- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
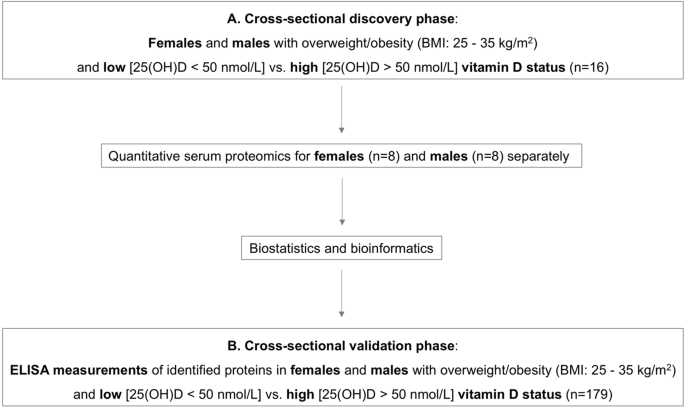
ABSTRACT OBJECTIVE Subjects with low vitamin D levels are at risk of cardiometabolic disease. The aim of this study was to identify novel serological markers linking vitamin D status with
cardiometabolic profile in non-diabetic adults with obesity. METHODS For the discovery phase, we used quantitative serum proteomics in sex-matched, age-matched and BMI-matched subjects with
obesity [BMI: 25–35 kg/m2] and low [25(OH)D < 50 nmol/L] vs. high vitamin D status [25(OH)D > 50 nmol/L] (_n_ = 16). For the validation phase, we performed ELISA in a larger cohort
with similar characteristics (_n_ = 179). RESULTS We identified 423 and 549 differentially expressed proteins in the high vs. low vitamin D groups of the male and female cohorts,
respectively. The _small molecule biochemistry_ protein networks and the _glycolysis|gluconeogenesis_ pathway were significantly enriched in the DEPs of both sexes. As surrogate markers to
these processes, the insulin-like growth factor binding protein -2 (IGFBP-2) was upregulated in males, whereas IGFBP-3 was upregulated in females from the high Vitamin D status. This
sex-specific trend was confirmed using Luminex ELISA to an independent but clinically analogous cohort of males (_n_ = 84, _p_ _=_ 0.002) and females (_n_ = 95, _p_ _=_ 0.03). CONCLUSIONS
The high Vitamin D status correlated with the serological upregulation of IGFBP-2 in males and IGFBP-3 in females with obesity and may constitute surrogate markers of risk reduction of
cardiometabolic disease. SIMILAR CONTENT BEING VIEWED BY OTHERS CARDIOMETABOLIC PROFILES AND PROTEOMICS ASSOCIATED WITH OBESITY PHENOTYPES IN A LONGITUDINAL COHORT OF YOUNG ADULTS Article
Open access 28 March 2024 EFFECTS OF ADIPOSITY ON THE HUMAN PLASMA PROTEOME: OBSERVATIONAL AND MENDELIAN RANDOMISATION ESTIMATES Article Open access 05 July 2021 COMPREHENSIVE ANALYSES OF
CIRCULATING CARDIOMETABOLIC PROTEINS AND OBJECTIVE MEASURES OF FAT MASS Article Open access 07 August 2023 INTRODUCTION Vitamin D is an ancient hormone, originally produced by
archaebacteria, phytoplankton and zooplankton dating back to over 500 million years1. Almost all mammalian cell types express the vitamin D receptor, suggesting that vitamin D may exhibit a
pleiotropic effect in addition to its well-established role in calcium and phosphorus homoeostasis2. A clinically relevant marker used to measure vitamin D status is circulating levels of
25(OH)D3. Although there is significant controversy in this field, vitamin D insufficiency is defined by the Institute of Medicine (IOM) as serum 25(OH)D levels lower than 50 nmol/L4.
Notably, the Endocrine Society suggested that circulating 25(OH)D should be maintained at higher concentrations (75 to 80 nmol/L) for extra-skeletal health benefits5 with no known toxicity
at this level6,7. Across countries in all continents, the mean serum concentration of 25(OH)D is around 50 nmol/L, suggesting that approximately 50% of these populations have vitamin D
insufficiency8,9. Emerging evidence suggests the extra-skeletal, pluripotent effects of vitamin D in reducing the risk of adverse cardiometabolic outcomes. A meta-analysis, including 65,000
prospectively monitored participants, showed that the group with the lowest compared to the highest serum 25(OH)D levels had a relative risk of 1.5 (1.3 to 1.8) for total incidents of
cardiovascular disease and 1.6 (1.3 to 2.1) for stroke10. Another meta-analysis of approximately 500,000 participants found an inverse association with all-cause mortality for circulating
25(OH)D levels up to 75 nmol/L11. An additional dose-response meta-analysis from 34 studies involving 180,667 participants demonstrated that serum 25(OH)D levels were inversely correlated
with total number of CVD events and CVD mortality12. Decreased 25(OH)D levels may affect cardiovascular risk either directly, for example by increasing blood pressure through the
renin-angiotensin system, or indirectly, by influencing inflammation, myocardial function, vascular calcification and parathyroid hormone levels13. An additional potential extra-skeletal
effect of vitamin D is its chemopreventive effects against type II diabetes mellitus (T2DM). The meta-analysis of well-powered clinical studies have shown that subjects with low 25(OH)D
levels have an increased risk of developing T2DM compared to those with a normal vitamin D status14. Results from a population study in Victoria, Australia showed an inverse association
between vitamin D status and risk factors of T2DM, including fasting plasma glucose and HbA1c levels15. A recent systematic review and meta-analysis that included almost 30,000 subjects,
found that lower 25(OH)D levels were significantly associated with an increased risk of developing diabetes among older adults16. Low vitamin D status may lead to insulin resistance by
impairing insulin secretion and compromising pancreatic beta-cell function, all hallmark features of T2DM17,18. Recent findings suggest that supplementation with vitamin D could positively
affect insulin secretion and glucose homoeostasis19,20. An additional recently published Mendelian randomisation study in European and Chinese adults provided first ever evidence for a
causal effect of higher 25(OH)D serum levels for the prevention of T2DM21. As a corollary to these observations, the chemoprevention of T2D prior to its clinical diagnosis in individuals
with obesity by means of Vitamin D status correction may also reduce the risk of cardiometabolic disease. However, surrogate protein markers that can easily be measured in serum to gauge
