- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Findings on the associations of dietary/tissue levels of omega-6 polyunsaturated fatty acids (n-6 PUFAs) with the risk of colorectal cancer (CRC) are conflicting. We conducted a
dose-response meta-analysis to assess the associations of dietary/tissue levels of n-6 PUFAs [total, linoleic acid (LA), and arachidonic acid (AA)] with CRC risk in adults. Twenty
prospective cohort studies with a total sample size of 787,490 participants were included. Comparing extreme intake levels of LA revealed the summary relative risks (RR) of 1.15 (95%
confidence interval (CI): 1.05–1.27) for CRC, and 1.30 (95% CI: 1.00–1.68) for rectal cancer, indicating a significant positive association for LA. However, neither total n-6 PUFAs nor AA
were associated with cancers. A significant positive association was also found between a 1 gr/day increase in dietary LA intake and risk of colon cancer (RR: 1.01, 95% CI: 1.00–1.02). There
were no significant associations between tissue levels of total n-6 PUFAs (RR: 0.94, 95% CI: 0.75–1.19), LA (RR: 0.93, 95% CI: 0.61–1.41), and AA (RR: 0.97, 95% CI: 0.70–1.33) and CRC risk.
In conclusion, these findings suggest that dietary intake, but not tissue levels, of LA was associated with an increased risk of colorectal, colon, and rectal cancers. (PROSPERO
registration: CRD42024516584). SIMILAR CONTENT BEING VIEWED BY OTHERS POPULATION ATTRIBUTABLE FRACTION OF DIETARY RISK FACTORS FOR CANCER MORTALITY WITH A FOCUS ON GASTROINTESTINAL CANCERS
IN A POPULATION BASED COHORT STUDY Article Open access 10 February 2025 INFLAMMATORY POTENTIAL OF DIET AND COLORECTAL CARCINOGENESIS: A PROSPECTIVE LONGITUDINAL COHORT Article 08 February
2022 SEX-SPECIFIC ASSOCIATIONS OF EMPIRICALLY DERIVED DIETARY PATTERNS WITH COLORECTAL CANCER RISK IN A KOREAN POPULATION: A CASE‒CONTROL STUDY Article Open access 20 March 2024 INTRODUCTION
Colorectal cancer (CRC) is a prevalent gastrointestinal cancer worldwide [1], with its incidence increasing at an alarming rate and predicted to reach 2.5 million new cases by 2035. CRC is
the fourth leading cause of cancer-related deaths worldwide, accounting for more than 900,000 deaths annually [1, 2]. This cancer also imposes a significant economic burden on healthcare
systems, highlighting the need for effective preventive strategies. It is well established that lifestyle factors, including both genetic and environmental influences, play an important role
in the etiology of CRC [3]. Of different environmental factors, diet has always been of particular interest. Emerging evidence from epidemiological studies indicates that adherence to a
Western-style or high-fat diet is associated with an increased risk of CRC [4], while adherence to Mediterranean or DASH diet is associated with a reduced risk [5, 6]. These dietary patterns
have been characterized by differing notably in their content of various fatty acids, particularly omega-6 polyunsaturated fatty acids (n-6 PUFA). However, it remains unclear whether the
increased risk of CRC is directly attributable to the effects of these fatty acids or other dietary factors also contribute. Vegetable oils like sunflower, safflower, soybean, corn, and
canola oils, nuts, and seeds are other dietary sources of these fatty acids, particularly linoleic acid (LA) [7]. Overall, due to the role of n-6 PUFAs in inflammatory responses, they may
increase the risk of some cancers [8, 9]. However, the findings of two systematic reviews reveal that increasing dietary intake of LA, the most abundant n-6 PUFA, does not have a significant
effect on inflammatory markers [10, 11]. On the other hand, it has been shown that the conversion rate of dietary LA to AA in humans is low [12]. Overall, the role of dietary LA on
inflammation is still unclear. In addition, findings from observational studies on the link between n-6 PUFAs and CRC risk are inconsistent
[13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32], with some studies indicated a significant positive association between dietary and tissue biomarkers of n-6 PUFA and risk of
CRC [17, 20, 22, 23, 29], while other studies did not report any significant association [13, 15, 20, 22, 24, 27, 28, 31] and even an inverse association [14]. A recent meta-analysis (Lu et
al. 2023) summarized available findings on the association between dietary/tissue biomarkers of n-6 PUFA and CRC [33], revealing that the n-6/n-3 PUFA ratio is related to a higher risk of
CRC. However, several eligible studies were not included in that meta-analysis [23, 32], and it did not assess the dose-response relationship between dietary n-6 PUFA and CRC risk, focusing
only on comparisons between the highest and lowest intake levels. A dose-response analysis provides additional insights into the association between dietary n-6 PUFA and CRC risk,
recognizing that fat intake does vary substantially across different populations. Accordingly, we have now conducted a comprehensive systematic review and dose-response meta-analysis of all
existing prospective cohort studies to provide an improved understanding of association(s) between dietary/tissue biomarkers of n-6 PUFAs and risk of CRC in adults. METHODS This systematic
review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses protocol [34]. SEARCH STRATEGY We conducted a systematic search
using online databases, including PubMed, Scopus, and ISI Web of Science until March 5, 2024, to identify prospective cohort studies that examined the associations between dietary intake and
tissue biomarkers of n-6 PUFAs (total, LA, and AA) and risk of CRC. Supplementary Table 1 presented the medical subject heading terms (MeSH) and non-MeSH terms used in the search strategy.
