- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
ABSTRACT DNA methylation patterns at specific cytosine-phosphate-guanine (CpG) sites predictably change with age and can be used to derive “epigenetic age”, an indicator of biological age,
as opposed to merely chronological age. A relatively new estimator, called “DNAm GrimAge”, is notable for its superior predictive ability in older populations regarding numerous age-related
metrics like time-to-death, time-to-coronary heart disease, and time-to-cancer. PTSD is associated with premature mortality and frequently has comorbid physical illnesses suggestive of
accelerated biological aging. This is the first study to assess DNAm GrimAge in PTSD patients. We investigated the acceleration of GrimAge relative to chronological age, denoted
“AgeAccelGrim” in combat trauma-exposed male veterans with and without PTSD using cross-sectional and longitudinal data from two independent well-characterized veteran cohorts. In both
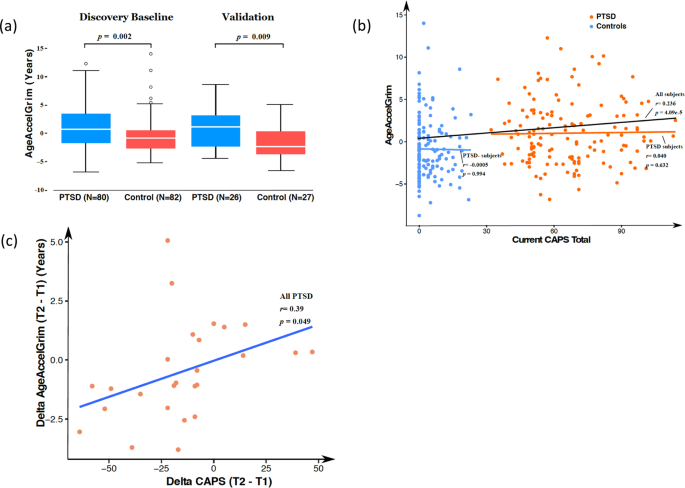
cohorts, AgeAccelGrim was significantly higher in the PTSD group compared to the control group (_N_ = 162, 1.26 vs −0.57, _p_ = 0.001 and _N_ = 53, 0.93 vs −1.60 Years, _p_ = 0.008),
suggesting accelerated biological aging in both cohorts with PTSD. In 3-year follow-up study of individuals initially diagnosed with PTSD (_N_ = 26), changes in PTSD symptom severity were
correlated with AgeAccelGrim changes (_r_ = 0.39, _p_ = 0.049). In addition, the loss of CD28 cell surface markers on CD8 + T cells, an indicator of T-cell senescence/exhaustion that is
associated with biological aging, was positively correlated with AgeAccelGrim, suggesting an immunological contribution to the accelerated biological aging. Overall, our findings delineate
cellular correlates of biological aging in combat-related PTSD, which may help explain the increased medical morbidity and mortality seen in this disease. Access through your institution Buy
or subscribe This is a preview of subscription content, access via your institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and
online access $259.00 per year only $21.58 per issue Learn more Buy this article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes
which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in * Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY
OTHERS DNA METHYLATION GRIMAGE ACCELERATION IN US MILITARY VETERANS WITH PTSD Article 01 February 2023 PTSD IS ASSOCIATED WITH ACCELERATED TRANSCRIPTIONAL AGING IN WORLD TRADE CENTER
RESPONDERS Article Open access 24 May 2021 POSTTRAUMATIC STRESS DISORDER, TRAUMA, AND ACCELERATED BIOLOGICAL AGING AMONG POST-9/11 VETERANS Article Open access 06 January 2024 DATA
AVAILABILITY All datasets for selected cohorts are available with permission through the SysBioCube, at https://sysbiocube-abcc.ncifcrf.gov. CHANGE HISTORY * _ 10 JULY 2020 A Correction to
this paper has been published: https://doi.org/10.1038/s41380-020-0837-y _ REFERENCES * McLeay SC, Harvey W, Romaniuk NM, Crawford D, Colquhoun DM, Young R, et al. Physical comorbidities of
post-traumatic stress disorder in Australian Vietnam War veterans. Med J Aust. 2017;206:251–7. Article Google Scholar * Wolf EJ, Schnurr PP. Posttraumatic stress disorder-related
cardiovascular disease and accelerated cellular aging. Psychiatr Ann. 2016;46:527–32. Article Google Scholar * Cohen BE, Marmar C, Ren L, Bertenthal D, Seal KH. Association of
cardiovascular risk factors with mental health diagnoses in Iraq and Afghanistan war veterans using VA health care. JAMA. 2009;302:489–92. Article CAS Google Scholar * Schlenger WE, Corry
NH, Williams CS, Kulka RA, Mulvaney-Day N, DeBakey S, et al. A prospective study of mortality and trauma-related risk factors among a nationally representative sample of vietnam veterans.
Am J Epidemiol. 2015;182:980–90. PubMed Google Scholar * Lohr JB, Palmer BW, Eidt CA, Aailaboyina S, Mausbach BT, Wolkowitz OM, et al. Is post-traumatic stress disorder associated with
premature senescence? A review of the literature. Am J Geriatr Psychiatry. 2015;23:709–25. Article Google Scholar * Wolf EJ, Morrison FG. Traumatic stress and accelerated cellular aging:
from epigenetics to cardiometabolic disease. Curr psychiatry Rep. 2017;19:75. Article Google Scholar * Darrow SM, Verhoeven JE, Révész D, Lindqvist D, Penninx BWJH, Delucchi KL, et al. The
Association between psychiatric disorders and telomere length: a meta-analysis involving 14,827 persons. Psychosom Med. 2016;78:776–87. Article Google Scholar * Aiello AE, Dowd JB,
Jayabalasingham B, Feinstein L, Uddin M, Simanek AM, et al. PTSD is associated with an increase in aged T cell phenotypes in adults living in Detroit. Psychoneuroendocrinology.
2016;67:133–41. Article CAS Google Scholar * Jylhävä J, Pedersen NL, Hägg S. Biological age predictors. EBioMed. 2017;21:29–36. Article Google Scholar * Weng N-P, Akbar AN, Goronzy J.
CD28(-) T cells: their role in the age-associated decline of immune function. Trends Immunol. 2009;30:306–12. Article CAS Google Scholar * Wolf EJ, Maniates H, Nugent N, Maihofer AX,
Armstrong D, Ratanatharathorn A, et al. Traumatic stress and accelerated DNA methylation age: a meta-analysis. Psychoneuroendocrinology. 2018;92:123–34. Article CAS Google Scholar *
Hannum G, Guinney J, Zhao L, Zhang L, Hughes G, Sadda S, et al. Genome-wide methylation profiles reveal quantitative views of human aging rates. Mol Cell. 2013;49:359–67. Article CAS
Google Scholar * Horvath S. DNA methylation age of human tissues and cell types. Genome Biol. 2013;14:R115–R115. Article Google Scholar * Levine ME, Lu AT, Quach A, Chen BH, Assimes TL,
Bandinelli S, et al. An epigenetic biomarker of aging for lifespan and healthspan. Aging. 2018;10:573–91. Article Google Scholar * Lu AT, Quach A, Wilson JG, Reiner AP, Aviv A, Raj K, et
al. DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging. 2019;11:303–27. Article CAS Google Scholar * Horvath S, Oshima J, Martin GM, Lu AT, Quach A, Cohen H, et al.
Epigenetic clock for skin and blood cells applied to Hutchinson Gilford Progeria Syndrome and ex vivo studies. Aging. 2018;10:1758–75. Article CAS Google Scholar * Marioni RE, Shah S,
McRae AF, Chen BH, Colicino E, Harris SE, et al. DNA methylation age of blood predicts all-cause mortality in later life. Genome Biol. 2015;16:25. Article Google Scholar * Dean KR,
Hammamieh R, Mellon SH, Abu-Amara D, Flory JD, Guffanti G, et al. Multi-omic biomarker identification and validation for diagnosing warzone-related post-traumatic stress disorder. Mol
Psychiatry. 2019;1–13. * Flory JD, Yehuda R. Comorbidity between post-traumatic stress disorder and major depressive disorder: alternative explanations and treatment considerations.
Dialogues Clin Neurosci. 2015;17:141–50. Article Google Scholar * Houseman EA, Accomando WP, Koestler DC, Christensen BC, Marsit CJ, Nelson HH, et al. DNA methylation arrays as surrogate
measures of cell mixture distribution. BMC Bioinforma. 2012;13:86. Article Google Scholar * Chen X, Liu Q, Xiang AP. CD8+CD28- T cells: not only age-related cells but a subset of
regulatory T cells. Cell Mol Immunol. 2018;15:734–6. Article CAS Google Scholar * Verhoeven JE, Yang R, Wolkowitz OM, Bersani FS, Lindqvist D, Mellon SH, et al. Epigenetic age in male
combat-exposed war veterans: associations with posttraumatic stress disorder status. Mol Neuropsychiatry. 2018;4:90–99. Article CAS Google Scholar * Kievit R, Frankenhuis WE, Waldorp L,
Borsboom D. Simpson’s paradox in psychological science: a practical guide. Front Psychol. 2013;4:513. Article Google Scholar * Wolf EJ, Bovin MJ, Green JD, Mitchell KS, Stoop TB, Barretto
KM, et al. Longitudinal associations between post-traumatic stress disorder and metabolic syndrome severity. Psychological Med. 2016;46:2215–26. Article CAS Google Scholar * Ahmadi N,
Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am J Cardiol. 2011;108:29–33. Article Google Scholar *
Yaffe K, Vittinghoff E, Lindquist K, Barnes D, Covinsky KE, Neylan T, et al. Posttraumatic stress disorder and risk of dementia among US veterans. Arch Gen Psychiatry. 2010;67:608–13.
Article Google Scholar * Wolf EJ, Logue MW, Stoop TB, Schichman SA, Stone A, Sadeh N, et al. Accelerated DNA methylation age: associations with PTSD and mortality. Psychosomatic Med.
2017;80:42–8. Article Google Scholar * Chou JP, Effros RB. T cell replicative senescence in human aging. Curr Pharm Des. 2013;19:1680–98. CAS PubMed PubMed Central Google Scholar *
Cesari M, Pahor M, Incalzi RA. REVIEW: plasminogen activator inhibitor-1 (PAI-1): a key factor linking fibrinolysis and age-related subclinical and clinical conditions. Cardiovascular
Therapeutics. 2010;28:e72–e91. Article CAS Google Scholar * Ferguson TW, Komenda P, Tangri N. Cystatin C as a biomarker for estimating glomerular filtration rate. Curr Opin Nephrol
Hypertension. 2015;24:295–300. Article CAS Google Scholar * Levin A, Lan JH. Cystatin C and cardiovascular disease. J Am Coll Cardiol. 2016;68:946. Article Google Scholar * Ashutosh,
Chao C, Borgmann K, Brew K, Ghorpade A. Tissue inhibitor of metalloproteinases-1 protects human neurons from staurosporine and HIV-1-induced apoptosis: mechanisms and relevance to
HIV-1-associated dementia. _Cell Death Dis._ 2012; 3:e332. * Song G, Xu S, Zhang H, Wang Y, Xiao C, Jiang T, et al. TIMP1 is a prognostic marker for the progression and metastasis of colon
cancer through FAK-PI3K/AKT and MAPK pathway. J Exp Clin Cancer Res. 2016;35:148. Article Google Scholar * Rivera S, Tremblay E, Timsit S, Canals O, Ben-Ari Y, Khrestchatisky M. Tissue
inhibitor of metalloproteinases-1 (TIMP-1) is differentially induced in neurons and astrocytes after seizures: evidence for developmental, immediate early gene, and lesion response. J
Neurosci. 1997;17:4223. Article CAS Google Scholar * Mills JA, Beach SRH, Dogan M, Simons RL, Gibbons FX, Long JD, et al. A direct comparison of the relationship of epigenetic aging and
epigenetic substance consumption markers to mortality in the framingham heart study. Genes (Basel). 2019;10:51. Article Google Scholar * Stejskalova L, Dvorak Z, Pavek P. Endogenous and
exogenous ligands of aryl hydrocarbon receptor: current state of art. Curr Drug Metab. 2011;12:198–212. Article CAS Google Scholar * Hammamieh R, Chakraborty N, Gautam A, Muhie S, Yang R,
Donohue D, et al. Whole-genome DNA methylation status associated with clinical PTSD measures of OIF/OEF veterans. Transl Psychiatry. 2017;7:e1169. Article CAS Google Scholar * Triche TJ
Jr, Weisenberger DJ, Van Den Berg D, Laird PW, Siegmund KD. Low-level processing of Illumina Infinium DNA methylation beadarrays. Nucleic Acids Res. 2013;41:e90–e90. Article CAS Google
Scholar * Blom G. Statistical estimates and transformed beta-variables. New York, Stockholm: J. Wiley & sons; Almqvist & Wiksell; 1958. Google Scholar * Quach A, Levine ME, Tanaka
T, Lu AT, Chen BH, Ferrucci L, et al. Epigenetic clock analysis of diet, exercise, education, and lifestyle factors. Aging. 2017;9:419–46. Article CAS Google Scholar Download references
ACKNOWLEDGEMENTS This work was supported by funding from the U.S. Army Research Office, through award numbers W911NF-13-1-0376, W911NF-17-2-0086, W911NF-18-2-0056, by the Army Research
Laboratory under grant number W911NF-17-1-0069, and from the U.S. Department of Defense under W81XWH-10-1-0021, W81XWH-09-2-0044, and W81XWH-14-1-0043. Additional members of the PTSD Systems
Biology Consortium are acknowledged in Supplementary information Appendix. AUTHOR INFORMATION Author notes * These authors jointly supervised this work: Rasha Hammamieh, Synthia H. Mellon,
Owen M. Wolkowitz AUTHORS AND AFFILIATIONS * Medical Readiness Systems Biology, Walter Reed Army Institute of Research, Silver Spring, MD, USA Ruoting Yang, Aarti Gautam, Marti Jett &
Rasha Hammamieh * Weill Institute for Neurosciences and Department of Psychiatry, University of California San Francisco (UCSF) School of Medicine, San Francisco, CA, USA Gwyneth W. Y. Wu,
Victor I. Reus & Owen M. Wolkowitz * Amsterdam UMC, Vrije Universiteit Amsterdam, Department of Psychiatry, Amsterdam Public Health Research Institute, Amsterdam, The Netherlands Josine
E. Verhoeven * Institute of Behavioral Science in Medicine & Department of Psychiatry, Yonsei University College of Medicine, Seoul, South Korea Jee In Kang * James J Peters VA Medical
Center, Bronx, NY, USA Janine D. Flory & Rachel Yehuda * Icahn School of Medicine at Mount Sinai, New York, NY, USA Janine D. Flory & Rachel Yehuda * Department of Psychiatry, New
York University School of Medicine, New York, NY, USA Duna Abu-Amara & Charles R. Marmar * Institute for Systems Biology, Seattle, WA, USA Leroy Hood * John A. Paulson School of
Engineering & Applied Sciences, Harvard University, Cambridge, MA, USA Francis J. Doyle III * Department of OB-GYN and Reproductive Sciences, UCSF School of Medicine, San Francisco, CA,
USA Synthia H. Mellon Authors * Ruoting Yang View author publications You can also search for this author inPubMed Google Scholar * Gwyneth W. Y. Wu View author publications You can also
search for this author inPubMed Google Scholar * Josine E. Verhoeven View author publications You can also search for this author inPubMed Google Scholar * Aarti Gautam View author
publications You can also search for this author inPubMed Google Scholar * Victor I. Reus View author publications You can also search for this author inPubMed Google Scholar * Jee In Kang
View author publications You can also search for this author inPubMed Google Scholar * Janine D. Flory View author publications You can also search for this author inPubMed Google Scholar *
Duna Abu-Amara View author publications You can also search for this author inPubMed Google Scholar * Leroy Hood View author publications You can also search for this author inPubMed Google
Scholar * Francis J. Doyle III View author publications You can also search for this author inPubMed Google Scholar * Rachel Yehuda View author publications You can also search for this
author inPubMed Google Scholar * Charles R. Marmar View author publications You can also search for this author inPubMed Google Scholar * Marti Jett View author publications You can also
search for this author inPubMed Google Scholar * Rasha Hammamieh View author publications You can also search for this author inPubMed Google Scholar * Synthia H. Mellon View author
publications You can also search for this author inPubMed Google Scholar * Owen M. Wolkowitz View author publications You can also search for this author inPubMed Google Scholar CONSORTIA
PTSD SYSTEMS BIOLOGY CONSORTIUM CONTRIBUTIONS RY, GWYW, RH, OMW, and SHM designed research; all authors performed research and proofed or contributed to the manuscript; RY and GWYW analyzed
data; and RY, GWYW, SHM, and OMW wrote the paper. CORRESPONDING AUTHOR Correspondence to Ruoting Yang. ETHICS DECLARATIONS CONFLICT OF INTEREST The authors declare that they have no conflict
of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations. Members of the
PTSD Systems Biology Consortium are listed in Supplementary information. SUPPLEMENTARY INFORMATION SUPPLEMENTAL MATERIAL SUPPORTING DATASET 1 SUPPORTING DATASET 2 RIGHTS AND PERMISSIONS
Reprints and permissions ABOUT THIS ARTICLE CITE THIS ARTICLE Yang, R., Wu, G.W.Y., Verhoeven, J.E. _et al._ A DNA methylation clock associated with age-related illnesses and mortality is
accelerated in men with combat PTSD. _Mol Psychiatry_ 26, 4999–5009 (2021). https://doi.org/10.1038/s41380-020-0755-z Download citation * Received: 29 January 2020 * Revised: 20 March 2020 *
Accepted: 23 April 2020 * Published: 07 May 2020 * Issue Date: September 2021 * DOI: https://doi.org/10.1038/s41380-020-0755-z SHARE THIS ARTICLE Anyone you share the following link with
will be able to read this content: Get shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt
content-sharing initiative