such a risk reduction due to Vitamin D status improvement are currently lacking. The aim of the present study was to perform quantitative serum proteomics in a cross-sectional cohort of
non-diabetic adults with obesity and low vs. high vitamin D status in order to identify novel serological markers linking vitamin D status to cardiometabolic disease risk. The relevant
differentially expressed proteins chosen as candidate markers were further verified against a larger cross-sectional cohort with the same inclusion/exclusion criteria as for the discovery
phase. An overview of the study design is presented in Fig. 1. MATERIALS AND METHODS DISCOVERY PHASE: SERUM PROTEOMICS IN CROSS-SECTIONAL SAMPLES The study received ethics approval by the
King Saud University Ethics Committee. Reporting of the cross-sectional study conforms to the STROBE statement and the broader EQUATOR guidelines22. For the cross-sectional serum proteomics
study, participants were randomly selected from an existing cohort, the Riyadh cohort 223. Written informed consent was obtained from all participants. Adults with overweight/obesity (BMI
between 25 and 35 kg/m2) and with normal fasting plasma glucose levels (3.9 to 5.5 mmol/L) and serum 25(OH)D < 50nmol/L or >50 nmol/L were included in the study. Subjects pregnant or
breastfeeding, with morbid obesity (BMI > 35 kg/m2), diagnosed with type 1 or type 2 diabetes mellitus, or with non-alcoholic fatty liver disease were excluded from the study. An
abdominal ultrasound was performed to exclude non-alcoholic fatty liver disease among the study participants. Anthropometry and morning blood withdrawal was performed after overnight
fasting. Blood collection for all participants took place during the winter months (December to February). The discovery cohort comprised 16 participants (four males with serum 25(OH)D <
50nmol/L; four males with serum 25(OH)D > 80 nmol/L; four females with serum 25(OH)D < 50nmol/L; four females with serum 25(OH)D > 80 nmol/L). Participants were asked about their
sun exposure habits using a questionnaire as reported before24. Total energy and micronutrient (vitamin D, calcium and EPA/DHA) intake was estimated using food frequency questionnaires as
reported before24. Physical activity levels of the participants were assessed using the WHO Global Physical Activity Questionnaire, as reported previously24. SERUM PROCUREMENT AND PROTEOMIC
ANALYSIS The procurement and handling of sera was in accordance with the recommendations of the Standard Operating Procedure Integration Working Group (SOPIWG) as adopted by the author’s
method25. Two eight-plex serum proteomics experiments were performed for male and female subjects separately, as we have shown previously that vitamin D has sex-specific non-skeletal
cardiovascular effects24. The serum specimens were freshly thawed and vortexed for 2 min. For each participant, 100 µL of unprocessed serum were immediately mixed with 400 µL 6 M Guanidine
Hydrochloride and subjected to global quantitative serum proteomic analysis using our previously published method24,25. In summary, high-performance Size Exclusion Chromatography using three
serially connected Waters KW-804 columns at 0.75 ml/min flow rate and 30 °C was used to separate the proteins based on their molecular weight differences. The separated low-molecular weight
protein segments (molecular weight cutoff 3 kDa) were dialysis purified and lyophilised to dryness at 4 °C. One-hundred μg of protein from each sample was subjected to trypsin proteolysis
and the peptides were chemically labelled using the eight-plex isobaric Tag for Relative and Absolute Quantitation (iTRAQ) reagents, pooled, and offline fractionated with C4 reverse phase
chromatography at pH 8.0 (2.1 mm X 150 mm, 3 μm particle, 120 Å pore, Kromasil, Germany). As a multiplex proteomics workflow, samples were analysed under the same exact experimental
conditions. Each fraction was analysed using ultra-high performance C18 nano-liquid chromatography at pH 3.5 (75μm ID x 50 cm, 2 μm particle, 100 Å pore, Hypersil, USA) hyphenated with
high-resolution tandem mass spectrometry using the FT-Orbitrap Elite platform. Unprocessed raw files were submitted to Proteome Discoverer 1.4 for target decoy search against the UniProtKB
Homo Sapiens database (release date 10-Jan-2015) using SequestHT. Only reporter ion ratios from unique peptides were considered for the quantitation of the respective protein. Median
normalisation and log2 transformation was performed for the reporter ion quantification ratios. A protein was considered differentially expressed between the high vs. low vitamin D status
when its one-sample, two-tailed, _T_-test _p_-value was ≤0.05 and the mean iTRAQ log2ratio of high vs. low vitamin D status was higher than ±0.3. All mass spectrometry proteomics data have
been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD009606. PRINCIPAL COMPONENT ANALYSIS AND BIOINFORMATICS WITH BIOSTATISTICS
INFORMED SELECTION OF CANDIDATE VERIFICATION MARKERS Principal component analysis using the reporter ion ratios of the all analysed proteins in the male and female cohorts, respectively was
performed using ClustVis (https://biit.cs.ut.ee/clustvis/). DAVID (https://david.ncifcrf.gov/) and Ingenuity Pathway Analysis (IPA) (Qiagen, Hilden, Germany) software tools were used to
identify canonical pathways and protein networks significantly over-represented in the differentially expressed proteins between high and low vitamin D status groups of the male and female
cohorts. Significance was set at _p_-value ≤0.05. Surrogate markers to the identified enriched pathways were further evaluated as candidate verification markers with bibliographic research.
