- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
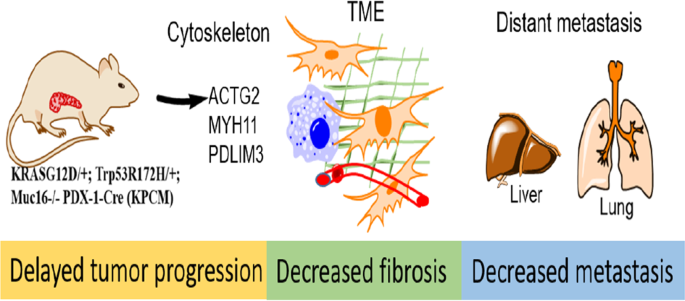
MUC16, membrane-bound mucin, plays an oncogenic role in pancreatic ductal adenocarcinoma (PDAC). However, the pathological role of MUC16 in the PDAC progression, tumor microenvironment, and
metastasis in cooperation with KrasG12D and Trp53R172H mutations remains unknown. Deletion of Muc16 with activating mutations KrasG12D/+ and Trp53R172H/+ in mice significantly decreased
progression and prolonged overall survival in KrasG12D/+; Trp53R172H/+; Pdx-1-Cre; Muc16−/− (KPCM) and KrasG12D/+; Pdx-1-Cre; Muc16−/− (KCM), as compared to KrasG12D/+; Trp53R172H/+;
Pdx-1-Cre (KPC) and KrasG12D/+; Pdx-1-Cre (KC) mice, respectively. Muc16 knockout pancreatic tumor (KPCM) displays decreased tumor microenvironment factors and significantly reduced
incidence of liver and lung metastasis compared to KPC. Furthermore, in silico data analysis showed a positive correlation of MUC16 with activated stroma and metastasis-associated genes.
KPCM mouse syngeneic cells had significantly lower metastatic and endothelial cell binding abilities than KPC cells. Similarly, KPCM organoids significantly decreased the growth rate
compared to KPC organoids. Interestingly, RNA-seq data revealed that the cytoskeletal proteins Actg2, Myh11, and Pdlim3 were downregulated in KPCM tumors. Further knockdown of these genes
showed reduced metastatic potential. Overall, our results demonstrate that Muc16 alters the tumor microenvironment factors during pancreatic cancer progression and metastasis by changing the
expression of Actg2, Myh11, and Pdlim3 genes.
The accession number for RNA sequencing data of Muc16 knockout pancreatic tumor tissues is GSE212777, and materials associated with the current study are available from the corresponding
author upon reasonable request.
We thank Dr. Richard R. Behringer, Department of Genetics, Division of Basic Science Research, The University of Texas MD Anderson Cancer Center, Houston, Texas, USA, for providing the Muc16
whole-body knockout (C57BL/6J) mice. We thank Corinn Grabow for all the technical support. We thank the University of Nebraska Medical Center core facilities such as Advanced Microscopy
Core Facility, Genomics Core Facility, and Tissue Science Facility. This work was, in parts, supported by the support from the National Institutes of Health P01 CA217798, R01 CA263575, R01
CA256973, R01 CA273349, R01 CA247471, R01 CA254036, R01 CA206444, R01 CA210637, R01 CA228524, F99 CA234962, U01 CA200466, and U01 CA210240, and the Nebraska Department of Health and Human
Services LB595, US Department of Veterans Affairs (I01 BX004676).
These authors contributed equally: Imayavaramban Lakshmanan, Saravanakumar Marimuthu.
Department of Biochemistry and Molecular Biology, University of Nebraska Medical Center, Omaha, NE, 68198-5870, USA
Imayavaramban Lakshmanan, Saravanakumar Marimuthu, Sanjib Chaudhary, Parthasarathy Seshacharyulu, Satyanarayana Rachagani, Sakthivel Muniyan, Ramakanth Chirravuri-Venkata, Pranita Atri,
Sanchita Rauth, Rama Krishna Nimmakayala, Jawed Akhtar Siddiqui, Shailendra K. Gautam, Ashu Shah, Gopalakrishnan Natarajan, Seema Parte, Namita Bhyravbhatla, Kavita Mallya, Dhanya Haridas,
Sushil Kumar, Maneesh Jain, Moorthy P. Ponnusamy & Surinder K. Batra
Department of Pathology and Microbiology, University of Nebraska Medical Center, Omaha, NE, 68198-5900, USA
Department of Biostatistics, College of Public Health, University of Nebraska Medical Center, Omaha, NE, 68198-4375, USA
Fred & Pamela Buffett Cancer Center, University of Nebraska Medical Center, Omaha, NE, 68198-5870, USA
Apar Kishor Ganti, Maneesh Jain, Moorthy P. Ponnusamy & Surinder K. Batra
Division of Oncology-Hematology, Department of Internal Medicine, VA Nebraska Western Iowa Health Care System, and University of Nebraska Medical Center, Omaha, NE, 68105-1850, USA
Eppley Institute for Research in Cancer and Allied Diseases, University of Nebraska Medical Center, Omaha, NE, 68198-5870, USA
IL, MPP, and SKB conceived and designed the experiments. IL and SM performed the experiments. IL, SM, PS, and SC assisted with in vivo experiments. Data were collected and analyzed by IL,
SM, SC, PS, SR, SM, RCV, PA, SR, RKN, JAS, SKG, AS, GN, SP, NB, KM, DH, GAT, LMS, SK, AKG, MJ, MPP. The manuscript was written by IL and SM with input from MPP, MJ, and SKB and reviewed by
all authors. Statistical analysis and IHC scoring were done by LMS and GAT, respectively. All authors have read and approved the final manuscript.
SKB is one of the co-founders of Sanguine. Diagnostics and Therapeutics, Inc. The other authors declare no potential conflicts of interest.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author
self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
Anyone you share the following link with will be able to read this content:









