- Select a language for the TTS:
- UK English Female
- UK English Male
- US English Female
- US English Male
- Australian Female
- Australian Male
- Language selected: (auto detect) - EN
Play all audios:
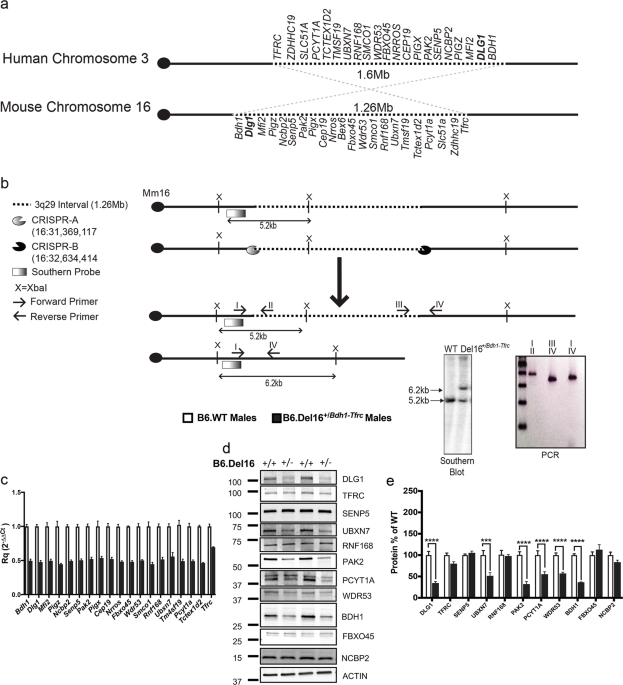
ABSTRACT The 3q29 deletion confers increased risk for neuropsychiatric phenotypes including intellectual disability, autism spectrum disorder, generalized anxiety disorder, and a >40-fold
increased risk for schizophrenia. To investigate consequences of the 3q29 deletion in an experimental system, we used CRISPR/Cas9 technology to introduce a heterozygous deletion into the
syntenic interval on C57BL/6 mouse chromosome 16. mRNA abundance for 20 of the 21 genes in the interval was reduced by ~50%, while protein levels were reduced for only a subset of these,
suggesting a compensatory mechanism. Mice harboring the deletion manifested behavioral impairments in multiple domains including social interaction, cognitive function, acoustic startle, and
amphetamine sensitivity, with some sex-dependent manifestations. In addition, 3q29 deletion mice showed reduced body weight throughout development consistent with the phenotype of 3q29
deletion syndrome patients. Of the genes within the interval, _DLG1_ has been hypothesized as a contributor to the neuropsychiatric phenotypes. However, we show that _Dlg1__+/-_ mice did not
exhibit the behavioral deficits seen in mice harboring the full 3q29 deletion. These data demonstrate the following: the 3q29 deletion mice are a valuable experimental system that can be
used to interrogate the biology of 3q29 deletion syndrome; behavioral manifestations of the 3q29 deletion may have sex-dependent effects; and mouse-specific behavior phenotypes associated
with the 3q29 deletion are not solely due to haploinsufficiency of _Dlg1_. Access through your institution Buy or subscribe This is a preview of subscription content, access via your
institution ACCESS OPTIONS Access through your institution Subscribe to this journal Receive 12 print issues and online access $259.00 per year only $21.58 per issue Learn more Buy this
article * Purchase on SpringerLink * Instant access to full article PDF Buy now Prices may be subject to local taxes which are calculated during checkout ADDITIONAL ACCESS OPTIONS: * Log in
* Learn about institutional subscriptions * Read our FAQs * Contact customer support SIMILAR CONTENT BEING VIEWED BY OTHERS COMPARISON OF MOUSE MODELS REVEALS A MOLECULAR DISTINCTION BETWEEN
PSYCHOTIC ILLNESS IN PWS AND SCHIZOPHRENIA Article Open access 20 August 2021 A GENETICS-FIRST APPROACH TO UNDERSTANDING AUTISM AND SCHIZOPHRENIA SPECTRUM DISORDERS: THE 22Q11.2 DELETION
SYNDROME Article Open access 03 October 2022 DISSECTING 16P11.2 HEMI-DELETION TO STUDY SEX-SPECIFIC STRIATAL PHENOTYPES OF NEURODEVELOPMENTAL DISORDERS Article 26 January 2024 REFERENCES *
Ballif BC, Theisen A, Coppinger J, Gowans GC, Hersh JH, Madan-Khetarpal S, et al. Expanding the clinical phenotype of the 3q29 microdeletion syndrome and characterization of the reciprocal
microduplication. Mol Cytogenet. 2008;1:8. Article Google Scholar * Glassford MR, Rosenfeld JA, Freedman AA, Zwick ME, Mulle JG, Unique Rare Chromosome Disorder Support G. Novel features
of 3q29 deletion syndrome: results from the 3q29 registry. Am J Med Genet A. 2016;170A:999–1006. Article Google Scholar * Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W,
Greer DS, et al. Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet. 2017;49:27–35. Article CAS Google Scholar * Mulle JG, Dodd
AF, McGrath JA, Wolyniec PS, Mitchell AA, Shetty AC, et al. Microdeletions of 3q29 confer high risk for schizophrenia. Am J Hum Genet. 2010;87:229–36. Article CAS Google Scholar * Mulle
JG, Gambello MJ, Cook EH, Rutkowski TP, Glassford M. 3q29 recurrent deletion. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al., editors. GeneReviews((R)).
Seattle, WA; University of Washington, Seattle; 1993–2019. Available from: https://www.ncbi.nlm.nih.gov/books/NBK385289/. * Sanders SJ, He X, Willsey AJ, Ercan-Sencicek AG, Samocha KE, Cicek
AE, et al. Insights into autism spectrum disorder genomic architecture and biology from 71 risk loci. Neuron. 2015;87:1215–33. Article CAS Google Scholar * Willatt L, Cox J, Barber J,
Cabanas ED, Collins A, Donnai D, et al. 3q29 microdeletion syndrome: clinical and molecular characterization of a new syndrome. Am J Hum Genet. 2005;77:154–60. Article CAS Google Scholar
* Grozeva D, Conrad DF, Barnes CP, Hurles M, Owen MJ, O’Donovan MC, et al. Independent estimation of the frequency of rare CNVs in the UK population confirms their role in schizophrenia.
Schizophr Res. 2012;135:1–7. Article Google Scholar * Mulle JG. The 3q29 deletion confers >40-fold increase in risk for schizophrenia. Mol Psychiatry. 2015;20:1028–9. Article CAS
Google Scholar * Korablev AN, Serova IA, Serov OL. Generation of megabase-scale deletions, inversions and duplications involving the Contactin-6 gene in mice by CRISPR/Cas9 technology. BMC
Genet. 2017;18(Suppl 1):112. Article Google Scholar * Carroll LS, Williams HJ, Walters J, Kirov G, O’Donovan MC, Owen MJ. Mutation screening of the 3q29 microdeletion syndrome candidate
genes DLG1 and PAK2 in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2011;156B:844–9. Article CAS Google Scholar * Rutkowski TP, Schroeder JP, Gafford GM, Warren ST, Weinshenker
D, Caspary T, et al. Unraveling the genetic architecture of copy number variants associated with schizophrenia and other neuropsychiatric disorders. J Neurosci Res. 2017;95:1144–60. Article
CAS Google Scholar * Leonard AS, Davare MA, Horne MC, Garner CC, Hell JW. SAP97 is associated with the alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor GluR1 subunit. J
Biol Chem. 1998;273:19518–24. Article CAS Google Scholar * Purcell SM, Moran JL, Fromer M, Ruderfer D, Solovieff N, Roussos P, et al. A polygenic burden of rare disruptive mutations in
schizophrenia. Nature. 2014;506:185–90. Article CAS Google Scholar * Uezato A, Kimura-Sato J, Yamamoto N, Iijima Y, Kunugi H, Nishikawa T. Further evidence for a male-selective genetic
association of synapse-associated protein 97 (SAP97) gene with schizophrenia. Behav Brain Funct. 2012;8:2. Article CAS Google Scholar * Toyooka K, Iritani S, Makifuchi T, Shirakawa O,
Kitamura N, Maeda K, et al. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J Neurochem. 2002;83:797–806. Article CAS Google
Scholar * Gupta P, Uner OE, Nayak S, Grant GR, Kalb RG. SAP97 regulates behavior and expression of schizophrenia risk enriched gene sets in mouse hippocampus. PLoS ONE. 2018;13:e0200477.
Article Google Scholar * Wang Y, Zeng C, Li J, Zhou Z, Ju X, Xia S, et al. PAK2 haploinsufficiency results in synaptic cytoskeleton impairment and autism-related behavior. Cell Rep.
2018;24:2029–41. Article CAS Google Scholar * Grice SJ, Liu JL, Webber C. Synergistic interactions between _Drosophila_ orthologues of genes spanned by de novo human CNVs support
multiple-hit models of autism. PLoS Genet. 2015;11:e1004998. Article Google Scholar * Mahoney ZX, Sammut B, Xavier RJ, Cunningham J, Go G, Brim KL, et al. Discs-large homolog 1 regulates
smooth muscle orientation in the mouse ureter. Proc Natl Acad Sci USA. 2006;103:19872–7. Article CAS Google Scholar * Yang M, Silverman JL, Crawley JN. Automated three-chambered social
approach task for mice. Curr Protoc Neurosci. 2011;Chapter 8: Unit 8 26. * Chalermpalanupap T, Schroeder JP, Rorabaugh JM, Liles LC, Lah JJ, Levey AI, et al. Locus coeruleus ablation
exacerbates cognitive deficits, neuropathology, and lethality in P301S tau transgenic mice. J Neurosci. 2018;38:74–92. Article CAS Google Scholar * Weinshenker D, Miller NS, Blizinsky K,
Laughlin ML, Palmiter RD. Mice with chronic norepinephrine deficiency resemble amphetamine-sensitized animals. Proc Natl Acad Sci USA. 2002;99:13873–7. Article CAS Google Scholar *
R-CoreTeam. A language and environment for statistical computing. Vienna, Austria: R foundation for Statistical Computing; 2017. http://www.R-project.org. * Bates D, Machler M, Bolker BM,
Walker SC. Fitting linear mixed-effects models using lme4. J Stat Softw. 2015;67:1–48. Article Google Scholar * Kuznetsova A, Brockhoff PB, Christensen RHB. lmerTest package: tests in
linear mixed effects models. J Stat Softw. 2017;82:1–26. Article Google Scholar * Cox DM, Butler MG. A clinical case report and literature review of the 3q29 microdeletion syndrome. Clin
Dysmorphol. 2015;24:89–94. Article Google Scholar * Nielsen J, Fejgin K, Sotty F, Nielsen V, Mork A, Christoffersen CT, et al. A mouse model of the schizophrenia-associated 1q21.1
microdeletion syndrome exhibits altered mesolimbic dopamine transmission. Transl Psychiatry. 2017;7:1261. Article Google Scholar * Takahashi H, Nakamura T, Kim J, Kikuchi H, Nakahachi T,
Ishitobi M, et al. Acoustic hyper-reactivity and negatively skewed locomotor activity in children with autism spectrum disorders: an exploratory study. Front Psychiatry. 2018;9:355. Article
Google Scholar * Kesby JP, Eyles DW, McGrath JJ, Scott JG. Dopamine, psychosis and schizophrenia: the widening gap between basic and clinical neuroscience. Transl Psychiatry. 2018;8:30.
Article CAS Google Scholar * Paval D. A dopamine hypothesis of autism spectrum disorder. Dev Neurosci. 2017;39:355–60. Article CAS Google Scholar * Kidd JM, Cooper GM, Donahue WF,
Hayden HS, Sampas N, Graves T, et al. Mapping and sequencing of structural variation from eight human genomes. Nature. 2008;453:56–64. Article CAS Google Scholar * Logue SF, Paylor R,
Wehner JM. Hippocampal lesions cause learning deficits in inbred mice in the Morris water maze and conditioned-fear task. Behav Neurosci. 1997;111:104–13. Article CAS Google Scholar *
Morris RG, Hagan JJ, Rawlins JN. Allocentric spatial learning by hippocampectomised rats: a further test of the “spatial mapping” and “working memory” theories of hippocampal function. Q J
Exp Psychol B. 1986;38:365–95. CAS PubMed Google Scholar * Baez-Mendoza R, Schultz W. The role of the striatum in social behavior. Front Neurosci. 2013;7:233. Article Google Scholar *
Koch M. The neurobiology of startle. Prog Neurobiol. 1999;59:107–28. Article CAS Google Scholar * Murphy MM, Lindsey Burrell T, Cubells JF, Espana RA, Gambello MJ, Goines KCB, et al.
Study protocol for The Emory 3q29 Project: evaluation of neurodevelopmental, psychiatric, and medical symptoms in 3q29 deletion syndrome. BMC Psychiatry. 2018;18:183. Article Google Scholar
* Halladay AK, Bishop S, Constantino JN, Daniels AM, Koenig K, Palmer K, et al. Sex and gender differences in autism spectrum disorder: summarizing evidence gaps and identifying emerging
areas of priority. Mol Autism. 2015;6:36. Article Google Scholar * Kirkovski M, Enticott PG, Fitzgerald PB. A review of the role of female gender in autism spectrum disorders. J Autism Dev
Disord. 2013;43:2584–603. Article Google Scholar * Lai MC, Lombardo MV, Baron-Cohen S. Autism. Lancet. 2014;383:896–910. Article Google Scholar * Prendergast BJ, Onishi KG, Zucker I.
Female mice liberated for inclusion in neuroscience and biomedical research. Neurosci Biobehav Rev. 2014;40:1–5. Article Google Scholar Download references ACKNOWLEDGEMENTS The work was
supported by R01GM097331 (TC, DW, and STW), R56MH116994 (TC, JGM, DW, and STW) and R01MH110701 along with funds from the Department of Human Genetics at Emory. This study was supported in
part by the Mouse Transgenic and Gene Targeting Core (TMF) and the Rodent Behavioral Core, which are subsidized by the Emory University School of Medicine and are part of the Emory
Integrated Core Facilities. Additional support was provided by the Georgia Clinical and Translational Science Alliance of the National Institutes of Health under Award Number UL1TR002378.
The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. We are grateful to Dr. Jeffrey Miner,
Washington University in St Louis, for providing _Dlg1_ mutant mice. This study was also supported by the Emory Winship Research Pathology Core Lab. AUTHOR CONTRIBUTIONS GJB, TC, JGM, STW,
and DW designed the research. TPR, RHP, and RMP performed research with help from GMG, SMG, UAK, RMP, TM, and JPS. MPE, TPR, and RMP analyzed data. TC, JGM, TPR, and DW wrote the manuscript.
All authors provided edits and approved final manuscript. AUTHOR INFORMATION Author notes * These authors contributed equally: David Weinshenker, Tamara Caspary, Jennifer Gladys Mulle
AUTHORS AND AFFILIATIONS * Department of Human Genetics, Emory University School of Medicine, Atlanta, GA, 30322, USA Timothy P. Rutkowski, Rebecca M. Pollak, Stephanie M. Grewenow,
Georgette M. Gafford, Tamika Malone, Uswa A. Khan, Jason P. Schroeder, Michael P. Epstein, Stephen T. Warren, David Weinshenker, Tamara Caspary & Jennifer Gladys Mulle * Department of
Cell Biology, Emory University School of Medicine, Atlanta, GA, 30322, USA Ryan H. Purcell & Gary J. Bassell Authors * Timothy P. Rutkowski View author publications You can also search
for this author inPubMed Google Scholar * Ryan H. Purcell View author publications You can also search for this author inPubMed Google Scholar * Rebecca M. Pollak View author publications
You can also search for this author inPubMed Google Scholar * Stephanie M. Grewenow View author publications You can also search for this author inPubMed Google Scholar * Georgette M.
Gafford View author publications You can also search for this author inPubMed Google Scholar * Tamika Malone View author publications You can also search for this author inPubMed Google
Scholar * Uswa A. Khan View author publications You can also search for this author inPubMed Google Scholar * Jason P. Schroeder View author publications You can also search for this author
inPubMed Google Scholar * Michael P. Epstein View author publications You can also search for this author inPubMed Google Scholar * Gary J. Bassell View author publications You can also
search for this author inPubMed Google Scholar * Stephen T. Warren View author publications You can also search for this author inPubMed Google Scholar * David Weinshenker View author
publications You can also search for this author inPubMed Google Scholar * Tamara Caspary View author publications You can also search for this author inPubMed Google Scholar * Jennifer
Gladys Mulle View author publications You can also search for this author inPubMed Google Scholar CORRESPONDING AUTHOR Correspondence to Jennifer Gladys Mulle. ETHICS DECLARATIONS CONFLICT
OF INTEREST The authors declare that they have no conflict of interest. ADDITIONAL INFORMATION PUBLISHER’S NOTE: Springer Nature remains neutral with regard to jurisdictional claims in
published maps and institutional affiliations. SUPPLEMENTARY INFORMATION B6.3Q29 SUPPLEMENTAL MATERIALS SUPPLEMENTAL FIGURE 1 SUPPLEMENTAL FIGURE 2 SUPPLEMENTAL FIGURE 3 SUPPLEMENTAL FIGURE
4 SUPPLEMENTAL FIGURE 5 SUPPLEMENTAL FIGURE 6 SUPPLEMENTAL FIGURE 7 SUPPLEMENTAL FIGURE 8 SUPPLEMENTAL FIGURE 9 RIGHTS AND PERMISSIONS Reprints and permissions ABOUT THIS ARTICLE CITE THIS
ARTICLE Rutkowski, T.P., Purcell, R.H., Pollak, R.M. _et al._ Behavioral changes and growth deficits in a CRISPR engineered mouse model of the schizophrenia-associated 3q29 deletion. _Mol
Psychiatry_ 26, 772–783 (2021). https://doi.org/10.1038/s41380-019-0413-5 Download citation * Received: 26 November 2018 * Revised: 13 February 2019 * Accepted: 18 March 2019 * Published: 11
April 2019 * Issue Date: March 2021 * DOI: https://doi.org/10.1038/s41380-019-0413-5 SHARE THIS ARTICLE Anyone you share the following link with will be able to read this content: Get
shareable link Sorry, a shareable link is not currently available for this article. Copy to clipboard Provided by the Springer Nature SharedIt content-sharing initiative