In addition to the mentioned databases, we conducted a web-based search in Google Scholar using a combination of “omega-6 fatty acid” and “colorectal cancer” terms. In this search engine, we
screened the first 500 papers ranked based on relevancy. In the systematic search, no restrictions were considered for publication time or the language of articles. We also searched the
reference lists of the included articles and recent reviews to ensure comprehensive inclusion. INCLUSION CRITERIA We included prospective studies (e.g., prospective cohort, nested
case-control, case-cohort studies), those that recruited adults (≥ 18 years), and reported relative risk (RR) estimates, including hazard ratio (HR), risk ratio (RR), and odds ratio (OR),
with 95% confidence interval (CI) for the associations of dietary/tissue biomarkers of n-6 PUFAs (total, LA, AA) with colorectal, colon, or rectal cancers risk or presenting required data
for the calculation of these effect sizes. If findings from one dataset were published in more than one article, we selected the one with the greatest number of cases or longer follow-up
duration. EXCLUSION CRITERIA We excluded letters, comments, reviews, meta-analyses, animal studies, abstracts, citations, and those studies with insufficient data. We also did not include
studies that were conducted on children and adolescents, recruited critically ill participants, had a retrospective design, and considered genetically predicted dietary of n-6 PUFAs. In
addition, studies that assessed dietary intake of fatty acids, without considering n-6 PUFAs (total, LA, and AA), were excluded. DATA EXTRACTION Study selection and data extraction were
conducted by two independent investigators (NA and NE) and any disagreement between them was resolved by discussion with a third researcher (OS). For the meta-analysis, we extracted any
reported relative risk estimates, including RR, HR, and OR, along with 95% CI for the associations of dietary intake and tissue biomarkers of n-6 PUFA (total, LA, and AA) with colorectal,
colon, and rectal cancer risk. In the case of studies that reported several risk estimates, we selected the one with the most adjustments. In addition to the risk estimates, we extracted
additional information on the first author’s name, publication year, cohort name, sample size, number of cases, gender and age of participants, study location, follow-up duration (years),
methods used to assess dietary or tissue levels of n-6 PUFA and colorectal cancer diagnosis, and confounding variables adjusted in the statistical analysis. For studies that reported their
findings by gender, we considered that study as two separate studies. For the studies that reported main analyses for total population as well as stratified analyses for gender or any other
variables, we used the effect size for total population in our primary analysis. QUALITY ASSESSMENT The quality of studies included in the current meta-analysis was evaluated using the
Newcastle Ottawa Scale (NOS) [35]. According to this scale, a maximum of 9 points would be given to each study according to the following parameters: 4 points for selection of participants,
2 points for comparability, and 3 points for the assessment of outcomes. Since the median score of included studies in the current meta-analysis was 7, we considered studies with a score of
≥7 as high-quality studies. STATISTICAL ANALYSIS We included the relative risks (RRs, HRs, and ORs) of colorectal, colon, and rectal cancers, reported for the comparison between the highest
and lowest intakes/tissue levels of n-6 PUFA (total, LA, and AA), into the meta-analysis. Since the relative risks are non-normally distributed variables, we included the natural log form
(and its standard error) of these risk estimates into the statistical analyses. To calculate the summary relative risk, a random-effects model was used to take between-study heterogeneity
into account. To assess heterogeneity among the included studies, both _Q_-statistic and _I_2 values were used. For the _I_2 statistic, values of >50% were considered as significant
heterogeneity between studies. In addition to the main analysis, subgroup analyses were conducted based on gender, study location, and study quality. To assess publication bias, we used
Egger’s regression asymmetry test [36]. For the significant publication bias, the trim-and-fill method was used to detect the effect of probable missing studies on the overall relative risk
[37]. Furthermore, sensitivity analysis was conducted to evaluate the dependency of overall effect size on one study. In this analysis, each study was excluded to assess the influence of
that study on the overall estimate. For the linear dose-response analysis, we used the generalized least squares trend (glst command in STATA) estimation method [38, 39]. Firstly, study
specific slopes were estimated, and then, these slopes were combined to obtain an overall average slope. Using a random-effects model, the study specific slopes were combined. In the glst
method, outcome distribution, the total number of participants, and the effect sizes with the variance estimates for ≥3 quantitative categories of exposure were required. For each study, we
assigned the median or mean amount of n-6 PUFA (total, LA, and AA) in each category to the corresponding effect size. For studies that reported the intake of n-6 PUFAs (total, LA, and AA) as
the percent of energy (%E), we converted them to gr/day. For studies that reported n-6 PUFA as ranges, the midpoint in each category was estimated. When the highest and lowest categories
were open ended, we assumed the length of the open-ended interval to be the same as that of the adjacent interval. Also, a possible non-linear dose-response association was examined using
restricted cubic splines with 3 knots at centiles of 10%, 50%, and 90% of the distribution. The correlation within each set of provided risk estimates was accounted for and the study
specific estimates were combined by using a linear mixed effects meta-analysis. This method estimates the study specific slopes and combines them to obtain an overall average slope in a
single stage. The significance for non-linearity was calculated by null hypothesis testing, in which we considered the coefficient of the second spline to be equal to zero. Statistical
analyses were performed using STATA version 14.0. For all tests, including Cochran’s _Q_ test, _P_ < 0.05 was considered statistically significant. RESULT LITERATURE SEARCH 4005 articles
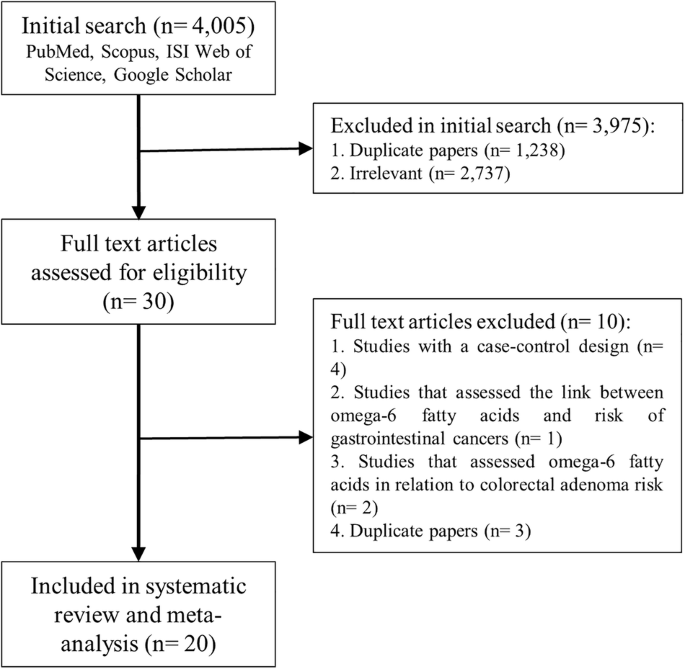
were retrieved from the initial search (Fig. 1). Following the exclusion of duplicate papers (_n_ = 1238) and those that did not meet the inclusion criteria (_n_ = 2737), 30 potentially
relevant full-text articles were identified. Of the 30 papers, a further 7 articles were excluded. These studies used a case-control design (_n_ = 4) [40,41,42,43], reporting an overall risk
estimate of gastrointestinal cancer, but not CRC (_n_ = 1) [44], and reporting colorectal adenoma as an outcome (_n_ = 2) [45, 46]. Five duplicate articles were identified, of which two
were related to the Netherlands cohort study [31, 47], three to the Nurses’ Health Study and Health Professionals Follow-up Study [30, 48, 49]. As these articles evaluated similar exposure
variables, we included only the one with higher quality, with the greatest number of cases, or higher follow-up period [30, 31], and excluded others [47,48,49]. Two other duplicate articles
were also identified, and all were included, given that different outcome variables (CRC, colon cancer, and rectal cancer) were investigated [13, 19]. Accordingly, 20 prospective cohort
studies were included in the current systematic review and meta-analysis [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32], of which fourteen evaluated the association between
n-6 PUFA and CRC (10 on dietary n-6 PUFA intake [13, 15, 18, 21, 22, 29, 30], 3 on tissue levels of n-6 PUFA [16, 20, 23], and one on both [17]), eleven assessed the link between LA and CRC
(7 on dietary LA intake [22, 24, 26, 27, 29, 30, 32], 3 on tissue levels of LA [14, 20, 23], and one on both [17]), nine assessed the relation between AA and CRC (5 on dietary AA intake [22,
24, 26, 29, 30], 3 on tissue levels of AA [14, 20, 23], and one on both [17]). In addition, three studies investigated the relationship between dietary or tissue levels of n-6 PUFA and
colon or rectal cancers without considering CRC in relation to dietary or tissue levels of n-6 PUFA [19, 28, 31]. CHARACTERISTICS OF INCLUDED STUDIES The characteristics of included studies,
published between 1999 and 2021, are summarized in Supplementary Table 2. The number of participants ranged from 460 to 134,017, totaling 787,490, with an age range between 27 and 84 years.
Duration of the follow-up ranged from 6 to 26 years, with a total of 10,694 CRC, 5417 colon cancer, and 2533 rectal cancer cases were recorded. Three studies recruited men only [16, 26,
27], five were conducted solely on women [22, 24, 25, 29, 32], and the remaining studies enrolled both genders [23, 28, 30, 31], of which, only four reported sex-stratified effect sizes [15,
20, 28, 30]. Five studies were from the United States (US) [15, 16, 22, 25, 30], one from Australia [17], and seven from European populations [18, 21, 23, 27, 29, 31, 32], and Asian [13,
14, 19, 20, 24, 26, 28] countries. In addition to the assessment of dietary intake at the study baseline, 5 studies repeated this assessment during the follow-up period [15, 24, 26, 28, 30].
Of sixteen studies on dietary intake of n-6 PUFAs, fifteen used food frequency questionnaire [13, 15, 17, 19, 21, 22, 24,25,26,27,28,29,30,31,32] and one used food diaries [18] for dietary
assessment. Two studies collected dietary data through a face-to-face interview [19, 26], and others used self-reported data in their analysis [13, 15, 17, 21, 22, 24, 25,
27,28,29,30,31,32]. In all studies on tissue levels of n-6 PUFAs, fatty acids were measured in blood [14, 16, 17, 20, 23], and all used chromatography methods to measure n-6 fatty acids
concentrations. CRC and its subtypes were determined using data from medical records or cancer registries in thirteen studies [13, 14, 24, 31, 32], self-reported data in five [15, 16, 22,
25, 30], and both medical records and self-reported data in two studies [23, 26]. In the majority of studies, relative risk estimates were adjusted for some important confounders including
family history of CRC (_n_ = 7), age (_n_ = 17), body mass index (_n_ = 17), smoking (_n_ = 17), alcohol consumption (_n_ = 17), physical activity (_n_ = 14), energy intake (_n_ = 16), and
other dietary variables, including processed meat, fiber and calcium (_n_ = 8). The NOS scores of the studies ranged from 6 to 9, with a median of 7 Sixteen studies had a score of ≥7, and
were, accordingly, considered high-quality studies [13, 14, 32] (Supplementary Tables 3 and 4). FINDINGS FROM THE SYSTEMATIC REVIEW None of the included studies revealed a significant
association between dietary intake or tissue levels of n-6 PUFAs and the risk of CRC and colon cancer. Of eight studies, investigating the association between dietary intake of n-6 PUFAs and
risk of rectal cancer, two reported a positive association [17, 29]. Of studies assessing dietary intake of LA, one study showed a positive association with CRC [29] and two indicated a
positive association with rectal cancer [17, 29]. No significant association was found for colon cancer. In contrast, one study revealed a significant inverse association between dietary
intake of LA and risk of CRC and colon cancer [14]. No other significant association was seen for tissue levels of LA and AA with risk of CRC, colon, and rectal cancer. META-ANALYSIS ON N-6
PUFAS AND RISK OF CRC DIETARY N-6 PUFA Sixteen studies evaluated the association between dietary intake of n-6 PUFAs and risk of CRC [13, 15, 21, 22, 28,29,30]. Among 738,604 participants
included in these studies, 9152 cases of CRC, 3797 cases of colon cancer, and 1676 cases of rectal cancer were recorded during the follow-up period. Comparing the highest categories of
dietary n-6 PUFAs intake with the lowest, RR for CRC, was 1.05 (95% CI: 0.94–1.17, _P_ = 0.38, _I_2 = 48.9%; _P_ = 0.02 for heterogeneity), indicating no significant association between
dietary n-6 PUFAs intake and CRC risk (Table 1 and Supplementary Fig. 1). Such non-significant association was seen for colon (RR: 1.02, 95% CI: 0.91–1.15, _P_ = 0.71, _I_2 = 0.0%; _P_ =
0.88 for heterogeneity) and rectal (RR: 1.19, 95% CI: 0.93–1.52, _P_ = 0.18, _I_2 = 47.6%; _P_ = 0.06 for heterogeneity) cancers. Fifteen studies were eligible for the linear dose-response
analysis of dietary n-6 PUFAs intake and risk of CRC [13, 15, 21, 22, 24, 25, 28,29,30]. There was no significant linear association between a 1 gr/day increase in dietary n-6 PUFAs intake
and the risk of colorectal (RR: 1.00, 95% CI: 0.99–1.01, _P_ = 0.97, _I_2 = 41.4%; _P_ = 0.07 for heterogeneity), colon (RR: 1.00, 95% CI: 0.99–1.01, _P_ = 0.88, _I_2 = 0%; _P_ = 1.00 for
heterogeneity), and rectal (RR: 1.01, 95% CI: 0.98–1.03, _P_ = 0.51, _I_2 = 61.3%; _P_ = 0.02 for heterogeneity) cancers (Table 1 and Supplementary Fig. 2). Eleven studies were included in
the non-linear dose-response analysis [13, 15, 18, 19, 21, 22, 24, 25, 28,29,30], revealing no evidence of a non-linear association for colorectal (_P_ = 0.39 for non-linearity), colon (_P_
= 0.70 for non-linearity), and rectal (_P_ = 0.64 for non-linearity) cancers in relation to dietary n-6 PUFAs intake (Fig. 2). TISSUE N-6 PUFAS Four studies assessed the relationship between
tissue levels of n-6 PUFAs and CRC risk [16, 17, 20, 23], with a total of 7453 participants and 1811 cases of CRC. There was no significant association between tissue levels of n-6 PUFAs
and risk of CRC (RR: 0.94, 95% CI: 0.75–1.19, _P_ = 0.62, _I_2 = 0%, _P_ = 0.58 for heterogeneity) (Table 1 and Supplementary Fig. 3). The number of studies for n-6 PUFAs levels and
colon/rectal cancer was not sufficient for a meta-analysis. Also, due to the limited number of studies, performing the dose-response analysis was not possible for all outcomes. META-ANALYSIS
ON LA AND RISK OF CRC DIETARY LA Ten studies in 9 articles, with a total sample size of 438,873 participants, 5304 cases of CRC, 3485 cases of colon cancer, and 1468 cases of rectal cancer,
were included [17, 22, 24, 26, 27, 29,30,31,32], for dietary LA analysis. RR for the risk of CRC, comparing the highest categories of dietary LA intake with the lowest categories, was 1.15
(95% CI: 1.05–1.27, _P_ = 0.003, _I_2 = 0%, _P_ = 0.44 for heterogeneity), indicating a significant positive association (Table 2 and Supplementary Fig. 4). Such positive association was
seen for colon (RR: 1.10, 95% CI: 0.99–1.23, _P_ = 0.09, _I_2 = 0%, _P_ = 0.78 for heterogeneity) and rectal (RR: 1.30, 95% CI: 1.00–1.68, _P_ = 0.05, _I_2 = 57.0%, _P_ = 0.03 for
heterogeneity) cancers. However, these associations were marginally significant. Nine studies in 8 articles were eligible for the linear dose-response analysis [17, 22, 24, 27, 29,30,31,32],
and there was no significant linear association between a 1 gr/day increase in dietary LA intake and risk of CRC (RR: 1.01, 95% CI: 0.99–1.03, _P_ = 0.12, _I_2 = 42.1%; _P_ = 0.11 for
heterogeneity) and rectal cancer (RR: 1.03, 95% CI: 1.00–1.06, _P_ = 0.09, _I_2 = 76.0; _P_ = 0.001 for heterogeneity). In contrast, a 1 gr/day increase in dietary LA intake was associated
with a 1% increased risk of colon cancer (RR: 1.01, 95% CI: 1.00–1.02, _P_ = 0.02, _I_2 = 0%; _P_ = 0.60 for heterogeneity) (Table 2 and Supplementary Fig. 5). Eight studies in 7 articles
were included in the non-linear dose-response analysis [22, 24, 27, 29,30,31,32], revealing no evidence of non-linearity for the associations between dietary LA intake and risk of colorectal
(_P_ = 0.88 for non-linearity), colon (_P_ = 0.44 for non-linearity) and rectal (_P_ = 0.41 for non-linearity) cancers (Fig. 3). TISSUE LA Five studies in 4 papers assessed the relationship
between tissue levels of LA and CRC risk [14, 17, 20, 23], with a total of 7693 participants and recorded 1983 cases of CRC, 211 cases of colon cancer, and 139 cases of rectal cancer.
Combining the results from these articles, we found no significant association between tissue levels of LA and CRC risk (RR: 0.93, 95% CI: 0.61–1.41, _P_ = 0.74, _I_2 = 69.0%, _P_ = 0.01 for
heterogeneity) (Table 2 and Supplementary Fig. 6). Due to the limited number of studies, we were not able to perform a meta-analysis for colon/rectal cancer and also the dose-response
analysis. META-ANALYSIS ON AA AND RISK OF CRC DIETARY AA Seven studies, with a total sample size of 346,743 participants and 4659 CRC cases, 2746 colon cancer cases, and 1149 rectal cancer
cases, evaluated the association between dietary AA intake and CRC risk [17, 22, 24, 26, 29, 30]. Comparing the highest and lowest categories of dietary AA intake, RR for CRC was 0.97 (95%
CI: 0.86–1.10, _P_ = 0.66, _I_2 = 25.7%; _P_ = 0.23 for heterogeneity), indicating a non-significant association between dietary AA intake and CRC risk (Table 3 and Supplementary Fig. 7).
Such non-significant association was also observed for colon (RR: 0.97, 95% CI: 0.84–1.10, _P_ = 0.61, _I_2 = 0.90%; _P_ = 0.40 for heterogeneity) and rectal (RR: 0.89, 95% CI: 0.74–1.08,
_P_ = 0.24, _I_2 = 0%; _P_ = 0.74 for heterogeneity) cancers. Six studies were eligible for the linear dose-response analysis [17, 22, 24, 29, 30], and there was no linear association
between a 100 mg/day increase in dietary AA intake and the risk of colorectal (RR: 1.02, 95% CI: 0.94–1.11, _P_ = 0.59, _I_2 = 53.3%; _P_ = 0.07 for heterogeneity), colon (RR: 1.01, 95% CI:
0.94–1.09, _P_ = 0.76, _I_2 = 23.5%; _P_ = 0.27 for heterogeneity), and rectal (RR: 0.98, 95% CI: 0.93–1.04, _P_ = 0.52, _I_2 = 0%; _P_ = 0.80 for heterogeneity) cancers (Table 3 and
Supplementary Fig. 8). The non-linear dose-response also showed no evidence of non-linearity for colorectal (_P_ = 0.09 for non-linearity), colon cancer (_P_ = 0.19 for non-linearity), and
rectal (_P_ = 0.76 for non-linearity) cancers in relation to dietary AA intake [22, 24, 29, 30] (Fig. 4). TISSUE AA Five studies assessed the relationship between tissue levels of AA and CRC
risk [14, 17, 20, 23], with a total of 7693 participants, of which 1983 cases of CRC, 881 cases of colon cancer, and cases of 538 rectal cancer were recorded. Combining the results from
these studies revealed no significant association between tissue levels of AA and risks of colorectal (RR: 0.97, 95% CI: 0.70–1.33, _P_ = 0.83, _I_2 = 45.8%, _P_ = 0.12 for heterogeneity)
and rectal (RR: 1.35, 95% CI: 0.83–2.18, _P_ = 0.23, _I_2 = 0%, _P_ = 0.84 for heterogeneity) cancers (Table 3 and Supplementary Fig. 9). However, we found a marginally significant positive
association between tissue levels of AA and colon cancer risk (RR: 1.42, 95% CI: 1.00–2.03, _P_ = 0.05, _I_2 = 0%, _P_ = 0.80 for heterogeneity). Due to the limited number of studies, we did
not perform a dose-response analysis. SUBGROUP AND SENSITIVITY ANALYSES, AND PUBLICATION BIAS Tables 1, 2, and 3 show findings from the subgroup analyses of dietary intake/tissue levels of
omega-6 (total, LA, and AA) and risk of CRC and its subtypes. According to these analyses, no significant association was found between dietary intake/tissue levels of n-6 PUFAs and the risk
of CRC, colon and rectal cancer in all subgroups (Table 1). However, a significant positive association was seen between dietary LA intake (for the highest versus lowest comparison) and CRC
risk in men and non-US population. For rectal cancer, a significant positive association was seen with dietary LA intake in subgroups of non-US populations and high-quality studies (Table
2). For dietary intake/tissue levels of AA, we found no significant association in any subgroups (Table 3). The sensitivity analysis showed that the exclusion of any single study from the
analysis did not change the pooled effect sizes significantly. According to the visual inspection of funnel plots and both Begg’s test and Egger’s regression, there was no significant
publication bias in the associations evaluated (_P_ > 0.10). DISCUSSION This review is, to our knowledge, the first dose-response meta-analysis exploring the associations between
dietary/tissue biomarkers of n-6 PUFAs and risk of CRC. Our analysis revealed that higher intake of LA was associated with an increased risk of colorectal and rectal cancers. Also, in the
dose-response analysis, each 1 gr/day increase in dietary LA intake was associated with a 1% higher risk of colon cancer. There was no evidence of any associations with tissue levels of LA,
or dietary intake/tissue levels of n-6 PUFAs and AA. CRC is one of the most common gastrointestinal cancers [1], with dietary factors playing an important role in its pathogenesis [50]. Of
different dietary factors, n-6 PUFAs have received much attention due to their roles in inflammatory responses through the production of inflammatory prostaglandins [12]. However, findings
on the association between dietary intake and tissue levels of n-6 PUFAs and CRC risk are conflicting [14, 17, 20, 31]. In this meta-analysis, we found a significant positive association
between dietary LA intake and colorectal, colon, and rectal cancers. Contrary to our finding, a recent meta-analysis, conducted by Lu et al., showed no significant association between
dietary LA intake and risk of CRC [33]. While this could be due to missing out some eligible studies [23, 32], Lu et al. also combined the risk estimates from cohort studies with those from
case-control studies. However, our meta-analysis included only prospective studies, avoiding recall or selection biases, which could be the subject of concern in case-control studies.
Similar to our findings, experimental studies have shown that high-LA and high-glucose diets increase the levels of advanced glycation end products (AGE) and the receptor of advanced
glycation end products (RAGE), which are associated with CRC progression [51]. High-fat corn oil was also shown to promote colon tumorigenesis by up-regulating the cyclooxygenase-2
expression [52]. In the current meta-analysis, we found no significant association between tissue levels of LA and CRC risk. Nevertheless, a positive association was seen for dietary LA
intake. The disparity might be due to LA changes during food cooking or processing. On the other hand, oils high in LA, when exposed to food processing methods such as frying and high-heat
cooking, can undergo oxidation, leading to the formation of harmful compounds like lipid peroxides and aldehydes [53], which may be associated with an increased risk of CRC [54]. Moreover, a
higher intake of LA, which is commonly found in vegetable oils, can lead to increased energy consumption and contribute to obesity [55], a known risk factor for CRC [56, 57]. Despite this,
evidence suggests anti-cancer properties of LA levels in tissues or blood [14], indicating that dietary LA might be associated with CRC independently of tissue LA levels. Similar to our
findings, Lu et al. also reported that tissue levels of LA were not associated with CRC risk [33]. Also, it should be noted that the amount of LA in foods is low and therefore the estimation
of its intake might be affected by measurement error. In addition, the positive association between dietary LA and CRC might be due to the effect of confounding variables such as other
dietary factors rather than LA. Therefore, our findings on the relation between LA and CRC should be considered with caution, warranting further studies. We found that dietary intakes of
total n-6 PUFAs and AA were not associated with risk of colorectal, colon, and rectal cancers. Similarly, Lu et al. also showed no significant association between dietary intakes of total
n-6 PUFAs and AA and risk of colorectal, colon, and rectal cancers [33]. The observed disparity between dietary intake of n-6 PUFAs and LA might be explained by the different effects of n-6
PUFAs (LA and long-chain n-6 PUFAs) on cancer incidence. Therefore, considering the different effects of n-6 PUFAs on cancer etiology, the cumulative effects of these fatty acids on cancer
incidence might be null. For dietary intake of AA, the observed null connection may be due to AA metabolites that are produced by different pathways. Lipoxin A4 (LXA4) is produced by the
lipoxygenase pathway and is considered as a tumor growth suppressor due to its anti-angiogenic properties [58]. However, PGE2 produced via the cyclooxygenase pathway plays an important role
in the development of CRC [59]. Therefore, a combination of AA metabolites with different health benefits might justify the null association between dietary AA intake and risk of CRC. Our
findings should be interpreted with caution due to several limitations, most of which are common to observational studies and meta-analyses. These include being unable to perform a
dose-response meta-analysis due to the limited number of studies in some associations. In addition, since the current meta-analysis was conducted on observational studies, causality cannot
be established. Also, the existence of measurement and reporting errors in the estimation of food and nutrient intake is inevitable in observational studies. Moreover, previous studies have
not examined the influence of processing and cooking methods or the sources of n-6 PUFAs on the association between these fatty acids and CRC risk. The source of n-6 PUFAs, whether it is
derived from plant-based foods or from fast foods and snacks, may have different effects. Therefore, future studies should assess the influence of different food sources of n-6 PUFAs on CRC
risk. In addition, differences in n-6 PUFAs intake among geographical regions could have affected the highest and lowest levels of n-6 PUFAs intake and the results from these comparisons. To
control these differences, we conducted the subgroup and dose-response analyses. In conclusion, we found that dietary intake of LA is associated with an increased risk of colorectal, colon,
and rectal cancers. However, no significant association was found for total n-6 PUFAs and AA, either in the highest versus lowest comparison or in the dose-response meta-analysis. Also, in
the dose-response analysis, each 1 gr/day increase in dietary LA intake (equal to 3.67 grams of sunflower oil, 1.6 grams of walnut oil, or 2.35 grams of pumpkin oil [60]) was associated with
a 1% higher risk of colon cancer. Although the increased risk of 1% with a 1 gr/day more intake of LA might be clinically unimportant, this risk might be large in the higher dosages of LA
intake. Among the studies included in the current meta-analysis, the range of LA intake was between 0 and 20 gr/day. Therefore, higher intakes of LA can provide higher risk of colon cancer.
In terms of tissue levels of n-6 PUFAs, LA, and AA, no significant association was found with CRC risk. Future studies, particularly well-designed prospective cohort studies, should assess
the influence of dietary/tissue levels of n-6 PUFAs on the risk of CRC mortality. Further studies are also needed to investigate whether specific foods rich in n-6 PUFAs are differentially
associated with CRC risk. DATA AVAILABILITY The data that support the findings of this study are available from the corresponding author upon reasonable request. REFERENCES * Dekker E, Tanis
PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394:1467–80. Article PubMed Google Scholar * Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F.
Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–91. Article PubMed Google Scholar * Ionescu VA, Gheorghe G, Bacalbasa N, Chiotoroiu AL, Diaconu
C. Colorectal cancer: from risk factors to oncogenesis. Medicina (Kaunas). 2023;59:1646–56. Article PubMed Google Scholar * Mehta, Song RS, Nishihara M, Drew DA R, Wu K, Qian ZR, et al.
Dietary patterns and risk of colorectal cancer: analysis by tumor location and molecular subtypes. Gastroenterology. 2017;152:1944–53.e1. Article CAS PubMed Google Scholar * Bamia C,
Lagiou P, Buckland G, Grioni S, Agnoli C, Taylor AJ, et al. Mediterranean diet and colorectal cancer risk: results from a European cohort. Eur J Epidemiol. 2013;28:317–28. Article CAS
PubMed Google Scholar * Fung TT, Hu FB, Wu K, Chiuve SE, Fuchs CS, Giovannucci E. The mediterranean and dietary approaches to stop hypertension (DASH) diets and colorectal cancer. Am J
Clin Nutr. 2010;92:1429–35. Article CAS PubMed PubMed Central Google Scholar * Spector AA, Kim HY. Discovery of essential fatty acids. J Lipid Res. 2015;56:11–21. Article CAS PubMed
PubMed Central Google Scholar * Smith WL, DeWitt DL, Garavito RM. Cyclooxygenases: structural, cellular, and molecular biology. Annu Rev Biochem. 2000;69:145–82. Article CAS PubMed
Google Scholar * Romagnolo DF, Donovan MG, Doetschman TC, Selmin OI. n-6 linoleic acid induces epigenetics alterations associated with colonic inflammation and cancer. Nutrients.
2019;11:171–81. Article CAS PubMed PubMed Central Google Scholar * Su H, Liu R, Chang M, Huang J, Wang X. Dietary linoleic acid intake and blood inflammatory markers: a systematic
review and meta-analysis of randomized controlled trials. Food Funct. 2017;8:3091–103. Article CAS PubMed Google Scholar * Johnson GH, Fritsche K. Effect of dietary linoleic acid on
markers of inflammation in healthy persons: a systematic review of randomized controlled trials. J Acad Nutr Diet. 2012;112:1029–41, 41.e1-15. Article CAS PubMed Google Scholar * Kumar
NG, Contaifer D, Madurantakam P, Carbone S, Price ET, Tassell BV, et al. Dietary bioactive fatty acids as modulators of immune function: implications on human health. Nutrients.
2019;11:2974. Article CAS PubMed PubMed Central Google Scholar * Butler LM, Wang R, Koh WP, Stern MC, Yuan JM, Yu MC. Marine n-3 and saturated fatty acids in relation to risk of
colorectal cancer in Singapore Chinese: a prospective study. Int J Cancer. 2009;124:678–86. Article CAS PubMed PubMed Central Google Scholar * Butler LM, Yuan JM, Huang JY, Su J, Wang
R, Koh WP, et al. Plasma fatty acids and risk of colon and rectal cancers in the Singapore Chinese Health Study. NPJ Precis Oncol. 2017;1:38. Article PubMed PubMed Central Google Scholar
* Daniel CR, McCullough ML, Patel RC, Jacobs EJ, Flanders WD, Thun MJ, et al. Dietary intake of ω-6 and ω-3 fatty acids and risk of colorectal cancer in a prospective cohort of US men and
women. Cancer Epidemiol Biomarkers Prev. 2009;18:516–25. Article CAS PubMed Google Scholar * Hall MN, Campos H, Li H, Sesso HD, Stampfer MJ, Willett WC, et al. Blood levels of long-chain
polyunsaturated fatty acids, aspirin, and the risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2007;16:314–21. Article CAS PubMed Google Scholar * Hodge AM, Williamson EJ,
Bassett JK, MacInnis RJ, Giles GG, English DR. Dietary and biomarker estimates of fatty acids and risk of colorectal cancer. Int J Cancer. 2015;137:1224–34. Article CAS PubMed Google
Scholar * Key TJ, Appleby PN, Masset G, Brunner EJ, Cade JE, Greenwood DC, et al. Vitamins, minerals, essential fatty acids and colorectal cancer risk in the United Kingdom Dietary Cohort
Consortium. Int J Cancer. 2012;131:E320–5. Article CAS PubMed Google Scholar * Koh WP, Yuan JM, van den Berg D, Lee HP, Yu MC. Interaction between cyclooxygenase-2 gene polymorphism and
dietary n-6 polyunsaturated fatty acids on colon cancer risk: the Singapore Chinese Health Study. Br J Cancer. 2004;90:1760–4. Article CAS PubMed PubMed Central Google Scholar * Kojima
M, Wakai K, Tokudome S, Suzuki K, Tamakoshi K, Watanabe Y, et al. Serum levels of polyunsaturated fatty acids and risk of colorectal cancer: a prospective study. Am J Epidemiol.
2005;161:462–71. Article PubMed Google Scholar * Kraja B, Muka T, Ruiter R, de Keyser CE, Hofman A, Franco OH, et al. Dietary fiber intake modifies the positive association between n–3
PUFA intake and colorectal cancer risk in a caucasian population. J Nutr. 2015;145:1709–16. Article CAS PubMed Google Scholar * Lin J, Zhang SM, Cook NR, Lee IM, Buring JE. Dietary fat
and fatty acids and risk of colorectal cancer in women. Am J Epidemiol. 2004;160:1011–22. Article PubMed Google Scholar * Linseisen J, Grundmann N, Zoller D, Kühn T, Jansen EH, Chajès V,
et al. Red blood cell fatty acids and risk of colorectal cancer in the European prospective investigation into cancer and nutrition (EPIC). Cancer Epidemiol Biomarkers Prev. 2021;30:874–85.
Article CAS PubMed Google Scholar * Murff HJ, Shu XO, Li H, Dai Q, Kallianpur A, Yang G, et al. A prospective study of dietary polyunsaturated fatty acids and colorectal cancer risk in
Chinese women. Cancer Epidemiol Biomarkers Prev. 2009;18:2283–91. Article CAS PubMed PubMed Central Google Scholar * Navarro SL, Neuhouser ML, Cheng TYD, Tinker LF, Shikany JM,
Snetselaar L, et al. The interaction between dietary fiber and fat and risk of colorectal cancer in the women’s health initiative. Nutrients. 2016;8:779. Article PubMed PubMed Central
Google Scholar * Nguyen S, Li H, Yu D, Cai H, Gao J, Gao Y, et al. Dietary fatty acids and colorectal cancer risk in men: a report from the Shanghai Men’s Health Study and a meta-analysis.
Int J Cancer. 2021;148:77–89. Article CAS PubMed Google Scholar * Pietinen P, Malila N, Virtanen M, Hartman TJ, Tangrea JA, Albanes D, et al. Diet and risk of colorectal cancer in a
cohort of Finnish men. Cancer Causes Control. 1999;10:387–96. Article CAS PubMed Google Scholar * Sasazuki S, Inoue M, Iwasaki M, Sawada N, Shimazu T, Yamaji T, et al. Intake of n-3 and
n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int J Cancer. 2011;129:1718–29. Article CAS PubMed
Google Scholar * Shin A, Cho S, Sandin S, Lof M, Oh MY, Weiderpass E. Omega-3 and -6 fatty acid intake and colorectal cancer risk in Swedish Women’s Lifestyle and Health Cohort. Cancer Res
Treat. 2020;52:848–54. Article CAS PubMed PubMed Central Google Scholar * Song M, Chan AT, Fuchs CS, Ogino S, Hu FB, Mozaffarian D, et al. Dietary intake of fish, ω-3 and ω-6 fatty
acids and risk of colorectal cancer: a prospective study in US men and women. Int J Cancer. 2014;135:2413–23. Article CAS PubMed PubMed Central Google Scholar * Brink M, Weijenberg MP,
De Goeij AF, Schouten LJ, Koedijk FD, Roemen GM, et al. Fat and K-ras mutations in sporadic colorectal cancer in The Netherlands Cohort Study. Carcinogenesis. 2004;25:1619–28. Article CAS
PubMed Google Scholar * Terry P, Bergkvist L, Holmberg L, Wolk A. No association between fat and fatty acids intake and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev.
2001;10:913–4. CAS PubMed Google Scholar * Lu Y, Li D, Wang L, Zhang H, Jiang F, Zhang R, et al. Comprehensive investigation on associations between dietary intake and blood levels of
fatty acids and colorectal cancer risk. Nutrients. 2023;15:730. Article CAS PubMed PubMed Central Google Scholar * Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD,
et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906. Article PubMed Google Scholar * Stang A. Critical evaluation of the
Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. Article PubMed Google Scholar * van Enst WA, Ochodo E,
Scholten RJ, Hooft L, Leeflang MM. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol. 2014;14:70. Article
PubMed PubMed Central Google Scholar * Lin L. Hybrid test for publication bias in meta-analysis. Stat Methods Med Res. 2020;29:2881–99. Article PubMed PubMed Central Google Scholar *
Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–9. Article CAS PubMed Google
Scholar * Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose–response data. stata journal. 2006;6:40–57. Article Google Scholar * Shao
S, Kao TC, Eckhaus J, Bourgeois J, Perera K, Zhu K. The association of percentage energy from fat and colon cancer risk among members of the US military. Eur J Cancer Prev. 2015;24:188–94.
Article PubMed PubMed Central Google Scholar * Tu K, Ma T, Zhou R, Xu L, Fang Y, Zhang C. Association between dietary fatty acid patterns and colorectal cancer risk: a large-scale
case-control study in China. Nutrients. 2022;14:4375. Article CAS PubMed PubMed Central Google Scholar * Williams CD, Satia JA, Adair LS, Stevens J, Galanko J, Keku TO, et al.
Associations of red meat, fat, and protein intake with distal colorectal cancer risk. Nutr Cancer. 2010;62:701–9. Article CAS PubMed PubMed Central Google Scholar * Wu Q, Shi D, Dong T,
Zhang Z, Ou Q, Fang Y, et al. Serum saturated fatty acids including very long-chain saturated fatty acids and colorectal cancer risk among Chinese Population. Nutrients. 2023;15:1917.
Article CAS PubMed PubMed Central Google Scholar * Sellem L, Srour B, Gueraud F, Pierre F, Kesse-Guyot E, Fiolet T, et al. Saturated, mono- and polyunsaturated fatty acid intake and
cancer risk: results from the French prospective cohort NutriNet-Sante. Eur J Nutr. 2019;58:1515–27. Article CAS PubMed Google Scholar * Cottet V, Collin M, Gross AS, Boutron-Ruault MC,
Morois S, Clavel-Chapelon F, et al. Erythrocyte membrane phospholipid fatty acid concentrations and risk of colorectal adenomas: a case-control nested in the French E3N-EPIC cohort study.
Cancer Epidemiol Biomarkers Prev. 2013;22:1417–27. Article CAS PubMed Google Scholar * Oh K, Willett WC, Fuchs CS, Giovannucci E. Dietary marine n-3 fatty acids in relation to risk of
distal colorectal adenoma in women. Cancer Epidemiol Biomarkers Prev. 2005;14:835–41. Article CAS PubMed Google Scholar * Weijenberg MP, Luchtenborg M, de Goeij AF, Brink M, van Muijen
GN, de Bruïne AP, et al. Dietary fat and risk of colon and rectal cancer with aberrant MLH1 expression, APC or KRAS genes. Cancer Causes Control. 2007;18:865–79. Article PubMed PubMed
Central Google Scholar * Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Speizer FE. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women.
N Engl J Med. 1990;323:1664–72. Article CAS PubMed Google Scholar * Wan Y, Wu K, Wang L, Yin K, Song M, Giovannucci EL, et al. Dietary fat and fatty acids in relation to risk of
colorectal cancer. Eur J Nutr. 2022;61:1863–73. Article CAS PubMed Google Scholar * Kato I, Majumdar AP, Land SJ, Barnholtz-Sloan JS, Severson RK. Dietary fatty acids, luminal modifiers,
and risk of colorectal cancer. Int J Cancer. 2010;127:942–51. Article CAS PubMed PubMed Central Google Scholar * Shimomoto T, Luo Y, Ohmori H, Chihara Y, Fujii K, Sasahira T, et al.
Advanced glycation end products (AGE) induce the receptor for AGE in the colonic mucosa of azoxymethane-injected Fischer 344 rats fed with a high-linoleic acid and high-glucose diet. J
Gastroenterol. 2012;47:1073–83. Article CAS PubMed Google Scholar * Singh J, Hamid R, Reddy BS. Dietary fat and colon cancer: modulation of cyclooxygenase-2 by types and amount of
dietary fat during the postinitiation stage of colon carcinogenesis. Cancer Res. 1997;57:3465–70. CAS PubMed Google Scholar * Choe E, Min DB. Mechanisms and factors for edible oil
oxidation. Compr Rev Food Sci. 2006;5:169–86. Article CAS Google Scholar * Guo C, Li X, Wang R, Yu J, Ye M, Mao L, et al. Association between oxidative DNA damage and risk of colorectal
cancer: sensitive determination of urinary 8-Hydroxy-2’-deoxyguanosine by UPLC-MS/MS analysis. Sci Rep. 2016;6:32581. Article CAS PubMed PubMed Central Google Scholar * Naughton SS,
Mathai ML, Hryciw DH, McAinch AJ. Linoleic acid and the pathogenesis of obesity. Prostaglandins Other Lipid Mediat. 2016;125:90–9. Article CAS PubMed Google Scholar * Miranda BCJ,
Tustumi F, Nakamura ET, Shimanoe VH, Kikawa D, Waisberg J. Obesity and colorectal cancer: a narrative review. Medicina (Kaunas). 2024;60:1218. Article PubMed Google Scholar * Ma Y, Yang
Y, Wang F, Zhang P, Shi S, Zou Y, et al. Obesity and risk of colorectal cancer: a systematic review of prospective studies. PLoS ONE. 2013;8:e53916. Article CAS PubMed PubMed Central
Google Scholar * Chen Y, Hao H, He S, Cai L, Li Y, Hu S, et al. Lipoxin A4 and its analogue suppress the tumor growth of transplanted H22 in mice: the role of antiangiogenesis. Mol Cancer
Ther. 2010;9:2164–74. Article CAS PubMed Google Scholar * Rifkin SB, Shrubsole MJ, Cai Q, Smalley WE, Ness RM, Swift LL, et al. PUFA levels in erythrocyte membrane phospholipids are
differentially associated with colorectal adenoma risk. Br J Nutr. 2017;117:1615–22. Article CAS PubMed PubMed Central Google Scholar * Ivanova S, Marinova G, Batchvarov V. Comparison
of fatty acid composition of various types of edible oils. Bulg J Agric Sci. 2016;22:5–15. Google Scholar Download references FUNDING The study was financially supported by Abadan
University of Medical Sciences, Abadan, Iran (code: 1746). The funder had no role in the design and conduct of the study; collection of the data; preparation, review, or approval of the
manuscript; or the decision to submit the manuscript for publication. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Center for Exercise, Nutrition & Health Sciences, School for Policy
Studies, University of Bristol, Bristol, UK Negin Atashi * Student Research Committee, Isfahan University of Medical Sciences, Isfahan, Iran Niloofar Eshaghian * Adelaide Medical School and
Centre of Research Excellence in Translating Nutritional Science to Good Health, University of Adelaide, Adelaide, Australia Javad Anjom-Shoae * Nutrition and Food Security Research Center,
Department of Community Nutrition, School of Nutrition and Food Science, Isfahan University of Medical Sciences, Isfahan, Iran Gholamreza Askari & Omid Sadeghi * Department of Operating
Room Nursing, Abadan University of Medical Sciences, Abadan, Iran Masoomeh Asadi * Nutrition and Food Security Research Center, Shahid Sadoughi University of Medical Sciences, Yazd, Iran
Omid Sadeghi * Research Center for Food Hygiene and Safety, School of Public Health, Shahid Sadoughi University of Medical Sciences, Yazd, Iran Omid Sadeghi Authors * Negin Atashi View
author publications You can also search for this author inPubMed Google Scholar * Niloofar Eshaghian View author publications You can also search for this author inPubMed Google Scholar *
Javad Anjom-Shoae View author publications You can also search for this author inPubMed Google Scholar * Gholamreza Askari View author publications You can also search for this author
inPubMed Google Scholar * Masoomeh Asadi View author publications You can also search for this author inPubMed Google Scholar * Omid Sadeghi View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS NA contributed to the literature search. NA and NE contributed to data extraction and data analysis. NA and OS drafted the manuscript which
was critically revised for important intellectual content by all authors. MA contributed to the manuscript editing and obtained funding. JAS and GA contributed to the manuscript editing. OS
supervised the study. All authors have read and approved the final manuscript. CORRESPONDING AUTHORS Correspondence to Masoomeh Asadi or Omid Sadeghi. ETHICS DECLARATIONS COMPETING INTERESTS
The authors declare no competing interests. ETHICS APPROVAL AND CONSENT TO PARTICIPATE All analyses were based on previously published studies; thus, no ethical approval is required.
ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. SUPPLEMENTARY INFORMATION
SUPPLEMENTAL MATERIAL RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License, which permits
any non-commercial use, sharing, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the
Creative Commons licence, and indicate if you modified the licensed material. You do not have permission under this licence to share adapted material derived from this article or parts of
it. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material
is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission
directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc-nd/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE
Atashi, N., Eshaghian, N., Anjom-Shoae, J. _et al._ Dietary intake and tissue biomarkers of omega-6 fatty acids and risk of colorectal cancer in adults: a systematic review and dose-response
meta-analysis of prospective cohort studies. _Nutr. Diabetes_ 15, 17 (2025). https://doi.org/10.1038/s41387-025-00367-w Download citation * Received: 21 June 2024 * Revised: 08 February
2025 * Accepted: 12 February 2025 * Published: 18 April 2025 * DOI: https://doi.org/10.1038/s41387-025-00367-w SHARE THIS ARTICLE Anyone you share the following link with will be able to
read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing
initiative