- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
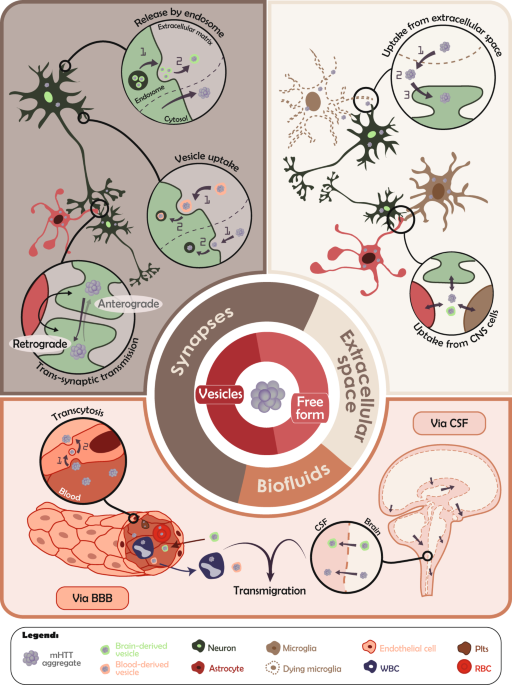
ABSTRACT If theories postulating that pathological proteins associated with neurodegenerative disorders behave similarly to prions were initially viewed with reluctance, it is now
well-accepted that this occurs in several disease contexts. Notably, it has been reported that protein misfolding and subsequent prion-like properties can actively participate in
neurodegenerative disorders. While this has been demonstrated in multiple cellular and animal model systems related to Alzheimer’s and Parkinson’s diseases, the prion-like properties of the
mutant huntingtin protein (mHTT), associated with Huntington’s disease (HD), have only recently been considered to play a role in this pathology, a concept our research group has contributed
to extensively. In this review, we summarize the last few years of in vivo research in the field and speculate on the relationship between prion-like events and human HD. By interpreting
observations primarily collected in in vivo models, our discussion will aim to discriminate which experimental factors contribute to the most efficient types of prion-like activities of mHTT
and which routes of propagation may be more relevant to the human condition. A look back at nearly a decade of experimentation will inform future research and whether therapeutic strategies
may emerge from this new knowledge. Access through your institution Buy or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through
your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant
access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions *
Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS NUCLEAR AND CYTOPLASMIC HUNTINGTIN INCLUSIONS EXHIBIT DISTINCT BIOCHEMICAL COMPOSITION, INTERACTOME AND
ULTRASTRUCTURAL PROPERTIES Article Open access 12 November 2021 THE ROLE OF TDP-43 PROPAGATION IN NEURODEGENERATIVE DISEASES: INTEGRATING INSIGHTS FROM CLINICAL AND EXPERIMENTAL STUDIES
Article Open access 13 October 2020 HTRA1 DISAGGREGATES Α-SYNUCLEIN AMYLOID FIBRILS AND CONVERTS THEM INTO NON-TOXIC AND SEEDING INCOMPETENT SPECIES Article Open access 18 March 2024
REFERENCES * Venkatraman P, Wetzel R, Tanaka M, Nukina N, Goldberg AL. Eukaryotic proteasomes cannot digest polyglutamine sequences and release them during degradation of
polyglutamine-containing proteins. Mol Cell. 2004;14:95–104. Article CAS PubMed Google Scholar * Jana NR, Zemskov EA, Wang G, Nukina N. Altered proteasomal function due to the expression
of polyglutamine-expanded truncated N-terminal huntingtin induces apoptosis by caspase activation through mitochondrial cytochrome c release. Hum Mol Genet. 2001;10:1049–59. Article CAS
PubMed Google Scholar * Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA, et al. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice
transgenic for the HD mutation. Cell. 1997;90:537–48. Article CAS PubMed Google Scholar * Jeon I, Cicchetti F, Cisbani G, Lee S, Li E, Bae J, et al. Human-to-mouse prion-like
propagation of mutant huntingtin protein. Acta Neuropathol. 2016;132:577–92. Article CAS PubMed PubMed Central Google Scholar * Wild EJ, Boggio R, Langbehn D, Robertson N, Haider S,
Miller JR, et al. Quantification of mutant huntingtin protein in cerebrospinal fluid from Huntington’s disease patients. J Clin Investig. 2015;125:1979–86. Article PubMed PubMed Central
Google Scholar * Cicchetti F, Lacroix S, Cisbani G, Vallieres N, Saint-Pierre M, St-Amour I, et al. Mutant huntingtin is present in neuronal grafts in Huntington disease patients. Ann
Neurol. 2014;76:31–42. Article CAS PubMed Google Scholar * Liu X, Valentine SJ, Plasencia MD, Trimpin S, Naylor S, Clemmer DE. Mapping the human plasma proteome by SCX-LC-IMS-MS. J Am
Soc Mass Spectrom. 2007;18:1249–64. Article CAS PubMed PubMed Central Google Scholar * Gutekunst CA, Li SH, Yi H, Mulroy JS, Kuemmerle S, Jones R, et al. Nuclear and neuropil aggregates
in Huntington’s disease: relationship to neuropathology. J Neurosci. 1999;19:2522–34. Article CAS PubMed PubMed Central Google Scholar * Reiner A, Dragatsis I, Dietrich P. Genetics and
neuropathology of Huntington’s disease. Int Rev Neurobiol. 2011;98:325–72. Article CAS PubMed PubMed Central Google Scholar * Vonsattel JP, Keller C, Del Pilar Amaya M. Neuropathology
of Huntington’s disease. Handb Clin Neurol. 2008;89:599–618. * Tang C, Feigin A. Monitoring Huntington’s disease progression through preclinical and early stages. Neurodegenerative Dis
Manag. 2012;2:421–35. Article Google Scholar * Maxan A, Mason S, Saint-Pierre M, Smith E, Ho A, Harrower T, et al. Outcome of cell suspension allografts in a patient with Huntington’s
disease. Ann. Neurol. 2018;84:950–6. Article PubMed PubMed Central Google Scholar * Pecho-Vrieseling E, Rieker C, Fuchs S, Bleckmann D, Esposito MS, Botta P, et al. Transneuronal
propagation of mutant huntingtin contributes to non-cell autonomous pathology in neurons. Nat Neurosci. 2014;17:1064–72. Article CAS PubMed Google Scholar * Tan Z, Dai W, van Erp TG,
Overman J, Demuro A, Digman MA, et al. Huntington’s disease cerebrospinal fluid seeds aggregation of mutant huntingtin. Mol Psychiatry. 2015;20:1286–93. Article CAS PubMed PubMed Central
Google Scholar * Ast A, Buntru A, Schindler F, Hasenkopf R, Schulz A, Brusendorf L, et al. mHTT Seeding Activity: A Marker of Disease Progression and Neurotoxicity in Models of
Huntington’s Disease. Mol Cell. 2018;71:675–88. Article CAS PubMed Google Scholar * Imran M, Mahmood S. An overview of human prion diseases. Virol J. 2011;8:559. Article CAS PubMed
PubMed Central Google Scholar * Hewitt PE, Llewelyn CA, Mackenzie J, Will RG. Creutzfeldt-Jakob disease and blood transfusion: results of the UK Transfusion Medicine Epidemiological Review
study. Vox Sang. 2006;91:221–30. Article CAS PubMed Google Scholar * Ironside JW. Neuropathological findings in new variant CJD and experimental transmission of BSE. FEMS Immunol Med
Microbiol. 1998;21:91–95. Article CAS PubMed Google Scholar * Lang CJ, Heckmann JG, Neundorfer B. Creutzfeldt-Jakob disease via dural and corneal transplants. J Neurol Sci.
1998;160:128–39. Article CAS PubMed Google Scholar * Bonda DJ, Manjila S, Mehndiratta P, Khan F, Miller BR, Onwuzulike K, et al. Human prion diseases: surgical lessons learned from
iatrogenic prion transmission. Neurosurg Focus. 2016;41:E10. Article PubMed PubMed Central Google Scholar * de Pedro Cuesta J, Ruiz Tovar M, Ward H, Calero M, Smith A, Verduras CA, et
al. Sensitivity to biases of case-control studies on medical procedures, particularly surgery and blood transfusion, and risk of Creutzfeldt-Jakob disease. Neuroepidemiology. 2012;39:1–18.
Article PubMed Google Scholar * Rieux M, Alpaugh M, Sciacca G, Saint-Pierre M, Masnata M, Denis HL, et al. Shedding a new light on Huntington’s disease: how blood can both propagate and
ameliorate disease pathology. Mol Psychiatry. 2020:1-23. * Bu XL, Xiang Y, Jin WS, Wang J, Shen LL, Huang ZL, et al. Blood-derived amyloid-beta protein induces Alzheimer’s disease
pathologies. Mol Psychiatry. 2018;23:1948–56. Article PubMed Google Scholar * Sundaram SM, Garg P. The gut-brain axis in Parkinson’s disease: a focus on the transport of alpha-Synuclein.
Mov Disord. 2019;34:1479. Article PubMed Google Scholar * Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s
disease. Neurobiol Aging. 2003;24:197–211. Article PubMed Google Scholar * Brahic M, Bousset L, Bieri G, Melki R, Gitler AD. Axonal transport and secretion of fibrillar forms of
alpha-synuclein, Abeta42 peptide and HTTExon 1. Acta Neuropathol. 2016;131:539–48. Article CAS PubMed PubMed Central Google Scholar * Poudel GR, Harding IH, Egan GF,
Georgiou-Karistianis N. Network spread determines severity of degeneration and disconnection in Huntington’s disease. Hum Brain Mapp. 2019;40:4192–201. Article PubMed PubMed Central
Google Scholar * Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, et al. Exogenous alpha-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction
and neuron death. Neuron. 2011;72:57–71. Article CAS PubMed PubMed Central Google Scholar * Freundt EC, Maynard N, Clancy EK, Roy S, Bousset L, Sourigues Y, et al. Neuron-to-neuron
transmission of alpha-synuclein fibrils through axonal transport. Ann Neurol. 2012;72:517–24. Article CAS PubMed PubMed Central Google Scholar * Wu JW, Herman M, Liu L, Simoes S, Acker
CM, Figueroa H, et al. Small misfolded Tau species are internalized via bulk endocytosis and anterogradely and retrogradely transported in neurons. J Biol Chem. 2013;288:1856–70. Article
CAS PubMed Google Scholar * Maxan A, Sciacca G, Alpaugh M, Tao Z, Breger L, Dehay B, et al. Use of adeno-associated virus-mediated delivery of mutant huntingtin to study the spreading
capacity of the protein in mice and non-human primates. Neurobiol Dis. 2020;141:104951. Article CAS PubMed Google Scholar * Babcock DT, Ganetzky B. Transcellular spreading of huntingtin
aggregates in the Drosophila brain. Proc Natl Acad Sci USA. 2015;112:E5427–5433. Article CAS PubMed PubMed Central Google Scholar * Donnelly KM, DeLorenzo OR, Zaya AD, Pisano GE, Thu
WM, Luo L, et al. Phagocytic glia are obligatory intermediates in transmission of mutant huntingtin aggregates across neuronal synapses. Elife. 2020;9:e58499. Article CAS PubMed PubMed
Central Google Scholar * Masnata M, Sciacca G, Maxan A, Bousset L, Denis HL, Lauruol F, et al. Demonstration of prion-like properties of mutant huntingtin fibrils in both in vitro and in
vivo paradigms. Acta Neuropathol. 2019;137:981–1001. Article CAS PubMed PubMed Central Google Scholar * Pearce MMP, Spartz EJ, Hong W, Luo L, Kopito RR. Prion-like transmission of
neuronal huntingtin aggregates to phagocytic glia in the Drosophila brain. Nat Commun. 2015;6:6768. Article CAS PubMed Google Scholar * Gosset P, Maxan A, Alpaugh M, Breger L, Dehay B,
Tao Z, et al. Evidence for the spread of human-derived mutant huntingtin protein in mice and non-human primates. Neurobiol Dis. 2020;141:104941. Article CAS PubMed Google Scholar * Choi
I, Zhang Y, Seegobin SP, Pruvost M, Wang Q, Purtell K, et al. Microglia clear neuron-released alpha-synuclein via selective autophagy and prevent neurodegeneration. Nat Commun. 2020;11:1386.
Article CAS PubMed PubMed Central Google Scholar * Sung K, Jimenez-Sanchez M. Autophagy in Astrocytes and its Implications in Neurodegeneration. J Mol Biol. 2020;432:2605–21. Article
CAS PubMed Google Scholar * Mishra PS, Boutej H, Soucy G, Bareil C, Kumar S, Picher-Martel V, et al. Transmission of ALS pathogenesis by the cerebrospinal fluid. Acta Neuropathol Commun.
2020;8:65. Article CAS PubMed PubMed Central Google Scholar * Weiss A, Abramowski D, Bibel M, Bodner R, Chopra V, DiFiglia M, et al. Single-step detection of mutant huntingtin in animal
and human tissues: a bioassay for Huntington’s disease. Anal Biochem. 2009;395:8–15. Article CAS PubMed Google Scholar * Byrne LM, Rodrigues FB, Johnson EB, Wijeratne PA, De Vita E,
Alexander DC, et al. Evaluation of mutant huntingtin and neurofilament proteins as potential markers in Huntington’s disease. Sci Transl Med. 2018;10:eaat7108. Article PubMed Google
Scholar * Lee CYD, Wang N, Shen K, Stricos M, Langfelder P, Cheon KH, et al. Disease-related Huntingtin seeding activities in cerebrospinal fluids of Huntington’s disease patients. Sci Rep.
2020;10:20295. Article CAS PubMed PubMed Central Google Scholar * Caron NS, Banos R, Yanick C, Aly AE, Byrne LM, Smith ED, et al. Mutant Huntingtin Is Cleared from the Brain via Active
Mechanisms in Huntington Disease. J Neurosci. 2021;41:780–96. Article CAS PubMed PubMed Central Google Scholar * Sharma M, Subramaniam S. Rhes travels from cell to cell and transports
Huntington disease protein via TNT-like protrusion. J Cell Biol. 2019;218:1972–93. Article CAS PubMed PubMed Central Google Scholar * Costanzo M, Abounit S, Marzo L, Danckaert A,
Chamoun Z, Roux P, et al. Transfer of polyglutamine aggregates in neuronal cells occurs in tunneling nanotubes. J Cell Sci. 2013;126:3678–85. CAS PubMed Google Scholar * Raposo G,
Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. Article CAS PubMed PubMed Central Google Scholar * Gyorgy B, Szabo TG, Pasztoi
M, Pal Z, Misjak P, Aradi B, et al. Membrane vesicles, current state-of-the-art: emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. Article CAS PubMed PubMed
Central Google Scholar * S ELA, Mager I, Breakefield XO, Wood MJ. Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57. Article
Google Scholar * Wang Y, Balaji V, Kaniyappan S, Kruger L, Irsen S, Tepper K, et al. The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener. 2017;12:5. Article
PubMed PubMed Central Google Scholar * Vella LJ, Sharples RA, Lawson VA, Masters CL, Cappai R, Hill AF. Packaging of prions into exosomes is associated with a novel pathway of PrP
processing. J Pathol. 2007;211:582–90. Article CAS PubMed Google Scholar * Sardar Sinha M, Ansell-Schultz A, Civitelli L, Hildesjo C, Larsson M, Lannfelt L, et al. Alzheimer’s disease
pathology propagation by exosomes containing toxic amyloid-beta oligomers. Acta Neuropathol. 2018;136:41–56. Article CAS PubMed PubMed Central Google Scholar * Harischandra DS, Rokad D,
Neal ML, Ghaisas S, Manne S, Sarkar S, et al. Manganese promotes the aggregation and prion-like cell-to-cell exosomal transmission of alpha-synuclein. Sci Signal. 2019;12:eaau4543. Article
CAS PubMed PubMed Central Google Scholar * Wang JKT, Langfelder P, Horvath S, Palazzolo MJ. Exosomes and Homeostatic Synaptic Plasticity Are Linked to Each other and to Huntington’s,
Parkinson’s, and Other Neurodegenerative Diseases by Database-Enabled Analyses of Comprehensively Curated Datasets. Front Neurosci. 2017;11:149. Article PubMed PubMed Central Google
Scholar * Deng J, Koutras C, Donnelier J, Alshehri M, Fotouhi M, Girard M, et al. Neurons Export Extracellular Vesicles Enriched in Cysteine String Protein and Misfolded Protein Cargo. Sci
Rep. 2017;7:956. Article PubMed PubMed Central Google Scholar * Diaz-Hidalgo L, Altuntas S, Rossin F, D’Eletto M, Marsella C, Farrace MG, et al. Transglutaminase type 2-dependent
selective recruitment of proteins into exosomes under stressful cellular conditions. Biochim Biophys Acta. 2016;1863:2084–92. Article CAS PubMed Google Scholar * Denis HL,
Lamontagne-Proulx J, St-Amour I, Mason SL, Weiss A, Chouinard S, et al. Platelet-derived extracellular vesicles in Huntington’s disease. J Neurol. 2018;265:2704–12. Article CAS PubMed
Google Scholar * Heng MY, Duong DK, Albin RL, Tallaksen-Greene SJ, Hunter JM, Lesort MJ, et al. Early autophagic response in a novel knock-in model of Huntington disease. Hum Mol Genet.
2010;19:3702–20. Article CAS PubMed PubMed Central Google Scholar * Trajkovic K, Jeong H, Krainc D. Mutant Huntingtin Is Secreted via a Late Endosomal/Lysosomal Unconventional Secretory
Pathway. J Neurosci : Off J Soc Neurosci. 2017;37:9000–12. Article CAS Google Scholar * Waldvogel HJ, Kim EH, Tippett LJ, Vonsattel JP, Faull RL. The Neuropathology of Huntington’s
Disease. Curr Top Behav Neurosci. 2015;22:33–80. Article CAS PubMed Google Scholar * Jones DR, Delenclos M, Baine AT, DeTure M, Murray ME, Dickson DW, et al. Transmission of Soluble and
Insoluble alpha-Synuclein to Mice. J Neuropathol Exp Neurol. 2015;74:1158–69. CAS PubMed Google Scholar * Watts JC, Condello C, Stohr J, Oehler A, Lee J, DeArmond SJ, et al. Serial
propagation of distinct strains of Abeta prions from Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2014;111:10323–8. Article CAS PubMed PubMed Central Google Scholar * Ascari
LM, Rocha SC, Goncalves PB, Vieira T, Cordeiro Y. Challenges and Advances in Antemortem Diagnosis of Human Transmissible Spongiform Encephalopathies. Front Bioeng Biotechnol. 2020;8:585896.
Article PubMed PubMed Central Google Scholar * Van Den Berge N, Ferreira N, Mikkelsen TW, Alstrup AKO, Tamguney G, Karlsson P, et al. Ageing promotes pathological alpha-synuclein
propagation and autonomic dysfunction in wild-type rats. Brain. 2021;144:1853–68. Article Google Scholar * Wegmann S, Bennett RE, Delorme L, Robbins AB, Hu M, McKenzie D, et al.
Experimental evidence for the age dependence of tau protein spread in the brain. Sci Adv. 2019;5:eaaw6404. Article CAS PubMed PubMed Central Google Scholar * Moron-Oset J, Super T,
Esser J, Isaacs AM, Gronke S, Partridge L. Glycine-alanine dipeptide repeats spread rapidly in a repeat length- and age-dependent manner in the fly brain. Acta Neuropathol Commun.
2019;7:209. Article CAS PubMed PubMed Central Google Scholar * Courte J, Bousset L, Boxberg YV, Villard C, Melki R, Peyrin JM. The expression level of alpha-synuclein in different
neuronal populations is the primary determinant of its prion-like seeding. Sci Rep. 2020;10:4895. Article CAS PubMed PubMed Central Google Scholar * Alpaugh M, Cicchetti F. Huntington’s
disease: lessons from prion disorders. J Neurol. 2021;68:3493–504. Article Google Scholar * Manassero G, Guglielmotto M, Monteleone D, Vasciaveo V, Butenko O, Tamagno E, et al. Dual
Mechanism of Toxicity for Extracellular Injection of Tau Oligomers versus Monomers in Human Tau Mice. J Alzheimers Dis. 2017;59:743–51. Article CAS PubMed Google Scholar *
Masuda-Suzukake M, Nonaka T, Hosokawa M, Oikawa T, Arai T, Akiyama H, et al. Prion-like spreading of pathological alpha-synuclein in brain. Brain. 2013;136:1128–38. Article PubMed PubMed
Central Google Scholar * Pinotsi D, Michel CH, Buell AK, Laine RF, Mahou P, Dobson CM, et al. Nanoscopic insights into seeding mechanisms and toxicity of alpha-synuclein species in
neurons. Proc Natl Acad Sci USA. 2016;113:3815–9. Article CAS PubMed PubMed Central Google Scholar * Katzmarski N, Ziegler-Waldkirch S, Scheffler N, Witt C, Abou-Ajram C, Nuscher B, et
al. Abeta oligomers trigger and accelerate Abeta seeding. Brain Pathol. 2020;30:36–45. Article CAS PubMed Google Scholar * Saudou F, Humbert S. The Biology of Huntingtin. Neuron.
2016;89:910–26. Article CAS PubMed Google Scholar * Morozova OA, Gupta S, Colby DW. Prefibrillar huntingtin oligomers isolated from HD brain potently seed amyloid formation. FEBS Lett.
2015;589:1897–903. Article CAS PubMed Google Scholar * Pieri L, Madiona K, Bousset L, Melki R. Fibrillar alpha-synuclein and huntingtin exon 1 assemblies are toxic to the cells. Biophys
J. 2012;102:2894–905. Article CAS PubMed PubMed Central Google Scholar * Lau HHC, Ingelsson M, Watts JC. The existence of Abeta strains and their potential for driving phenotypic
heterogeneity in Alzheimer’s disease. Acta Neuropathol. 2020;142:17–39. Article PubMed Google Scholar * Sathasivam K, Neueder A, Gipson TA, Landles C, Benjamin AC, Bondulich MK, et al.
Aberrant splicing of HTT generates the pathogenic exon 1 protein in Huntington disease. Proc Natl Acad Sci USA. 2013;110:2366–70. Article CAS PubMed PubMed Central Google Scholar *
Barbaro BA, Lukacsovich T, Agrawal N, Burke J, Bornemann DJ, Purcell JM, et al. Comparative study of naturally occurring huntingtin fragments in Drosophila points to exon 1 as the most
pathogenic species in Huntington’s disease. Hum Mol Genet. 2015;24:913–25. Article CAS PubMed Google Scholar * Schindler F, Praedel N, Neuendorf N, Kunz S, Schnoegl S, Mason MA, et al.
Small, Seeding-Competent Huntingtin Fibrils Are Prominent Aggregate Species in Brains of zQ175 Huntington’s Disease Knock-in Mice. Front Neurosci. 2021;15:682172. Article PubMed PubMed
Central Google Scholar * Langbehn DR, Stout JC, Gregory S, Mills JA, Durr A, Leavitt BR, et al. Association of CAG Repeats With Long-term Progression in Huntington Disease. JAMA Neurol.
2019;76:1375–85. Article PubMed PubMed Central Google Scholar * Koshy BT, Zoghbi HY. The CAG/polyglutamine tract diseases: gene products and molecular pathogenesis. Brain Pathol.
1997;7:927–42. Article CAS PubMed Google Scholar * Scherzinger E, Sittler A, Schweiger K, Heiser V, Lurz R, Hasenbank R, et al. Self-assembly of polyglutamine-containing huntingtin
fragments into amyloid-like fibrils: implications for Huntington’s disease pathology. Proc Natl Acad Sci USA. 1999;96:4604–9. Article CAS PubMed PubMed Central Google Scholar * Kim DK,
Cho KW, Ahn WJ, Perez-Acuna D, Jeong H, Lee HJ, et al. Cell-to-cell Transmission of Polyglutamine Aggregates in C. elegans. Exp Neurobiol. 2017;26:321–8. Article PubMed PubMed Central
Google Scholar * de Rus Jacquet A, Denis HL, Cicchetti F, Alpaugh M. Current and future applications of induced pluripotent stem cell-based models to study pathological proteins in
neurodegenerative disorders. Mol Psychiatry. 2021;26:2685–706. Article PubMed PubMed Central Google Scholar * Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, et al. Ageing as
a risk factor for neurodegenerative disease. Nat Rev Neurol. 2019;15:565–81. Article PubMed Google Scholar * Scahill RI, Zeun P, Osborne-Crowley K, Johnson EB, Gregory S, Parker C, et al.
Biological and clinical characteristics of gene carriers far from predicted onset in the Huntington’s disease Young Adult Study (HD-YAS): a cross-sectional analysis. Lancet Neurol.
2020;19:502–12. Article CAS PubMed PubMed Central Google Scholar * Del Tredici K, Braak H. To stage, or not to stage. Curr Opin Neurobiol. 2020;61:10–22. Article PubMed Google Scholar
* Nitta A, Itoh A, Hasegawa T, Nabeshima T. beta-Amyloid protein-induced Alzheimer’s disease animal model. Neurosci Lett. 1994;170:63–66. Article CAS PubMed Google Scholar * Nakamura
S, Murayama N, Noshita T, Annoura H, Ohno T. Progressive brain dysfunction following intracerebroventricular infusion of beta(1-42)-amyloid peptide. Brain Res. 2001;912:128–36. Article CAS
PubMed Google Scholar * Ramsingh AI, Manley K, Rong Y, Reilly A, Messer A. Transcriptional dysregulation of inflammatory/immune pathways after active vaccination against Huntington’s
disease. Hum Mol Genet. 2015;24:6186–97. Article CAS PubMed PubMed Central Google Scholar * Miller TW, Shirley TL, Wolfgang WJ, Kang X, Messer A. DNA vaccination against mutant
huntingtin ameliorates the HDR6/2 diabetic phenotype. Mol Ther. 2003;7:572–9. Article CAS PubMed Google Scholar * Bartl S, Oueslati A, Southwell AL, Siddu A, Parth M, David LS, et al.
Inhibiting cellular uptake of mutant huntingtin using a monoclonal antibody: Implications for the treatment of Huntington’s disease. Neurobiol Dis. 2020;141:104943. Article CAS PubMed
Google Scholar * Bartl S, Xie Y, Potluri N, Gordon B, Willenberg A, Balazs K, et al. In vivo targeting and reduction of mtHTT protein by passive immunization with the monoclonal antibody
C6-17. _CHDI_2021;poster I09;36. * Aoyagi A, Condello C, Stohr J, Yue W, Rivera BM, Lee JC, et al. Abeta and tau prion-like activities decline with longevity in the Alzheimer’s disease human
brain. Sci Transl Med. 2019;11:eaat8462. Article CAS PubMed PubMed Central Google Scholar * Dujardin S, Commins C, Lathuiliere A, Beerepoot P, Fernandes AR, Kamath TV, et al. Tau
molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat Med. 2020;26:1256–63. Article CAS PubMed PubMed Central Google Scholar * Busch A, Engemann S, Lurz
R, Okazawa H, Lehrach H, Wanker EE. Mutant huntingtin promotes the fibrillogenesis of wild-type huntingtin: a potential mechanism for loss of huntingtin function in Huntington’s disease. J
Biol Chem. 2003;278:41452–61. Article CAS PubMed Google Scholar * Isas JM, Langen A, Isas MC, Pandey NK, Siemer AB. Formation and Structure of Wild Type Huntingtin Exon-1 Fibrils.
Biochemistry. 2017;56:3579–86. Article CAS PubMed Google Scholar * Shahnawaz M, Mukherjee A, Pritzkow S, Mendez N, Rabadia P, Liu X, et al. Discriminating alpha-synuclein strains in
Parkinson’s disease and multiple system atrophy. Nature. 2020;578:273–7. Article CAS PubMed PubMed Central Google Scholar * Nekooki-Machida Y, Kurosawa M, Nukina N, Ito K, Oda T, Tanaka
M. Distinct conformations of in vitro and in vivo amyloids of huntingtin-exon1 show different cytotoxicity. Proc Natl Acad Sci USA. 2009;106:9679–84. Article CAS PubMed PubMed Central
Google Scholar * Monckton DG. The Contribution of Somatic Expansion of the CAG Repeat to Symptomatic Development in Huntington’s Disease: A Historical Perspective. J Huntingt Dis.
2021;10:7–33. Article CAS Google Scholar * Neueder A, Landles C, Ghosh R, Howland D, Myers RH, Faull RLM, et al. The pathogenic exon 1 HTT protein is produced by incomplete splicing in
Huntington’s disease patients. Sci Rep. 2017;7:1307. Article PubMed PubMed Central Google Scholar * Rauch JN, Luna G, Guzman E, Audouard M, Challis C, Sibih YE, et al. LRP1 is a master
regulator of tau uptake and spread. Nature. 2020;580:381–5. Article CAS PubMed PubMed Central Google Scholar Download references ACKNOWLEDGEMENTS FC is a recipient of a Researcher Chair
from the Fonds de Recherche du Québec en Santé (FRQS) (grant # 28941) providing salary support and operating funds and receives funding from the Canadian Institutes of Health Research
(CIHR) to conduct her HD-related research (Grant # PJT-168865 and PJT-162164). MA was supported by post-doctoral fellowships from CIHR, FRQS and the Huntington Disease Society of America
(HDSA) during the course of the work described herein. HLD is supported by an FRQS doctoral research award. AUTHOR INFORMATION AUTHORS AND AFFILIATIONS * Centre de Recherche du CHU de Québec
- Université Laval, Axe Neurosciences, Québec, QC, G1V 4G2, Canada Melanie Alpaugh, Hélèna L. Denis & Francesca Cicchetti * Département de Psychiatrie & Neurosciences, Université
Laval, Québec, QC, G1V 0A6, Canada Melanie Alpaugh, Hélèna L. Denis & Francesca Cicchetti Authors * Melanie Alpaugh View author publications You can also search for this author inPubMed
Google Scholar * Hélèna L. Denis View author publications You can also search for this author inPubMed Google Scholar * Francesca Cicchetti View author publications You can also search for
this author inPubMed Google Scholar CONTRIBUTIONS MA conceptualization; roles/writing—original draft; writing—review and editing. HLD conceptualization; visualization; writing—review and
editing. FC conceptualization; writing—review and editing; supervision. CORRESPONDING AUTHOR Correspondence to Francesca Cicchetti. ETHICS DECLARATIONS COMPETING INTERESTS The authors
declare no competing interests. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Alpaugh, M., Denis, H.L. & Cicchetti, F. Prion-like properties of the mutant huntingtin protein in
living organisms: the evidence and the relevance. _Mol Psychiatry_ 27, 269–280 (2022). https://doi.org/10.1038/s41380-021-01350-4 Download citation * Published: 28 October 2021 * Issue Date:
January 2022 * DOI: https://doi.org/10.1038/s41380-021-01350-4 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get shareable link Sorry, a
shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative







