- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT BACKGROUND Hepcidin is a master regulator of iron metabolism. Recently, it has been shown that vitamin D suppresses hepcidin expression. Our hypothesis was that hepcidin levels
inversely correlate with vitamin D levels in anemic children during acute infection. METHODS A prospective study was performed on 90 patients (45 females, 45 males, mean age 7.3 ± 5 years)
who were admitted to the pediatric ward. Sixty-two patients had infectious disease (32 with coexisting anemia, 30 without anemia), and 28 patients were hospitalized for noninfectious causes.
Blood samples for IL-6, hepcidin, iron status parameters, and 25-hydroxyvitamin D (25-OHD) were obtained within 72 h after admission. RESULTS Serum concentrations of IL-6 and hepcidin were
significantly higher and 25-OHD, iron, and transferrin were significantly lower in anemic children with infectious disease compared with controls. Children with a serum 25-OHD level < 20
ng/ml had significantly increased odds of having anemia than those with a level > 20 ng/ml (OR: 6.1, CI: 1.15–32.76). Correlation analyses found positive associations between hepcidin
levels and ferritin (_R_2 = 0.47, _P_ < 0.001) and negative associations between hepcidin and transferrin (_R_2 = 0.57, _P_ < 0.001). CONCLUSION Higher IL-6 and lower 25-OHD levels may
lead to higher hepcidin levels and subsequently to hypoferremia and anemia in children with acute infection. You have full access to this article via your institution. Download PDF SIMILAR
CONTENT BEING VIEWED BY OTHERS CHARACTERIZATION OF ACQUIRED ANEMIA IN CHILDREN BY IRON METABOLISM PARAMETERS Article Open access 17 February 2022 SERUM ERYTHROFERRONE LEVELS DURING THE FIRST
MONTH OF LIFE IN PREMATURE INFANTS Article 10 August 2021 DYNAMICS OF IRON METABOLISM IN PATIENTS WITH BLOODSTREAM INFECTIONS: A TIME-COURSE CLINICAL STUDY Article Open access 06 November
2023 INTRODUCTION Anemia is known to occur in the setting of chronic infection as well as in inflammatory disease.1,2 Hepcidin, a master regulator of iron metabolism, is an important factor
in the development of anemia associated with inflammation.3,4,5,6 Hepcidin functions as a regulator of cellular iron export by decreasing the amount of ferroportin, a membrane protein that
is the major exporter of iron from cells, including macrophages that recycle iron, duodenal enterocytes that absorb dietary iron, and hepatocytes that store iron.5,7 During inflammatory
states or infection, hepcidin expression is upregulated by several proinflammatory cytokines, including interleukin-6 (IL-6) and IL-18,9 via both the BMP/SMAD and the JAK STAT3 inflammatory
signaling pathways.5,10 This upregulation of hepcidin limits the pool of extracellular iron by preventing iron release from the cells and its availability for erythropoiesis, causing
iron-restrictive anemia.5,7,8 Hepcidin was initially described as an antimicrobial protein because hepcidin-induced hypoferremia is thought to be a defense against bacterial infection by
withholding iron from invading pathogens.5 It has recently been demonstrated that vitamin D is a potent regulator of hepcidin in humans.11,12 Treatment of cultured hepatocytes or monocytes
with 25-OHD3 or 1,25(OH)2D3 decreased the expression of hepcidin mRNA by 0.5-fold. Promotor–receptor and chromatin immunoprecipitation analysis indicated that direct transcriptional
expression of hepcidin gene by 1,25(OH)2D3 caused a decrease in hepcidin mRNA levels. Furthermore, two pilot studies on healthy volunteers reported that supplementation with a single oral
high dose of vitamin D significantly reduced the circulating levels of hepcidin.11,13 Indeed, the association between vitamin D deficiency and anemia has been described in a number of
observational studies in both healthy and diseased populations.14,15,16,17 In the present study, we aimed to investigate the association between anemia associated with infection, hepcidin,
and vitamin D status in hospitalized pediatric patients, and to examine the role of hepcidin and vitamin D in the setting of anemia during acute inflammation in children. METHODS SUBJECTS We
