- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Long-term memory formation leads to enduring alterations in synaptic efficacy and neuronal responses that may be created by changes in neuronal morphology. We show that fear
conditioning leads to a long-lasting increase in the volume of the primary and secondary dendritic branches, but not of distal branches, of neurons located at the basolateral amygdala (BLA).
The length of the dendritic branches is not affected by fear conditioning. Fear conditioning leads to an enduring increase in the length and volume of dendritic spines, especially in the
length of the spine neck and the volume of the spine head. Fear conditioning does not affect dendritic spine density. We further reveal that activation of Rac1 in BLA during fear
conditioning impairs long-term auditory, but not contextual, fear conditioning memory. Activation of Rac1 during fear conditioning prevents the enduring increase in the dendritic primary
branch volume and dendritic spines length and volume. Rac1 activation per se has no effect on neuronal morphology. These results show that fear conditioning induces changes known to reduce
the inhibition of signal propagation along the dendrite and the increase in synaptic efficacy whereas preventing these changes, by Rac1 activation, impairs fear memory formation. You have
full access to this article via your institution. Download PDF SIMILAR CONTENT BEING VIEWED BY OTHERS NCK1 ACTIVITY IN LATERAL AMYGDALA REGULATES LONG-TERM FEAR MEMORY FORMATION Article Open
access 12 November 2022 EPHRINB2 IN EXCITATORY NEURONS AND ASTROCYTES IN THE BASOLATERAL AMYGDALA CONTROLS LONG-TERM FEAR MEMORY FORMATION Article Open access 17 September 2024 INTERCALATED
AMYGDALA CLUSTERS ORCHESTRATE A SWITCH IN FEAR STATE Article 26 May 2021 INTRODUCTION Evidence indicates that long-term memory (LTM) formation involves alterations in synaptic efficacy and
transmission of the signal along the neuron [1,2,3,4]. These changes may be mediated by modifying the morphology of neurons in particular of dendritic spines [4, 5] but further studies need
to unveil the role of these alterations in long-term memory. In this study, we explored whether learning leads to changes in neuronal morphology and if altering such changes affects
long-term memory. Toward that end, we used auditory fear conditioning. In this paradigm, an association is formed between an auditory tone (conditioned stimulus (CS)) and an aversive mild
footshock (unconditioned stimulus (US)) [6,7,8,9,10]. The site of fear conditioning memory, within the basolateral amygdala (BLA; that is comprised of the lateral amygdala and the basal
amygdala nuclei), is identified [6, 7, 11,12,13]. Toward elucidating whether changes in neuronal morphology are involved in memory we aimed to affect them by manipulating Rac1 GTPase
activity. Rac1 GTPase is a member of the Rho GTPases family of molecular switches that cycle between an inactive GDP-bound state and an active GTP-bound form to regulate downstream
effectors. Rac1 GTPase has been shown to be involved in neuronal morphogenesis and is intimately involved in the regulation of dendritic spine structure formation [14,15,16,17,18]. Rac1 is
involved in memory formation and erasure [19,20,21]. To study whether Rac1 activation can affect neuronal changes induced by learning, we have employed a novel approach to photoactivate Rac1
GTPase in BLA by light with a high temporal and spatial resolution [21]. In this photoactivatable Rac1 (PA-Rac1), a complete _Avena sativa_ Phototropin1 LOV2-Jα domain (sequence 404–547) is
conjugated to the N-terminus of a constitutively active Rac1 [22]. LOV2 interacts with a C-terminal helical extension (Jα) in the dark and blocks the binding of Rac1 to its effectors. Blue
light (473 nm) induces unwinding of the Jα helix and releases its steric inhibition, leading to Rac1 activation [22]. PA-Rac1 activation in the brain leads to the phosphorylation of its
effector p21-activated kinase (PAK) [21]. We also revealed that activation of PA-Rac1 during fear conditioning impaired long-term fear memory but not short-term fear memory. Moreover,
shining light per se or activation of PA-Rac1 after fear conditioning learning had no effect [21]. Here we study whether fear conditioning leads to morphological changes in neurons in BLA
and whether activation of Rac1 in BLA affects fear conditioning memory and structural plasticity. MATERIALS AND METHODS ANIMALS Male C57BL6 mice (8–10 weeks) were used in this study (Harlan
Laboratories). Following surgery, mice were housed separately at 22 ± 2 °C in a 12 h light/dark cycle, with _ad libitum_ access to food and water. All experiments were done following the
instructions and approval of the University of Haifa animal ethics committee for animal experiments observing National Institutes of Health guidelines and all experiments were performed in
accordance with the relevant guidelines and regulations. FEAR CONDITIONING On the day of training, mice were placed in a training chamber (Coulbourn Instruments). Mice were allowed to
acclimate in the chamber for 2 min and then subjected to 3 pairs of tone (conditioned stimulus (CS) - 20 s, 2.8 kHz, 85 dB) that co-terminated with a foot shock (unconditioned stimulus (US)
- 2 s, 0.8 mA). The inter-trial interval was 120 s. Mice were tested for contextual fear conditioning in the same context 24 h after training for long-term memory (placed for 9 min in the
chamber and the first 5 min were analyzed). Mice were tested for auditory fear conditioning in a different context 48 h after training for long-term memory. Behavior was recorded and the
video images were transferred to a computer equipped with an analysis program (FreezeFrame). The percentage of changed pixels between two adjacent 0.25 s images was used as a measure of
activity. AAV PRODUCTION AND MICROINJECTION hSyn-mCherry-PARac1 containing AAVs at high titer (2.63E + 13) was produced by ELSC Vector Core Facility (Hebrew University of Jerusalem, Israel).
pTriEx-mCherry-PA-Rac1 was a gift from Klaus Hahn (Addgene plasmid # 22027; http://n2t.net/addgene:22027; RRID: Addgene_22027) (Wu et al., 2009). Animals were anesthetized with Medetomidine
(Domitor) 1 mg/ml and Ketamine 100 mg/ml cocktail, diluted in sterile isotonic saline (administered doses: Ketamine 50 mg/kg; Domitor 0.5 mg/kg; 100 µl/10 gm of animal body weight).
Carprofen (5 mg/kg) was injected for analgesia before surgery and consecutive 3 days after surgery. AAV was injected (0.5 µl/hemisphere, 0.1 µl/min) into BLA following a stereotaxic surgery
(Neurostar stereo drive). After virus injection, intracranial optic fiber (Thorlabs, Fiber Optic Cannula, Ø1.25 mm Stainless Ferrule, Ø200 µm Core, 0.39 NA) was implanted on the same line
and 0.5 mm above virus injection place. After surgery, animals received the antibiotic Baytril (5 mg/kg; Enrofloxacin) for 3 consecutive days. Animals were allowed to recuperate for 4 weeks
before behavioral experiments. LIGHT STIMULATION Blue light (473 nm) from a laser was subjected at the indicated time to activate PA-Rac1. Optic fibers were connected to a 473-nm blue laser
diode (Shanghai Dreamlasers) via an FC/PC adaptor. The light intensity ~15 mW/mm2 was measured at the tip of the fiber. A control group of animals got an equal amount of virus microinjection
into BLA along with the fiber optic implantation but did not receive light stimulation. SCALES Immediately after the auditory fear conditioning test the animals were anaesthetized using
isoflurane followed by perfusion with 4% PFA in PBS. Brains were removed and post-fixed in 1% PFA and 30% sucrose. Brains were kept in −80 °C until use. After slicing, each brain slice
sample (250 µm in thickness) was placed in a volume of more than 25 ml/g of tissue in each step of the protocol [23]. First, samples were placed in the S0 solution (D-(–)-sorbitol % (w/v)-
20; Glycerol % (w/v)- 5; 1 mM Methyl-ß-cyclodextrin); 1 mM α-Cyclodextrin, N-acetyl-L-hydroxyproline % (w/v)- 1, Dimethylsulfoxide % (v/v)- 3; 1X PBS (–); pH = 7.2) at 37 °C overnight for 12
h after which they were transferred to S1 solution (D-(–)-sorbitol % (w/v)-20; Glycerol % (w/v)-10; 4 M Urea; Triton X-100 % (w/v)- 0.2; pH 8.3), S2 solution (D-(–)-sorbitol % (w/v)- 27;
2.7 M Urea; Triton X-100 % (w/v)- 0.1; Dimethylsulfoxide % (v/v)-8.3; pH=8.3) and S3 solution (D-(–)-sorbitol % (w/v)- 36.4; 2.7 M Urea; Dimethylsulfoxide % (v/v)- 9.1; pH = 7.9) with an
incubation period of 4 h each at 37 °C. Following these incubations, samples were placed in a PBS solution at 4 °C overnight, for an incubation period of 12 h. Finally, the samples were
transferred to a S4 solution (D-(–)-sorbitol % (w/v)- 40; Glycerol % (w/v)-10; 4 M Urea; Triton X-100 % (w/v)- 0.2; Dimethylsulfoxide % (v/v)- 20; pH = 7.9) at 37 °C for 12 h. The images
acquisition was made using 10× and 20× objectives for the elaboration of maps of the brain slices and region of interest and 60x objectives to visualize dendritic spine morphology. Z-stack
images of 0.15 μm increments were made. The photographs were analyzed using the Imaris program (Oxford Instruments). For branch levels, the Imaris software calculates it as follows: At each
branching point, the dendrite segment with a smaller mean diameter sequentially increases the branch level, while the dendrite segment with a greater diameter maintains the same branch
level. In the case of two dendrite segments with the same diameter, the segment with a smaller branching angle keeps the branch level, while the dendrite segment with a greater branching
angle sequentially raises its branch level. STATISTICS Repeated measure ANOVA and t-test analysis were done for the auditory or contextual fear conditioning memory tests, respectively.
Kruskal–Wallis analysis that followed with Dunn’s test with Bonferroni correction was performed for the morphological experiments. The effect size was determined (Partial Eta squared (ɳp2)
for ANOVA analysis and epsilon squared (ϵ2) for Kruskal–Wallis analysis). α level is 0.05 (also after Bonferonni corrections so that when multiplying the unadjusted _p_ value by the number
of comparisons we never exceeded 0.05). RESULTS ACTIVATION OF PA-RAC1 IN THE BASOLATERAL AMYGDALA IMPAIRS AUDITORY, BUT NOT CONTEXTUAL, FEAR MEMORY FORMATION We activated PA-Rac1 in BLA
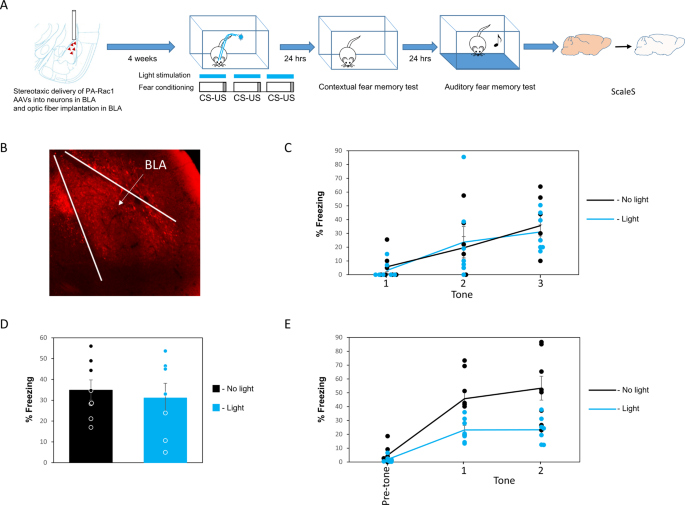
during CS-US pairing in fear conditioning and examined the effects on both contextual fear conditioning 24 h after training, and auditory fear conditioning 48 h after training (see Fig. 1A
for a schematic presentation of the experiment and Fig. 1B for AAV expression in BLA). Activation of PA-Rac1 during fear conditioning has no significant effect on freezing responses during
training (_F_(1,12) = 0.024, _p_ = 0.880; ɳp2 = 0.002). There is no treatment × tone trial interaction (_F_(2,24) = 0.262, _p_ = 0.772) (Fig. 1C). Activation of PA-Rac1 (_n_ = 7) during
training does not affect contextual fear conditioning, tested 24 h after training when compared to animals that expressed PA-Rac1 in BLA but that were not activated by light (_n_ = 8) (_p_ =
0.66; ɳp2 = 0.015) (Fig. 1D). Activation of Rac1 GTPase during fear conditioning (_n_ = 7) inhibited fear LTM compared to the no-light control group (_n_ = 8) when tested 48 h after
training (_F_(1,13) = 10.753, _p_ = 0.006; ɳp2 = 0.453) (Fig. 1E). There is no treatment × tone trial interaction (_F_(1,13) = 1.022, _p_ = 0.33). These results show that the activation of
PA-Rac1 in BLA neurons had no effect on contextual fear conditioning but specifically impaired long-term auditory fear conditioning memory 48 h after training. FEAR CONDITIONING ALTERS
DENDRITIC SHAFT MORPHOLOGY IN BASOLATERAL AMYGDALA NEURONS AND SUCH CHANGES ARE PREVENTED BY RAC1 GTPASE ACTIVATION The results above show that activation of Rac1 GTPase in BLA impairs fear
conditioning LTM. Previous studies have shown an association between Rac1 activity and alterations in dendritic morphology [14,15,16,17,18]. We were therefore interested to further study
whether activation of Rac1 GTPase can affect morphological changes induced by learning. We first examined whether fear conditioning leads to alterations in dendritic shaft morphology in BLA
and whether PA-Rac1 activation affects these changes. Animals were injected with AAV expressing PA-Rac1 into the BLA and divided into 4 groups: 1) Naïve animals that were subjected to the
training box with no light stimulation. 2) Naïve animals that were subjected to the training box with light stimulation in the BLA (same light stimulation protocol as in fear-conditioned
animals). 3) Fear-conditioned animals with no light stimulation. 4) Fear conditioning with light stimulation. Animals were perfused immediately after the last memory test (48 h after
training) and subjected to ScaleS clearing procedure and imaging. We examined the effect of the different treatments on dendritic shaft morphology. We found an effect on dendritic volume
(H(3) = 14.663, _p_ = 0.002; ϵ2 = 0.167). Posthoc analysis revealed that fear conditioning (_n_ = 18 neurons; _n_ = 4 animals) led to a significant increase in the total volume of the
dendritic shaft when compared to the naïve group (_n_ = 17 neurons; _n_ = 3 animals) (_p_ = 0.001) (Fig. 2A). We further analyzed the volume of the dendrite per dendritic branch (Fig. 2B).
We revealed a significant effect in the first dendritic branch (H(3) = 19.792, _p_ < 0.001, ϵ2 = 0.225) and the second dendritic branch (H(3) = 10.104, _p_ = 0.018, ϵ2 = 0.117). Posthoc
analysis revealed that fear conditioning led to an increase in the dendritic volume in the first (_p_ < 0.001) and second (p = 0.02) dendritic branch when compared to naïve animals (Fig.
2C). The increase in dendritic volume was significantly blocked by PA-Rac1 activation during fear conditioning (_n_ = 15 neurons; _n_ = 4 animals) in the primary first branch (_p_ = 0.035)
but not in the secondary branch (_p_ = 1) (Fig. 2C). Rac1 activation per se has no significant effect on dendritic morphology (_p_ = 0.228) (Fig. 2C) (_n_ = 17 neurons; _n_ = 5 animals).
There are no significant changes in other dendritic branches (_p_ > 0.2). There is no difference in the volume of the soma between all groups (H(3) = 2.723, _p_ = 0.436) (Fig. 2D). Next,
we examined the effects of fear conditioning on the length of the dendrites. We revealed that fear conditioning does not affect the total length of the dendrites in BLA (H(3) = 2.622; _p_ =
0.454; ϵ2 = 0.03) (Fig. 2E) (Fear conditioning- _n_ = 18 neurons; _n_ = 4 animals; Naïve- _n_ = 17 neurons; _n_ = 3 animals; Fear conditioning PA-Rac1 activation- _n_ = 15 neurons; _n_ = 4
animals; Naïve Rac1 activation; _n_ = 17 neurons; _n_ = 5 animals). Additional analysis revealed that there is a general effect on length in the first branch (H(3) = 3, _p_ = 0.047, ϵ2 =
0.09) but an in-depth post-hoc analysis revealed that the groups are not different from each other (Fig. 2F). There are no significant changes in other branches (_p_ > 0.3). Cumulatively,
these results show that fear conditioning leads to an increase in the volume but not the length of the primary and secondary branches. In addition, Rac1 activation per se does not affect
dendritic shaft morphology but prevents the morphological changes induced by fear conditioning. We further analyzed the general structure of the dendritic tree and its complexity (Fear
conditioning- _n_ = 18 neurons; _n_ = 4 animals; Naïve- _n_ = 17 neurons; _n_ = 3 animals; Fear conditioning PA-Rac1 activation- _n_ = 15 neurons; _n_ = 4 animals; Naïve Rac1 activation; _n_
= 17 neurons; _n_ = 5 animals). There is no difference in the space that the neurons occupy (convex hull analysis) between the different groups (H(3) = 0.291, _p_ = 0.962; ϵ2 = 0.161) (Fig.
3A) and in the number of dendrites extending from the neurons (H(3) = 3.2, _p_ = 0.362; ϵ2 = 0.036) (Fig. 3B). There is an overall effect on the number of dendritic segments (H(3) = 7.896,
_p_ = 0.048; ϵ2 = 0.09) where fear conditioning showed the lower scores (Fig. 3C). However, an in-depth posthoc analysis revealed that the groups are not different from each other. There is
an effect on the number of dendritic branch points (H(3) = 8.163, _p_ = 0.043; ϵ2 = 0.093) (Fig. 3D) where neurons of fear conditioning groups showed the lowest score. Posthoc analysis
reveals that activation of PA-Rac1 during fear conditioning leads to an increase in branch points compared to fear conditioning where PA-Rac1 is not activated (_p_ = 0.04) (Fig. 3D). These
results show that PA-Rac1 activation increases the number of dendritic branch points of fear-conditioned animals to the level of the naïve animals. We observed an effect on dendritic
branching 65 μm, 70 μm, 75 μm and 80 μm from the soma as determined by intersections in Sholl analysis (H(3) = 11.142, _p_ = 0.011, ϵ2 = 0.155; H(3) = 8.405, _p_ = 0.038, ϵ2 = 0.122; H(3) =
9.859, _p_ = 0.02, ϵ2 = 0.149; H(3) = 8.010, _p_ = 0.046, ϵ2 = 0.075) (Fig. 3E). Posthoc analysis revealed that the effect is caused by differences between the naïve no PA-Rac1 activation
and naïve PA-Rac1 activation in the 65 μm, 75 μm and 80 μm distances (_p_ = 0.011, _p_ = 0.019, _p_ = 0.035, respectively). There was no significant difference between the groups at the 70
μm distance. Thus, such changes are not induced by fear conditioning or have an effect on fear conditioning. Cumulatively, fear conditioning leads to structural changes in dendritic shaft
morphology. These morphological alterations may relieve the constraints on signal propagation in the dendrite (see discussion). Rac1 activation prevents these morphological changes and
inhibits the formation of fear memory. FEAR CONDITIONING LEADS TO CHANGES IN SPINES MORPHOLOGY THAT ARE PREVENTED BY RAC1 GTPASE ACTIVATION The aforementioned results show that fear
conditioning increases dendritic shaft volume in BLA neurons and that Rac1 activity prevents this morphological change. We were interested next to examine the effects of fear conditioning on
dendritic spines in BLA neurons and the response of these changes to Rac1 activity that impairs fear memory formation. Toward that end, we measured spines density along the dendrite and
their morphology (see Fig. 4A for examples of labeled spines). We found that fear conditioning does not increase significantly the spine density in BLA neurons in different dendritic
branches (Fig. 4B). We next explored the effect of fear conditioning on dendritic spines morphology. We found an effect on spine length (H(3) = 21.796, _p_ < 0.001; ϵ2 = 0.248). Posthoc
analysis revealed that fear conditioning led to an increase in the average length of spines measured from the dendritic shaft (_n_ = 18; _n_ = 4 animals) when compared to naïve animals (_n_
= 17; _n_ = 3 animals) (_p_ = 0.028) (Fig. 4C). In addition, we found that Rac1 activity during fear conditioning blocked the ability of fear conditioning to increase spines length (_n_ =
15; _n_ = 4 animals) when compared to animals where fear conditioning was performed without Rac1 activation (_p_ = 0.008). The spines length in Rac1 activated neurons in fear conditioning
animals was kept at its basal level and was not different from spines length in the naïve no light group (p = 1). Spine length in BLA neurons in the naïve light group where Rac1 is activated
(_n_ = 17 neurons; _n_ = 5 animals) was not different from spine length in the naïve no light group (_p_ = 1). The spine neck in particular was increased significantly between the four
groups (H(3) = 22.587, _p_ < 0.001; ϵ2 = 0.260). Posthoc analysis found that fear conditioning (no light) increases the length of the spine neck when compared to naïve animals (no light)
(_p_ = 0.019) (Fig. 4D). The increase in the length of the spine neck following fear conditioning was prevented by Rac1 activation during fear conditioning training (_p_ = 0.014). Spines
neck length in Rac1 activation fear-conditioned mice was not different from spines neck length in naïve mice with no light (basal level) (_p_ = 1). Rac1 activation per se in naïve mice had
no effect on spines neck length when compared to naïve no light (_p_ = 1). We next examined spines volume differences between the four groups and found an effect (H(3) = 12.887, _p_ <
0.001; ϵ2 = 0.146) (Fear conditioning- _n_ = 18 neurons; _n_ = 4 animals; Naïve- _n_ = 17 neurons; _n_ = 3 animals; Fear conditioning PA-Rac1 activation- _n_ = 15 neurons; _n_ = 4 animals;
Naïve Rac1 activation; _n_ = 17 neurons; _n_ = 5 animals). Posthoc analysis found that fear conditioning increased the volume of spines when compared with the naïve group (light and no light
combined) (_p_ < 0.006) (Fig. 4E). Rac1 activation during training abolished the ability of fear conditioning to increase spine volume and spine volume was significantly different
between fear conditioning with no Rac1 activation and those with Rac1 activation (_p_ = 0.01). Spine volume in BLA neurons in the fear conditioning light group where Rac1 is activated was
not different from spine volume in the naïve no light group (basal level) (_p_ = 1). Spine volume in BLA neurons in naïve light group where Rac1 is activated was not different from spine
volume in the naïve no light group (_p_ = 0.471). Spine head volume is different between the groups (H(3) = 10.277, _p_ = 0.006; ϵ2 = 0.136). Posthoc analysis found that spine head volume is
increased in fear conditioning when compared with the naïve group (light and no light combined) (_p_ = 0.029) (Fig. 4F). Rac1 activation during training abolished the ability of fear
conditioning to increase spine head volume and spine head volume was significantly different between fear conditioning with no Rac1 activation and those with Rac1 activation (_p_ = 0.006).
Spine head volume in BLA neurons in the fear conditioning light group where Rac1 is activated was not different from spine volume in the naïve no light group (basal level) (_p_ = 0.602). We
examined possible alteration in the mean spine diameter per dendritic branch and revealed an effect in branches 1, 2 and 3 (H(3) = 26.26, _p_ < 0.001, ϵ2 = 0.298; H(3) = 24.743, _p_ <
0.001, ϵ2 = 0.288; H(3) = 13.772, _p_ = 0.003, ϵ2 = 0.203) (Fear conditioning- _n_ = 18 neurons; _n_ = 4 animals; Naïve- _n_ = 17 neurons; _n_ = 3 animals; Fear conditioning PA-Rac1
activation- _n_ = 15 neurons; _n_ = 4 animals; Naïve Rac1 activation; _n_ = 17 neurons; _n_ = 5 animals). Posthoc analysis found that fear conditioning (no light) increased the mean
diameters of spines when compared to naïve no light or naïve light groups in branches 1, 2 and 3 (_p_ < 0.001; _p_ < 0.001; _p_ < 0.025) (Fig. 4G). However, if fear conditioning was
performed in conjunction with PA-Rac1 activation no significant increase in spine diameter was observed when compared to the basal levels in the naïve groups (_p_ = 1). There are no
significant changes in other dendritic branches. Cumulatively, these results show that fear conditioning does not lead to changes in spine density but in the length and volume of the spine
in particular the length of the neck and volume of the head of the spine. Rac1 activation per se has no effect on spines morphology but if activated during fear conditioning training it
prevented the fear conditioning-induced spines morphogenesis. DISCUSSION In this study, we explored whether fear conditioning leads to long-lasting changes in neuronal morphology in the
basolateral amygdala (BLA). Moreover, we examined whether the activation of Rac1 GTPase in these neurons will affect long-term memory and such structural changes. Prevention of neuronal
morphology and memory formation by Rac1 activation will strongly imply that these neuronal morphological alterations are involved in fear conditioning long-term memory formation. We show
that Rac1 GTPase activation in BLA during fear conditioning impairs auditory long-term fear memory formation but not contextual fear conditioning. This difference is not caused because of
examination of the contextual and auditory fear memories at different time points (24 h and 48 h, respectively) as when we activated PA-Rac1 during fear conditioning we see impairments in
auditory fear conditioning memory also when we tested it 24 h after training [21]. We observed that fear conditioning leads to an increase in dendritic shaft volume in the proximal dendrite
(primary and secondary branches), but not in more distal dendritic branches. The alteration in dendritic volume is prevented in the primary dendritic branch by activation of Rac1 during fear
conditioning training. Dendritic branches were not different in length. In addition, fear conditioning increased spines length and volume, especially the length of the spine neck and the
volume of the spine head, and Rac1 activation prevented such alterations. Spine density was not affected by fear conditioning. Fear conditioning affects neuronal morphology in the amygdala.
Fear conditioning alters the morphology of BLA neurons that show an increase in spines and synapses size [24,25,26]. This is consistent with our observation. Alteration in spine head volume
is linked to changes in synaptic transmission. For example, it was shown that spines with large postsynaptic densities (PSDs) tend to have a higher level of
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors (AMPARs) than spines with smaller PSDs [27]. Since the area of PSDs is correlated with that of the dimensions of the spine head
[28] it is implied that spines with larger heads express more glutamate receptors than spines with smaller heads. In addition, a study found a correlation between the amplitudes of currents
in the spine and the spine head volume showing that the distribution of functional AMPARs is approximately proportional to the spine head volume [29]. Thus, synaptic efficacy mediated by
AMPA receptors is correlated with spine head volume from silent synapses in small spines to highly responsive larger spines. Thus, enduring increase in spine head volume induced by fear
conditioning can increase the efficacy in transmission between neurons in BLA and be part of the memory trace. We also show that fear conditioning leads to an increase in spines length which
is contributed mainly to changes in the spine’s neck. Spine neck plasticity appears to mainly affect local voltage amplification in spines and biochemical compartmentalization, such as of
Ca2+, within the spine head [30] that may affect signal transduction and bidirectional diffusion of material from dendrite to spines [31, 32]. Spines with longer thinner spine necks confine
more molecules. Thus, changes in the spine neck may affect synaptic efficacy and also neuronal function [33, 34]. For example, spines with long necks have small somatic voltage
contributions. We found here that activation of Rac1 during learning prevents both fear memory formation and alterations in spines morphology indicating that changes in spines morphology,
namely increase spines volume and length, are required for fear memory formation. We also observed that fear conditioning leads to an increase in dendritic shaft volume at the primary and
secondary branches. The length of the dendrites is not affected. The geometry of the dendrite can affect its electrical properties. For example, the diameter of the dendrite can affect the
input impedance where small-diameter dendrites have a high input impedance that can affect and increase local synaptic potentials and thus increase the activation of voltage-gated
conductance [35]. It was demonstrated that an increase in the diameter of proximal dendrites increased the synaptic efficiency of distal dendrites [36]. We revealed that the alteration in
dendrite volume at the primary branch induced by fear conditioning is reduced by Rac1 activation which also impairs fear memory indicating that this morphological change is needed for memory
formation. In addition, fear conditioning showed the smallest number of dendritic branches in BLA neurons that were increased back to the naïve level by PA-Rac1 activation during fear
conditioning. Reducing the branch points in the dendritic shaft facilitates signal propagation along the dendrite [37, 38]. Thus, increasing both the volume of the dendrite and reducing its
branch points can facilitate the propagation (and backpropagation) of signals along the dendrite and preventing such changes, by Rac1 activation, can reduce such propagation and impair
memory. Sholl analysis did not detect fear conditioning-induced changes in intersections. However, some differences were detected between Naïve no PA-Rac1 activation and Naïve PA-Rac1
activation at distal dendritic locations from the soma. Interestingly, Rac1 inhibition activity can reduce dendritic complexity (as measured by sholl analysis) at more distal dendritic
locations [39]. It could be that there are different Rac1 effectors in these distal dendritic areas. The results of the study indicate that the morphological changes that are responsive to
PA-Rac1 are specific to auditory fear conditioning. We show that fear conditioning leads to alterations in neuronal morphology. PA-Rac1 reduces the morphological alterations (in some cases
up to the basal level) and impairs auditory fear conditioning long-term memory. The animals do learn contextual fear conditioning when they receive shock only (also in the context of the
auditory-shock protocol). If the morphological changes are caused by shock only, leading to contextual fear conditioning, we would expect to see an effect of PA-Rac1 activation also on
contextual fear conditioning memory. However, we do not see any effect of PA-Rac1 activation on contextual fear conditioning. Therefore, the changes in morphology affected by PA-Rac1 are
associated with auditory fear conditioning induced by the tone-shock pairing and not contextual fear conditioning memory induced by the shock only. PA-Rac1 also cannot relieve the effects of
the footshock as short-term memory is intact [21]. However, although both groups respond to the shock similarly, the PA-Rac1 activated animals show a reduction in auditory fear conditioning
long-term memory and changes in morphology. Therefore, the reduction of morphological alteration by PA-Rac1 is not caused by the relief of effects of shock and reduction in stress. In
addition, stress affects contextual fear conditioning memory [40]. If PA-Rac1 activation reduces stress and neuronal morphogenesis, then we would expect to see an effect on contextual fear
conditioning as well but we do not see an effect. Taken together, our observations show that shock per se, stress and contextual fear learning do not contribute to the effects on PA-Rac1
responsive morphological changes. Interestingly, we saw that Rac1 activation per se did not reduce the volume and length of the spines or led to a reduction in dendritic volume in the naïve
group but rather specifically prevented the increase in spine volume and length and dendritic volume induced by fear conditioning. Thus, it seems that Rac1 activation inhibits signaling that
leads to an increase in spine volume and length and dendritic shaft volume by fear conditioning. Both spines and dendritic shafts contain actin cytoskeleton and actin cytoskeleton can
affect dendritic spines and dendritic shaft morphology [41,42,43,44]. Rac1 regulates actin regulatory proteins that in turn affect the actin cytoskeleton and spine morphology [45]. It is
possible that fear conditioning leads to an increase in actin polymerization through the regulation of actin regulatory proteins and that Rac1 activation inhibits the actin regulatory
proteins and counteracts fear conditioning-induced morphogenesis. We think that subsequent studies should be also conducted in female mice as behavioral outcomes induced by alterations of
proteins located at the Rac1 pathway may be different between males and female mice (e.g., as seen with Dock4 a Rac1 guanine nucleotide exchange factor [46]). Moreover, neuronal properties
and morphology and morphological responses to inputs in the BLA can be different between males and females [47, 48]. The observations in the study show that: 1) Fear conditioning leads to
specific changes in neuronal morphology in BLA neurons. 2) These alterations are long-lasting and are preserved 48 h after training. 3) Rac1 activation during learning prevents the dendritic
morphological changes induced by fear conditioning. Since the activation of Rac1 is effective during fear conditioning training the cellular signaling for dendritic changes is fast. 4) The
prevention of both fear conditioning long-term memory and fear conditioning-induced morphological alteration by Rac1 activation indicates that these neuronal enduring morphological
alterations are needed for fear conditioning memory formation and form the memory trace. REFERENCES * Hebb DO. The organization of behavior: a neuropsychological theory. New York, NY: Wiley;
1949. * Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–9. CAS PubMed Google Scholar * Kandel ER. The molecular
biology of memory storage: a dialogue between genes and synapses. Science. 2001;294:1030–8. CAS PubMed Google Scholar * Lamprecht R, LeDoux J. Structural plasticity and memory. Nat Rev
Neurosci. 2004;5:45–54. CAS PubMed Google Scholar * Bailey CH, Kandel ER, Harris KM. Structural components of synaptic plasticity and memory consolidation. Cold Spring Harb Perspect Biol.
2015;7:a021758. PubMed PubMed Central Google Scholar * Fanselow MS, LeDoux JE. Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron.
1999;23:229–32. CAS PubMed Google Scholar * LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–84. CAS PubMed Google Scholar * Davis M, Whalen PJ. The amygdala:
vigilance and emotion. Mol Psychiatry. 2001;6:13–34. CAS PubMed Google Scholar * Sah P, Faber ES, Lopez De Armentia M, Power J. The amygdaloid complex: anatomy and physiology. Physiol
Rev. 2003;83:803–34. CAS PubMed Google Scholar * Maren S. Synaptic mechanisms of associative memory in the amygdala. Neuron. 2005;47:783–6. CAS PubMed Google Scholar * Schafe GE, Nader
K, Blair HT, LeDoux JE. Memory consolidation of Pavlovian fear conditioning: a cellular and molecular perspective. Trends Neurosci. 2001;24:540–6. CAS PubMed Google Scholar * Rodrigues
SM, Schafe GE, LeDoux JE. Molecular mechanisms underlying emotional learning and memory in the lateral amygdala. Neuron. 2004;44:75–91. CAS PubMed Google Scholar * Johansen JP, Cain CK,
Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147:509–24. CAS PubMed PubMed Central Google Scholar * Luo L, Hensch TK, Ackerman L, Barbel S, Jan LY,
Jan YN. Differential effects of the Rac GTPase on Purkinje cell axons and dendritic trunks and spines. Nature. 1996;379:837–40. CAS PubMed Google Scholar * Nakayama AY, Harms MB, Luo L.
Small GTPases Rac and Rho in the maintenance of dendritic spines and branches in hippocampal pyramidal neurons. J Neurosci. 2000;20:5329–38. CAS PubMed PubMed Central Google Scholar *
Luo L. Rho GTPases in neuronal morphogenesis. Nat Rev Neurosci. 2000;1:173–80. CAS PubMed Google Scholar * Pilpel Y, Segal M. Activation of PKC induces rapid morphological plasticity in
dendrites of hippocampal neurons via Rac and Rho-dependent mechanisms. Eur J Neurosci. 2004;19:3151–64. PubMed Google Scholar * Newey SE, Velamoor V, Govek EE, Van Aelst L. Rho GTPases,
dendritic structure, and mental retardation. J Neurobiol. 2005;64:58–74. CAS PubMed Google Scholar * Gao Q, Yao W, Wang J, Yang T, Liu C, Tao Y, et al. Post-training activation of Rac1 in
the basolateral amygdala is required for the formation of both short-term and long-term auditory fear memory. Front Mol Neurosci. 2015;8:65. PubMed PubMed Central Google Scholar *
Hayashi-Takagi A, Yagishita S, Nakamura M, Shirai F, Wu YI, Loshbaugh AL, et al. Labelling and optical erasure of synaptic memory traces in the motor cortex. Nature. 2015;525:333–8. CAS
PubMed PubMed Central Google Scholar * Das A, Dines M, Alapin JM, Lamprecht R. Affecting long-term fear memory formation through optical control of Rac1 GTPase and PAK activity in lateral
amygdala. Sci Rep. 2017;7:13930. PubMed PubMed Central Google Scholar * Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, et al. A genetically encoded photoactivatable Rac
controls the motility of living cells. Nature. 2009;461:104–8. CAS PubMed PubMed Central Google Scholar * Hama H, Hioki H, Namiki K, Hoshida T, Kurokawa H, Ishidate F, et al. ScaleS: an
optical clearing palette for biological imaging. Nat Neurosci. 2015;18:1518–29. CAS PubMed Google Scholar * Lamprecht R, Farb CR, Rodrigues SM, LeDoux JE. Fear conditioning drives
profilin into amygdala dendritic spines. Nat Neurosci. 2006;9:481–3. CAS PubMed Google Scholar * Ostroff LE, Cain CK, Bedont J, Monfils MH, Ledoux JE. Fear and safety learning
differentially affect synapse size and dendritic translation in the lateral amygdala. Proc Natl Acad Sci USA. 2010;107:9418–23. CAS PubMed PubMed Central Google Scholar * Choi DI, Kim J,
Lee H, Kim JI, Sung Y, Choi JE, et al. Synaptic correlates of associative fear memory in the lateral amygdala. Neuron. 2021;109:2717–2726.e3. CAS PubMed Google Scholar * Takumi Y,
Ramírez-León V, Laake P, Rinvik E, Ottersen OP. Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nat Neurosci. 1999;2:618–24. CAS PubMed Google Scholar *
Harris KM, Stevens JK. Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J Neurosci.
1989;9:2982–97. CAS PubMed PubMed Central Google Scholar * Noguchi J, Nagaoka A, Watanabe S, Ellis-Davies GC, Kitamura K, Kano M, et al. In vivo two-photon uncaging of glutamate
revealing the structure-function relationships of dendritic spines in the neocortex of adult mice. J Physiol. 2011;589:2447–57. CAS PubMed PubMed Central Google Scholar * Noguchi J,
Matsuzaki M, Ellis-Davies GC, Kasai H. Spine-neck geometry determines NMDA receptor-dependent Ca2+ signaling in dendrites. Neuron. 2005;46:609–22. CAS PubMed PubMed Central Google Scholar
* Bloodgood BL, Sabatini BL. Neuronal activity regulates diffusion across the neck of dendritic spines. Science. 2005;310:866–9. CAS PubMed Google Scholar * Santamaria F, Wils S, De
Schutter E, Augustine GJ. Anomalous diffusion in Purkinje cell dendrites caused by spines. Neuron. 2006;52:635–48. CAS PubMed PubMed Central Google Scholar * Araya R, Jiang J, Eisenthal
KB, Yuste R. The spine neck filters membrane potentials. Proc Natl Acad Sci USA. 2006;103:17961–6. CAS PubMed PubMed Central Google Scholar * Araya R, Vogels TP, Yuste R.
Activity-dependent dendritic spine neck changes are correlated with synaptic strength. Proc Natl Acad Sci USA. 2014;111:E2895–904. CAS PubMed PubMed Central Google Scholar * Spruston N.
Pyramidal neurons: dendritic structure and synaptic integration. Nat Rev Neurosci. 2008;9:206–21. CAS PubMed Google Scholar * Wolf E, Birinyi A, Székely G. Simulation of the effect of
synapses: the significance of the dendritic diameter in impulse propagation. Eur J Neurosci. 1992;4:1013–21. PubMed Google Scholar * Williams SR, Stuart GJ. Action potential
backpropagation and somato-dendritic distribution of ion channels in thalamocortical neurons. J Neurosci Off J Soc Neurosci. 2000;20:1307–17. CAS Google Scholar * Vetter P, Roth A, Häusser
M. Propagation of action potentials in dendrites depends on dendritic morphology. J Neurophysiol. 2001;85:926–37. CAS PubMed Google Scholar * Vadodaria KC, Brakebusch C, Suter U,
Jessberger S. Stage-specific functions of the small Rho GTPases Cdc42 and Rac1 for adult hippocampal neurogenesis. J Neurosci. 2013;33:1179–89. CAS PubMed PubMed Central Google Scholar *
Cordero MI, Venero C, Kruyt ND, Sandi C. Prior exposure to a single stress session facilitates subsequent contextual fear conditioning in rats. Evidence for a role of corticosterone. Horm
Behav. 2003;44:338–45. CAS PubMed Google Scholar * Dillon C, Goda Y. The actin cytoskeleton: integrating form and function at the synapse. Annu Rev Neurosci. 2005;28:25–55. * Leite SC,
Sousa MM. The neuronal and actin commitment: why do neurons need rings? Cytoskeleton. 2016;73:424–34. CAS PubMed Google Scholar * Lanoue V, Cooper HM. Branching mechanisms shaping
dendrite architecture. Dev Biol. 2019;451:16–24. CAS PubMed Google Scholar * Bucher M, Fanutza T, Mikhaylova M. Cytoskeletal makeup of the synapse: shaft versus spine. Cytoskeleton.
2020;77:55–64. CAS PubMed Google Scholar * Costa JF, Dines M, Lamprecht R. The role of Rac GTPase in dendritic spine morphogenesis and memory. Front Synaptic Neurosci. 2020;12:12. CAS
PubMed PubMed Central Google Scholar * Guo D, Peng Y, Wang L, Sun X, Wang X, Liang C, et al. Autism-like social deficit generated by Dock4 deficiency is rescued by restoration of Rac1
activity and NMDA receptor function. Mol Psychiatry. 2021;26:1505–19. CAS PubMed Google Scholar * Guadagno A, Wong TP, Walker CD. Morphological and functional changes in the preweaning
basolateral amygdala induced by early chronic stress associate with anxiety and fear behavior in adult male, but not female rats. Prog Neuropsychopharmacol Biol Psychiatry. 2018;81:25–37.
PubMed Google Scholar * Guily P, Lassalle O, Chavis P, Manzoni OJ. Sex-specific divergent maturational trajectories in the postnatal rat basolateral amygdala. iScience. 2022;25:103815. CAS
PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS We would like to thank Boris Shklyar from the Bioimaging Unit, Faculty of Natural Sciences, University of
Haifa, for his help with the imaging. This research was supported by a grant from the Ministry of Science and Technology, Israel and the Israel Science Foundation for RL. AUTHOR INFORMATION
AUTHORS AND AFFILIATIONS * Sagol Department of Neurobiology, Faculty of Natural Sciences, University of Haifa, Haifa, Israel Joana Freitas Costa, Monica Dines, Karishma Agarwal & Raphael
Lamprecht Authors * Joana Freitas Costa View author publications You can also search for this author inPubMed Google Scholar * Monica Dines View author publications You can also search for
this author inPubMed Google Scholar * Karishma Agarwal View author publications You can also search for this author inPubMed Google Scholar * Raphael Lamprecht View author publications You
can also search for this author inPubMed Google Scholar CONTRIBUTIONS JFC performed the experiments and analyzed the data, MD performed part of the behavioral experiments, KA performed part
of the behavioral experiments and RL performed the statistical analysis and wrote the paper with inputs from all authors. CORRESPONDING AUTHOR Correspondence to Raphael Lamprecht. ETHICS
DECLARATIONS COMPETING INTERESTS The authors declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. RIGHTS AND PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a
publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing
agreement and applicable law. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Costa, J.F., Dines, M., Agarwal, K. _et al._ Rac1 GTPase activation impairs fear
conditioning-induced structural changes in basolateral amygdala neurons and long-term fear memory formation. _Neuropsychopharmacol._ 48, 1338–1346 (2023).
https://doi.org/10.1038/s41386-022-01518-8 Download citation * Received: 28 June 2022 * Revised: 29 November 2022 * Accepted: 30 November 2022 * Published: 15 December 2022 * Issue Date:
August 2023 * DOI: https://doi.org/10.1038/s41386-022-01518-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative






