. A. Psychophysical bases of perceived exertion. _Med. Sci. Sports Exerc._ 14, 377–381 (1982). Article CAS PubMed Google Scholar * Parak, J. et al. Evaluation of the beat-to-beat
detection accuracy of PulseOn wearable optical heart rate monitor. In _2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society_ 8099–8102 (IEEE,
2015). * Parak, J. & Korhonen, I. Accuracy of Firstbeat Bodyguard 2 beat-to-beat heart rate monitor. White Paper.
https://www.firstbeat.com/en/accuracy-firstbeat-bodyguard-2-heart-rate-monitor/ (2013). * European Society of Cardiology. Heart rate variability: standards of measurement, physiological
interpretation and clinical use. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. _Circulation_ 93, 1043–1065 (1996). Article
Google Scholar * Sami, S., Mikko, S. & Antti, K. Advanced methods for processing bioelectrical signals artefact correction for heart beat interval data.
https://www.firstbeat.com/app/uploads/2015/10/saalasti_et_al_probisi_2004_congress.pdf (2004). * Routledge, F. S., Campbell, T. S., McFetridge-Durdle, J. A. & Bacon, S. L. Improvements
in heart rate variability with exercise therapy. _Can. J. Cardiol._ 26, 303–312 (2010). Article PubMed PubMed Central Google Scholar * Cachadiña, E. S. et al. Heart rate variability is
lower in patients with intermittent claudication: a preliminary study. _Arch. Med. Deport._ 35, 218–221 (2018). Google Scholar * von Scheidt, F. et al. Heart rate response during treadmill
exercise test in children and adolescents with congenital heart disease. _Front. Pediatr._ 7, 65 (2019). Article Google Scholar * Easley, E. A. et al. Recovery responses to maximal
exercise in healthy-weight children and children with obesity. _Res. Q Exerc. Sport_ 89, 38–46 (2018). Article PubMed Google Scholar * Hager, A. Normal values for cardiopulmonary exercise
testing in children. _Eur. J. Prev. Cardiol._ 18, 675 (2011). Article Google Scholar * Peçanha, T., Silva-Júnior, N. D. & Forjaz, C. LdeM. Heart rate recovery: autonomic determinants,
methods of assessment and association with mortality and cardiovascular diseases. _Clin. Physiol. Funct. Imaging_ 34, 327–339 (2014). Article PubMed Google Scholar * Jouven, X. et al.
Heart-rate profile during exercise as a predictor of sudden death. _N. Engl. J. Med._ 352, 1951–1958 (2005). Article CAS PubMed Google Scholar * Cheng, Y. J. et al. Heart rate recovery
following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. _Diabetes Care_ 26, 2052–2057 (2003). Article PubMed Google
Scholar * Young, F. L. S. & Leicht, A. S. Short-term stability of resting heart rate variability: influence of position and gender. _Appl. Physiol. Nutr. Metab._ 36, 210–218 (2011).
Article PubMed Google Scholar * Speer, K. E., Semple, S., Naumovski, N. & McKune, A. J. Measuring heart rate variability using commercially available devices in healthy children: a
validity and reliability study. _Eur. J. Investig. Health Psychol. Educ._ 10, 390–404 (2020). PubMed PubMed Central Google Scholar * Buchheit, M. et al. Supramaximal training and
postexercise parasympathetic reactivation in adolescents. _Med. Sci. Sport Exerc._ 40, 362–371 (2008). Article Google Scholar * Bentley, R. F. et al. Heart rate variability and recovery
following maximal exercise in endurance athletes and physically active individuals. _Appl. Physiol. Nutr. Metab._ 45, 1138–1144 (2020). Article CAS PubMed Google Scholar * Hopkins, W.
G., Marshall, S. W., Batterham, A. M. & Hanin, J. Progressive statistics for studies in sports medicine and exercise science. _Med. Sci. Sports Exerc._ 41, 3–13 (2009). Article PubMed
Google Scholar * Kwok, S. Y. et al. Resting heart rate in children and adolescents: association with blood pressure, exercise and obesity. _Arch. Dis. Child._ 98, 287–291 (2013). Article
PubMed Google Scholar * Fernandes, R. A. et al. Resting heart rate is associated with blood pressure in male children and adolescents. _J. Pediatr._ 158, 634–637 (2011). Article PubMed
Google Scholar * Silva, C. F. et al. Relationship between cardiometabolic parameters and elevated resting and effort heart rate in schoolchildren. _Arq. Bras. Cardiol._ 109, 191–198 (2017).
PubMed PubMed Central Google Scholar * Lindgren, M. et al. Resting heart rate in late adolescence and long term risk of cardiovascular disease in Swedish men. _Int. J. Cardiol._ 259,
109–115 (2018). Article PubMed Google Scholar * Ørntoft, C. et al. Physical fitness and body composition in 10-12-year-old danish children in relation to leisure-time club-based sporting
activities. _Biomed. Res. Int._ 27, 9807569 (2018). Google Scholar * Sarganas, G., Schaffrath Rosario, A. & Neuhauser, H. K. Resting heart rate percentiles and associated factors in
children and adolescents. _J. Pediatr._ 187, 174.e3–181.e3 (2017). Article Google Scholar * Salameh, A. et al. Normal limits for heart rate as established using 24-hour ambulatory
electrocardiography in children and adolescents. _Cardiol. Young._ 18, 467–472 (2008). Article PubMed Google Scholar * Baruteau, A. E., Perry, J. C., Sanatani, S., Horie, M. & Dubin,
A. M. Evaluation and management of bradycardia in neonates and children. _Eur. J. Pediatr._ 175, 151–161 (2016). Article CAS PubMed Google Scholar * Alom, M. M. et al. Physical training
induced resting bradycardia and its association with cardiac autonomic nervous activities. _Mymensingh Med. J._ 20, 665–670 (2011). CAS PubMed Google Scholar * Obert, P. et al. Effect of
aerobic training and detraining on left ventricular dimensions and diastolic function in prepubertal boys and girls. _Int. J. Sports Med._ 22, 90–96 (2001). Article CAS PubMed Google
Scholar * Rowland, T. W. in _The Young Athlete_ 39-49 (Blackwell, 2008). * Triposkiadis, F. et al. Cardiac adaptation to intensive training in prepubertal swimmers. _Eur. J. Clin. Invest._
32, 16–23 (2002). Article CAS PubMed Google Scholar * Fleming, S. et al. Normal ranges of heart rate and respiratory rate in children from birth to 18 years of age: a systematic review
of observational studies. _Lancet_ 19, 1011–1018 https://doi.org/10.1016/S0140-6736(10)62226-X (2011). * Andersen, K. L. & Ghesquiere, J. Sex differences in maximal oxygen uptake, heart
rate and oxygen pulse at 10 and 14 years in Norwegian children. _Hum. Biol._ 44, 413–431 (1972). CAS PubMed Google Scholar * Fomin, Å. et al. Sex differences in response to maximal
exercise stress test in trained adolescents. _BMC Pediatr._ 12, 127 (2012). Article PubMed PubMed Central Google Scholar * Verschuren, O., Maltais, D. B. & Takken, T. The 220-age
equation does not predict maximum heart rate in children and adolescents. _Dev. Med. Child Neurol._ 53, 861–864 (2011). Article PubMed Google Scholar * Paridon, S. M. et al. Clinical
stress testing in the pediatric age group: a statement from the American Heart Association Council on Cardiovascular Disease in the Young, Committee on Atherosclerosis, Hypertension, and
Obesity in Youth. _Circulation_ 113, 1905–1920 (2006). Article PubMed Google Scholar * Van Brussel, M., Bongers, B. C., Hulzebos, E. H. J., Burghard, M. & Takken, T. A systematic
approach to interpreting the cardiopulmonary exercise test in pediatrics. _Pediatr. Exerc. Sci._ 31, 194–203 (2019). Article PubMed Google Scholar * Gelbart, M., Ziv-Baran, T., Williams,
C. A., Yarom, Y. & Dubnov-Raz, G. Prediction of maximal heart rate in children and adolescents. _Clin. J. Sport Med._ 27, 139–144 (2017). Article PubMed Google Scholar * Baba, R.,
Iwagaki, S., Tauchi, N. & Tsurusawa, M. Is the chronotropic index applicable to children and adolescents? _Circ. J._ 69, 471–474 (2005). Article PubMed Google Scholar * Franssen, W.
M. A. et al. Chronotropic incompetence is more frequent in obese adolescents and relates to systemic inflammation and exercise intolerance. _J. Sport Health Sci_. 12, 194–201 (2023). *
Nikolaidis, P. et al. The effect of body mass index on acute cardiometabolic responses to graded exercise testing in children: a narrative review. _Sports_ 6, 103 (2018). Article PubMed
PubMed Central Google Scholar * Karvonen, J. & Vuorimaa, T. Heart rate and exercise intensity during sports activities. _Sport Med._ 5, 303–312 (1988). Article CAS Google Scholar *
Delgado-Floody, P., Alvarez, C., Caamaño-Navarrete, F., Jerez-Mayorga, D. & Latorre-Román, P. Influence of Mediterranean diet adherence, physical activity patterns, and weight status on
cardiovascular response to cardiorespiratory fitness test in Chilean school children. _Nutrition_ 71, 110621 (2020). Article PubMed Google Scholar * Hansen, D. et al. Exercise tolerance
in obese vs. lean adolescents: a systematic review and meta‐analysis. _Obes. Rev._ 15, 894–904 (2014). Article CAS PubMed Google Scholar * Lin, L.-Y. et al. Inverse correlation between
heart rate recovery and metabolic risks in healthy children and adolescents: insight from the National Health and Nutrition Examination Survey 1999-2002. _Diabetes Care_ 31, 1015–1020
https://doi.org/10.2337/dc07-2299 (2008). Article PubMed PubMed Central Google Scholar * Guilkey, J. P., Dykstra, B., Erichsen, J. & Mahon, A. D. Heart rate response and variability
following maximal exercise in overweight children. _Pediatr. Exerc. Sci._ 29, 341–349 (2017). Article PubMed Google Scholar * Laguna, M., Aznar, S., Lara, M. T., Lucía, A. & Ruiz, J.
R. Heart rate recovery is associated with obesity traits and related cardiometabolic risk factors in children and adolescents. _Nutr. Metab. Cardiovasc. Dis._ 23, 995–1001 (2013). Article
CAS PubMed Google Scholar * Guilkey, J. P., Overstreet, M. & Mahon, A. D. Heart rate recovery and parasympathetic modulation in boys and girls following maximal and submaximal
exercise. _Eur. J. Appl. Physiol._ 115, 2125–2133 (2015). Article CAS PubMed Google Scholar * Dimkpa, U. Post-exercise heart rate recovery: an index of cardiovascular fitness. _J. Exerc.
Physiol. Online_ 12, 10–22 (2009). Google Scholar * Fernando, R. J., Ravichandran, K. & Vaz, M. Aerobic fitness, heart rate recovery and heart rate recovery time in indian school
children. _Indian J. Physiol. Pharm._ 59, 407–413 (2015). Google Scholar * Sharma, V. K., Subramanian, S. K., Arunachalam, V. & Rajendran, R. Heart rate variability in
adolescents–normative data stratified by sex and physical activity. _J. Clin. Diagn. Res._ 9, CC08 (2015). PubMed PubMed Central Google Scholar * Vanderlei, L. C. M., Pastre, C. M.,
Junior, I. F. F. & de Godoy, M. F. Analysis of cardiac autonomic modulation in obese and eutrophic children. _Clinics_ 65, 789–792 (2010). Article PubMed PubMed Central Google Scholar
* Jarrin, D. C. et al. Short-term heart rate variability in a population-based sample of 10-year-old children. _Pediatr. Cardiol._ 36, 41–48 (2015). Article PubMed Google Scholar *
Gasior, J. S. et al. Interaction between heart rate variability and heart rate in pediatric population. _Front. Physiol._ 18, 385 (2015). Google Scholar * Bobkowski, W. et al. Measures of
heart rate variability in 24-h ECGs depend on age but not gender of healthy children. _Front. Physiol._ 8, 311 (2017). Article PubMed PubMed Central Google Scholar * Kaufman, C. L.,
Kaiser, D. R., Steinberger, J., Kelly, A. S. & Dengel, D. R. Relationships of cardiac autonomie function with metabolic abnormalities in childhood. _Obes. Obes._ 15, 1164–1171 (2007).
CAS Google Scholar * Leppänen, M. H. et al. Associations of cardiometabolic risk factors with heart rate variability in 6- to 8-year-old children: the PANIC study. _Pediatr. Diabetes_ 21,
251–258 (2020). Article PubMed Google Scholar * Subramanian, S. K., Sharma, V. K., Arunachalam, V., Rajendran, R. & Gaur, A. Comparison of baroreflex sensitivity and cardiac autonomic
function between adolescent athlete and non-athlete boys–a cross-sectional study. _Front. Physiol._ 10, 1043 (2019). Article PubMed PubMed Central Google Scholar * Chen, H. et al.
Effects of physical activity on heart rate variability in children and adolescents: a systematic review and meta-analysis. _Cien Saude Colet._ 27, 1827–1842 (2022). Article PubMed Google
Scholar * Grant, C. C., Murray, C., Janse van Rensburg, D. C. & Fletcher, L. A comparison between heart rate and heart rate variability as indicators of cardiac health and fitness.
_Front. Physiol._ 20, 337 (2013). Google Scholar * Gamelin, F. X. et al. Effect of high intensity intermittent training on heart rate variability in prepubescent children. _Eur. J. Appl.
Physiol._ 105, 731–738 (2009). Article PubMed Google Scholar * Da Silva, C. C., Pereira, L. M., Cardoso, J. R., Moore, J. P. & Nakamura, F. Y. The effect of physical training on heart
rate variability in healthy children: a systematic review with meta-analysis. _Pediatr. Exerc. Sci._ 26, 147–158 (2014). Article PubMed Google Scholar * Mahon, A. D., Anderson, C. S.,
Hipp, M. J. & Hunt, K. A. Heart rate recovery from submaximal exercise in boys and girls. _Med. Sci. Sports Exerc._ 35, 2093–2097 (2003). Article PubMed Google Scholar * Desai, M. Y.,
De La Peña-Almaguer, E. & Mannting, F. Abnormal heart rate recovery after exercise as a reflection of an abnormal chronotropic response. _Am. J. Cardiol._ 15, 1164–1169 (2001). Article
Google Scholar * Legantis, C. D. et al. Role of cardiorespiratory fitness and obesity on hemodynamic responses in children. _J. Sport Med. Phys. Fit._ 52, 311–318 (2012). CAS Google
Scholar * Kelishadi, R., Mirmoghtadaee, P., Najafi, H. & Keikha, M. Systematic review on the association of abdominal obesity in children and adolescents with cardio-metabolic risk
factors. _J. Res. Med. Sci. J. Isfahan Univ. Med. Sci._ 20, 294 (2015). Google Scholar * Proudfoot, N. A. et al. Physical activity and trajectories of cardiovascular health indicators
during early childhood. _Pediatrics_ 144, e20182242 (2019). Article PubMed Google Scholar * Dangardt, F. et al. Reduced cardiac vagal activity in obese children and adolescents. _Clin.
Physiol. Funct. Imaging_ 31, 108–113 (2011). PubMed Google Scholar Download references AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Didactic of Music, Plastic and Corporal
Expression, University of Jaen, 23071, Jaen, Spain Pedro Á. Latorre-Román, Ana de la Casa Pérez, David Pancorbo-Serrano, Pedro J. Consuegra-Gonzalez, Marcos Muñoz-Jiménez, Juan M. Ramírez
Lucas, José Carlos Cabrera-Linares & Juan A. Párraga-Montilla * Universidad Autónoma de Chile, Temuco, Chile Jesús Salas-Sánchez * Facultad de Educación, Universidad Internacional de la
Rioja, Logroño, Spain Jesús Salas-Sánchez * Department of Health Sciences, Area of Physiology, University of Jaen, Jaen, Spain Jerónimo Aragón-Vela Authors * Pedro Á. Latorre-Román View
author publications You can also search for this author inPubMed Google Scholar * Ana de la Casa Pérez View author publications You can also search for this author inPubMed Google Scholar *
David Pancorbo-Serrano View author publications You can also search for this author inPubMed Google Scholar * Pedro J. Consuegra-Gonzalez View author publications You can also search for
this author inPubMed Google Scholar * Jesús Salas-Sánchez View author publications You can also search for this author inPubMed Google Scholar * Marcos Muñoz-Jiménez View author publications
You can also search for this author inPubMed Google Scholar * Jerónimo Aragón-Vela View author publications You can also search for this author inPubMed Google Scholar * Juan M. Ramírez
Lucas View author publications You can also search for this author inPubMed Google Scholar * José Carlos Cabrera-Linares View author publications You can also search for this author inPubMed
Google Scholar * Juan A. Párraga-Montilla View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS All the authors made substantial contributions
to conception and design, acquisition of data, or analysis and interpretation of data; drafted the article or revised it critically for important intellectual content; and gave final
approval of the version to be published. CORRESPONDING AUTHOR Correspondence to Jerónimo Aragón-Vela. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE Parents voluntarily signed an informed consent for the participation of their children in this study. ADDITIONAL INFORMATION PUBLISHER’S NOTE
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society
or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of
this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Latorre-Román, P.Á., de la Casa
Pérez, A., Pancorbo-Serrano, D. _et al._ Influence of physical fitness and weight status on autonomic cardiac modulation in children. _Pediatr Res_ 94, 1754–1763 (2023).
https://doi.org/10.1038/s41390-023-02676-1 Download citation * Received: 07 February 2023 * Revised: 31 March 2023 * Accepted: 20 April 2023 * Published: 07 June 2023 * Issue Date: November
2023 * DOI: https://doi.org/10.1038/s41390-023-02676-1 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable
link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative








