- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
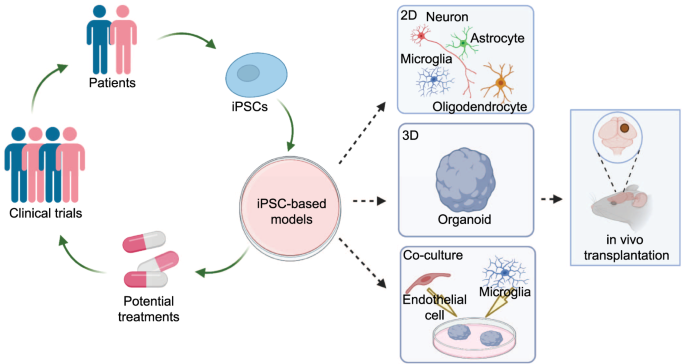
ABSTRACT Neuropsychiatric disorders affect a large proportion of the global population and there is an urgent need to understand the pathogenesis and to develop novel and improved treatments
of these devastating disorders. However, the diverse symptomatology combined with complex polygenic etiology, and the limited access to disorder-relevant cell types in human brains
represent a major obstacle for mechanistic disease research. Conventional animal models, such as rodents, are limited by inherent species differences in brain development, architecture, and
function. Advances in human induced pluripotent stem cells (hiPSCs) technologies have provided platforms for new discoveries in neuropsychiatric disorders. First, hiPSC-based disease models
enable unprecedented investigation of psychiatric disorders at the molecular, cellular, and structural levels. Second, hiPSCs derived from patients with known genetics, symptoms, and drug
response profiles offer an opportunity to recapitulate pathogenesis in relevant cell types and provide novel approaches for understanding disease mechanisms and for developing effective
treatments. Third, genome-editing technologies have extended the potential of hiPSCs for generating models to elucidate the genetic basis of rare monogenetic and complex polygenic
psychiatric disorders and to establish the causality between genotype and phenotype. Here we review opportunities and limitations for studying psychiatric disorders using various
hiPSC-derived model systems. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your
institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access
to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read
our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS PEERING INTO THE MIND: UNRAVELING SCHIZOPHRENIA’S SECRETS USING MODELS Article 08 September 2024 A RESOURCE OF
INDUCED PLURIPOTENT STEM CELL (IPSC) LINES INCLUDING CLINICAL, GENOMIC, AND CELLULAR DATA FROM GENETICALLY ISOLATED FAMILIES WITH MOOD AND PSYCHOTIC DISORDERS Article Open access 16 December
2023 FUNCTIONAL PATIENT-DERIVED CELLULAR MODELS FOR NEUROPSYCHIATRIC DRUG DISCOVERY Article Open access 17 February 2021 REFERENCES * Wen Z, Christian KM, Song H, Ming GL. Modeling
psychiatric disorders with patient-derived iPSCs. Curr Opin Neurobiol. 2016;36:118–27. Article CAS PubMed Google Scholar * Amin ND, Paşca SP. Building models of brain disorders with
three-dimensional organoids. Neuron. 2018;100:389–405. Article CAS PubMed Google Scholar * Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of
pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. Article CAS PubMed Google Scholar * Jacob F, Schnoll JG, Song H, Ming GL. Building the
brain from scratch: Engineering region-specific brain organoids from human stem cells to study neural development and disease. Curr Top Dev Biol. 2021;142:477–530. Article PubMed PubMed
Central Google Scholar * Wang M, Zhang L, Gage FH. Modeling neuropsychiatric disorders using human induced pluripotent stem cells. Protein Cell. 2020;11:45–59. Article CAS PubMed Google
Scholar * Zhang DY, Song H, Ming GL. Modeling neurological disorders using brain organoids. Semin Cell Dev Biol. 2021;111:4–14. Article PubMed Google Scholar * Zhou Y, Su Y, Li S,
Kennedy BC, Zhang DY, Bond AM, et al. Molecular landscapes of human hippocampal immature neurons across lifespan. Nature. 2022;607:527–33. Article CAS PubMed PubMed Central Google
Scholar * Tao Y, Zhang SC. Neural subtype specification from human pluripotent stem cells. Cell Stem Cell. 2016;19:573–86. Article CAS PubMed PubMed Central Google Scholar * Coghlan S,
Horder J, Inkster B, Mendez MA, Murphy DG, Nutt DJ. GABA system dysfunction in autism and related disorders: from synapse to symptoms. Neurosci Biobehav Rev. 2012;36:2044–55. Article CAS
PubMed PubMed Central Google Scholar * Marín O. Interneuron dysfunction in psychiatric disorders. Nat Rev Neurosci. 2012;13:107–20. Article PubMed Google Scholar * Chao HT, Chen H,
Samaco RC, Xue M, Chahrour M, Yoo J, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–9. Article CAS PubMed
PubMed Central Google Scholar * Javitt DC. Glutamatergic theories of schizophrenia. Isr J Psychiatry Relat Sci. 2010;47:4–16. PubMed Google Scholar * Lewis DA, Hashimoto T, Volk DW.
Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–24. Article CAS PubMed Google Scholar * Li XJ, Zhang X, Johnson MA, Wang ZB, Lavaute T, Zhang SC. Coordination
of sonic hedgehog and Wnt signaling determines ventral and dorsal telencephalic neuron types from human embryonic stem cells. Development. 2009;136:4055–63. Article CAS PubMed PubMed
Central Google Scholar * Shi Y, Kirwan P, Livesey FJ. Directed differentiation of human pluripotent stem cells to cerebral cortex neurons and neural networks. Nat Protoc. 2012;7:1836–46.
Article CAS PubMed Google Scholar * Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ. Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses.
Nat Neurosci. 2012;15:477–86. Article CAS PubMed Google Scholar * Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7:687–96. Article
CAS PubMed Google Scholar * Liu Y, Liu H, Sauvey C, Yao L, Zarnowska ED, Zhang SC. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat Protoc.
2013;8:1670–9. Article CAS PubMed PubMed Central Google Scholar * Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, et al. Directed differentiation and functional maturation
of cortical interneurons from human embryonic stem cells. Cell Stem Cell. 2013;12:559–72. Article CAS PubMed PubMed Central Google Scholar * Nicholas CR, Chen J, Tang Y, Southwell DG,
Chalmers N, Vogt D, et al. Functional maturation of hPSC-derived forebrain interneurons requires an extended timeline and mimics human neural development. Cell Stem Cell. 2013;12:573–86.
Article CAS PubMed PubMed Central Google Scholar * Abi-Dargham A. Schizophrenia: overview and dopamine dysfunction. J Clin Psychiatry. 2014;75:e31. Article PubMed Google Scholar *
Whitton AE, Treadway MT, Pizzagalli DA. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr Opin Psychiatry. 2015;28:7–12. Article PubMed PubMed
Central Google Scholar * Kriks S, Shim JW, Piao J, Ganat YM, Wakeman DR, Xie Z, et al. Dopamine neurons derived from human ES cells efficiently engraft in animal models of Parkinson’s
disease. Nature. 2011;480:547–51. Article CAS PubMed PubMed Central Google Scholar * Swistowski A, Peng J, Liu Q, Mali P, Rao MS, Cheng L, et al. Efficient generation of functional
dopaminergic neurons from human induced pluripotent stem cells under defined conditions. Stem Cells. 2010;28:1893–904. Article CAS PubMed Google Scholar * Ma L, Liu Y, Zhang SC. Directed
differentiation of dopamine neurons from human pluripotent stem cells. Methods Mol Biol. 2011;767:411–8. Article CAS PubMed Google Scholar * Yu DX, Di Giorgio FP, Yao J, Marchetto MC,
Brennand K, Wright R, et al. Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Rep. 2014;2:295–310. Article CAS Google Scholar * Mertens J, Wang QW, Kim Y,
Yu DX, Pham S, Yang B, et al. Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature. 2015;527:95–99. Article CAS PubMed PubMed Central
Google Scholar * Sakaguchi H, Kadoshima T, Soen M, Narii N, Ishida Y, Ohgushi M, et al. Generation of functional hippocampal neurons from self-organizing human embryonic stem cell-derived
dorsomedial telencephalic tissue. Nat Commun. 2015;6:8896. Article CAS PubMed Google Scholar * Ming GL, Song H. Adult neurogenesis in the mammalian brain: significant answers and
significant questions. Neuron. 2011;70:687–702. Article CAS PubMed PubMed Central Google Scholar * Reif A, Fritzen S, Finger M, Strobel A, Lauer M, Schmitt A, et al. Neural stem cell
proliferation is decreased in schizophrenia, but not in depression. Mol Psychiatry. 2006;11:514–22. Article CAS PubMed Google Scholar * Tamminga CA, Thomas BP, Chin R, Mihalakos P,
Youens K, Wagner AD, et al. Hippocampal novelty activations in schizophrenia: disease and medication effects. Schizophr Res. 2012;138:157–63. Article PubMed Google Scholar * Sarkar A, Mei
A, Paquola ACM, Stern S, Bardy C, Klug JR, et al. Efficient generation of CA3 neurons from human pluripotent stem cells enables modeling of hippocampal connectivity in vitro. Cell Stem
Cell. 2018;22:684–697.e689. Article CAS PubMed PubMed Central Google Scholar * Barres BA. The mystery and magic of glia: a perspective on their roles in health and disease. Neuron.
2008;60:430–40. Article CAS PubMed Google Scholar * Molofsky AV, Krencik R, Ullian EM, Tsai HH, Deneen B, Richardson WD, et al. Astrocytes and disease: a neurodevelopmental perspective.
Genes Dev. 2012;26:891–907. Article CAS PubMed PubMed Central Google Scholar * Santos R, Vadodaria KC, Jaeger BN, Mei A, Lefcochilos-Fogelquist S, Mendes APD, et al. Differentiation of
inflammation-responsive astrocytes from glial progenitors generated from human induced pluripotent stem cells. Stem Cell Rep. 2017;8:1757–69. Article CAS Google Scholar * Simons M, Nave
KA. Oligodendrocytes: Myelination and axonal support. Cold Spring Harb Perspect Biol. 2015;8:a020479. Article PubMed Google Scholar * Hu BY, Du ZW, Zhang SC. Differentiation of human
oligodendrocytes from pluripotent stem cells. Nat Protoc. 2009;4:1614–22. Article CAS PubMed PubMed Central Google Scholar * Wang S, Bates J, Li X, Schanz S, Chandler-Militello D,
Levine C, et al. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12:252–64. Article CAS
PubMed PubMed Central Google Scholar * Rodrigues GMC, Gaj T, Adil MM, Wahba J, Rao AT, Lorbeer FK, et al. Defined and scalable differentiation of human oligodendrocyte precursors from
pluripotent stem cells in a 3D culture system. Stem Cell Rep. 2017;8:1770–83. Article CAS Google Scholar * Hammond TR, Robinton D, Stevens B. Microglia and the brain: complementary
partners in development and disease. Annu Rev Cell Dev Biol. 2018;34:523–44. Article CAS PubMed Google Scholar * Ginhoux F, Greter M, Leboeuf M, Nandi S, See P, Gokhan S, et al. Fate
mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–5. Article CAS PubMed PubMed Central Google Scholar * Kierdorf K, Erny D, Goldmann
T, Sander V, Schulz C, Perdiguero EG, et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat Neurosci. 2013;16:273–80. Article CAS PubMed
Google Scholar * Abud EM, Ramirez RN, Martinez ES, Healy LM, Nguyen CHH, Newman SA, et al. iPSC-derived human microglia-like cells to study neurological diseases. Neuron.
2017;94:278–293.e279. Article CAS PubMed PubMed Central Google Scholar * Pandya H, Shen MJ, Ichikawa DM, Sedlock AB, Choi Y, Johnson KR, et al. Differentiation of human and murine
induced pluripotent stem cells to microglia-like cells. Nat Neurosci. 2017;20:753–9. Article CAS PubMed PubMed Central Google Scholar * Muffat J, Li Y, Yuan B, Mitalipova M, Omer A,
Corcoran S, et al. Efficient derivation of microglia-like cells from human pluripotent stem cells. Nat Med. 2016;22:1358–67. Article CAS PubMed PubMed Central Google Scholar * McQuade
A, Coburn M, Tu CH, Hasselmann J, Davtyan H, Blurton-Jones M. Development and validation of a simplified method to generate human microglia from pluripotent stem cells. Mol Neurodegener.
2018;13:67. Article CAS PubMed PubMed Central Google Scholar * Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, et al. Rapid single-step induction of functional neurons from human
pluripotent stem cells. Neuron. 2013;78:785–98. Article CAS PubMed PubMed Central Google Scholar * Yang N, Chanda S, Marro S, Ng YH, Janas JA, Haag D, et al. Generation of pure
GABAergic neurons by transcription factor programming. Nat Methods. 2017;14:621–8. Article CAS PubMed PubMed Central Google Scholar * Ng YH, Chanda S, Janas JA, Yang N, Kokubu Y, Südhof
TC, et al. Efficient generation of dopaminergic induced neuronal cells with midbrain characteristics. Stem Cell Rep. 2021;16:1763–76. Article CAS Google Scholar * Powell SK, O’Shea C,
Townsley K, Prytkova I, Dobrindt K, Elahi R, et al. Induction of dopaminergic neurons for neuronal subtype-specific modeling of psychiatric disease risk. Mol Psychiatry. 2021.
https://doi.org/10.1038/s41380-021-01273-0. Online ahead of print. * Canals I, Ginisty A, Quist E, Timmerman R, Fritze J, Miskinyte G, et al. Rapid and efficient induction of functional
astrocytes from human pluripotent stem cells. Nat Methods. 2018;15:693–6. Article CAS PubMed Google Scholar * Ehrlich M, Mozafari S, Glatza M, Starost L, Velychko S, Hallmann AL, et al.
Rapid and efficient generation of oligodendrocytes from human induced pluripotent stem cells using transcription factors. Proc Natl Acad Sci USA. 2017;114:E2243–e2252. Article CAS PubMed
PubMed Central Google Scholar * Chen SW, Hung YS, Fuh JL, Chen NJ, Chu YS, Chen SC, et al. Efficient conversion of human induced pluripotent stem cells into microglia by defined
transcription factors. Stem Cell Rep. 2021;16:1363–80. Article CAS Google Scholar * Dräger NM, Sattler SM, Huang CT, Teter OM, Leng K, Hashemi SH, et al. A CRISPRi/a platform in human
iPSC-derived microglia uncovers regulators of disease states. Nat Neurosci. 2022;25:1149–62. Article PubMed PubMed Central Google Scholar * Pasca SP, Arlotta P, Bateup HS, Camp JG,
Cappello S, Gage FH, et al. A nomenclature consensus for nervous system organoids and assembloids. Nature. 2022;609:907–10. Article CAS PubMed Google Scholar * Lancaster MA, Renner M,
Martin CA, Wenzel D, Bicknell LS, Hurles ME, et al. Cerebral organoids model human brain development and microcephaly. Nature. 2013;501:373–9. Article CAS PubMed Google Scholar * Paşca
AM, Sloan SA, Clarke LE, Tian Y, Makinson CD, Huber N, et al. Functional cortical neurons and astrocytes from human pluripotent stem cells in 3D culture. Nat Methods. 2015;12:671–8. Article
PubMed PubMed Central Google Scholar * Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL. Generation of human brain region-specific organoids using a miniaturized spinning
bioreactor. Nat Protoc. 2018;13:565–80. Article CAS PubMed PubMed Central Google Scholar * Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, et al. Brain-region-specific
organoids using mini-bioreactors for Modeling ZIKV exposure. Cell. 2016;165:1238–54. Article CAS PubMed PubMed Central Google Scholar * Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando
S, Eiraku M, et al. Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci USA.
2013;110:20284–9. Article CAS PubMed PubMed Central Google Scholar * Muguruma K, Nishiyama A, Kawakami H, Hashimoto K, Sasai Y. Self-organization of polarized cerebellar tissue in 3D
culture of human pluripotent stem cells. Cell Rep. 2015;10:537–50. Article CAS PubMed Google Scholar * Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, Sun P, et al. hESC-derived
thalamic organoids form reciprocal projections when fused with cortical organoids. Cell Stem Cell. 2019;24:487–497.e487. Article CAS PubMed PubMed Central Google Scholar * Huang WK,
Wong SZH, Pather SR, Nguyen PTT, Zhang F, Zhang DY, et al. Generation of hypothalamic arcuate organoids from human induced pluripotent stem cells. Cell Stem Cell. 2021;28:1657–1670.e1610.
Article CAS PubMed PubMed Central Google Scholar * Monzel AS, Smits LM, Hemmer K, Hachi S, Moreno EL, van Wuellen T, et al. Derivation of human midbrain-specific organoids from
neuroepithelial stem cells. Stem Cell Rep. 2017;8:1144–54. Article CAS Google Scholar * Qian X, Su Y, Adam CD, Deutschmann AU, Pather SR, Goldberg EM, et al. Sliced human cortical
organoids for modeling distinct cortical layer formation. Cell Stem Cell. 2020;26:766–781.e769. Article CAS PubMed PubMed Central Google Scholar * Marton RM, Pasca SP. Organoid and
assembloid technologies for investigating cellular crosstalk in human brain development and disease. Trends Cell Biol. 2020;30:133–43. Article CAS PubMed Google Scholar * Bagley JA,
Reumann D, Bian S, Lévi-Strauss J, Knoblich JA. Fused cerebral organoids model interactions between brain regions. Nat Methods. 2017;14:743–51. Article CAS PubMed PubMed Central Google
Scholar * Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, et al. Assembly of functionally integrated human forebrain spheroids. Nature. 2017;545:54–59. Article CAS PubMed
PubMed Central Google Scholar * Bennett ML, Song H, Ming GL. Microglia modulate neurodevelopment in human neuroimmune organoids. Cell Stem Cell. 2021;28:2035–6. Article CAS PubMed
Google Scholar * Song L, Yuan X, Jones Z, Vied C, Miao Y, Marzano M, et al. Functionalization of brain region-specific spheroids with isogenic Microglia-like cells. Sci Rep. 2019;9:11055.
Article PubMed PubMed Central Google Scholar * Shen Q, Goderie SK, Jin L, Karanth N, Sun Y, Abramova N, et al. Endothelial cells stimulate self-renewal and expand neurogenesis of neural
stem cells. Science. 2004;304:1338–40. Article CAS PubMed Google Scholar * Yin X, Mead BE, Safaee H, Langer R, Karp JM, Levy O. Engineering stem cell organoids. Cell Stem Cell.
2016;18:25–38. Article CAS PubMed PubMed Central Google Scholar * Raybaud C. Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg Clin N. Am.
2010;21:399–426. Article PubMed Google Scholar * Paredes I, Himmels P, Ruiz de Almodovar C. Neurovascular communication during CNS development. Dev Cell. 2018;45:10–32. Article CAS
PubMed Google Scholar * Santander N, Lizama CO, Meky E, McKinsey GL, Jung B, Sheppard D, et al. Lack of Flvcr2 impairs brain angiogenesis without affecting the blood-brain barrier. J Clin
Invest. 2020;130:4055–68. CAS PubMed PubMed Central Google Scholar * Shi Y, Sun L, Wang M, Liu J, Zhong S, Li R, et al. Vascularized human cortical organoids (vOrganoids) model cortical
development in vivo. PLoS Biol. 2020;18:e3000705. Article CAS PubMed PubMed Central Google Scholar * Pham MT, Pollock KM, Rose MD, Cary WA, Stewart HR, Zhou P, et al. Generation of
human vascularized brain organoids. Neuroreport. 2018;29:588–93. Article PubMed PubMed Central Google Scholar * Chen HI, Wolf JA, Blue R, Song MM, Moreno JD, Ming GL, et al.
Transplantation of human brain organoids: revisiting the science and ethics of brain chimeras. Cell Stem Cell. 2019;25:462–72. Article CAS PubMed PubMed Central Google Scholar * Mansour
AA, Gonçalves JT, Bloyd CW, Li H, Fernandes S, Quang D, et al. An in vivo model of functional and vascularized human brain organoids. Nat Biotechnol. 2018;36:432–41. Article CAS PubMed
PubMed Central Google Scholar * Dong X, Xu SB, Chen X, Tao M, Tang XY, Fang KH, et al. Human cerebral organoids establish subcortical projections in the mouse brain after transplantation.
Mol Psychiatry. 2021;26:2964–76. Article CAS PubMed Google Scholar * Revah O, Gore F, Kelley KW, Andersen J, Sakai N, Chen X, et al. Maturation and circuit integration of transplanted
human cortical organoids. Nature. 2022;610:319–26. Article CAS PubMed PubMed Central Google Scholar * Sacco R, Cacci E, Novarino G. Neural stem cells in neuropsychiatric disorders. Curr
Opin Neurobiol. 2018;48:131–8. Article CAS PubMed Google Scholar * Villa C, Combi R, Conconi D, Lavitrano M. Patient-derived Induced Pluripotent Stem Cells (iPSCs) and cerebral
organoids for drug screening and development in autism spectrum disorder: opportunities and challenges. Pharmaceutics. 2021;13:280. Article CAS PubMed PubMed Central Google Scholar *
Kahn RS, Sommer IE, Murray RM, Meyer-Lindenberg A, Weinberger DR, Cannon TD, et al. Schizophrenia. Nat Rev Dis Prim. 2015;1:15067. Article PubMed Google Scholar * Chiang CH, Su Y, Wen Z,
Yoritomo N, Ross CA, Margolis RL, et al. Integration-free induced pluripotent stem cells derived from schizophrenia patients with a DISC1 mutation. Mol Psychiatry. 2011;16:358–60. Article
CAS PubMed PubMed Central Google Scholar * Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, et al. Modelling schizophrenia using human induced pluripotent stem cells.
Nature. 2011;473:221–5. Article CAS PubMed PubMed Central Google Scholar * Robicsek O, Karry R, Petit I, Salman-Kesner N, Muller FJ, Klein E, et al. Abnormal neuronal differentiation
and mitochondrial dysfunction in hair follicle-derived induced pluripotent stem cells of schizophrenia patients. Mol Psychiatry. 2013;18:1067–76. Article CAS PubMed Google Scholar *
Stertz L, Di ReJ, Pei G, Fries GR, Mendez E, Li S, et al. Convergent genomic and pharmacological evidence of PI3K/GSK3 signaling alterations in neurons from schizophrenia patients.
Neuropsychopharmacology. 2021;46:673–82. Article CAS PubMed Google Scholar * Wen Z, Nguyen HN, Guo Z, Lalli MA, Wang X, Su Y, et al. Synaptic dysregulation in a human iPS cell model of
mental disorders. Nature. 2014;515:414–8. Article CAS PubMed PubMed Central Google Scholar * Page SC, Sripathy SR, Farinelli F, Ye Z, Wang Y, Hiler DJ, et al. Electrophysiological
measures from human iPSC-derived neurons are associated with schizophrenia clinical status and predict individual cognitive performance. Proc Natl Acad Sci USA. 2022;119:e2109395119. Article
CAS PubMed PubMed Central Google Scholar * Lord C, Brugha TS, Charman T, Cusack J, Dumas G, Frazier T, et al. Autism spectrum disorder. Nat Rev Dis Prim. 2020;6:5. Article PubMed
Google Scholar * Geschwind DH. Autism: many genes, common pathways? Cell. 2008;135:391–5. Article CAS PubMed PubMed Central Google Scholar * Marchetto MC, Belinson H, Tian Y, Freitas
BC, Fu C, Vadodaria K, et al. Altered proliferation and networks in neural cells derived from idiopathic autistic individuals. Mol Psychiatry. 2017;22:820–35. Article CAS PubMed Google
Scholar * Mariani J, Coppola G, Zhang P, Abyzov A, Provini L, Tomasini L, et al. FOXG1-dependent dysregulation of GABA/Glutamate neuron differentiation in autism spectrum disorders. Cell.
2015;162:375–90. Article CAS PubMed PubMed Central Google Scholar * Wang M, Wei PC, Lim CK, Gallina IS, Marshall S, Marchetto MC, et al. Increased neural progenitor proliferation in a
hiPSC Model of autism induces replication stress-associated genome instability. Cell Stem Cell. 2020;26:221–233.e226. Article CAS PubMed PubMed Central Google Scholar * Malhotra D,
Sebat J. CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 2012;148:1223–41. Article CAS PubMed PubMed Central Google Scholar * Sebat J, Lakshmi B, Malhotra
D, Troge J, Lese-Martin C, Walsh T, et al. Strong association of de novo copy number mutations with autism. Science. 2007;316:445–9. Article CAS PubMed PubMed Central Google Scholar *
McCarthy SE, Makarov V, Kirov G, Addington AM, McClellan J, Yoon S, et al. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41:1223–7. Article CAS PubMed
PubMed Central Google Scholar * Pinto D, Pagnamenta AT, Klei L, Anney R, Merico D, Regan R, et al. Functional impact of global rare copy number variation in autism spectrum disorders.
Nature. 2010;466:368–72. Article CAS PubMed PubMed Central Google Scholar * Yoon KJ, Nguyen HN, Ursini G, Zhang F, Kim NS, Wen Z, et al. Modeling a genetic risk for schizophrenia in
iPSCs and mice reveals neural stem cell deficits associated with adherens junctions and polarity. Cell Stem Cell. 2014;15:79–91. Article CAS PubMed PubMed Central Google Scholar *
Sawada T, Chater TE, Sasagawa Y, Yoshimura M, Fujimori-Tonou N, Tanaka K, et al. Developmental excitation-inhibition imbalance underlying psychoses revealed by single-cell analyses of
discordant twins-derived cerebral organoids. Mol Psychiatry. 2020;25:2695–711. Article PubMed PubMed Central Google Scholar * Sellgren CM, Gracias J, Watmuff B, Biag JD, Thanos JM,
Whittredge PB, et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat Neurosci. 2019;22:374–85. Article CAS PubMed PubMed
Central Google Scholar * de Jong JO, Llapashtica C, Genestine M, Strauss K, Provenzano F, Sun Y, et al. Cortical overgrowth in a preclinical forebrain organoid model of CNTNAP2-associated
autism spectrum disorder. Nat Commun. 2021;12:4087. Article PubMed PubMed Central Google Scholar * Urresti J, Zhang P, Moran-Losada P, Yu NK, Negraes PD, Trujillo CA, et al. Cortical
organoids model early brain development disrupted by 16p11.2 copy number variants in autism. Mol Psychiatry. 2021;26:7560–80. Article CAS PubMed PubMed Central Google Scholar *
Martin-Brevet S, Rodríguez-Herreros B, Nielsen JA, Moreau C, Modenato C, Maillard AM, et al. Quantifying the effects of 16p11.2 copy number variants on brain structure: a multisite
genetic-first study. Biol Psychiatry. 2018;84:253–64. Article CAS PubMed Google Scholar * Sønderby IE, Gústafsson Ó, Doan NT, Hibar DP, Martin-Brevet S, Abdellaoui A, et al. Dose
response of the 16p11.2 distal copy number variant on intracranial volume and basal ganglia. Mol Psychiatry. 2020;25:584–602. Article PubMed Google Scholar * Ye F, Kang E, Yu C, Qian X,
Jacob F, Yu C, et al. DISC1 regulates neurogenesis via modulating Kinetochore attachment of Ndel1/Nde1 during Mitosis. Neuron. 2017;96:1041–1054.e1045. Article CAS PubMed PubMed Central
Google Scholar * Stoner R, Chow ML, Boyle MP, Sunkin SM, Mouton PR, Roy S, et al. Patches of disorganization in the neocortex of children with autism. N. Engl J Med. 2014;370:1209–19.
Article CAS PubMed PubMed Central Google Scholar * Stern S, Santos R, Marchetto MC, Mendes APD, Rouleau GA, Biesmans S, et al. Neurons derived from patients with bipolar disorder divide
into intrinsically different sub-populations of neurons, predicting the patients’ responsiveness to lithium. Mol Psychiatry. 2018;23:1453–65. Article CAS PubMed Google Scholar * Kamijo
S, Ishii Y, Horigane SI, Suzuki K, Ohkura M, Nakai J, et al. A critical neurodevelopmental role for L-type voltage-gated calcium channels in neurite extension and radial migration. J
Neurosci. 2018;38:5551–66. Article CAS PubMed PubMed Central Google Scholar * Bhat S, Dao DT, Terrillion CE, Arad M, Smith RJ, Soldatov NM, et al. CACNA1C (Cav1.2) in the
pathophysiology of psychiatric disease. Prog Neurobiol. 2012;99:1–14. Article CAS PubMed PubMed Central Google Scholar * Birey F, Li MY, Gordon A, Thete MV, Valencia AM, Revah O, et al.
Dissecting the molecular basis of human interneuron migration in forebrain assembloids from Timothy syndrome. Cell Stem Cell. 2022;29:248–264.e247. Article CAS PubMed Google Scholar *
Ruzzo EK, Pérez-Cano L, Jung JY, Wang LK, Kashef-Haghighi D, Hartl C, et al. Inherited and De Novo genetic risk for autism impacts shared networks. Cell. 2019;178:850–866.e826. Article CAS
PubMed PubMed Central Google Scholar * Sanders SJ, Murtha MT, Gupta AR, Murdoch JD, Raubeson MJ, Willsey AJ, et al. De novo mutations revealed by whole-exome sequencing are strongly
associated with autism. Nature. 2012;485:237–41. Article CAS PubMed PubMed Central Google Scholar * Grove J, Ripke S, Als TD, Mattheisen M, Walters RK, Won H, et al. Identification of
common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51:431–44. Article CAS PubMed PubMed Central Google Scholar * Satterstrom FK, Kosmicki JA, Wang J, Breen MS,
De Rubeis S, An JY, et al. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell. 2020;180:568–584.e523. Article CAS
PubMed PubMed Central Google Scholar * Cederquist GY, Tchieu J, Callahan SJ, Ramnarine K, Ryan S, Zhang C, et al. A multiplex human pluripotent stem cell platform defines molecular and
functional subclasses of autism-related genes. Cell Stem Cell. 2020;27:35–49.e36. Article CAS PubMed PubMed Central Google Scholar * Paulsen B, Velasco S, Kedaigle AJ, Pigoni M,
Quadrato G, Deo AJ, et al. Autism genes converge on asynchronous development of shared neuron classes. Nature. 2022;602:268–73. Article CAS PubMed PubMed Central Google Scholar *
Pardiñas AF, Holmans P, Pocklington AJ, Escott-Price V, Ripke S, Carrera N, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong
background selection. Nat Genet. 2018;50:381–9. Article PubMed PubMed Central Google Scholar * Stahl EA, Breen G, Forstner AJ, McQuillin A, Ripke S, Trubetskoy V, et al. Genome-wide
association study identifies 30 loci associated with bipolar disorder. Nat Genet. 2019;51:793–803. Article CAS PubMed PubMed Central Google Scholar * Forrest MP, Zhang H, Moy W, McGowan
H, Leites C, Dionisio LE, et al. Open chromatin profiling in hiPSC-derived neurons prioritizes functional noncoding psychiatric risk variants and highlights neurodevelopmental Loci. Cell
Stem Cell. 2017;21:305–318.e308. Article CAS PubMed PubMed Central Google Scholar * Schrode N, Ho SM, Yamamuro K, Dobbyn A, Huckins L, Matos MR, et al. Synergistic effects of common
schizophrenia risk variants. Nat Genet. 2019;51:1475–85. Article CAS PubMed PubMed Central Google Scholar * Das D, Feuer K, Wahbeh M, Avramopoulos D. Modeling psychiatric disorder
biology with stem cells. Curr Psychiatry Rep. 2020;22:24. Article PubMed PubMed Central Google Scholar * Brookhouser N, Tekel SJ, Standage-Beier K, Nguyen T, Schwarz G, Wang X, et al.
BIG-TREE: Base-edited isogenic hPSC line generation using a transient reporter for editing enrichment. Stem Cell Rep. 2020;14:184–91. Article CAS Google Scholar * Chang YJ, Xu CL, Cui X,
Bassuk AG, Mahajan VB, Tsai YT, et al. CRISPR base editing in induced pluripotent stem cells. Methods Mol Biol. 2019;2045:337–46. Article CAS PubMed Google Scholar * Sürün D, Schneider
A, Mircetic J, Neumann K, Lansing F, Paszkowski-Rogacz M, et al. Efficient generation and correction of mutations in human iPS cells utilizing mRNAs of CRISPR base editors and prime editors.
Genes. 2020;11:511. Article PubMed PubMed Central Google Scholar * Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, et al. Search-and-replace genome editing without
double-strand breaks or donor DNA. Nature. 2019;576:149–57. Article CAS PubMed PubMed Central Google Scholar * McTague A, Rossignoli G, Ferrini A, Barral S, Kurian MA. Genome editing in
iPSC-based neural systems: from disease models to future therapeutic strategies. Front Genome Ed. 2021;3:630600. Article PubMed PubMed Central Google Scholar * Matos MR, Ho SM, Schrode
N, Brennand KJ. Integration of CRISPR-engineering and hiPSC-based models of psychiatric genomics. Mol Cell Neurosci. 2020;107:103532. Article CAS PubMed PubMed Central Google Scholar *
Li Q, Barres BA. Microglia and macrophages in brain homeostasis and disease. Nat Rev Immunol. 2018;18:225–42. Article CAS PubMed Google Scholar * Ormel PR, Vieira de Sa R, van Bodegraven
EJ, Karst H, Harschnitz O, Sneeboer MAM, et al. Microglia innately develop within cerebral organoids. Nat Commun. 2018;9:4167. Article PubMed PubMed Central Google Scholar * Xu R,
Boreland AJ, Li X, Erickson C, Jin M, Atkins C, et al. Developing human pluripotent stem cell-based cerebral organoids with a controllable microglia ratio for modeling brain development and
pathology. Stem Cell Rep. 2021;16:1923–37. Article Google Scholar * Su Y, Zhou Y, Bennett ML, Li S, Carceles-Cordon M, Lu L, et al. A single-cell transcriptome atlas of glial diversity in
the human hippocampus across the postnatal lifespan. Cell Stem Cell. 2022;29:1594–1610.e1598. Article CAS PubMed Google Scholar * Kim NS, Wen Z, Liu J, Zhou Y, Guo Z, Xu C, et al.
Pharmacological rescue in patient iPSC and mouse models with a rare DISC1 mutation. Nat Commun. 2021;12:1398. Article CAS PubMed PubMed Central Google Scholar Download references
FUNDING Funding The research in the authors’ laboratory were supported by grants from the Institutes of Health (R35NS097370, U19AI131130, RF1MH123979, R01MH125528 to GLM, and R35NS116843,
U01HG012047, RF1AG079557, and U19MH106434 to H.S.) and from Dr. Miriam and Sheldon G. Adelson Medical Research Foundation (to GLM). AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Department
of Neuroscience and Mahoney Institute for Neurosciences, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA Yan Hong, Qian Yang, Hongjun Song & Guo-li Ming *
Department of Cell and Developmental Biology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA Hongjun Song & Guo-li Ming * Institute for Regenerative
Medicine, University of Pennsylvania, Philadelphia, PA, USA Hongjun Song & Guo-li Ming * The Epigenetics Institute, Perelman School of Medicine, University of Pennsylvania, Philadelphia,
PA, USA Hongjun Song * Department of Psychiatry, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, USA Guo-li Ming Authors * Yan Hong View author publications You
can also search for this author inPubMed Google Scholar * Qian Yang View author publications You can also search for this author inPubMed Google Scholar * Hongjun Song View author
publications You can also search for this author inPubMed Google Scholar * Guo-li Ming View author publications You can also search for this author inPubMed Google Scholar CONTRIBUTIONS YH
wrote the manuscript with contributions from all co-authors. CORRESPONDING AUTHOR Correspondence to Guo-li Ming. ETHICS DECLARATIONS COMPETING INTERESTS The authors declare no competing
interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. RIGHTS AND
PERMISSIONS Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s);
author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law. Reprints and permissions ABOUT THIS
ARTICLE CITE THIS ARTICLE Hong, Y., Yang, Q., Song, H. _et al._ Opportunities and limitations for studying neuropsychiatric disorders using patient-derived induced pluripotent stem cells.
_Mol Psychiatry_ 28, 1430–1439 (2023). https://doi.org/10.1038/s41380-023-01990-8 Download citation * Received: 21 September 2022 * Revised: 27 January 2023 * Accepted: 31 January 2023 *
Published: 13 February 2023 * Issue Date: April 2023 * DOI: https://doi.org/10.1038/s41380-023-01990-8 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this
content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative


