- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT Suicide is a significant public health problem worldwide, and several Asian countries including Japan have relatively high suicide rates on a world scale. Twin, family, and adoption
studies have suggested high heritability for suicide, but genetics lags behind due to difficulty in obtaining samples from individuals who died by suicide, especially in non-European
populations. In this study, we carried out genome-wide association studies combining two independent datasets totaling 746 suicides and 14,049 non-suicide controls in the Japanese
population. Although we identified no genome-wide significant single-nucleotide polymorphisms (SNPs), we demonstrated significant SNP-based heritability (35–48%; _P_ < 0.001) for
completed suicide by genomic restricted maximum-likelihood analysis and a shared genetic risk between two datasets (_P_best = 2.7 × 10−13) by polygenic risk score analysis. This study is the
first genome-wide association study for suicidal behavior in an East Asian population, and our results provided the evidence of polygenic architecture underlying completed suicide. SIMILAR
CONTENT BEING VIEWED BY OTHERS GENOME-WIDE ASSOCIATION STUDY META-ANALYSIS OF SUICIDE DEATH AND SUICIDAL BEHAVIOR Article Open access 17 October 2022 FUNCTIONAL AND MOLECULAR
CHARACTERIZATION OF SUICIDALITY FACTORS USING PHENOTYPIC AND GENOME-WIDE DATA Article Open access 06 January 2023 GENETIC AETIOLOGY OF SELF-HARM IDEATION AND BEHAVIOUR Article Open access 16
June 2020 INTRODUCTION Suicide is a significant public health problem that causes nearly one million deaths worldwide each year [1]. In addition, several Asian countries including Japan
have relatively high suicide rates on a world scale [2, 3]. There is evidence for high heritability in suicidal behavior, with an estimated _h_2 of 30–50% based on twin, family, and adoption
studies in European populations [4]. To investigate the genetic components of suicidal behavior, large-scale genetic studies focusing on “completed suicide” should be crucial because other
suicidal behaviors (suicide ideation or attempt) vary in terms of degree of lethality and suicidal intent, which may cause heterogeneity [5]. However, due to the difficulty in obtaining
samples from individuals who died by suicide, few genome-wide association studies (GWASs) have been reported (only European populations) [6,7,8,9]. The largest case–control GWAS to date
included 317 suicides, and revealed no significant findings [7]. In addition, no study has investigated single-nucleotide polymorphism (SNP)-based heritability or polygenic effects for
completed suicide. Consequently, genetic insights into suicide lag behind those of other mental problems, and no useful genetic biomarker of suicide risk has been found. On the other hand,
previous studies have associated different characteristics with suicidal behavior in different age groups; for instance, more impulsive, aggressive, and novelty seeking personality traits in
the young, while heightened harm avoidance and higher comorbidity rate of depression and physical diseases were associated with increasing age of suicidal behavior [10,11,12,13]. Family
studies have indicated strong familial transmission for early-onset suicidal behavior [14,15,16]. These studies encourage research focusing on the biological factors associated with age at
suicide. Here, we first conducted genome-wide association analyses using two independent datasets totaling ~746 suicides and 14,049 non-suicide controls in the Japanese population in order
to identify genetic variants affecting suicide risk (case–control study). We then utilized these genome-wide SNP data to investigate SNP-based heritability and polygenic effects for
completed suicide. Additionally, we also investigated individual variants and polygenicity affecting age at suicide (targeting only suicidal cohorts). MATERIALS AND METHODS SUBJECTS The
entire study design and procedures were performed in accordance with the Declaration of Helsinki. This study was approved by the Ethics Committee for Genetic Studies of Kobe University and
RIKEN. INDIVIDUALS WHO DIED BY SUICIDE Autopsies on suicide victims were conducted at the Division of Legal Medicine in the Department of Community Medicine and Social Health Science at the
Kobe University Graduate School of Medicine. The verdict of “completed suicide” was made through discussion with the Medical Examiner’s Office of the Hyogo Prefecture and the Division of
Legal Medicine in the Kobe University Graduate School of Medicine [17]. In order to gather background information on completed suicides, psychological autopsy through their medical records
and bereaved family interviews were conducted by professional staff from the Medical Examiner’s Office of the Hyogo Prefecture and the Division of Legal Medicine in the Kobe University,
where available. NON-SUICIDE CONTROLS As non-suicide controls, we used genome-wide genotype data from subjects in the Biobank Japan project who had been genotyped as case subjects for
non-psychiatric disorders and from healthy volunteers of the Osaka-Midosuji Rotary Club and the Pharma SNP consortium. The controls were not psychiatrically evaluated [18,19,20]. GENOTYPING,
QC, AND IMPUTATION We genotyped 434 individuals who died by suicide between June 1996 and July 2012 and 405 individuals who died by suicide between August 2012 and February 2017 using
Illumina HumanOmniExpress and HumanOmniExpressExome BeadChips for the first and second set, respectively. We obtained control data genotyped with the same arrays (_N_ = 7993 and 7136 for the
first and second set, respectively) (Table S1). We performed quality control (QC) using PLINK 1.9 [21]. First, we excluded SNPs with a call rate <0.98 and minor allele frequency (MAF)
<0.01, and those with _P_ < 1.0 × 10−6 for Hardy–Weinberg equilibrium (HWE) in controls. Related individuals were excluded (PI_HAT ≥0.175). We then performed principal component
analysis (PCA), and excluded samples outside the Japanese main islands cluster [22, 23]. The results of PCA are shown in Fig. S1. The final datasets included 386 suicides and 7458 controls
as the first set, and 360 suicides and 6591 controls as the second set. After estimating haplotypes using SHAPEIT2 (v2.r778) [24], we performed genotype imputation by Minimac3 (1.0.13) [25]
using ALL samples in the 1000 Genomes Project phase 3v5 [26] as a reference. To investigate the X chromosome, we called genotypes using GenomeStudio. First, we generated genoplots using only
female samples. After that, we added male samples to genoplots and called genotypes. Genotypes called as heterozygotes were treated as missing in male samples. For the genotyping QC, we
excluded SNPs with MAF <0.01 and SNP call rate <0.98 in either male or female samples. We also excluded SNPs with _P_ value for HWE <1.0 × 10−6 in female samples. Haplotype phasing
and imputation were performed separately for males and females. Allelic dosages were imputed from 0 to 2 in male samples under the assumption of full dosage compensation. The
pseudo-autosomal region was excluded from the reference before imputation. After imputation of autosomal and X chromosomes, we included only SNPs with high imputation quality (_r_2 ≥ 0.7)
and MAF ≥0.01 (Fig. S2). ASSOCIATION ANALYSIS AND META-ANALYSIS For all GWASs identifying genetic variants affecting suicide risk (case–control study; 746 suicides and 14,049 controls) and
age at suicide (only suicidal cohorts; 719 suicides), single variant association tests were performed for common variants (MAF ≥0.01) using logistic regression and linear regression based on
Wald test implemented in the Rvtests software [27] with a correction for the top 10 PCs as covariates. Meta-analysis was performed with the METAL software [28] using a fixed-effects model
with inverse-variance weighted approach. The significance level was set at _P_ < 2.5 × 10−8 due to correction for multiple comparison for two GWASs (case–control GWAS and GWAS for age at
suicide). _P_ for heterogeneity between two analyses (first set and second set) was calculated by Cochran’s _Q_ test. Regional association plots were generated using LocusZoom [29]. A QQ
plot for each GWAS is shown in Fig. S3. EVALUATION OF PREVIOUSLY IMPLICATED SNPS IN PRIOR GWAS OF SUICIDAL BEHAVIOR Among variants that have previously been associated with suicidal behavior
(suicide ideation, suicide attempt, and suicide completion) in the published literature [7, 9, 30,31,32,33,34,35,36], we identified 26 variants with _P_ < 1.0 × 10−6 in previous GWASs
and MAF >0.01 in JPT (Japanese in Tokyo, Japan) population (the 1000 Genomes Project phase 3). We looked up the association of each of these SNPs with the results from our case–control
GWAS. Only the top SNP in the same region in each reference was selected. For a candidate SNP that was not directly genotyped or imputed in our GWAS, we identified a proxy SNP (_r_2 > 0.8
in East Asian samples of the 1000 Genomes Project phase 3) whenever possible. For SNPs that reached _P_ < 0.05, we determined whether the direction of the association was consistent
between the prior and current studies. ESTIMATION OF THE PROPORTION OF THE VARIANCE IN COMPLETED SUICIDE EXPLAINED BY THE GENOTYPED SNPS (SNP-BASED HERITABILITY) To assess SNP-based
heritability (_h_2SNP), we used genome-wide complex trait analysis (GCTA) [37] to generate genetic relatedness matrices (GRMs) among 506,645 SNPs that passed QC in both genotyped datasets,
and then performed genomic restricted maximum-likelihood (GREML) analysis. This assumed prevalence rates of 0.1 and 0.5% for completed suicide, considering the reported incidence of
completed suicide in Japan (Ministry of Health, Labor, and Welfare of Japan) [3] and the estimates from previous papers [38,39,40]. We strictly controlled the cryptic relatedness of the
analyzed samples using the –grm-cutoff option (threshold of 0.05) implemented by GCTA, including 385 cases and 7409 controls for the first set, and 357 cases and 6560 controls for the second
set, respectively. We then estimated SNP-based heritability using reml function implemented by GCTA with top 10 PCs as covariates. PRS ANALYSIS Polygenic risk score (PRS) analyses were
performed using PRSice v1.23 [41]. The _P_ threshold (_P_t) for selecting “risk” SNPs was sequentially set at 0.1, 0.2, 0.3, 0.4, and 0.5 without SNPs in the major histocompatibility complex
region. We then performed linkage disequilibrium (LD) clumping (used by the default setting of the software) to select the eligible SNPs for PRS. To calculate the PRS, we analyzed two
discovery/target sets, using the first set (386 suicides and 7458 controls) as the discovery set and the second set (360 suicides and 6591 controls) as the target set, and vice versa. We
included the top 10 PCs derived from each genotyped dataset as covariates, respectively. The variance explained for the PRS was estimated based on Nagelkerke’s _R_2 from a logistic
regression model. For the analysis of polygenic effects on age at suicide (first set, 366 suicides; second set, 353 suicides), we also applied the same procedure as the above to the
case–control GWAS datasets. PATHWAY ENRICHMENT ANALYSIS Using the results of a meta-analysis of the case–control GWAS datasets, we ran PASCAL [42] for gene-based enrichment analysis using
1077 gene sets, including KEGG [43], REACTOME [44], and BIOCARTA (http://cgap.nci.nih.gov/Pathways/BioCarta_Pathways). The significance level was set at _χ_2 _P_ < 4.6 × 10−5 after
Bonferroni correction for 1077 tests. RESULTS GWAS FOR RISK OF COMPLETED SUICIDE Sample characteristics are shown in Table S1. We performed a meta-analysis of the case–control GWAS datasets
for both suicides and non-suicide controls (totaling 746 cases and 14,049 controls) using 8,625,325 SNPs (λ genomic control (GC) = 1.07; Fig. S3a). This analysis identified no genome-wide
significant SNPs (Fig. S4), although some loci, including SNPs on _GRM1_ and _CTPS2_, were suggestive with _P_meta < 1.0 × 10−6 (Table S2). In addition, we looked up the 26 variants
showing _P_ < 1.0 × 10−6 in prior European GWASs for suicidal behavior [7, 9, 30,31,32,33,34,35,36]. Among these, rs7989250, reported in the recent UK Biobank GWAS for ordinal
suicidality, reached the threshold for our replication analysis (_P_ < 0.05) with the same direction of allelic effect (Table S3). We ran PASCAL [42] using our meta-GWAS to estimate the
enrichments in 1077 gene sets, but no pathways were enriched at the Bonferroni level of significance (Results for all tested 1077 pathways are listed in an Excel spreadsheet that is included
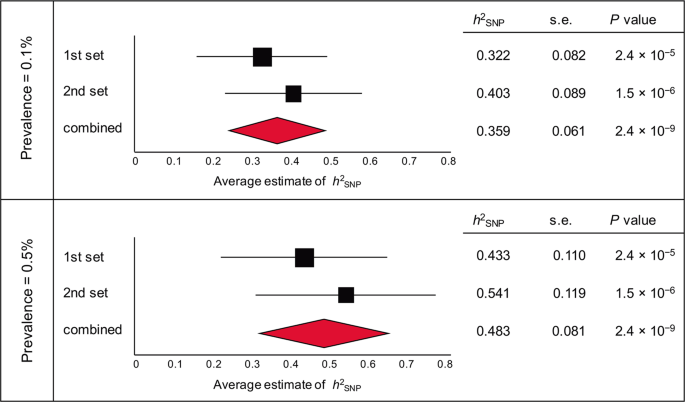
in the Supplementary Material). GREML ANALYSIS REVEALS HIGH SNP-BASED HERITABILITY FOR COMPLETED SUICIDE To investigate SNP-based heritability (_h_2SNP) for completed suicide, we used GCTA
[37] to GRMs using 506,645 directly genotyped SNPs, and then estimated SNP-based heritability with GREML. These analyses revealed significant SNP-based heritability in both datasets (_P_
< 0.001; Fig. 1). By combining the results, we estimated that 35.9% and 48.3% of phenotypic variance in the two datasets could be explained by SNPs with a prevalence of 0.1% and 0.5%,
respectively; this implies the underlying polygenic architecture composed by numerous “risk” SNPs for completed suicide. PRS ANALYSIS REVEALS POLYGENIC EFFECTS FOR COMPLETED SUICIDE To
investigate whether two independent datasets share genetic components conferring the risk of completed suicide, we performed PRS analyses by PRSice (v1.23) [41], setting the first set as the
discovery set and the second set as the target set, and vice versa. The analyses demonstrated significant polygenic effects on completed suicide (_P_ < 0.001) when the SNPs were
stratified by _P_ values, obtaining the most significant _P_ values (_P_best = 2.7 × 10−13) with an inclusion threshold of _P_t = 0.4 in each set, with explained 1.3–2.4% of the variance
(Fig. 2 and Table S4). These results strongly suggest shared genetic components for completed suicide between the two datasets, providing additional evidence of polygenic architecture
underlying completed suicide. GWAS FOR AGE AT SUICIDE Age-divided sample characteristics focusing on psychological background information, comorbid severe physical diseases, and distribution
of age at suicide in both the first and second set of our suicidal cohorts are shown in Table S5 and Fig. S5. We performed GWAS for the age at completed suicide in each dataset (only
suicides with accurate age information available; _N_ = 366 and 353 for the first and second set, respectively), and integrated these results by fixed-effects meta-analysis. The meta-GWAS
included 8,810,873 SNPs, and the genomic inflation factor suggested low possibility of bias from population stratification and cryptic relatedness (λ GC = 1.02; a QQ plot is shown in Fig.
S3b). We identified a novel suggestive locus on 7q11.23 in GTF2I repeat domain containing 1 (_GTF2IRD1_) (top SNP rs73135307 G > C; _P_ = 3.3 × 10−8, _β_ = −12.3 years; Fig. 3, Table 1,
Table S6, and Fig. S6) without significant heterogeneity between the two datasets (_P_het = 0.20). This means that the effect allele of rs73135307 (G allele) could lead to 12.3 years younger
of age at suicide in our suicidal cohort compared to C allele. G allele frequency variation of rs73135307 in divided groups based on age at suicide in the first and second set are shown in
Fig. S7. According to HaploReg (v4.1) [45], rs73135307 and the four variants in LD (_r_2 > 0.6 in East Asian samples of the 1000 Genomes Project [26]) with rs73135307 affect various
histone modifications (H3K4me1, H3K4me3, H3K27ac, and H3K9ac) in various brain regions, including the hippocampus and dorsolateral prefrontal cortex. However, neither nonsynonymous SNP nor
significant expression quantitative trait loci (eQTL) was found. Our PRS analysis showed no significant polygenic effects on age at suicide (Table S7). DISCUSSION Through the GWAS including
746 suicides in the Japanese population, we demonstrated SNP-based heritability (35–48%) and polygenic effects, indicating polygenic inheritance of completed suicide. We also showed that the
_GTF2IRD1_ locus is suggestively associated with age at completed suicide. Using the two case–control GWAS datasets, we estimated SNP-based heritability with GREML and conducted PRS
analyses. Our GREML analysis revealed that completed suicide has significant SNP-based heritability, with estimates of 35–48% in both individual GWAS sets. We estimated SNP-based
heritability using the GREML method with two different prevalence rates (0.1 and 0.5%) assumed from the reported incidence of completed suicide in Japan [3] and the previous studies
[38,39,40]. These estimates are qualified by the difficulty of estimating the lifetime prevalence of completed suicide, compared to those of “diseases,” presumably due to the influence of
historical and cultural contexts on the epidemiology of suicide, along with other national/regional differences [46]. Moreover, our PRS analyses demonstrated significant polygenic effects on
completed suicide, indicating that two independent cohorts of suicides shared genetic components conferring suicide risk. Consequently, to our knowledge, we provide the first evidence of
SNP-based heritability in completed suicide derived from case–control GWAS dataset, other than from epidemiological data such as twin, family, and adoption studies. Due to the lack of
well-designed epidemiological studies and GWAS for suicidal behavior in Asia, this is the first study demonstrating SNP-based heritability of suicide in an Asian population. This is a major
strength of the current study, since the dearth of GWASs in non-European populations compared with the abundance of European-descent studies is causing the disparity and poor
generalizability of genetic studies across populations [47]. The SNP-based heritability of completed suicide found in our analysis dramatically exceeded the previous estimates from past
GWASs of suicide attempt in European populations, and further comparable to twin heritability estimated by prior studies of European populations despite SNP-based heritability estimates by
GWASs usually explaining roughly half of those based on twin modeling [48]. In order to elucidate the reasons for the different levels of SNP-based heritability estimated by previous GWASs
for suicide attempt and our GWAS, we should focus on the following points regarding SNP-based heritability differences: (1) between suicide attempt and suicide completion and (2) between
Europeans and Asians. On the other hand, significant high SNP-based heritability for completed suicide shown here might be overestimated to some extent due to residual population
stratification (e.g., regional differences in Japan) or the genetic effects of comorbid psychiatric disorders. Indeed, genetic influences on suicidal behavior seem to be confounded by
genetic vulnerability for psychiatric disorders; for instance, a previous twin study reported lower heritability (~17%) for suicidal behavior after controlling for the inheritance of
psychiatric disorders [49]. Publicly available GWAS data focusing on psychiatric problems in East Asian population are limited, which makes difficult to perform replication of GWAS for
suicidal behavior and in-depth analyses (e.g., suicide GWAS divided into cases with and without psychiatric disorders, or genetic correlation analysis with various psychiatric
disorders/conditions) using GWAS datasets from the same Asian population. Since we have made our summary statistics of GWAS publicly available, our data will enable the above analyses in the
future. Our meta-analysis of two GWASs of age at completed suicide identified a suggestive locus, intronic SNPs in the _GTF2IRD1_ gene (top hit rs73135307, _P_ = 3.3 × 10−8) at 7q11.23.
This SNP and variants in LD with the lead SNP are associated with various histone modifications across brain regions related to suicide pathophysiology [5], although none of those SNPs were
nonsynonymous or overlapped with eQTLs. _GTF2IRD1_ has been reported as one of the promising genes for Williams syndrome (WS), particularly responsible for neurodevelopmental abnormalities
[50], which is known as a risk factor for suicidal behavior [51]. While the typical social phenotype of WS is characterized by optimistic personality, there is also evidence of diametric
characteristics in individuals with WS, such as heightened anxiety, social relationship difficulties, and higher prevalence of autism spectrum disorder (ASD) [52,53,54]. The associations of
_GTF2IRD1_ with anxiety and social impairment in WS have been previously reported [53, 55]. In addition, various copy number variations in 7q11.23 region have been strikingly implicated in
the genetic etiology of schizophrenia and ASD [56, 57], which are associated with high risk of youth suicide [58, 59]. Focusing on this chromosomal region by more detailed genome sequencing
is necessary to yield further insight into the genetic feature of suicide in young people. Indeed, the aberrant personality traits, lower comorbidity rate of depression and physical
diseases, and familial transmission previously linked with younger age at suicidal behavior [10,11,12,13,14,15,16] indicated the possibility that future genetic studies for suicide in young
people may identify genetic clues. It must be emphasized that our findings should be interpreted in the context of several other limitations. First, although our sample size was one of the
largest ever for research on completed suicide, larger sample sizes would be preferable in order to draw robust conclusions regarding SNP-based heritability and polygenicity for completed
suicide, and to detect reliable genetic markers. In particular, although we reported that the _GTF2IRD1_ locus is suggestively associated with age at completed suicide, GWAS for age at
suicide here contained too small sample size (_N_ = 719) and replication studies are indeed required. Second, our study cohort was restricted to only the Japanese population; thus, findings
from the present study might not be generalizable to other populations. Third, the subjects we used non-suicide controls who had not been psychiatrically screened. In addition, most of the
controls had various non-psychiatric disorders [18,19,20]. However, this approach has been already applied in the previous GWAS [20] of which results was replicable in other recent GWAS
[60], supporting the reliability of our results. Fourth, the biological interpretation of the significant polygenic effects for completed suicide and the variants reported here remains
largely unknown due to lack of functional evidence yielded by our pathway analysis and database searches, respectively. In conclusion, we provided the first evidence of SNP-based
heritability and polygenic effects in completed suicide, and polygenic effects in age at suicide. FUNDING AND DISCLOSURE This work was supported, in part, by JSPS KAKENHI Grant Number
JP17H04249, BBJ, and the Rotary Club of Osaka-Midosuji District 2660 Rotary International in Japan. The authors declare no competing interests. REFERENCES * World Health Organization.
Suicide fact sheet; 2017. http://www.who.int/mediacentre/factsheets/fs398/en/. Accessed 29 Aug 2017. * Värnik P. Suicide in the world. Int J Environ Res Public Health. 2012;9:760–71. Article
PubMed PubMed Central Google Scholar * Statistics and Information Department, Minister’s Secretariat, Ministry of Health, Labour and Welfare. Vital statistics of Japan; 2017.
https://www.mhlw.go.jp/wp/hakusyo/jisatsu/19/index.html. * Brent DA, Turecki G. Suicide and suicidal behavior. Lancet. 2017;387:1227–39. Google Scholar * Oquendo MA, Sullivan GM, Sudol K,
Baca-Garcia E, Stanley BH, Sublette E, et al. Toward a biosignature for suicide. Am J Psychiatry. 2014;171:1259–77. Article PubMed PubMed Central Google Scholar * Coon H, Darlington TM,
DiBlasi E, Callor WB, Ferris E, Fraser A, et al. Genome-wide significant regions in 43 Utah high-risk families implicate multiple genes involved in risk for completed suicide. Mol
Psychiatry. 2018. https://www.nature.com/articles/s41380-018-0282-3. [Epub ahead of print]. * Galfalvy H, Haghighi F, Hodgkinson C, Goldman D, Oquendo MA, Burke A, et al. A genome-wide
association study of suicidal behavior. Am J Med Genet B. 2015;168:557–63. Article CAS Google Scholar * Mirkovic B, Laurent C, Podlipski MA, Frebourg T, Cohen D, Gerardin P. Genetic
association studies of suicidal behavior: a review of the past 10 years, progress, limitations, and future directions. Front Psychiatry. 2016;7:158. Article PubMed PubMed Central Google
Scholar * Strawbridge RJ, Ward J, Ferguson A, Graham N, Shaw RJ.Cullen B, et al. Identification of novel genome-wide associations for suicidality in UK Biobank, genetic correlation with
psychiatric disorders and polygenic association with completed suicide. EBioMedicine. 2019;41:517–25. * Bozzay ML, Liu RT, Kleiman EM. Gender and age differences in suicide mortality in the
context of violent death: findings from a multi-state population-based surveillance system. Compr Psychiatry. 2014;55:1077–84. Article PubMed Google Scholar * Fässberg MM, Cheung G,
Canetto SS, Erlangsen A, Lapierre S, Lindner R, et al. A systematic review of physical illness, functional disability, and suicidal behaviour among older adults. Aging Ment Health.
2016;20:166–94. Article PubMed Google Scholar * McGirr A, Renaud J, Bureau A, Seguin M, Lesage A, Turecki G. Impulsive-aggressive behaviors and completed suicide across the life cycle: a
predisposition for younger age of suicide. Psychol Med. 2008;38:407–17. Article CAS PubMed Google Scholar * Rich CL, Young D, Fowler RC. San Diego suicide study. I. Young vs old
subjects. Arch Gen Psychiatry. 1986;43:577–82. Article CAS PubMed Google Scholar * Brent DA, Melhem NM, Oquendo M, Burke A, Birmaher B, Stanley B, et al. Familial pathways to early-onset
suicide attempt: a 5.6-year prospective study. JAMA Psychiatry. 2015;72:160–8. Article PubMed PubMed Central Google Scholar * Brent DA, Oquendo M, Birmaher B, Greenhill L, Kolko D,
Stanley B, et al. Familial pathways to early-onset suicide attempt: risk for suicidal behavior in offspring of mood-disordered suicide attempters. Arch Gen Psychiatry. 2002;59:801–7. Article
PubMed Google Scholar * Melhem N, Brent DA, Ziegler M, Iyengar S, Kolko D, Oquendo M, et al. Familial pathways to early-onset suicidal behavior: familial and individual antecedents of
suicidal behavior. Am J Psychiatry. 2007;164:1364–70. Article PubMed PubMed Central Google Scholar * Otsuka I, Izumi T, Boku S, Kimura A, Zhang Y, Mouri K, et al. Aberrant telomere
length and mitochondrial DNA copy number in suicide completers. Sci Rep. 2017;7:3176. Article CAS PubMed PubMed Central Google Scholar * Nagai A, Hirata M, Kamatani Y, Muto K, Matsuda
K, Kiyohara Y, et al. Overview of the BioBank Japan Project: study design and profile. J Epidemiol. 2017;27:S2–8. Article PubMed PubMed Central Google Scholar * Hirata M, Kamatani Y,
Nagai A, Kiyohara Y, Ninomiya T, Tamakoshi A, et al. Cross-sectional analysis of BioBank Japan clinical data: a large cohort of 200,000 patients with 47 common diseases. J Epidemiol.
2017;27:S9–21. Article PubMed PubMed Central Google Scholar * Ikeda M, Takahashi A, Kamatani Y, Okahisa Y, Kunugi H, Mori N, et al. A genome-wide association study identifies two novel
susceptibility loci and trans population polygenicity associated with bipolar disorder. Mol Psychiatry. 2018;23:639–47. Article CAS PubMed Google Scholar * Purcell S, Neale B, Todd-Brown
K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. Article CAS PubMed PubMed
Central Google Scholar * Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association
studies. Nat Genet. 2006;38:904–9. Article CAS PubMed Google Scholar * Yamaguchi-Kabata Y, Nakazono K, Takahashi A, Saito S, Hosono N, Kubo M, et al. Japanese population structure, based
on SNP genotypes from 7003 individuals compared to other ethnic groups: effects on population-based association studies. Am J Hum Genet. 2008;83:445–56. Article CAS PubMed PubMed Central
Google Scholar * Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2011;9:179–81. Article CAS PubMed Google Scholar * Li Y,
Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–34. Article PubMed PubMed
Central Google Scholar * Abecasis GR, Altshuler D, Auton A, Brooks LD, Durbin RM, Gibbs RA, et al. A map of human genome variation from population-scale sequencing. Nature.
2010;467:1061–73. Article CAS PubMed Google Scholar * Zhan X, Hu Y, Li B, Abecasis GR, Liu DJ. RVTESTS: an efficient and comprehensive tool for rare variant association analysis using
sequence data. Bioinformatics. 2016;32:1423–6. Article CAS PubMed PubMed Central Google Scholar * Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide
association scans. Bioinformatics. 2010;26:2190–1. Article CAS PubMed PubMed Central Google Scholar * Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom:
regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–7. Article CAS PubMed PubMed Central Google Scholar * Willour VL, Seifuddin F, Mahon PB,
Jancic D, Pirooznia M, Steele J, et al. A genome-wide association study of attempted suicide. Mol Psychiatry. 2012;17:433–44. Article CAS PubMed Google Scholar * Erlangsen A, Appadurai
V, Wang Y, Turecki G, Mors O, Werge T, et al. Genetics of suicide attempts in individuals with and without mental disorders: a population-based genome-wide association study. Mol Psychiatry.
2018. https://www.nature.com/articles/s41380-018-0218-y. [Epub ahead of print]. * Kimbrel NA, Garrett ME, Dennis MF, Hauser MA, Ashley-Koch AE, et al. A genome-wide association study of
suicide attempts and suicidal ideation in U.S. military veterans. Psychiatry Res. 2018;269:64–9. Article PubMed PubMed Central Google Scholar * Levey DF, Polimanti R, Cheng Z, Zhou H,
Nuñez YZ, Jain S, et al. Genetic associations with suicide attempt severity and genetic overlap with major depression. Transl Psychiatry. 2019;9:22. Article CAS PubMed PubMed Central
Google Scholar * Mullins N, Perroud N, Uher R, Butler AW, Cohen-Woods S, Rivera M, et al. Genetic relationships between suicide attempts, suicidal ideation and major psychiatric disorders:
a genome-wide association and polygenic scoring study. Am J Med Genet B. 2014;165B:428–37. Article Google Scholar * Perroud N, Uher R, Ng MY, Guipponi M, Hauser J, Henigsberg N, et al.
Genome-wide association study of increasing suicidal ideation during antidepressant treatment in the GENDEP project. Pharmacogenomics J. 2012;12:68–77. Article CAS PubMed Google Scholar
* Stein MB, Ware EB, Mitchell C, Chen CY, Borja S, Cai T, et al. Genomewide association studies of suicide attempts in US soldiers. Am J Med Genet B. 2017;174:786–97. Article CAS Google
Scholar * Yang J, Lee SH, Goddard ME, Visscher PM. GCTA: a tool for genome-wide complex trait analysis. Am J Hum Genet. 2011;88:76–82. Article CAS PubMed PubMed Central Google Scholar
* Kessler RC, Borges G, Walters EE. Prevalence of and risk factors for lifetime suicide attempts in the National Comorbidity Survey. Arch Gen Psychiatry. 1999;56:617–26. Article CAS PubMed
Google Scholar * Bostwick JM, Pabbati C, Geske JR, McKean AJ. Suicide attempt as a risk factor for completed suicide: even more lethal than we knew. Am J Psychiatry. 2016;173:1094–100.
Article PubMed PubMed Central Google Scholar * Nock MK, Green JG, Hwang I, McLaughlin KA, Sampson NA, Zaslavsky AM, et al. Prevalence, correlates, and treatment of lifetime suicidal
behavior among adolescents: results from the National Comorbidity Survey Replication Adolescent Supplement. JAMA Psychiatry. 2013;70:300–10. Article PubMed Google Scholar * Euesden J,
Lewis CM, O’Reilly PF. PRSice: Polygenic Risk Score software. Bioinformatics. 2015;31:1466–8. Article CAS PubMed Google Scholar * Lamparter D, Marbach D, Rueedi R, Kutalik Z, Bergmann S.
Fast and rigorous computation of gene and pathway scores from SNP-based summary statistics. PLoS Comput Biol. 2016;12:e1004714. Article CAS PubMed PubMed Central Google Scholar *
Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–14. Article CAS PubMed
Google Scholar * Croft D, O’Kelly G, Wu G, Haw R, Gillespie M, Matthews L, et al. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Res. 2011;39:D691–7.
Article CAS PubMed Google Scholar * Ward LD, Kellis M. HaploReg: a resource for exploring chromatin states, conservation, and regulatory motif alterations within sets of genetically
linked variants. Nucleic Acids Res. 2012;40:D930–4. Article CAS PubMed Google Scholar * Russell R, Metraux D, Tohen M. Cultural influences on suicide in Japan. Psychiatry Clin Neurosci.
2017;71:2–5. Article PubMed Google Scholar * Martin AR, Kanai M, Kamatani Y, Okada Y, Neale BM, Daly MJ. Clinical use of current polygenic risk scores may exacerbate health disparities.
Nat Genet. 2019;51:584–91. Article CAS PubMed PubMed Central Google Scholar * Yang J, Zeng J, Goddard ME, Wray NR, Visscher PM. Concepts, estimation and interpretation of SNP-based
heritability. Nat Genet. 2017;49:1304–10. Article CAS PubMed Google Scholar * Fu Q, Heath AC, Bucholz KK, Nelson EC, Glowinski AL, Goldberg J, et al. A twin study of genetic and
environmental influences on suicidality in men. Psychol Med. 2002;32:11–24. Article CAS PubMed Google Scholar * Fan CC, Schork AJ, Brown TT, Spencer BE, Akshoomoff N, Chen CH, et al.
Williams syndrome neuroanatomical score associates with GTF2IRD1 in large-scale magnetic resonance imaging cohorts: a proof of concept for multivariate endophenotypes. Transl Psychiatry.
2018;8:114. Article CAS PubMed PubMed Central Google Scholar * Sokolowski M, Wasserman J, Wasserman D. Polygenic associations of neurodevelopmental genes in suicide attempt. Mol
Psychiatry. 2016;21:1381–90. Article CAS PubMed Google Scholar * Klein-Tasman BP, Li-Barber KT, Magargee ET. Honing in on the social phenotype in Williams syndrome using multiple
measures and multiple raters. J Autism Dev Disord. 2010;41:341–51. Article Google Scholar * Kopp ND, Parrish PCR, Lugo M, Dougherty JD, Kozel BA. Exome sequencing of 85 Williams-Beuren
syndrome cases rules out coding variation as a major contributor to remaining variance in social behavior. Mol Genet Genom Med. 2018;6:749–65. Article CAS Google Scholar * Richards C,
Jones C, Groves L, Moss J, Oliver C. Prevalence of autism spectrum disorder phenomenology in genetic disorders: a systematic review and meta-analysis. Lancet Psychiatry. 2015;2:909–16.
Article PubMed Google Scholar * Schneider T, Skitt Z, Liu Y, Deacon RM, Flint J, Karmiloff-Smith A, et al. Anxious, hypoactive phenotype combined with motor deficits in Gtf2ird1 null
mouse model relevant to Williams syndrome. Behav Brain Res. 2012;233:458–73. Article CAS PubMed Google Scholar * Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS,
et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet. 2017;49:27–35. Article CAS PubMed Google Scholar * Sanders SJ, He X,
Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek AE, et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron. 2015;87:1215–33. Article CAS
PubMed PubMed Central Google Scholar * Radomsky ED, Haas GL, Mann JJ, Sweeney JA. Suicidal behavior in patients with schizophrenia and other psychotic disorders. Am J Psychiatry.
1999;156:1590–5. Article CAS PubMed Google Scholar * Chen MH, Pan TL, Lan WH, Hsu JW, Huang KL, Su TP, et al. Risk of Suicide attempts among adolescents and young adults with autism
spectrum disorder: a Nationwide Longitudinal Follow-Up Study. J Clin Psychiatry. 2017;78:e1174–9. Article PubMed Google Scholar * Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S,
Trubetskoy V, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51:793–803. Article CAS PubMed PubMed Central Google Scholar
Download references ACKNOWLEDGEMENTS We would like to acknowledge the staff of the Biobank Japan (BBJ) project for collecting samples and clinical information. We are grateful to Dr.
Tatsuhiro So, Dr. Woraphat Ratta-Apha, Yasuko Nagashima, and the staff of the RIKEN Center for Integrative Medical Sciences for genotyping and data management. AUTHOR INFORMATION AUTHORS AND
AFFILIATIONS * Department of Psychiatry, Kobe University Graduate School of Medicine, Kobe, Japan Ikuo Otsuka, Satoshi Okazaki, Shuken Boku, Ichiro Sora & Akitoyo Hishimoto * Laboratory
for Statistical Analysis, RIKEN Center for Integrative Medical Sciences, Yokohama, Japan Ikuo Otsuka, Masato Akiyama & Yoichiro Kamatani * Department of Ophthalmology, Graduate School
of Medical Sciences, Kyushu University, Fukuoka, Japan Masato Akiyama * Department of Neuropsychiatry, Kindai University Faculty of Medicine, Osaka, Japan Osamu Shirakawa * Laboratory for
Genotyping Development, RIKEN Center for Integrative Medical Sciences, Yokohama, Japan Yukihide Momozawa * Laboratory of Complex Trait Genomics, Department of Computational Biology and
Medical Sciences, Graduate School of Frontier Sciences, The University of Tokyo, Tokyo, Japan Yoichiro Kamatani * Department of Pharmacology, Pharmaceutical Sciences, Health Sciences
University of Hokkaido, Hokkaido, Japan Takeshi Izumi * Department of Psychiatry, Graduate School of Biomedical Sciences, Tokushima University, Tokushima, Japan Shusuke Numata * Division of
Legal Medicine, Kobe University Graduate School of Medicine, Kobe, Japan Motonori Takahashi & Yasuhiro Ueno * Department of Medical Biochemistry, Kurume University School of Medicine,
Kurume, Japan Ken Yamamoto * Department of Neurology, Graduate School of Medicine, University of Tokyo, Tokyo, Japan Tatsushi Toda * RIKEN Center for Integrative Medical Sciences, Yokohama,
Japan Michiaki Kubo Authors * Ikuo Otsuka View author publications You can also search for this author inPubMed Google Scholar * Masato Akiyama View author publications You can also search
for this author inPubMed Google Scholar * Osamu Shirakawa View author publications You can also search for this author inPubMed Google Scholar * Satoshi Okazaki View author publications You
can also search for this author inPubMed Google Scholar * Yukihide Momozawa View author publications You can also search for this author inPubMed Google Scholar * Yoichiro Kamatani View
author publications You can also search for this author inPubMed Google Scholar * Takeshi Izumi View author publications You can also search for this author inPubMed Google Scholar * Shusuke
Numata View author publications You can also search for this author inPubMed Google Scholar * Motonori Takahashi View author publications You can also search for this author inPubMed Google
Scholar * Shuken Boku View author publications You can also search for this author inPubMed Google Scholar * Ichiro Sora View author publications You can also search for this author
inPubMed Google Scholar * Ken Yamamoto View author publications You can also search for this author inPubMed Google Scholar * Yasuhiro Ueno View author publications You can also search for
this author inPubMed Google Scholar * Tatsushi Toda View author publications You can also search for this author inPubMed Google Scholar * Michiaki Kubo View author publications You can also
search for this author inPubMed Google Scholar * Akitoyo Hishimoto View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence
to Akitoyo Hishimoto. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES SUPPLEMENTARY TABLES SUPPLEMENTARY INFORMATION RIGHTS AND PERMISSIONS OPEN ACCESS This article is licensed under a Creative Commons
Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original
author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the
article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use
is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit
http://creativecommons.org/licenses/by/4.0/. Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Otsuka, I., Akiyama, M., Shirakawa, O. _et al._ Genome-wide association studies
identify polygenic effects for completed suicide in the Japanese population. _Neuropsychopharmacol._ 44, 2119–2124 (2019). https://doi.org/10.1038/s41386-019-0506-5 Download citation *
Received: 07 May 2019 * Revised: 07 August 2019 * Accepted: 24 August 2019 * Published: 02 September 2019 * Issue Date: November 2019 * DOI: https://doi.org/10.1038/s41386-019-0506-5 SHARE
THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to
clipboard Provided by the Springer Nature SharedIt content-sharing initiative