As an additional qualifier, retrospective statistical power analysis using the function _pwr.2p.test()_ available within R (https://www.R-project.org/) was applied to the suitable candidate
marker as reported26. The minimum statistical power threshold was set at 0.8, which factored in the _p_-value, variation, differential expression ratio, and the number of replicate
biological observations for the chosen protein analysed from the discovery experiment. VERIFICATION PHASE: ELISA MEASUREMENTS IN AN INDEPENDENT CROSS-SECTIONAL COHORT ELISA measurements of
selected proteins identified at the discovery phase were validated in a larger cross-sectional cohort of adults with obesity randomly selected from the Riyadh cohort 223, using the same
inclusion and exclusion criteria as described above for the discovery phase. In total, 179 adults were included, of which 84 males [_n_ = 40 with 25(OH) < 50 nmol/L; _n_ = 44 with 25(OH)
> 50 nmol/L] and 95 females [_n_ = 47 with 25(OH) < 50 nmol/L; _n_ = 48 with 25(OH) > 50 nmol/L]. The size of the verification cohort was based on the logistic regression models
requiring a minimum of 10 events per predictor variable27, which in our study included sex and 25(OH)D levels. All other clinical parameters listed in Table 2 were not considered, as they
remained constant between the cohorts examined. INSULIN-LIKE GROWTH FACTOR BINDING PROTEIN IGFBP-2 AND IGFBP-3 ELISA MEASUREMENTS IGFBP-2 and IGFBP-3 were targeted for measurement using the
commercially available Luminex ELISA kit (Catalog number: HIGFBMAG-53K; Millipore, Billerica, MA, USA) according to manufacturer’s instructions. The captured bead complexes were measured
with the FLEXMAP 3D system (Luminex Corporation, Austin, TX). The raw data (mean fluorescence intensity) were collected and further processed for calculating protein concentration. The
intra-assay and inter-assay coefficient of variation (CV) was <10 and <15%, respectively. BIOCHEMICAL ANALYSES Fasting glucose levels were measured using an enzymatic assay (hexokinase
coupled with glucose 6-phosphate dehydrogenase) with a chemical analyser (Konelab, Espoo, Finland). The inter-assay CV was 2.2%. Serum 25(OH)D levels were measured by a specific
enzyme-linked immunosorbent assay (IDS, Tyne and Wear, UK). The inter- and intra-assay variability of this assay was 5.1 and 4.6%, respectively. CLINICAL DATA ANALYSIS Clinical data were
analysed using SPSS (Version 25). An unpaired, two-tailed Student _T_-test was applied to compare the clinical and lifestyle characteristics of the low vs. high vitamin D status groups of
males and females in the discovery and validation cohorts. A Mann–Whitney _U_ Test was used to compare IGFBP-2 and IGFBP-3 levels in low vs. high vitamin D status groups of males and females
in the validation cohort. Parameters are presented as mean ± standard deviation or median (25th to 75th percentile). A _p_-value less than 0.05 was considered significant. RESULTS The
anthropometric, lifestyle and clinical characteristics of the participants in the discovery phase are presented in Table 1. The low and high vitamin D status groups of the male and female
participants were similar with regards to age, BMI, fasting glucose, sun exposure, physical activity and total energy, DHA/EPA and calcium intake. The two groups had significantly different
serum 25(OH)D levels (_p_ < 0.0001) as per the inclusion criteria and vitamin D intake (_p_ < 0.0001). In the male and female cohorts of the cross-sectional study, 1297 and 1114
protein groups were profiled, respectively (peptide level FDR _p_ < 0.05). Principal component analysis using the iTRAQ log2ratio of all analysed proteins in high vs. low vitamin D status
groups for male and female subjects of the cross-sectional study is presented in Fig. 2. Of the quantitatively analysed serum proteins, 423 and 549 proteins were differentially expressed in
the high vs. low vitamin D status conditions of the male and female cohorts, respectively (Supplementary Tables 1 and 2). IPA analysis showed that protein networks related to _small
molecule biochemistry_ were enriched in both male and female cohorts (score = 17 and 19 for males and females, respectively) (Fig. 3a, b). KEGG canonical pathway analysis showed that
_glycolysis|gluconeogenesis_ was significantly enriched in the differentially expressed proteins (DEPs) of the male and female cohorts (Fisher exact _p_ = 0.002 and 0.028, respectively for
males and females) (Fig. 3c). The secreted IGFBP2 and IGFBP3 proteins, as surrogate markers of glucose and fatty acid homeostasis through their engagement with the IGF/Insulin complex55,
were found to be differentially expressed between low and high Vitamin D status of both male and female cohorts. In particular, the discovery serum proteomics analysis showed that IGFBP-2
was over-expressed in men with high compared to low vitamin D status [Males: IGFBP-2 mean iTRAQ log2ratio in high vs. low vitamin D status (SD) = 0.6 (0.8), _p_ = 0.02] whereas IGFBP-3 was
expressed at higher levels in women with high compared to low vitamin D status [Females: IGFBP-3 mean iTRAQ log2ratio in high vs. low vitamin D status (SD) = 0.5 (0.5), _p_ = 0.001] (Fig.
4a). This sex-specific correlation of vitamin D status with IGFBP-2 and IGFBP-3 was further examined using Luminex ELISA analysis against an independent cross-sectional cohort. The clinical
characteristics of the validation cohort are presented in Table 2. As for the discovery cohort, the low and high vitamin D status groups of the male and female participants were similar with
regards to age, BMI, fasting glucose, sun exposure, physical activity and total energy, DHA/EPA and calcium intake. The two groups had significantly different vitamin D intake (_p_ <
0.0001) and vitamin D status (_p_ < 0.0001). ELISA measurements confirmed the sexually dimorphic correlation of IGFBP-2 and IGFBP-3 with vitamin D status among adults with obesity [Males:
IGFBP-2 Low vitamin D status median (25th to 75th percentile) = 5.1 (2.9–11.1), High vitamin D status median (25th to 75th percentile) = 10.0 (5.6–22.1), _p_ = 0.002; IGFBP-3 Low vitamin D
status median (25th to 75th percentile) = 5.4 (3.4–5.6), High vitamin D status median (25th to 75th percentile) = 5.5 (3.9–5.8), _p_ = 0.66] [Females: IGFBP-2 Low vitamin D status median
(25th to 75th percentile) = 11.4 (6.6–23.4), High vitamin D status median (25th to 75th percentile) = 13.4 (5.7–25.8), _p_ = 0.99; IGFBP-3 Low vitamin D status median (25th to 75th
percentile) = 2.4 (1.6–2.8), High vitamin D status median (25th to 75th percentile) = 2.8 (2.7–4.5), _p_ = 0.03] (Fig. 4b). DISCUSSION Individuals with obesity and low Vitamin D status
exhibit a substantially increased risk of cardiometabolic disease including its key component, T2D2,10,11,12,13,14,15,16,17,18,19,20,21. Such an increased risk is manifested well before the
clinical diagnosis of cardiometabolic disease. Identifying novel protein markers at the minimally invasive blood serum level may improve our understanding and aggressive management of such
at risk individuals. In this capacity, such serological protein markers may guage the effectiveness of Vitamin D intervention on reducing risk of cardiometabolic disease to individuals with
obesity and low Vitamin D status. To address this need, the main aim of this study was to identify novel serum proteins as surrogate markers that may correlate Vitamin D status with
cardiometabolic disease risk in non-diabetic adults with obesity using an agnostic quantitative serum proteomics approach (Fig. 1) based on the method developed by the authors24,25. The
Principle Component Analysis of the entire serum proteome observed (Fig. 2) captured the anticipated sexual dimorphic protein expression, as previouly reported24,25. Futher bioinformatics
interpretation (using Ingenuity and KEGG Pathway Analysis) of the differentially expressed proteins (_p<0.05_) between the high vs.low Vitamin D status conditions of the male and female
cohorts (Suppl. Tables 1 and 2) resulted in the sexual dimorphic enrichment of the _small molecule biochemistry_ protein networks (male and female enrichment scores of 17 and 19,
respectively, Fig. 3 a and b) and the _glycolysis|gluconeogenesis_ pathway (Fisher exact _p=0.02_ for males and _p=0.028_ for females, Fig. 3c). Interestingly, and consequent to the serum
proteomic method used in this study, key participatory proteins observed in these enriched metabolic processes were of exosomal origin, as listed in the highly curated ExoCarta database
(http://www.exocarta.org). Notable exosomal derived proteins found to be differentially exprerssed between the male high vs. low Vitamin D status conditions in the _small molecule
biochemistry_ network were the PDZ and LIM domain 1 (_PDLIM1_); acetyl-CoA carboxylase beta (_ACACB_); and UDP glycosyltransferace 1 family, polypeptide A8 (_UGT1A8_) (Fig. 3a), whereas the
differentially expressed proteins found in the female high vs. low Vitamin D status condition of the same network were carboxypeptidase N, polypeptide 2 (_CPN2_), protein C receptor,
endothelial (_PROCR_); and ADAM metallopeptidase with thrombospondin type 1 motif, 13 (_ADAMTS13_) (Fig. 3b). For the _glycolysis|gluconeogenesis_ pathway, key exosomal derived proteins
differentially expressed in the high vs. low Vitamin D status included aldolase A, fructose-bisphosphate (_ALDOA_) found to be upregulated in both sexes; pyruvate kinase liver and red blood
cell (_PKLR_) found upregulated in males; enolase 1, alpha (_ENO1_) found downregulated in females; and hexokinase 1 (_HK1_) found upregulated in females (Fig. 3c). An overarching regulatory
process controlling the above described sugar and fatty acid metabolic traits is the growth hormone--insulin-like growth factor axis28,29. More precisely, the growth hormone—insulin-like
growth factor axis is an evolutionary conserved system that controls somatic growth and metabolism28. Growth hormone (GH), a peptide hormone secreted by the anterior pituitary gland, is a
stress hormone that counteracts the action of insulin and directly increases the concentration of glucose in the blood29. GH administration has been shown to increase gluconeogenesis and
glycogenolysis from the kidney and liver30,31. Along these lines, patients with acromegaly exhibit increased gluconeogenic activity in the liver30 and are at risk of developing type 2
diabetes32. Furthermore, studies have found that GH suppresses glucose uptake by the adipose tissue, through the down-regulation of glucose transporter substrates on the plasma membrane of
adipocytes33. However, the effects of GH on glycaemic control are complex, since GH stimulates the production of insulin-like growth factor I (IGF1). Thus, GH deficiency is paradoxically
associated with insulin resistance and abdominal obesity, a phenomenon possibly attributed to decreased IGF1 activity34. IGF1, a hormone primarily produced by the liver, has growth-promoting
properties and insulin-like effects that are exerted through binding with the IGF1 receptor and insulin receptor34,35. Insulin-like growth factor II (IGF2) is a hormone closely related to
IGF1, that also exerts growth-regulating and insulin-like activities. The IGF1 and IGF2 components are carried in the systemic circulation by the soluble insulin-like growth factor binding
proteins (IGFBPs)36. There are six members in the IGFBP protein family (numbered 1 through 6) and their molecular weight varies from 24 to 45 kDa37. The primary role of IGFBPs is to extend
the half-life of IGFs in plasma38,55. However, studies have shown that IGFBPs can also inhibit the binding of IGF1 and IGF2 to their respective receptors39,40. Vitamin D and IGFBPs have been
suggested to act synergistically to affect insulin sensitivity, although the mechanism remains elusive40,41. IGFBPs have also been found to reflect risk for coronary heart disease and
stroke as part of a randomised control trial using hormone therapy42. A recent study demonstrated the relationships between IGF1 and its binding proteins, with cardiometabolic risk in
hypertensive perimenopausal females43. Furthermore, it has been reported in patients with T2DM, that the IGF system is strongly associated with cardiovascular disease damage44 and may
constitute alternative risk factor markers45. In this study, the non-targeted high-precision serum proteomics analysis of a cross-sectional discovery cohort with bioinformatics
interpretation, literature research and biostatistical assessment, identified a novel sexually dimorphic correlation of IGFBP-2 and IGFBP-3 with vitamin D status in non-diabetic males and
females with obesity (Fig. 4a). Additionally, the IGFBP-2 and IGFBP-3 proteomic findings were verified for relative quantitative accuracy with Luminex ELISA against a statistically
significant number of samples from an independent cohort (Fig. 4b) but with analogous clinical conditions with the discovery cohort (Tables 1 and 2). Although the precise function of
IGFBP-2 in cardiometabolic pathophysiology is unclear, it has been implicated in the decrease of the biological activity of IGF1, thus regulating insulin sensitivity46. A study by Hedbacker
et al.47 showed that the mRNA expression for IGFBP2 mediated the regulation of glucose metabolism, in response to the administration of physiologic and extra-physiologic doses of leptin in
mice. In particular, high mRNA levels of IGFBP2 were associated with reduced blood glucose in wild type, as well as diabetic mice, and also suppressed hepatic glucose production and reduced
expression of genes involved in hepatic fatty acid synthesis and gluconeogenesis. This study suggested that the induction of IGFBP2 as a result of leptin administration might play a
preventive role in the pathogenesis of T2DM. IGFBP-3 is the most prominent member of the IGFBP family, transporting 70–90% of the circulating IGF-1 and -239. Serum IGFBP-3 has been shown to
increase as a result of vitamin D administration48. Vitamin D may increase IGFBP-3 levels either through direct transcriptional induction in the liver, since IGFBP-3 is a transcriptional
target of the vitamin D receptor49, or through an indirect enhancement of growth hormone stimulation50. IGFBP-3 decreases insulin-mediated uptake of glucose in the adipocytes51 and inhibits
adipogenesis52. We have previously shown that the serum proteomic profile of men is distinct from that of women, with men over-expressing proteins associated with an increased risk of
cardiovascular disease25. Furthermore, sex hormones have been shown to affect circulating IGFBP levels53,54. Finally, we have described a sex-specific effect of vitamin D administration on
serum proteins related to cardiovascular risk24. The above-mentioned associations could partly explain the results of the present study on a sexually dimorphic correlation of IGFBP-2 and
IGFBP-3 with vitamin D status. Despite the significant statistical power of this pilot study, the present findings should be examined in larger cross-sectional cohorts and randomised
placebo-controlled studies to verify their translational importance. One study limitation is that some of the study participants were borderline hypertensive (systolic blood pressure up to
145 mmHg), an anticipated trend within a cohort of adults with overweight/obesity. Another study limitation is that serum IGF-1 levels were not measured in the present cohort. However,
examining the association between IGF-1 levels and sex, vitamin D status and BMI constitutes a future perspective. In conclusion, IGFBP-2 and IGFBP-3 were found to correlate with vitamin D
status in males and female adults with obesity, respectively. In this population group, IGFBP-2 and IGFBP-3 warrant further examination as potential sexually dimorphic serological markers
linking vitamin D status with cardiometabolic outcomes. REFERENCES * Bikle, D. D. Vitamin D: an ancient hormone. _Exp. Dermatol._ 20, 7–13 (2011). Article CAS Google Scholar * Reichrath,
J., Zouboulis, C. C., Vogt, T. & Holick, M. F. Targeting the vitamin D endocrine system (VDES) for the management of inflammatory and malignant skin diseases: an historical view and
outlook. _Rev. Endocr. Metab. Disord._ 17, 405–417 (2016). Article CAS Google Scholar * Lehmann, U. et al. Vitamin D3 supplementation: response and predictors of vitamin D3 metabolites-A
randomized controlled trial. _Clin. Nutr._ 35, 351–358 (2016). Article CAS Google Scholar * Ross, A. C. et al. The2011 report on dietary reference intakes for calcium and vitamin D from
the Institute of Medicine: what clinicians need to know. _J. Clin. Endocrinol. Metab._ 96, 53–58 (2011). Article CAS Google Scholar * Holick, M. F. et al. Evaluation, treatment, and
prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. _J. Clin. Endocrinol. Metab._ 96, 1911–1930 (2011). Article CAS Google Scholar * Lee, J. P., Tansey,
M., Jetton, J. G. & Krasowski, M. D. Vitamin D toxicity: a 16-year retrospective study at an academic medical center. _Lab. Med._ 49, 123–129 (2018). Article Google Scholar * Kimball,
S. M., Mirhosseini, N. & Holick, M. F. Evaluation of vitamin D3 intakes up to 15,000 international units/day and serum 25-hydroxyvitamin D concentrations up to 300 nmol/L on calcium
metabolism in a community setting. _Dermatoendocrinol_ 9, e1300213 (2017). Article CAS Google Scholar * Lips, P. Worldwide status of vitamin D nutrition. _J. Steroid Biochem. Mol. Biol._
121, 297–300 (2010). Article CAS Google Scholar * Cashman, K. D. et al. Vitamin D deficiency in Europe: pandemic? _Am. J. Clin. Nutr._ 103, 1033–1044 (2016). Article CAS Google Scholar
* Wang, L. et al. Circulating 25-hydroxy-vitamin D and risk of cardiovascular disease: a meta-analysis of prospective studies. _Circ. Cardiovasc Qual. Outcomes_ 5, 819–829 (2012). Article
CAS Google Scholar * Garland, C. F. et al. Meta-analysis of all-cause mortality according to serum 25-hydroxyvitamin D. _Am. J. Public Health_ 104, e43–e50 (2014). Article Google
Scholar * Zhang, R. et al. Serum 25-hydroxyvitamin D and the risk of cardiovascular disease: dose-response meta-analysis of prospective studies. _Am. J. Clin. Nutr._ 105, 810–819 (2017).
Article CAS Google Scholar * Lee, J. H., O’Keefe, J. H., Bell, D., Hensrud, D. D. & Holick, M. F. Vitamin D deficiency an important, common, and easily treatable cardiovascular risk
factor? _J. Am. Coll. Cardiol._ 52, 1949–1956 (2008). Article CAS Google Scholar * Song, Y. et al. Blood 25-hydroxy vitamin D levels and incident type 2 diabetes: a meta-analysis of
prospective studies. _Diabetes Care_ 36, 1422–1428 (2013). Article CAS Google Scholar * Pannu, P. K., Piers, L. S., Soares, M. J., Zhao, Y. & Ansari, Z. Vitamin D status is inversely
associated with markers of risk for type 2 diabetes: a population based study in Victoria, Australia. _PLoS ONE_ 12, e0178825 (2017). Article Google Scholar * Lucato, P. et al. Low vitamin
D levels increase the risk of type 2 diabetes in older adults: a systematic review and meta-analysis. _Maturitas_ 100, 8–15 (2017). Article CAS Google Scholar * Vangoitsenhoven, R. et
al. Effect of a transcriptional inactive or absent vitamin D receptor on beta-cell function and glucose homeostasis in mice. _J. Steroid Biochem. Mol. Biol._ 164, 309–317 (2016). Article
CAS Google Scholar * Garbossa, S. G. & Folli, F. Vitamin D, sub-inflammation and insulin resistance. A window on a potential role for the interaction between bone and glucose
metabolism. _Rev. Endocr. Metab. Disord._ 18, 243–258 (2017). Article CAS Google Scholar * Muscogiuri, G. et al. Mechanisms in endocrinology: vitamin D as a potential contributor in
endocrine health and disease. _Eur. J. Endocrinol._ 171, R101–R110 (2014). Article CAS Google Scholar * Gulseth, H. L., Wium, C., Angel, K., Eriksen, E. F. & Birkeland, K. I. Effects
of vitamin d supplementation on insulin sensitivity and insulin secretion in subjects with type 2 diabetes and vitamin d deficiency: a randomized controlled trial. _Diabetes Care_ 40,
872–878 (2017). Article Google Scholar * Lu, L. et al. Association of vitamin D with risk of type 2 diabetes: a Mendelian randomisation study in European and Chinese adults. _PLoS. Med._
15, e1002566 (2018). Article Google Scholar * von Elm, E. et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting
observational studies. _Lancet_ 370, 1453–1457 (2007). Article Google Scholar * Al-Daghri, N. M. et al. Diabetes mellitus type 2 and other chronic non-communicable diseases in the central
region, Saudi Arabia (Riyadh cohort 2): a decade of an epidemic. _Bmc. Med._ 9, 76 (2011). Article Google Scholar * Al-Daghri, N. M. et al. Sex-specific vitamin D effects on blood
coagulation among overweight adults. _Eur. J. Clin. Invest._ 46, 1031–1040 (2016). Article CAS Google Scholar * Al-Daghri, N. M. et al. Whole serum 3D LC-nESI-FTMS quantitative proteomics
reveals sexual dimorphism in the milieu interieur of overweight and obese adults. _J. Proteome Res._ 13, 5094–5105 (2014). Article CAS Google Scholar * Levin, Y. The role of statistical
power analysis in quantitative proteomics. _Proteomics_ 11, 2565–2567 (2011). Article CAS Google Scholar * Concato, J., Peduzzi, P., Holford, T. R. & Feinstein, A. R. Importance of
events per independent variable in proportional hazards analysis. I. Background, goals, and general strategy. _J. Clin. Epidemiol._ 48, 1495–1501 (1995). Article CAS Google Scholar * Le
Roith, D. Seminars in medicine of the Beth Israel Deaconess Medical Center. Insulin-like growth factors. _N. Engl. J. Med._ 336, 633–640 (1997). Article Google Scholar * Møller, N. &
Jørgensen, J. O. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. _Endocr. Rev._ 30, 152–177 (2009). Article Google Scholar * Schwarz, J. M. et al.
Effects of recombinant human growth hormone on hepatic lipidand carbohydrate metabolism in HIV-infected patients with fataccumulation. _J. Clin. Endocrinol. Metab._ 87, 942 (2002). Article
CAS Google Scholar * Höybye, C. et al. Contribution of gluconeogenesis and glycogenolysis to hepatic glucose production in acromegaly before and after pituitary microsurgery. _Horm. Metab.
Res._ 40, 498–501 (2008). Article Google Scholar * Vijayakumar, A., Yakar, S. & Leroith, D. The intricate role of growth hormone in metabolism. _Front Endocrinol. (Lausanne)._ 2, 32
(2011). PubMed PubMed Central Google Scholar * Kilgour, E., Baldwin, S. A. & Flint, D. J. Divergent regulation of rat adipocyte GLUT1 and GLUT4 glucose transporters by GH. _J.
Endocrinol._ 145, 27–33 (1995). Article CAS Google Scholar * Kim, S. H. & Park, M. J. Effects of growth hormone on glucose metabolism and insulin resistance in human. _Ann. Pediatr.
Endocrinol. Metab._ 22, 145–152 (2017). Article Google Scholar * Anisimov, V. N. & Bartke, A. The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. _Crit. Rev.
Oncol. Hematol._ 87, 201–223 (2013). Article Google Scholar * Shimasaki, S., Shimonaka, M., Zhang, H. P. & Ling, N. Identification of five different insulin-like growth factor binding
proteins (IGFBPs) from adult rat serum and molecular cloning of a novel IGFBP-5 in rat and human. _J. Biol. Chem._ 266, 10646–10653 (1991). CAS PubMed Google Scholar * Hwa, V., Oh, Y.
& Rosenfeld, R. G. The insulin-like growth factor-binding protein (IGFBP) superfamily. _Endocr. Rev._ 20, 761–787 (1999). CAS PubMed Google Scholar * Clemmons, D. R. et al. Role of
insulin-like growth factor binding proteins in the control of IGF actions. _Prog. Growth Factor Res._ 6, 357–366 (1995). Article CAS Google Scholar * Wetterau, L. A., Moore, M. G., Lee,
K. W., Shim, M. L. & Cohen, P. Novel aspects of the insulin-like growth factor binding proteins. _Mol. Genet. Metab._ 68, 161–181 (1999). Article CAS Google Scholar * Forouhi, N. G.,
Luan, J., Cooper, A., Boucher, B. J. & Wareham, N. J. Baseline serum 25-hydroxy vitamin d is predictive of future glycemic status and insulin resistance: the Medical Research Council Ely
Prospective Study 1990-2000. _Diabetes_ 57, 2619–2625 (2008). Article CAS Google Scholar * Kamycheva, E., Berg, V. & Jorde, R. Insulin-like growth factor I, growth hormone, and
insulin sensitivity: the effects of a one-year cholecalciferol supplementation in middle-aged overweight and obese subjects. _Endocrine_ 43, 412–418 (2013). Article CAS Google Scholar *
Prentice, R. L. et al. Proteomic risk markers for coronary heart disease and stroke: validation and mediation of randomized trial hormone therapy effects on these diseases. _Genome Med._ 5,
112 (2013). Article Google Scholar * Olszanecka, A., Dragan, A., Kawecka-Jaszcz, K., Fedak, D. & Czarnecka, D. Relationships of insulin-like growth factor-1, its binding proteins, and
cardiometabolic risk in hypertensive perimenopausal women. _Metabolism_ 69, 96–106 (2017). Article CAS Google Scholar * Hjortebjerg, R. et al. The IGF system in patients with type 2
diabetes: associations with markers of cardiovascular target organ damage. _Eur. J. Endocrinol._ 176, 521–531 (2017). Article CAS Google Scholar * Kaplan, R. C. et al. Insulinlike growth
factor binding protein-1 and ghrelin predict health outcomes among older adults: cardiovascular health study cohort. _J. Clin. Endocrinol. Metab._ 102, 267–278 (2017). PubMed Google Scholar
* Arafat, A. M. et al. The role of insulin-like growth factor (IGF) binding protein-2 in the insulin-mediated decrease in IGF-I bioactivity. _J. Clin. Endocrinol. Metab._ 94, 5093–5101
(2009). Article CAS Google Scholar * Hedbacker, K. et al. Antidiabetic effects of IGFBP2, a leptin-regulated gene. _Cell. Metab._ 11, 11–22 (2010). Article CAS Google Scholar *
Bereket, A. et al. Circulating insulin-like growth factor binding protein-4 (IGFBP-4) is not regulated by parathyroid hormone and vitamin D in vivo: evidence from children with rickets. _J.
Clin. Res. Pediatr. Endocrinol._ 2, 17–20 (2010). Article Google Scholar * Krishnan, A. V. et al. Analysis of vitamin D-regulated gene expression in LNCaP human prostate cancer cells using
cDNA microarrays. _Prostate_ 59, 243–251 (2004). Article CAS Google Scholar * Liao, L., Chen, X., Wang, S., Parlow, A. F. & Xu, J. Steroid receptor coactivator 3 maintains
circulating insulin-like growth factor I (IGF-I) by controlling IGF-binding protein 3 expression. _Mol. Cell. Biol._ 28, 2460–2469 (2008). Article CAS Google Scholar * Chan, S. S., Twigg,
S. M., Firth, S. M. & Baxter, R. C. Insulin-like growth factor binding protein-3 leads to insulin resistance in adipocytes. _J. Clin. Endocrinol. Metab._ 90, 6588–6595 (2005). Article
CAS Google Scholar * Kong, J. & Li, Y. C. Molecular mechanism of 1,25-dihydroxyvitamin D3 inhibition of adipogenesis in 3T3-L1 cells. _Am. J. Physiol. Endocrinol. Metab._ 290,
E916–E924 (2006). Article CAS Google Scholar * Münzer, T. et al. Effects of GH and/or sex steroids on circulating IGF-I and IGFBPs in healthy, aged women and men. _Am. J. Physiol.
Endocrinol. Metab._ 290, E1006–E1013 (2006). Article Google Scholar * Janssen, J. A. et al. Serum free IGF-I, total IGF-I, IGFBP-1 and IGFBP-3 levels in an elderly population: relation to
age and sex steroid levels. _Clin. Endocrinol._ 48, 471–478 (1998). Article CAS Google Scholar * Wenjing Ruan, Maode Lai, (2010) Insulin-like growth factor binding protein: a possible
marker for the metabolic syndrome?. Acta Diabetologica 47:5-14 Article CAS Google Scholar Download references ACKNOWLEDGEMENTS Funding: The Deanship of Scientific Research, Prince Mutaib
Chair for Biomarkers of Osteoporosis in King Saud University, Riyadh, Saudi Arabia. JT was supported by the China Scholarship Council and the China Postdoctoral Science Foundation
(2013T60260). AUTHOR INFORMATION Author notes * Spiros D. Garbis Present address: Proteome Exploration Laboratory, Beckman Institute, Division of Biology and Biological Engineering,
California Institute of Technology, Pasadena, CA, 91125, USA * These authors are contributed equally: Nasser M. Al-Daghri, Antigoni Manousopoulou AUTHORS AND AFFILIATIONS * Biochemistry
Department, College of Science, Biomarkers Research Program, King Saud University, Riyadh, Saudi Arabia Nasser M. Al-Daghri, Majed S. Alokail, Sobhy Yakout, Amal Alenad & Shaun Sabico *
Biochemistry Department, Prince Mutaib Chair for Biomarkers of Osteoporosis, King Saud University, Riyadh, Saudi Arabia Nasser M. Al-Daghri, Majed S. Alokail, Sobhy Yakout, Amal Alenad, Omar
Al-Attas, Yousef Al-Saleh & Shaun Sabico * Centre for Proteomic Research, Institute for Life Sciences, University of Southampton, Southampton, UK Antigoni Manousopoulou, Diana J.
Garay-Baquero, Miltiadis Fotopoulos, Jie Teng & Spiros D. Garbis * School of Pharmacy, Tianjin Medical University, Tianjin, China Jie Teng * 1st Department of Pediatrics, University of
Athens, Athens, Greece George P. Chrousos * Cancer Sciences Unit, Faculty of Medicine, University of Southampton, Southampton, UK Spiros D. Garbis Authors * Nasser M. Al-Daghri View author
publications You can also search for this author inPubMed Google Scholar * Antigoni Manousopoulou View author publications You can also search for this author inPubMed Google Scholar * Majed
S. Alokail View author publications You can also search for this author inPubMed Google Scholar * Sobhy Yakout View author publications You can also search for this author inPubMed Google
Scholar * Amal Alenad View author publications You can also search for this author inPubMed Google Scholar * Diana J. Garay-Baquero View author publications You can also search for this
author inPubMed Google Scholar * Miltiadis Fotopoulos View author publications You can also search for this author inPubMed Google Scholar * Jie Teng View author publications You can also
search for this author inPubMed Google Scholar * Omar Al-Attas View author publications You can also search for this author inPubMed Google Scholar * Yousef Al-Saleh View author publications
You can also search for this author inPubMed Google Scholar * Shaun Sabico View author publications You can also search for this author inPubMed Google Scholar * George P. Chrousos View
author publications You can also search for this author inPubMed Google Scholar * Spiros D. Garbis View author publications You can also search for this author inPubMed Google Scholar
CONTRIBUTIONS N.A.D., A.M., G.P.C., and S.D.G. concept and clinical design; A.M., D.G.B., M.F., J.T., and A.A. performed proteomics work; S.Y., Y.A.S., and S.S. performed verification work;
A.M., D.G.B., and S.D.G. contributed new reagents and analytic tools; A.M., S.Y., S.S. and S.D.G. analyzed data; N.A.D., O.A.A., G.P.C., and S.D.G. supervised research; A.M. and S.D.G.
writing-original draft; N.A.D., A.M., S.S., G.P.C. and S.D.G. writing-review and editing. CORRESPONDING AUTHOR Correspondence to Spiros D. Garbis. ETHICS DECLARATIONS CONFLICT OF INTEREST
The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER'S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in published
maps and institutional affiliations. ELECTRONIC SUPPLEMENTARY MATERIAL SUPPLEMENTARY TABLE 1. DIFFERENTIALLY EXPRESSED PROTEINS IN HIGH VS. LOW VITAMIN D STATUS (MALES) SUPPLEMENTARY TABLE
2. DIFFERENTIALLY EXPRESSED PROTEINS IN HIGH VS. LOW VITAMIN D STATUS (FEMALES) RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons Attribution 4.0
International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the
source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative
Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by
statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Al-Daghri, N.M., Manousopoulou, A., Alokail, M.S. _et al._ Sex-specific correlation
of IGFBP-2 and IGFBP-3 with vitamin D status in adults with obesity: a cross-sectional serum proteomics study. _Nutr & Diabetes_ 8, 54 (2018). https://doi.org/10.1038/s41387-018-0063-8
Download citation * Received: 27 May 2018 * Revised: 20 August 2018 * Accepted: 10 September 2018 * Published: 04 October 2018 * DOI: https://doi.org/10.1038/s41387-018-0063-8 SHARE THIS
ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard
Provided by the Springer Nature SharedIt content-sharing initiative