prospectively recruited male and female children aged 1–16 years who were admitted to the ward at Dana-Dwek Children’s Hospital of the Tel Aviv Medical Center between 1 February, 2016, and
3 December, 2016. The study population consisted of three groups: (1) children with acute bacterial infections (i.e., osteomyelitis/septic arthritis, pyelonephritis, pneumonia, mastoiditis,
etc.) and coexisting anemia (defined as hemoglobin levels < 11 mg/dl upon admission),13,18 (2) children with acute infection without anemia, and (3) children hospitalized for elective
surgery who were free of infection and served as controls. All patients in the infectious groups had high fever and all needed hospitalization for treatment with IV antibiotics. Children who
were receiving medications known to alter vitamin D metabolism or who had a malabsorption, hepatic, or renal disease, as well as those known to have nutritional deficiencies, such as B12 or
folic acid, were excluded from the study. The study protocol was approved by the institutional review board of the medical center. A written informed consent was obtained from the parents
of all the participants. MEASUREMENTS Blood samples for the measurement of IL-6, hepcidin, iron, ferritin, transferrin, complete blood count (CBC), C-reactive protein (CRP), and 25-OHD were
obtained from all children within 72 h after admission. Sera were separated and frozen at –80°C until analysis. The 25-OHD concentration was measured by a radioimmunoassay commercial kit
(25-hydroxyvitamin D RIH kit, 68100E, DiaSorin, Via Crescento, Italy) which has a sensitivity of 1.5 ng/ml and a coefficient of variance of 8.1%. Vitamin D deficiency was defined as a 25-OHD
level < 20 ng/ml. IL-6 was measured using Human IL-6 ELISA Ready-SET-Go!® eBioscience, Thermo Fisher Scientific (Paisley, UK) that has a sensitivity of 2 pg/ml. Hepcidin was measured by
DRG Hepcidin 25 (bioactive) HS ELISA (Springfield, NJ) with an analytical sensitivity of 0.143 ng/ml, an intra-assay variability of 5.3%, and an inter-assay variability of 9.5%. Blood
chemistry and CBC were measured by standard laboratory methods. DATA ANALYSIS The statistical analyses were performed using Minitab version 16 (Minitab Inc, State College, PA). Descriptive
statistics were examined for all variables. Continuous variables were expressed as median with range when they were not normally distributed, and as mean ± standard deviation (SD) for
normally distributed variables. Continuous variables that did not follow a normal distribution were logarithmically transformed (serum ferritin and IL-6) for modeling. Categorical variables
were presented as number and percentage. Differences in demographic and biochemical variables between the three studied groups were examined using one-way ANOVA for continuous variables and
the _χ_2 or Fisher’s exact test for categorical variables. The Pearson correlation and simple linear-regression analysis were performed to examine bivariate associations of vitamin D and
hepcidin with biomarkers related to anemia and inflammation. A _P_ value ≤ 0.05 was considered significant. RESULTS Ninety patients (45 females and 45 males, mean age 7.3 ± 5 years) were
enrolled after their admission to the pediatric ward. Sixty-two patients had infectious disease, 32 with coexisting anemia and 30 without anemia. Twenty-eight patients were hospitalized for
noninfectious causes (elective surgery) and served as controls. Table 1 depicts the distribution of the acute infectious diseases between the study groups. There was no correlation between
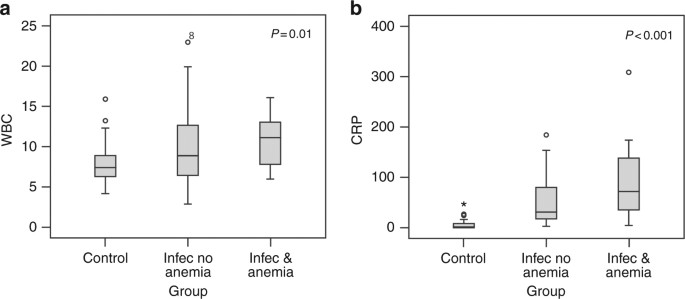
specific diseases and the development of anemia, although a more severe disease was observed in children with both infection and anemia, as reflected by higher mean CRP and WBC levels (_P_
_<_ 0.01 and _P_ < 0.001, respectively, Fig. 1). Table 2 displays the laboratory data of the patients with infection and anemia, those with infection without anemia, and controls. The
hepcidin, ferritin, IL-6 levels, and platelet count were significantly higher, and serum iron parameters (serum iron and transferrin) were significantly lower in patients with infections and
anemia compared with the other two groups of patients. Six patients with infection and anemia, two patients with infection without anemia, and none in the control group had vitamin D
deficiency. The mean 25-OHD levels were significantly lower among the infectious and anemic patients, and the patients with a 25-OHD level < 20 ng/ml had significantly increased odds of
having anemia than those with a 25-OHD level > 20 ng/ml (odds ratio (OR): 6.1, confidence interval (CI): 1.15–32.76). However, there was no significant correlation between serum 25-OHD
levels and circulating levels of hepcidin. There was significant positive correlation between serum hepcidin and ferritin levels (_R_2=0.47, _P_ < 0.001), and a significant negative
correlation between hepcidin and transferrin levels (_R_2 = 0.57, _P_ < 0.001, Fig. 2). DISCUSSION The findings of the present study demonstrate that the IL-6 and hepcidin levels were
significantly higher, and that the iron and transferrin levels were significantly lower in children with acute infectious disease and coexisting anemia than in children with infectious
disease and no anemia as well as in the noninfection controls. These results suggest that hepcidin-induced hypoferremia might be involved in the pathogenesis of anemia of acute infection in
children. It is now widely accepted that hepcidin has a pivotal role in the pathogenesis of iron-deficiency anemia of chronic infection and inflammatory disease.3,4,5 Anemia is also a
recognized feature of acute, moderate, and severe infection in previously healthy children who were hospitalized for a variety of acute infections,18 and in children with acute infection
seen in pediatric outpatient clinics.17 Children with moderately severe acute infections reportedly experienced a mean hemoglobin drop of 1.8 mg/dl within less than 1 week of illness onset,
regardless of the specific cause of infection.18 Anemia was reversible after resolution of the infection without the need for iron supplementation in the majority of children.17 The rapid
effect of acute bacterial infection on the IL-6-hepcidin axis and the development of hypoferremia have been studied in both humans and in mice models. Kemna et al.19 examined the temporal
association and responses of plasma IL-6 levels, hepcidin levels, and serum iron parameters in 10 healthy volunteers following injections of lipopolysaccharides. IL-6 was dramatically
reduced within 6 h, followed by a significant decrease in serum iron concentration. Darton et al.20 also demonstrated a strong early hepcidin upregulation and hypoferremia following the
injection of _Salmonella typhi_ to 50 healthy volunteers. Studies with models of wild-type and hepcidin knockout mice challenged with sublethal doses of either lipopolysaccharides21 or
heat-killed _Brucella abortus_22 demonstrated that hepcidin and ferroportin are important but not the sole mediators of acute hypoferremia. Those authors showed that the development of acute
hypoferremia during infection relied on both hepcidin-dependent and hepcidin-independent mechanisms. Furthermore, in addition to hepcidin-induced restrictive hypoferremia, those mouse
models showed a multifactorial pathogenesis of inflammatory anemia, including suppression of erythropoiesis and shortened erythrocyte lifespan.22,23 The association between vitamin D
deficiency and anemia—particularly anemia of inflammation—has been previously described by observational and epidemiologic studies.13,14,15,16 There are several possible mechanisms that
could explain this association. One is that vitamin D has both direct and indirect suppressive effects on hepcidin expression.11,12 Another is that 1,25(OH)2D3 interacts directly with
vitamin D response elements on the promotor of the hepcidin gene in monocytes and hepatocytes and suppresses hepcidin mRNA transcription.11,12 Vitamin D also has an indirect effect on
hepcidin expression by the suppression of proinflammatory cytokines which stimulate hepcidin production during inflammation.12 In addition, 1,25(OH)2D3 has been shown to directly support
erythropoiesis by increasing burst-forming unit erythroid proliferation and to have a synergistic effect with erythropoietin to further enhance erythroid progenitor cell proliferation.23
Serum 25-OHD levels were significantly lower and hepcidin levels were significantly higher in children with an infectious disease and coexisting anemia than in children with an infectious
disease without anemia and in patients without any infection. A serum 25-OHD level < 20 ng/ml held significantly increased odds of anemia than a serum 25-OHD level > 20 ng/ml. However,
there was no significant correlation between serum 25-OHD levels and circulating levels of hepcidin. One possible explanation for this is that although the intracellular production of
1,25(OH)2D3 in monocytes and macrophages appears to be sensitive to the availability of substrate 25-OHD in the serum, the hepcidin–ferroportin axis is suppressed by the 1,25(OH)2D3 produced
by intracellular 25-OHD3-1 alpha hydroxylase, and is actually regulated by several different cytokines and Toll-like receptors.24 Each of these factors may influence intracellular
1,25(OH)2D3 production and, subsequently, hepcidin expression. Moreover, hepcidin synthesis and release from hepatocytes and macrophages into the circulation are controlled by at least eight
different proteins.25 Interestingly, Adams et al.26 who studied the direct effect of vitamin D on the expression of cathelicidin, another antibacterial protein, in monocytes found that
although 1,25(OH)2D3 directly stimulates cathelicidin expression, there was a lack of any correlation between serum 25-OHD levels and circulating levels of cathelicidin. Those authors
emphasized the importance of a local intracrine mechanism regulating 1,25(OH)2D3 and cathelicidin production. In summary, the findings of the present study indicate that the
hepcidin–ferroportin axis is involved in the pathogenesis of hypoferremia and iron-restrictive anemia in children with acute infectious diseases, and suggests that a low vitamin D status may
be a contributing factor to anemia. REFERENCES * Weiss, G. & Goodnough, L. T. Anemia of chronic disease. _N. Engl. J. Med_ 352, 1011–1023 (2005). Article CAS Google Scholar * Weiss,
G. Iron metabolism in the anemia of chronic disease. _Biochim. Biophys. Acta_ 1790, 682–693 (2009). Article CAS Google Scholar * Ganz, T. & Nemeth, E. Hepcidin and iron homeostasis.
_Biochim. Biophys. Acta_ 1823, 1434–1443 (2012). Article CAS Google Scholar * Ganz, T. Hepcidin, a key regulator of iron metabolism and mediator of anemia of inflammation. _Blood_ 102,
783–788 (2003). Article CAS Google Scholar * Schmidt, P. J. Regulation of iron metabolism by hepcidin under condition of inflammation. _J. Biol. Chem._ 290, 18975–18983 (2015). Article
CAS Google Scholar * Girelli, D., Nemeth, E. & Swinkels, D. W. Hepcidin in the diagnosis of iron disorders. _Blood_ 127, 2809–1 (2016). Article CAS Google Scholar * Nemeth, E. et
al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. _Science_ 306, 2090–2093 (2004). Article CAS Google Scholar * Nemeth, E. et al.
IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. _J. Clin. Invest_ 113, 1271–1276 (2004). Article CAS Google Scholar * Lee,
P., Peng, H., Gelbart, T., Wang, L. & Beutler, E. Regulation of hepcidin transcription by interleukin-1 and interleukin-6. _Proc. Natl Acad. Sci. USA_ 102, 1906–1910 (2005). Article CAS
Google Scholar * Bacchetta, J. et al. Suppression of iron-regulatory hepcidin by vitamin D. _J. Am. Soc. Nephrol._ 25, 564–572 (2014). Article CAS Google Scholar * Zughaier, S. M.,
Alvarez, J. A., Sloan, J. H., Konrad, R. J. & Tangpricha, V. The role of vitamin D in regulating the iron-hepcidin-ferroportin axis in monocytes. _J. Clin. Transl. Endocrinol._ 1, 19–25
(2014). PubMed Google Scholar * Smith, E. M. et al. High-dose vitamin D3 reduces circulating hepcidin concentrations: a pilot, randomized, double-blind, placebo-controlled trial in healthy
adults. _Clin. Nutr._ 36, 980–985 (2017). Article CAS Google Scholar * Sim, J. J. et al. Vitamin D deficiency and anemia: a cross-sectional study. _Ann. Hematol._ 89, 447–452 (2010).
Article CAS Google Scholar * Lee, J. A. et al. Low vitamin D levels are associated with both iron deficiency and anemia in children and adolescents. _Pediatr. Hematol. Oncol._ 32, 99–108
(2015). Article CAS Google Scholar * Atkinson, M. A. et al. Vitamin D, race, and risk for anemia in children. _J. Pediatr._ 164, 153–158 (2014). Article CAS Google Scholar * Smith, E.
M. et al. Vitamin D deficiency is associated with anaemia among African Americans in a US cohort. _Br. J. Nutr._ 113, 1732–1740 (2015). Article CAS Google Scholar * Abshire, T. C. &
Reevers, J. D. Anemia of acute inflammation in children. _J. Pediatr._ 103, 868–871 (1983). Article CAS Google Scholar * Jansson, L. T., Kling, S. & Dallman, P. R. Anemia in children
with acute infections seen in a primary care pediatric outpatient clinic. _Pediatr. Infect. Dis._ 5, 424–427 (1986). Article CAS Google Scholar * Kemna, E., Pickkers, P., Nemeth, E., van
der Hoeven, H. & Swinkels, D. Time-course analysis of hepcidin, serum iron, and plasma cytokine levels in humans injected with LPS. _Blood_ 106, 1864–1866 (2005). Article CAS Google
Scholar * Darton, T. C. et al. Rapidly escalating hepcidin and associated serum iron starvation are features of the acute response to typhoid infection in humans. _PLoS Negl. Trop. Dis._ 9,
e0004029 (2015). Article Google Scholar * Deschemin, J. C. & Vaulont, S. Role of hepcidin in the setting of hypoferremia during acute inflammation. _PLoS ONE_ 8, e61050 (2013).
Article CAS Google Scholar * Kim, A. et al. A mouse model of anemia of inflammation: complex pathogenesis with partial dependence on hepcidin. _Blood_ 123, 1129–1136 (2014). Article CAS
Google Scholar * Alon, D. B. et al. Novel role of 1,25(OH)(2)D(3) in induction of erythroid progenitor cell proliferation. _Exp. Hematol._ 30, 403–409 (2002). Article Google Scholar *
Lagishetty, V., Liu, N. Q. & Hewison, M. Vitamin D metabolism and innate immunity. _Mol. Cell Endocrinol._ 347, 97–105 (2011). Article CAS Google Scholar * Zhao, N., Zhang, A. S.
& Enns, C. A. Iron regulation by hepcidin. _J. Clin. Invest_ 123, 2337–2343 (2013). Article CAS Google Scholar * Adams, J. S. et al. Vitamin d-directed rheostatic regulation of
monocyte antibacterial responses. _J. Immunol._ 182, 4289–4295 (2009). Article CAS Google Scholar Download references ACKNOWLEDGEMENTS Esther Eshkol is thanked for editorial assistance.
AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department of Pediatrics, Dana Dwek Children Hospital and The Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel Hadar
Moran-Lev, Yosef Weisman & Ronit Lubetzky * Department of Pediatric Gastroenterology, Dana Dwek Children Hospital and The Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv,
Israel Hadar Moran-Lev & Shlomi Cohen * Department of Hematology Laboratories, Tel Aviv Sourasky Medical Center and The Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel
Varda Deutsch & Michal Cipok * Preclinical Research Pulmonary Laboratory, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel Ekaterina Bondar * Department of Neonatology, Dana Dwek
Children Hospital and The Sackler Faculty of Medicine, Tel Aviv University, Tel Aviv, Israel Dror Mandel Authors * Hadar Moran-Lev View author publications You can also search for this
author inPubMed Google Scholar * Yosef Weisman View author publications You can also search for this author inPubMed Google Scholar * Shlomi Cohen View author publications You can also
search for this author inPubMed Google Scholar * Varda Deutsch View author publications You can also search for this author inPubMed Google Scholar * Michal Cipok View author publications
You can also search for this author inPubMed Google Scholar * Ekaterina Bondar View author publications You can also search for this author inPubMed Google Scholar * Ronit Lubetzky View
author publications You can also search for this author inPubMed Google Scholar * Dror Mandel View author publications You can also search for this author inPubMed Google Scholar
CORRESPONDING AUTHOR Correspondence to Hadar Moran-Lev. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER'S NOTE:
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE
THIS ARTICLE Moran-Lev, H., Weisman, Y., Cohen, S. _et al._ The interrelationship between hepcidin, vitamin D, and anemia in children with acute infectious disease. _Pediatr Res_ 84, 62–65
(2018). https://doi.org/10.1038/s41390-018-0005-0 Download citation * Received: 27 September 2017 * Revised: 17 January 2018 * Accepted: 21 January 2018 * Published: 23 May 2018 * Issue
Date: July 2018 * DOI: https://doi.org/10.1038/s41390-018-0005-0 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative






